The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology
Abstract
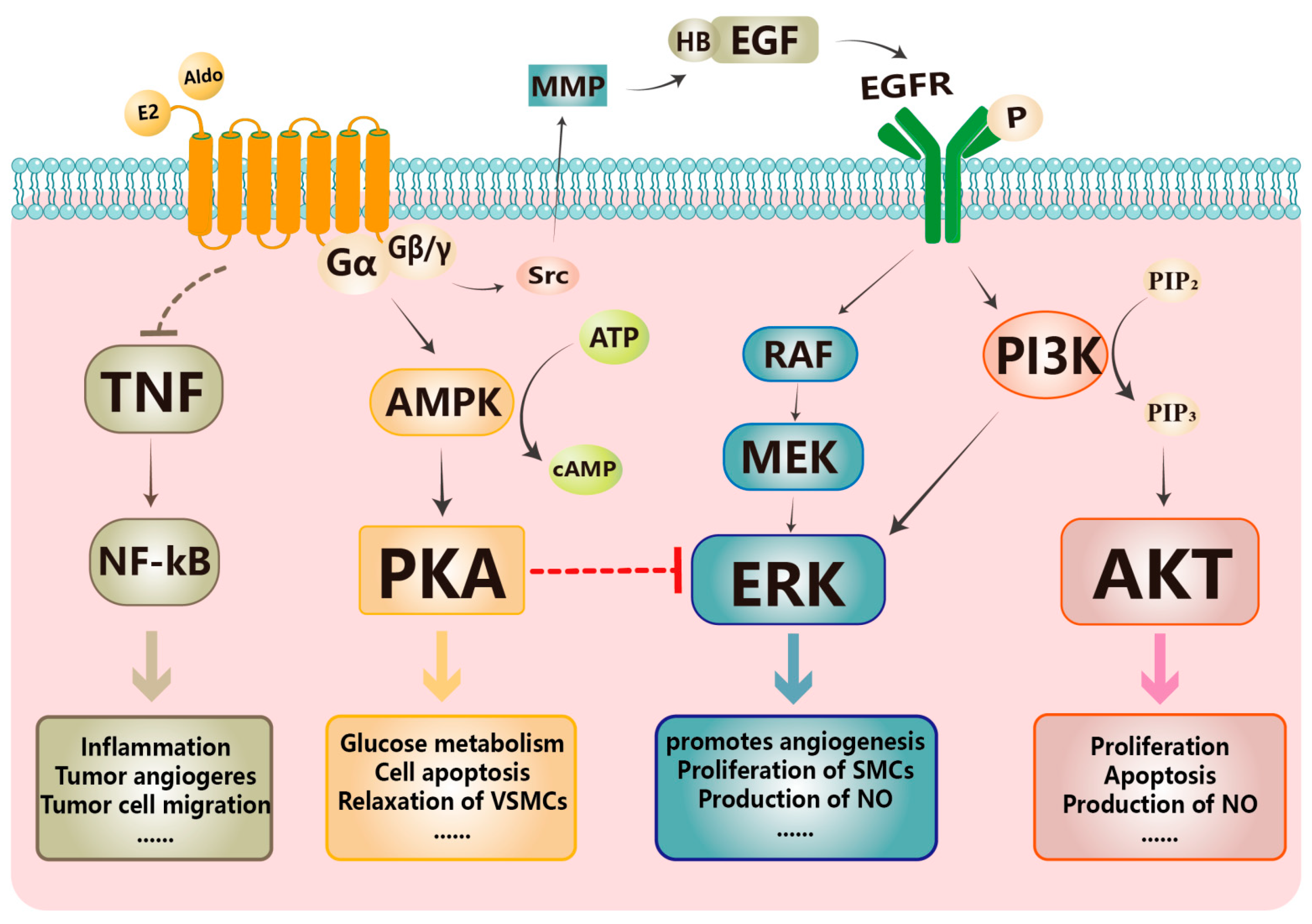
1. Introduction
2. Tissue, Cellular Localization, Ligands, and Classical Signaling Pathways of GPER
2.1. Tissue and Cellular Localization of GPER
2.2. Endogenous Ligands of GPER
2.2.1. Estrogen
2.2.2. Aldosterone
2.3. Exogenous Ligand of GPER
3. The Role of GPER in Vascular Pathology and Physiology
3.1. Blood Pressure Regulation and Hypertensive Disorders
3.2. Pulmonary Arterial Hypertension
3.3. Inflammation, Lipid Metabolism, and Atherosclerosis
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.X.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The Impact of Sex and Gender on Stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef]
- Yoon, S.S.; Carroll, M.D.; Fryar, C.D. Hypertension Prevalence and Control among Adults: United States, 2011–2014; NCHS Data Brief; US Department of Health and Human Services: Hyattsville, MD, USA, 2015; pp. 1–8.
