Mind the Gap: Model-Based Switching from Selatogrel to Maintenance Therapy with Oral P2Y12 Receptor Antagonists
Abstract
:1. Introduction
1.1. P2Y12 Receptor Antagonists
1.2. Selatogrel
1.3. Clinical Study Data
1.4. Drug-Drug Interaction Modeling
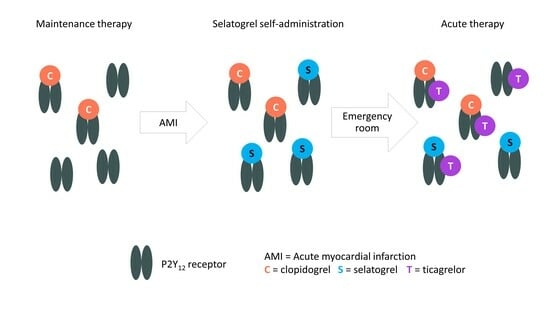
1.5. Research Question
2. Materials and Methods
3. Results
3.1. Selatogrel/Placebo Followed by Clopidogrel Loading Dose Administration
3.2. Ticagrelor Maintenance Therapy
3.3. Prasugrel Maintenance Therapy
3.4. Ticagrelor and Prasugrel Maintenance Therapy
3.5. Clopidogrel Maintenance Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016, 134, e123–e155. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease Developed in Collaboration with EACTS. Eur. J. Cardio-Thorac. Surg. 2018, 53, 34–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Chew, D.P.; Abdul Kader, M.A.S.K.; Ako, J.; Bahl, V.K.; Chan, M.; Park, K.W.; Chandra, P.; Hsieh, I.-C.; Huan, D.Q.; et al. 2020 Asian Pacific Society of Cardiology Consensus Recommendations on the Use of P2Y12 Receptor Antagonists in the Asia-Pacific Region. Eur. Cardiol. Rev. 2021, 16, e02. [Google Scholar] [CrossRef]
- US Food and Drug Administration and Center for Drug Evaluation and Research Plavix Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020839s070lbl.pdf (accessed on 24 July 2023).
- US Food and Drug Administration and Center for Drug Evaluation and Research Effient Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022307s016lbl.pdf (accessed on 4 September 2023).
- US Food and Drug Administration Center for Drug Evaluation and Research Brilinta Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022433s022lbl.pdf (accessed on 4 September 2023).
- Kim, K.; Lee, T.A.; Touchette, D.R.; DiDomenico, R.J.; Ardati, A.K.; Walton, S.M. Contemporary Trends in Oral Antiplatelet Agent Use in Patients Treated with Percutaneous Coronary Intervention for Acute Coronary Syndrome. J. Manag. Care Spec. Pharm. 2017, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dayoub, E.J.; Seigerman, M.; Tuteja, S.; Kobayashi, T.; Kolansky, D.M.; Giri, J.; Groeneveld, P.W. Trends in Platelet Adenosine Diphosphate P2Y 12 Receptor Inhibitor Use and Adherence Among Antiplatelet-Naive Patients After Percutaneous Coronary Intervention, 2008–2016. JAMA Intern. Med. 2018, 178, 943. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, T.; Brown, A.; Pol, D. Prescribing Trends and Health Care Expenditure of P2Y12 Prescriptions in Australia Over the Last Decade. Heart Lung Circ. 2022, 31, 1369–1375. [Google Scholar] [CrossRef]
- Ijaz, S.H.; Baron, S.J.; Shahnawaz, A.; Kulbak, G.; Levy, M.; Resnic, F.; Ganatra, S.; Dani, S.S. Utilization Trends in Platelet Adenosine Diphosphate P2Y12 Receptor Inhibitor and Cost among Medicare Beneficiaries. Curr. Probl. Cardiol. 2023, 48, 101608. [Google Scholar] [CrossRef]
- Ford, N.F.; Taubert, D. Clopidogrel, CYP2C19, and a Black Box. J. Clin. Pharmacol. 2013, 53, 241–248. [Google Scholar] [CrossRef]
