GMP Synthetase: Allostery, Structure, and Function
Abstract
:1. Introduction
2. GMPS: An Essential Metabolic Enzyme
Moonlighting Functions of GMPS
3. Biochemical and Kinetic Characteristics of GMPSs
4. Crystal Structures of Single- and Two-Chain GMPS
4.1. Structure of the GATase Domain/Subunit and Catalysis of Gln Hydrolysis
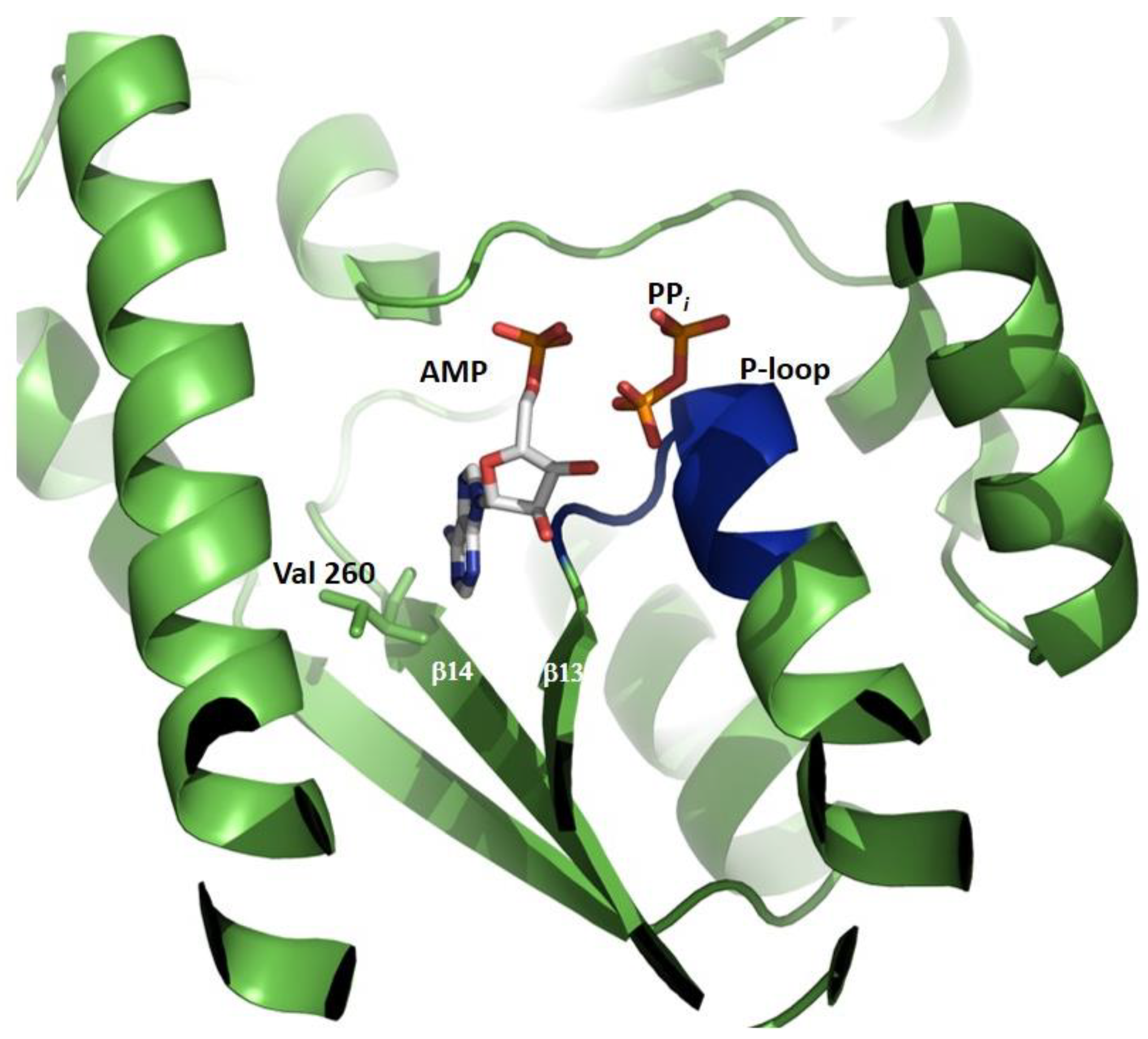
4.2. Structure of the ATPPase Domain/Subunit
4.3. The GATase and ATPPase Domains Are Flexible Relative to Each Other
5. Substrate Contacts and Catalysis in the ATPPase Domain/Subunit
5.1. C-Terminal Loop
5.2. Catalysis and Allostery: Role of Lid Loop Residues
5.3. Domain Rotation
5.4. Domain Reorganization and Ammonia Tunneling
6. Determinants of Domain Cross-Talk and Allostery
Residues on Interdomain Interface Helices α1, α11, and α12
7. Structure of Two-Subunit M. jannaschii GMPS
7.1. Structure of the MjGATase–MjATPPase Complex
7.2. Stable Succinimide (SNN) in MjGATase
8. Conserved Catalytic Mechanism
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bushell, E.; Gomes, A.R.; Sanderson, T.; Anar, B.; Girling, G.; Herd, C.; Metcalf, T.; Modrzynska, K.; Schwach, F.; Martin, R.E.; et al. Functional Profiling of a Plasmodium Genome Reveals an Abundance of Essential Genes. Cell 2017, 170, 260–272. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the Essential Genes of the Human Malaria Parasite Plasmodium falciparum by Saturation Mutagenesis. Science 2018, 360, 7847. [Google Scholar] [CrossRef]
- Webster, H.K.; Whaun, J.M. Antimalarial Properties of Bredinin: Prediction based on identification of differences in human host-parasite purine metabolism. J. Clin. Investig. 1982, 70, 461–469. [Google Scholar] [CrossRef]
- Queen, S.A.; Vander Jagt, D.L.; Reyes, P. In Vitro Susceptibilities of Plasmodium falciparum to Compounds Which Inhibit Nucleotide Metabolism. Antimicrob. Agents Chemother. 1990, 34, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Leija, C.; Rijo-Ferreira, F.; Chen, J.; Cestari, I.; Stuart, K.; Tu, B.P.; Phillips, M.A. GMP Synthase Is Essential for Viability and Infectivity of Trypanosoma brucei despite a Redundant Purine Salvage Pathway. Mol. Microbiol. 2015, 97, 1006–1020. [Google Scholar] [CrossRef]
- Rodriguez-Suarez, R.; Xu, D.; Veillette, K.; Davison, J.; Sillaots, S.; Kauffman, S.; Hu, W.; Bowman, J.; Martel, N.; Trosok, S.; et al. Article Mechanism-of-Action Determination of GMP Synthase Inhibitors and Target Validation in Candida albicans and Aspergillus Fumigatus. Chem. Biol. 2007, 14, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Chitty, J.L.; Tatzenko, T.L.; Williams, S.J.; Andre, Y.Q.; Koh, E.; Corfield, E.C.; Butler, M.S.; Robertson, A.A.B.; Cooper, M.A.; Kappler, U.; et al. GMP Synthase Is Required for Virulence Factor Production and Infection by Cryptococcus neoformans. J. Biol. Chem. 2017, 292, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Pasetti, M.F.; Barry, E.M.; Nataro, J.P.; Wasserman, S.S.; Sztein, M.B.; Picking, W.D.; Levine, M.M. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J. Infect. Dis. 2004, 190, 1745–1754. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Simon, J.K.; Pasetti, M.F.; Sztein, M.B.; Wooden, S.L.; Livio, S.; Nataro, J.P.; Blackwelder, W.C.; Barry, E.M.; Picking, W.; et al. Safety and Immunogenicity of CVD 1208S, a Live, Oral ΔguaBA Δsen Δset Shigella flexneri 2a Vaccine Grown on Animal-Free Media. Hum. Vaccines 2007, 3, 268–275. [Google Scholar] [CrossRef]
- Drumond Villela, A.; Eichler, P.; Frederico, A.; Pinto, M.; Rodrigues-Junior, V.; Yates Iii, J.R.; Valim Bizarro, C.; Basso, L.A.; Santos, S. Gene Replacement and Quantitative Mass Spectrometry Approaches Validate Guanosine Monophosphate Synthetase as Essential for Mycobacterium tuberculosis Growth. Biochem. Biophys. Rep. 2015, 4, 277–282. [Google Scholar]
- Smith-Peter, E.; Séguin, D.L.; St-Pierre, É.; Sekulovic, O.; Jeanneau, S.; Tremblay-Tétreault, C.; Lamontagne, A.-M.; Jacques, P.-É.; Lafontaine, D.A.; Fortier, L.-C. Inactivation of the Riboswitch-Controlled GMP Synthase GuaA in Clostridioides difficile is Associated with Severe Growth Defects and Poor Infectivity in a Mouse Model of Infection. RNA Biol. 2021, 18 (Suppl. 2), 699–710. [Google Scholar] [CrossRef]
- Bianchi-Smiraglia, A.; Wawrzyniak, J.A.; Bagati, A.; Marvin, E.K.; Ackroyd, J.; Moparthy, S.; Bshara, W.; Fink, E.E.; Foley, C.E.; Morozevich, G.E.; et al. Pharmacological Targeting of Guanosine Monophosphate Synthase Suppresses Melanoma Cell Invasion and Tumorigenicity. Cell Death Differ. 2015, 22, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Li, Y.; Wang, Y.; Shen, F.; Zhou, J.; Chen, Y. Guanosine Monophosphate Synthase Upregulation Mediates Cervical Cancer Progression by Inhibiting the Apoptosis of Cervical Cancer Cells via the Stat3/P53 Pathway. Int. J. Oncol. 2021, 58, 3. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, L. IMP Dehydrogenase: Structure, Mechanism, and Inhibition. Chem. Rev. 2009, 109, 2903–2928. [Google Scholar] [CrossRef]
- Ishikawa, H. Mizoribine and Mycophenolate Mofetil. Curr. Med. Chem. 1999, 6, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.A.; Van Der Knaap, J.A.; Bot, A.G.M.; Mohd-Sarip, A.; Dekkers, D.H.W.; Timmermans, M.A.; Martens, J.W.M.; Demmers, J.A.A.; Peter Verrijzer, C. Nucleotide Biosynthetic Enzyme GMP Synthase Is a TRIM21-Controlled Relay of P53 Stabilization. Mol. Cell 2014, 53, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Faesen, A.C.; Dirac, A.M.G.; Shanmugham, A.; Ovaa, H.; Perrakis, A.; Sixma, T.K. Mechanism of USP7/HAUSP Activation by its C-Terminal Ubiquitin-like Domain and Allosteric Regulation by GMP-Synthetase. Mol. Cell 2011, 44, 147–159. [Google Scholar] [CrossRef]