- Li, Y.G.; Ni, L.; Brandt, J.T.; Small, D.S.; Payne, C.D.; Ernest II, C.S.; Rohatagi, S.; Farid, N.A.; Jakubowski, J.A.; Winters, K.J. Inhibition of Platelet Aggregation with Prasugrel and Clopidogrel: An Integrated Analysis in 846 Subjects. Platelets 2009, 20, 316–327. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Butler, K.; Antonino, M.J.; Wei, C.; Teng, R.; Rasmussen, L.; Storey, R.F.; Nielsen, T.; Eikelboom, J.W.; et al. Response to Ticagrelor in Clopidogrel Nonresponders and Responders and Effect of Switching Therapies: The RESPOND Study. Circulation 2010, 121, 1188–1199. [Google Scholar] [CrossRef]
- Brandt, J.T.; Payne, C.D.; Wiviott, S.D.; Weerakkody, G.; Farid, N.A.; Small, D.S.; Jakubowski, J.A.; Naganuma, H.; Winters, K.J. A Comparison of Prasugrel and Clopidogrel Loading Doses on Platelet Function: Magnitude of Platelet Inhibition Is Related to Active Metabolite Formation. Am. Heart J. 2007, 153, 66.e9–66.e16. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Trenk, D.; Frelinger, A.L.; O’Donoghue, M.; Neumann, F.-J.; Michelson, A.D.; Angiolillo, D.J.; Hod, H.; Montalescot, G.; Miller, D.L.; et al. Prasugrel Compared With High Loading- and Maintenance-Dose Clopidogrel in Patients With Planned Percutaneous Coronary Intervention. Circulation 2007, 116, 2923–2932. [Google Scholar] [CrossRef] [PubMed]
- Dobesh, P.P.; Oestreich, J.H. Ticagrelor: Pharmacokinetics, Pharmacodynamics, Clinical Efficacy, and Safety. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2014, 34, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Husted, S.; van Giezen, J.J.J. Ticagrelor: The First Reversibly Binding Oral P2Y12 Receptor Antagonist. Cardiovasc. Ther. 2009, 27, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Butler, K. Pharmacokinetics, Pharmacodynamics, Tolerability and Safety of Single Ascending Doses of Ticagrelor, a Reversibly Binding Oral P2Y12 Receptor Antagonist, in Healthy Subjects. Eur. J. Clin. Pharmacol. 2010, 66, 487–496. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes: Commentary. Rev. Port. Cardiol. 2007, 26, 1297–1298. [Google Scholar] [CrossRef]
- Scott, S.; Sangkuhl, K.; Gardner, E.E.; Stein, C.M.; Hulot, J.; Johnson, J.A.; Roden, D.M.; Klein, T.E.; Shuldiner, A.R. Clinical Pharmacogenetics Implementation Consortium Guidelines for Cytochrome P450–2C19 (CYP2C19) Genotype and Clopidogrel Therapy. Clin. Pharmacol. Ther. 2011, 90, 328–332. [Google Scholar] [CrossRef]
- Sinnaeve, P.; Fahrni, G.; Schelfaut, D.; Spirito, A.; Mueller, C.; Frenoux, J.-M.; Hmissi, A.; Bernaud, C.; Ufer, M.; Moccetti, T.; et al. Subcutaneous Selatogrel Inhibits Platelet Aggregation in Patients With Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 75, 2588–2597. [Google Scholar] [CrossRef]
- Storey, R.F.; Gurbel, P.A.; ten Berg, J.; Bernaud, C.; Dangas, G.D.; Frenoux, J.-M.; Gorog, D.A.; Hmissi, A.; Kunadian, V.; James, S.K.; et al. Pharmacodynamics, Pharmacokinetics, and Safety of Single-Dose Subcutaneous Administration of Selatogrel, a Novel P2Y12 Receptor Antagonist, in Patients with Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 3132–3140. [Google Scholar] [CrossRef]