- Sarkari, F.; Sanchez-Alcaraz, T.; Wang, S.; Holowaty, M.N.; Sheng, Y. EBNA1-Mediated Recruitment of a Histone H2B Deubiquitylating Complex to the Epstein-Barr Virus Latent Origin of DNA Replication. PLoS Pathog. 2009, 5, 1000624. [Google Scholar] [CrossRef]
- van Der Knaap, J.A.; Kumar, B.R.P.; Moshkin, Y.M.; Langenberg, K.; Krijgsveld, J.; Heck, A.J.R.; Karch, F.; Verrijzer, C.P. GMP Synthetase Stimulates Histone H2B Deubiquitylation by the Epigenetic Silencer USP7. Mol. Cell 2005, 17, 695–707. [Google Scholar] [CrossRef]
- van der Knaap, J.A.; Kozhevnikova, E.; Langenberg, K.; Moshkin, Y.M.; Verrijzer, C.P. Biosynthetic Enzyme GMP Synthetase Cooperates with Ubiquitin-Specific Protease 7 in Transcriptional Regulation of Ecdysteroid Target Genes. Mol. Cell. Biol. 2010, 30, 736–744. [Google Scholar] [CrossRef]
- Zhou, Z.; Yao, X.; Li, S.; Zhao, Y.; Jiang, J.; Zhang, Q. Deubiquitination of Ci/Gli by Usp7/HAUSP Regulates Hedgehog Signaling. Dev. Cell 2015, 34, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Pegram, L.D.; Megonigal, M.D.; Lange, B.J.; Nowell, P.C.; Rowley, J.D.; Rappaport, E.F.; Felix, C.A. T(3;11) Translocation in Treatment-Related Acute Myeloid Leukemia Fuses MLL with the GMPS (Guanosine 5′ Monophosphate Synthetase) Gene. Blood 2000, 96, 4360–4362. [Google Scholar] [CrossRef] [PubMed]
- List, F.; Vega, M.C.; Razeto, A.; Häger, M.C.; Sterner, R.; Wilmanns, M. Catalysis Uncoupling in a Glutamine Amidotransferase Bienzyme by Unblocking the Glutaminase Active Site. Chem. Biol. 2012, 19, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Massie, F.; Badet-Denisot, M.A. The Mechanism of Glutamine-Dependent Amidotransferases. Cell. Mol. Life Sci. CMLS 1998, 54, 205–222. [Google Scholar] [CrossRef]
- Zalkin, H.; Smith, J.L. Enzymes Utilizing Glutamine as an Amide Donor. Adv. Enzymol. Relat. Areas Mol. Biol. 1998, 72, 87–144. [Google Scholar]
- Zalkin, H. The Amidotransferases. Adv. Enzymol. Relat. Areas Mol. Biol. 1993, 66, 203–309. [Google Scholar]
- Mouilleron, S.; Golinelli-Pimpaneau, B. Conformational Changes in Ammonia-Channeling Glutamine Amidotransferases. Curr. Opi. Struct. Biol. 2007, 17, 653–664. [Google Scholar] [CrossRef]
- Bhat, J.Y.; Venkatachala, R.; Balaram, H. Substrate-Induced Conformational Changes in Plasmodium falciparum Guanosine Monophosphate Synthetase. FEBS J. 2011, 278, 3756–3768. [Google Scholar] [CrossRef]
- Isupov, M.N.; Obmolova, G.; Butterworth, S.; Badet-Denisot, M.A.; Badet, B.; Polikarpov, I.; Littlechild, J.A.; Teplyakov, A. Substrate Binding Is Required for Assembly of the Active Conformation of the Catalytic Site in Ntn Amidotransferases: Evidence from the 1.8 Å Crystal Structure of the Glutaminase Domain of Glucosamine 6-Phosphate Synthase. Structure 1996, 4, 801–810. [Google Scholar] [CrossRef]
- Oliver, J.C.; Gudihal, R.; Burgner, J.W.; Pedley, A.M.; Zwierko, A.T.; Davisson, V.J.; Linger, R.S. Conformational Changes Involving Ammonia Tunnel Formation and Allosteric Control in GMP Synthetase. Arch. Biochem. Biophys. 2014, 545, 22–32. [Google Scholar] [CrossRef]
- Oliver, J.C.; Linger, R.S.; Chittur, S.V.; Davisson, V.J. Substrate Activation and Conformational Dynamics of Guanosine 5′-Monophosphate Synthetase. Biochemistry 2013, 52, 5225–5235. [Google Scholar] [CrossRef]
- Ballut, L.; Violot, S.; Shivakumaraswamy, S.; Thota, L.P.; Sathya, M.; Kunala, J.; Dijkstra, B.W.; Terreux, R.; Haser, R.; Balaram, H.; et al. Active Site Coupling in Plasmodium falciparum GMP Synthetase Is Triggered by Domain Rotation. Nat. Commun. 2015, 6, 8930. [Google Scholar] [CrossRef] [PubMed]
- Ballut, L.; Violot, S.; Galisson, F.; Gonçalves, I.R.; Martin, J.; Shivakumaraswamy, S.; Carrique, L.; Balaram, H.; Aghajari, N. Tertiary and Quaternary Structure Organization in GMP Synthetases: Implications for Catalysis. Biomolecules 2022, 12, 871. [Google Scholar] [CrossRef]
- Nakamura, J.; Lou, L. Biochemical Characterization of Human GMP Synthetase. J. Biol. Chem. 1995, 270, 7347–7353. [Google Scholar] [CrossRef] [PubMed]
- Zalkin, H. GMP Synthetase. Methods Enzymol. 1985, 113, 273–278. [Google Scholar]
- Maruoka, S.; Horita, S.; Lee, W.C.; Nagata, K.; Tanokura, M. Crystal Structure of the ATPPase Subunit and its Substrate-Dependent Association with the GATase Subunit: A Novel Regulatory Mechanism for a Two-Subunit-Type GMP Synthetase from Pyrococcus horikoshii OT3. J. Mol. Biol. 2010, 395, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Shivakumaraswamy, S.; Kumar, S.; Bellur, A.; Polisetty, S.D.; Balaram, H. Mechanistic Insights into the Functioning of a Two-Subunit GMP Synthetase, an Allosterically Regulated, Ammonia Channeling Enzyme. Biochemistry 2022, 61, 1988–2006. [Google Scholar] [CrossRef]
- Bhat, J.Y.; Shastri, B.G.; Balaram, H. Kinetic and Biochemical Characterization of Plasmodium falciparum GMP Synthetase. Biochem. J. 2008, 409, 263–273. [Google Scholar] [CrossRef]
- Bhat, J.Y.; Venkatachala, R.; Singh, K.; Gupta, K.; Sarma, S.P.; Balaram, H. Ammonia Channeling in Plasmodium falciparum GMP Synthetase: Investigation by NMR Spectroscopy and Biochemical Assays. Biochemistry 2011, 50, 3346–3356. [Google Scholar] [CrossRef]
- Franco, T.M.A.; Rostirolla, D.C.; Ducati, R.G.; Lorenzini, D.M.; Basso, L.A.; Santos, D.S. Biochemical Characterization of Recombinant GuaA-Encoded Guanosine Monophosphate Synthetase (EC 6.3.5.2) from Mycobacterium tuberculosis H37Rv Strain. Arch. Biochem. Biophys. 2012, 517, 1–11. [Google Scholar] [CrossRef]