- Baumann, M.; Lack, B.; Guillaumat, I.; Murphy, M.J.; Riederer, M.A. The Potency of Selatogrel, a Reversible Antagonist of the P2Y12 Receptor, Is Affected by Calcium Concentration. Platelets 2022, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, D.; Bruderer, S.; Krause, A.; Gutierrez, M.; Gueret, P.; Astruc, B.; Dingemanse, J. A New Reversible and Potent P2Y12 Receptor Antagonist (ACT-246475): Tolerability, Pharmacokinetics, and Pharmacodynamics in a First-in-Man Trial. Clin. Drug Investig. 2014, 34, 807–818. [Google Scholar] [CrossRef]
- Juif, P.; Boehler, M.; Dobrow, M.; Ufer, M.; Dingemanse, J. Clinical Pharmacology of the Reversible and Potent P2Y 12 Receptor Antagonist ACT-246475 After Single Subcutaneous Administration in Healthy Male Subjects. J. Clin. Pharmacol. 2019, 59, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ufer, M.; Huynh, C.; van Lier, J.J.; Caroff, E.; Fischer, H.; Dingemanse, J. Absorption, Distribution, Metabolism and Excretion of the P2Y12 Receptor Antagonist Selatogrel after Subcutaneous Administration in Healthy Subjects. Xenobiotica 2020, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Schilling, U.; Dingemanse, J.; Voors-Pette, C.; Romeijn, C.; Dogterom, P.; Ufer, M. Effect of Rifampin-mediated OATP1B1 and OATP1B3 Transporter Inhibition on the Pharmacokinetics of the P2Y12 Receptor Antagonist Selatogrel. Clin. Transl. Sci. 2020, 13, cts.12774. [Google Scholar] [CrossRef]
- Schilling, U.; Dingemanse, J.; Dobrow, M.; Baumann, M.; Riederer, M.A.; Juif, P.-E.; Ufer, M. Insights from In Vitro and Clinical Data to Guide Transition from the Novel P2Y12 Antagonist Selatogrel to Clopidogrel, Prasugrel, and Ticagrelor. Thromb. Haemost. 2021, 121, 755–766. [Google Scholar] [CrossRef]
- Zenklusen, I.; Hsin, C.-H.; Schilling, U.; Kankam, M.; Krause, A.; Ufer, M.; Dingemanse, J. Transition from Syringe to Autoinjector Based on Bridging Pharmacokinetics and Pharmacodynamics of the P2Y12 Receptor Antagonist Selatogrel in Healthy Subjects. Clin. Pharmacokinet. 2022, 61, 687–695. [Google Scholar] [CrossRef]
- Schilling, U.; Hsin, C.H.; Delahaye, S.; Krause, A.; Wuelfrath, H.; Halabi, A.; Dingemanse, J. Influence of Hepatic Impairment on the Pharmacokinetics and Pharmacodynamics of the P2Y12 Receptor Antagonist Selatogrel. Clin. Transl. Sci. 2022, 15, 1906–1915. [Google Scholar] [CrossRef]
- Crescence, L.; Kramberg, M.; Baumann, M.; Rey, M.; Roux, S.; Panicot-Dubois, L.; Dubois, C.; Riederer, M.A. The P2Y12 Receptor Antagonist Selatogrel Dissolves Preformed Platelet Thrombi In Vivo. J. Clin. Med. 2021, 10, 5349. [Google Scholar] [CrossRef]
- Crescence, L.; Darbousset, R.; Caroff, E.; Hubler, F.; Riederer, M.A.; Panicot-Dubois, L.; Dubois, C. Selatogrel, a Reversible P2Y12 Receptor Antagonist, Has Reduced off-Target Interference with Haemostatic Factors in a Mouse Thrombosis Model. Thromb. Res. 2021, 200, 133–140. [Google Scholar] [CrossRef]