- Tesmer, J.J.G.; Klem, T.J.; Deras, M.L.; Davisson, V.J.; Smith, J.L. The Crystal Structure of GMP Synthetase Reveals a Novel Catalytic Triad and is a Structural Paradigm for Two Enzyme Families. Nat. Struct. Biol. 1996, 3, 74–86. [Google Scholar] [CrossRef]
- Welin, M.; Lehtiö, L.; Johansson, A.; Flodin, S.; Nyman, T.; Trésaugues, L.; Hammarström, M.; Gräslund, S.; Nordlund, P. Substrate Specificity and Oligomerization of Human GMP Synthetase. J. Mol. Biol. 2013, 425, 4323–4333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Jovcevski, B.; Pukala, T.L.; Bruning, J.B. Structural Insights into the Antifungal Drug Target Guanosine Monophosphate Synthase from Aspergillus fumigatus. Acta Crystallogr. Sect. D Struct. Biol. 2022, 78, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.L.; Newell, J.M.; Lightcap, C.M.; Olanich, M.E.; Loughlin, D.T.; Weller, M.A.; Lam, G.; Pollack, S.; Patton, W.A. The Effects of Removing the GAT Domain from E. coli GMP Synthetase. Protein J. 2006, 25, 483–491. [Google Scholar] [CrossRef]
- Ali, R.; Kumar, S.; Balaram, H.; Sarma, S.P. Solution Nuclear Magnetic Resonance Structure of the GATase Subunit and Structural Basis of the Interaction between GATase and ATPPase Subunits in a Two-Subunit-Type GMPS from Methanocaldococcus jannaschii. Biochemistry 2013, 52, 4308–4323. [Google Scholar] [CrossRef]
- von der Saal, W.; Crysler, C.S.; Villafranca, J.J. Positional Isotope Exchange and Kinetic Experiments with Escherichia coli Guanosine-5′-Monophosphate Synthetase. Biochemistry 1985, 24, 5343–5350. [Google Scholar] [CrossRef]
- Fresquet, V.; Thoden, J.B.; Holden, H.M.; Raushel, F.M. Kinetic Mechanism of Asparagine Synthetase from Vibrio cholerae. Bioorg. Chem. 2004, 32, 63–75. [Google Scholar] [CrossRef]
- Tesson, A.R.; Soper, T.S.; Ciustea, M.; Richards, N.G.J. Revisiting the Steady State Kinetic Mechanism of Glutamine-Dependent Asparagine Synthetase from Escherichia coli. Arch. Biochem. Biophys. 2003, 413, 23–31. [Google Scholar] [CrossRef]
- Nakamura, J.; Straub, K.; Wu, J.; Lou, L. The Glutamine Hydrolysis Function of Human GMP Synthetase. Identification of an Essential Active Site Cysteine. J. Biol. Chem. 1995, 270, 23450–23455. [Google Scholar] [CrossRef] [PubMed]
- Chittur, S.V.; Klem, T.J.; Shafer, C.M.; Jo Davisson, V. Mechanism for Acivicin Inactivation of Triad Glutamine Amidotransferases. Biochemistry 2001, 40, 876–887. [Google Scholar] [CrossRef]
- Zalkin, H.; Truitt, C.D. Characterization of the Glutamine Site of Escherichia coli Guanosine 5′ Monophosphate Synthetase. J. Biol. Chem. 1977, 252, 5431–5436. [Google Scholar] [CrossRef] [PubMed]