- Henrich, A.; Claussen, C.H.; Dingemanse, J.; Krause, A. Pharmacokinetic/Pharmacodynamic Modeling of Drug Interactions at the P2Y12 Receptor between Selatogrel and Oral P2Y12 Antagonists. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 735–747. [Google Scholar] [CrossRef]
- Gaglia, M.A.; Torguson, R.; Pakala, R.; Xue, Z.; Sardi, G.; Suddath, W.O.; Kent, K.M.; Satler, L.F.; Pichard, A.D.; Waksman, R. Correlation between Light Transmission Aggregometry, VerifyNow P2Y12, and VASP-P Platelet Reactivity Assays Following Percutaneous Coronary Intervention. J. Interv. Cardiol. 2011, 24, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. Comparison of Methods to Evaluate Aspirin-Mediated Platelet Inhibition after Percutaneous Intervention with Stent Implantation. Platelets 2011, 22, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mould, D.R.; Upton, R.N. Basic Concepts in Population Modeling, Simulation, and Model-Based Drug Development—Part 2: Introduction to Pharmacokinetic Modeling Methods. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e38. [Google Scholar] [CrossRef] [PubMed]
- Bonate, P.L. Pharmacokinetic-Pharmacodynamic Modeling and Simulation, 2nd ed.; Springer: Boston, MA, USA, 2011. [Google Scholar]
- Upton, R.N.; Mould, D.R. Basic Concepts in Population Modeling, Simulation, and Model-Based Drug Development: Part 3—Introduction to Pharmacodynamic Modeling Methods. CPT Pharmacomet. Syst. Pharmacol. 2014, 3, e88. [Google Scholar] [CrossRef] [PubMed]
- Lixoft Simulx Documentation. Available online: https://simulx.lixoft.com/ (accessed on 4 September 2023).
- R Development Core Team R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 19 June 2023).
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; Antman, E.M. Time Delay to Treatment and Mortality in Primary Angioplasty for Acute Myocardial Infarction. Circulation 2004, 109, 1223–1225. [Google Scholar] [CrossRef]
- Björklund, E.; Stenestrand, U.; Lindbäck, J.; Svensson, L.; Wallentin, L.; Lindahl, B. Pre-Hospital Thrombolysis Delivered by Paramedics Is Associated with Reduced Time Delay and Mortality in Ambulance-Transported Real-Life Patients with ST-Elevation Myocardial Infarction. Eur. Heart J. 2006, 27, 1146–1152. [Google Scholar] [CrossRef]
- Hulot, J.-S.; Montalescot, G. Do We Need a New P2Y12 Receptor Antagonist? Eur. Heart J. 2020, 41, 3141–3143. [Google Scholar] [CrossRef]
- Chowdhury, I.Z.; Amin, M.N.; Chowdhury, M.Z.; Rahman, S.M.; Ahmed, M.; Cader, F.A. Pre Hospital Delay and Its Associated Factors in Acute Myocardial Infarction in a Developing Country. PLoS ONE 2021, 16, e0259979. [Google Scholar] [CrossRef]
- Idorsia Pharmaceuticals Ltd Selatogrel Outcome Study in Suspected Acute Myocardial Infarction (SOS-AMI). Available online: https://clinicaltrials.gov/ct2/show/NCT04957719 (accessed on 24 July 2023).