- Willemoës, M.; Sigurskjold, B.W. Steady-state kinetics of the glutaminase reaction of CTP synthase from Lactococcus lactis. Eur. J. Biochem. 2002, 269, 4772–4779. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Hatfield, G.W.; Moyed, H.S. Physical Properties and Subunit Structure of Xanthosine 5′-Phosphate Aminase. J. Biol. Chem. 1972, 247, 5880–5887. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Nakamura, J.; Tsing, S.; Nguyen, B.; Chow, J.; Straub, K.; Chan, H.; Barnett, J. High-Level Production from a Baculovirus Expression System and Biochemical Characterization of Human GMP Synthetase. Protein Expr. Purif. 1995, 6, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.V.; Das, S.; Bellur, A.; Kumar, S.; Chandrashekarmath, A.; Karmakar, T.; Balaram, P.; Balasubramanian, S.; Balaram, H. Structural Basis for the Hyperthermostability of an Archaeal Enzyme Induced by Succinimide Formation. Biophys. J. 2021, 120, 3732–3746. [Google Scholar] [CrossRef]
- Maruoka, S.; Lee, W.C.; Kamo, M.; Kudo, N.; Nagata, K.; Tanokura, M. Crystal Structure of Glutamine Amidotransferase from Pyrococcus horikoshii OT3. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2005, 81, 459–462. [Google Scholar] [CrossRef]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.; Frolow, F.; Franken, S.M.; Harel, M.; Remington, S.J.; Silman, I.; Schrag, J. The Alpha/Beta Hydrolase Fold. Protein Eng. 1992, 5, 197–211. [Google Scholar] [CrossRef]
- Yee, V.C.; Pedersen, L.C.; Le Trong, I.; Bishop, P.D.; Stenkamp, R.E.; Teller, D.C. Three-Dimensional Structure of a Transglutaminase: Human Blood Coagulation Factor XIII. Proc. Natl. Acad. Sci. USA 1994, 91, 7296–7300. [Google Scholar] [CrossRef]
- Saeed-Kothe, A.; Powers-Lee, S.G. Gain of Glutaminase Function in Mutants of the Ammonia-Specific Frog Carbamoyl Phosphate Synthetase. J. Biol. Chem. 2003, 278, 26722–26726. [Google Scholar] [CrossRef]
- Mouilleron, S.; Badet-Denisot, M.A.; Badet, B.; Golinelli-Pimpaneau, B. Dynamics of Glucosamine-6-Phosphate Synthase Catalysis. Arch. Biochem. Biophys. 2011, 505, 1–12. [Google Scholar] [CrossRef]
- Goto, M.; Omi, R.; Nakagawa, N.; Miyahara, I.; Hirotsu, K. Crystal Structures of CTP Synthetase Reveal ATP, UTP, and Glutamine Binding Sites. Structure 2004, 12, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.T. Formation of an Adenyl Xanthosine Monophosphate Intermediate by Xanthosine 5′-Phosphate Aminase and its Inhibition by Psicofuranine. J. Biol. Chem. 1966, 241, 4745–4749. [Google Scholar] [CrossRef] [PubMed]
- Shivakumaraswamy, S.; Pandey, N.; Ballut, L.; Violot, S.; Aghajari, N.; Balaram, H. Helices on Interdomain Interface Couple Catalysis in the ATPPase Domain with Allostery in Plasmodium falciparum GMP Synthetase. ChemBioChem 2020, 21, 2805–2817. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prakash, S.; Gupta, K.; Dongre, A.; Balaram, P.; Balaram, H. Unexpected Functional Implication of a Stable Succinimide in the Structural Stability of Methanocaldococcus jannaschii Glutaminase. Nat. Commun. 2016, 7, 12798. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]











| Organism [ref] | kcat (s−1) | Km | ||||
|---|---|---|---|---|---|---|
| ATP (μM) | XMP (μM) (Hill Coefficient n) | Gln (μM) | NH4+ (mM) | Mg2+ | ||
| E. coli [44] | --- | 104 ± 44 | 166 ± 43 | --- | 103 | --- |
| E. coli [30] (Gln as NH3 source) | 23 ± 1 (XMP) 13.7 ± 0.5 (Gln) | 53 ± 7 | 1.6 ± 0.3 mM | |||
| E. coli [30] (NH4Cl) | 13.3 ± 0.3 | 43 ± 4 | ||||
| H. sapiens [34] | 5.4 | 132 ± 7 | K0.5 35.6 ± 1.8 (n = 1.48) | 406 ± 49 μM | 174 ± 21 mM | K0.5 1780 ± 70 μM n = 4.14 ± 0.77 |
| T. brucei [5] | 4.7 | 200 | 8.8 | 240 μM | ||
| M tuberculosis [40] | 2.3 | 27 ± 2 | K0.5 45 ± 1 (n = 2.4) | 1.24 mM | K0.5 26 mM | K0.5 1.18 ± 0.03 mM n = 2.67 |
| P. falciparum [28,38] | 0.43 (Gln) | 260 ± 38 | 16.8 ± 2 | 472 ± 69 μM | 10.8 mM | K0.5 2090 ± 30 μM n = 4.4 |
| M. jannaschii (70 °C) | 1.94 ± 0.02 (Gln) | 452 ± 3 | 61 ± 3 | 520 ± 8 μM | K0.5 1.84 ± 0.01 mM n = 2.05 ± 0.07 | |
| MjATPPase (70 °C) [37] | 1.91 ± 0.02 (NH4+) | 447 ± 5 | 30 ± 2 | NA | 4.1 ± 0.2 mM | K0.5 1.75 ± 0.02 mM n = 2.45 ± 0.05 |
| C. neoformans [7] | 0.4 | 77.5 ± 6.0 | 65.9 ± 13.0 | 1130 ± 162 μM | K0.5 1289.0 ± 66.0 μM n = 2.2 ± 0.2 | |
| A. fumigatus [43] | 1.6 ± 0.1 | 245 ± 15 | ND | 2693 ± 119 μM | Km = 1230 ± 140 μM | |
| Organism | Native/Mutant | Ligand Bound | GATase Domain | GATase Ligand Bound |
|---|---|---|---|---|
| Single chain | ||||
| A. baumannii AB5075-UW | Mg2+, PO43−, Cl−; 7SBC | |||
| A. fumigatus Af293 | 7MO6 | |||
| C. burnetii | 3TQI | |||
| E. coli | AMP, citrate, Mg2+, PO43−, PPi; 1GPM | |||
| H. sapiens | XMP; 2VXO | 2VPI | ||
| N. gonorrhoeae | Mg2+; 5TW7 | |||
| P. falciparum | 4WIM C89A_C113A; 7ZU9 | C89A with Gln; 4WIO XMP & Ca2+; 3UOW | NO3−; 4WIN | |
| T. thermophilus | 2YWB | XMP; 2YWC | ||
| Double chain | ||||
| M. jannaschii | 2LXN | XMP; 6JP9 | 7D40 D110G; 7D96 and N109P; 7D97 | Acivicin; 7D95 |
| P. horikoshiiOT3 | 3A4I/2DPL | 2D7J/1WL8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballut, L.; Violot, S.; Kumar, S.; Aghajari, N.; Balaram, H. GMP Synthetase: Allostery, Structure, and Function. Biomolecules 2023, 13, 1379. https://doi.org/10.3390/biom13091379
Ballut L, Violot S, Kumar S, Aghajari N, Balaram H. GMP Synthetase: Allostery, Structure, and Function. Biomolecules. 2023; 13(9):1379. https://doi.org/10.3390/biom13091379
Chicago/Turabian StyleBallut, Lionel, Sébastien Violot, Sanjeev Kumar, Nushin Aghajari, and Hemalatha Balaram. 2023. "GMP Synthetase: Allostery, Structure, and Function" Biomolecules 13, no. 9: 1379. https://doi.org/10.3390/biom13091379
APA StyleBallut, L., Violot, S., Kumar, S., Aghajari, N., & Balaram, H. (2023). GMP Synthetase: Allostery, Structure, and Function. Biomolecules, 13(9), 1379. https://doi.org/10.3390/biom13091379