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef]
- Rollini, F.; Franchi, F.; Angiolillo, D.J. Switching P2Y12-Receptor Inhibitors in Patients with Coronary Artery Disease. Nat. Rev. Cardiol. 2016, 13, 11–27. [Google Scholar] [CrossRef]
- Rollini, F.; Franchi, F.; Angiolillo, D.J. Switching P2Y12 Receptor Inhibiting Therapies. Interv. Cardiol. Clin. 2017, 6, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Ernest, C.S.; Small, D.S.; Rohatagi, S.; Salazar, D.E.; Wallentin, L.; Winters, K.J.; Wrishko, R.E. Population Pharmacokinetics and Pharmacodynamics of Prasugrel and Clopidogrel in Aspirin-Treated Patients with Stable Coronary Artery Disease. J. Pharmacokinet. Pharmacodyn. 2008, 35, 593–618. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.; Jiang, X.L.; Peletier, L.A.; Shuldiner, A.R.; Horenstein, R.B.; Lewis, J.P.; Lesko, L.J.; Schmidt, S. Identifying Clinically Relevant Sources of Variability: The Clopidogrel Challenge. Clin. Pharmacol. Ther. 2017, 101, 264–273. [Google Scholar] [CrossRef] [PubMed]



| Scenario | P2Y12 Receptor Antagonist Administration Schedule | |||
|---|---|---|---|---|
| Selatogrel/Placebo | Clopidogrel | Ticagrelor | Prasugrel | |
| Scenarios without loading dose of ticagrelor/prasugrel | ||||
| 1A | 16 mg at 0 h | 600 mg at 4 h | 90 mg at 16 h and every 12 h subsequently | |
| 1B | 16 mg at 0 h | 600 mg at 4 h | 10 mg at 16 h and every 24 h subsequently | |
| 2A | 16 mg at 0 h | 600 mg at 12 h | 90 mg at 24 h and every 12 h subsequently | |
| 2B | 16 mg at 0 h | 600 mg at 12 h | 10 mg at 24 h and every 24 h subsequently | |
| Scenarios with loading dose of ticagrelor/prasugrel | ||||
| 3A | 16 mg at 0 h | 600 mg at 4 h | 180 mg at 16 h and 90 mg every 12 h subsequently | |
| 3B | 16 mg at 0 h | 600 mg at 4 h | 60 mg at 16 h and 10 mg every 24 h subsequently | |
| 4A | 16 mg at 0 h | 600 mg at 12 h | 180 mg at 24 h and 90 mg every 12 h subsequently | |
| 4B | 16 mg at 0 h | 600 mg at 12 h | 60 mg at 24 h and 10 mg every 24 h subsequently | |
| Scenarios with clopidogrel only | ||||
| 5 | 16 mg at 0 h | 600 mg at 4 h 75 mg at 16 h and every 24 h subsequently | ||
| 6 | 16 mg at 0 h | 600 mg at 12 h 75 mg at 24 h and every 24 h subsequently | ||
| Scenario | Treatment | Number of MD | Max. IPA (%) | IPA > 80% per Dosing Interval (h) | Time (%) with IPA > 80% per Dosing Interval |
|---|---|---|---|---|---|
| Scenarios without loading dose of ticagrelor/prasugrel | |||||
| 1A | 16 mg selatogrel (0 h) 600 mg clopidogrel (4 h) 90 mg ticagrelor b.i.d. | 1 | 86.4 | 7.5/12.0 | 62.5 |
| 2 | 92.8 | 11.7/12.0 | 97.5 | ||
| 3 | 94.9 | 12.0/12.0 | 100.0 | ||
| 4 | 95.7 | 12.0/12.0 | 100.0 | ||
| 5 | 96.0 | 12.0/12.0 | 100.0 | ||
| 1B | 16 mg selatogrel (0 h) 600 mg clopidogrel (4 h) 10 mg prasugrel o.d. | 1 | 80.8 | 1.7/24.0 | 7.1 |
| 2 | 97.8 | 23.4/24.0 | 97.5 | ||
| 3 | 99.6 | 24.0/24.0 | 100.0 | ||
| 2A | 16 mg selatogrel (0 h) 600 mg clopidogrel (12 h) 90 mg ticagrelor b.i.d. | 1 | 89.6 | 10.6/12.0 | 88.3 |
| 2 | 95.3 | 12.0/12.0 | 100.0 | ||
| 3 | 96.7 | 12.0/12.0 | 100.0 | ||
| 4 | 97.1 | 12.0/12.0 | 100.0 | ||
| 5 | 92.7 | 12.0/12.0 | 100.0 | ||
| 2B | 16 mg selatogrel (0 h) 600 mg clopidogrel (12 h) 10 mg prasugrel o.d. | 1 | 88.7 | 18.0/24.0 | 75.0 |
| 2 | 99.1 | 23.7/24.0 | 98.8 | ||
| 3 | 91.9 | 24.0/24.0 | 100.0 | ||
| Scenarios with loading dose of ticagrelor/prasugrel | |||||
| 3A | 16 mg selatogrel (0 h) 600 mg clopidogrel (4 h) 180 mg ticagrelor (16 h) 90 mg ticagrelor b.i.d. | 0 (LD only) | 96.2 | 11.3/12.0 | 94.2 |
| 1 | 96.1 | 12.0/12.0 | 100.0 | ||
| 2 | 96.1 | 12.0/12.0 | 100.0 | ||
| 3 | 96.2 | 12.0/12.0 | 100.0 | ||
| 3B | 16 mg selatogrel (0 h) 600 mg clopidogrel (4 h) 60 mg prasugrel (16 h) 10 mg prasugrel o.d. | 0 (LD only) | 100.0 | 23.6/24.0 | 98.3 |
| 1 | 99.9 | 24.0/24.0 | 100.0 | ||
| 2 | 99.8 | 24.0/24.0 | 100.0 | ||
| 4A | 16 mg selatogrel (0 h) 600 mg clopidogrel (12 h) 180 mg ticagrelor (24 h) 90 mg ticagrelor b.i.d. | 0 (LD only) | 97.3 | 11.4/12.0 | 95.0 |
| 1 | 97.5 | 12.0/12.0 | 100.0 | ||
| 2 | 97.5 | 12.0/12.0 | 100.0 | ||
| 3 | 97.4 | 12.0/12.0 | 100.0 | ||
| 4 | 93.4 | 12.0/12.0 | 100.0 | ||
| 4B | 16 mg selatogrel (0 h) 600 mg clopidogrel (12 h) 60 mg prasugrel (24 h) 10 mg prasugrel o.d. | 0 (LD only) | 100.0 | 23.6/24.0 | 98.3 |
| 1 | 99.9 | 24.0/24.0 | 100.0 | ||
| 2 | 94.8 | 24.0/24.0 | 100.0 | ||
| Scenarios with clopidogrel only | |||||
| 5 | 16 mg selatogrel (0 h) 600 mg clopidogrel (4 h) 75 mg clopidogrel o.d. | 1 | 50.7 | 0.0/24.0 | 0.0 |
| 2 | 19.0 | 0.0/24.0 | 0.0 | ||
| 3 | 23.5 | 0.0/24.0 | 0.0 | ||
| 6 | 16 mg selatogrel (0 h) 600 mg clopidogrel (12 h) 75 mg clopidogrel o.d. | 1 | 37.4 | 0.0/24.0 | 0.0 |
| 2 | 28.8 | 0.0/24.0 | 0.0 | ||
| 3 | 26.5 | 0.0/24.0 | 0.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsin, C.-h.; Dingemanse, J.; Henrich, A.; Bernaud, C.; Gehin, M.; Krause, A. Mind the Gap: Model-Based Switching from Selatogrel to Maintenance Therapy with Oral P2Y12 Receptor Antagonists. Biomolecules 2023, 13, 1365. https://doi.org/10.3390/biom13091365
Hsin C-h, Dingemanse J, Henrich A, Bernaud C, Gehin M, Krause A. Mind the Gap: Model-Based Switching from Selatogrel to Maintenance Therapy with Oral P2Y12 Receptor Antagonists. Biomolecules. 2023; 13(9):1365. https://doi.org/10.3390/biom13091365
Chicago/Turabian StyleHsin, Chih-hsuan, Jasper Dingemanse, Andrea Henrich, Corine Bernaud, Martine Gehin, and Andreas Krause. 2023. "Mind the Gap: Model-Based Switching from Selatogrel to Maintenance Therapy with Oral P2Y12 Receptor Antagonists" Biomolecules 13, no. 9: 1365. https://doi.org/10.3390/biom13091365
APA StyleHsin, C.-h., Dingemanse, J., Henrich, A., Bernaud, C., Gehin, M., & Krause, A. (2023). Mind the Gap: Model-Based Switching from Selatogrel to Maintenance Therapy with Oral P2Y12 Receptor Antagonists. Biomolecules, 13(9), 1365. https://doi.org/10.3390/biom13091365