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Farhat, M.Y.; Lavigne, M.C.; Ramwell, P.W. The vascular protective effects of estrogen. FASEB J. 1996, 10, 615–624. [Google Scholar] [CrossRef]
- Li, H.Y.; Bian, J.S.; Kwan, Y.W.; Wong, T.M. Enhanced responses to 17beta-estradiol in rat hearts treated with isoproterenol: Involvement of a cyclic AMP-dependent pathway. J. Pharmacol. Exp. Ther. 2000, 293, 592–598. [Google Scholar]
- Ullrich, N.D.; Krust, A.; Collins, P.; MacLeod, K.T. Genomic deletion of estrogen receptors ERalpha and ERbeta does not alter estrogen-mediated inhibition of Ca2+ influx and contraction in murine cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2421–H2427. [Google Scholar] [CrossRef]
- Sharma, G.; Hu, C.; Staquicini, D.I.; Brigman, J.L.; Liu, M.; Mauvais-Jarvis, F.; Pasqualini, R.; Arap, W.; Arterburn, J.B.; Hathaway, H.J.; et al. Preclinical efficacy of the GPER-selective agonist G-1 in mouse models of obesity and diabetes. Sci. Transl. Med. 2020, 12, eaau5956. [Google Scholar] [CrossRef]
- Xu, F.; Wang, X.; Wu, N.; He, S.; Yi, W.; Xiang, S.; Zhang, P.; Xie, X.; Ying, C. Bisphenol A induces proliferative effects on both breast cancer cells and vascular endothelial cells through a shared GPER-dependent pathway in hypoxia. Environ. Pollut. 2017, 231, 1609–1620. [Google Scholar] [CrossRef]
- Fredette, N.C.; Meyer, M.R.; Prossnitz, E.R. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J. Steroid Biochem. Mol. Biol. 2018, 176, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, S.H.; Cohen, J.A.; Brosnihan, K.B.; Gallagher, P.E.; Chappell, M.C. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology 2009, 150, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Arterburn, J.B. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol. Rev. 2015, 67, 505–540. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Alexander, S.P.; Mathie, A.; Peters, J.A. Guide to Receptors and Channels (GRAC), 5th edition. Br. J. Pharmacol. 2011, 164 (Suppl. 1), S1–S324. [Google Scholar] [CrossRef]
- Molina, L.; Figueroa, C.D.; Bhoola, K.D.; Ehrenfeld, P. GPER-1/GPR30 a novel estrogen receptor sited in the cell membrane: Therapeutic coupling to breast cancer. Expert. Opin. Ther. Targets 2017, 21, 755–766. [Google Scholar] [CrossRef]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef]
- Plante, B.J.; Lessey, B.A.; Taylor, R.N.; Wang, W.; Bagchi, M.K.; Yuan, L.; Scotchie, J.; Fritz, M.A.; Young, S.L. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod. Sci. 2012, 19, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Luca, A.; Nocito, M.C.; Avena, P.; La Padula, D.; Zavaglia, L.; Pezzi, V. Role of GPER-Mediated Signaling in Testicular Functions and Tumorigenesis. Cells 2020, 9, 2115. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Romeo, F.; Giordano, F.; Ferraro, A.; Carpino, A. Identification of the G protein-coupled estrogen receptor (GPER) in human prostate: Expression site of the estrogen receptor in the benign and neoplastic gland. Andrology 2016, 4, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-de-Arellano, A.; Pereira-Suarez, A.L.; Rico-Fuentes, C.; Lopez-Pulido, E.I.; Villegas-Pineda, J.C.; Sierra-Diaz, E. Distribution and Effects of Estrogen Receptors in Prostate Cancer: Associated Molecular Mechanisms. Front. Endocrinol. 2021, 12, 811578. [Google Scholar] [CrossRef] [PubMed]
- Kolkova, Z.; Noskova, V.; Ehinger, A.; Hansson, S.; Casslen, B. G protein-coupled estrogen receptor 1 (GPER, GPR 30) in normal human endometrium and early pregnancy decidua. Mol. Hum. Reprod. 2010, 16, 743–751. [Google Scholar] [CrossRef]
- Harding, A.T.; Goff, M.A.; Froggatt, H.M.; Lim, J.K.; Heaton, N.S. GPER1 is required to protect fetal health from maternal inflammation. Science 2021, 371, 271–276. [Google Scholar] [CrossRef]
- Hugo, E.R.; Brandebourg, T.D.; Woo, J.G.; Loftus, J.; Alexander, J.W.; Ben-Jonathan, N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. 2008, 116, 1642–1647. [Google Scholar] [CrossRef]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in vivo? Front. Endocrinol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Barton, M. Position paper: The membrane estrogen receptor GPER–Clues and questions. Steroids 2012, 77, 935–942. [Google Scholar] [CrossRef]
- Meyer, M.R.; Fredette, N.C.; Howard, T.A.; Hu, C.; Ramesh, C.; Daniel, C.; Amann, K.; Arterburn, J.B.; Barton, M.; Prossnitz, E.R. G protein-coupled estrogen receptor protects from atherosclerosis. Sci. Rep. 2014, 4, 7564. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.; Meyer, M.R.; Schurr, U.; Bhattacharya, I.; Minotti, R.; Nguyen, H.H.; Heigl, A.; Lachat, M.; Genoni, M.; Barton, M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 2007, 49, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Kockelkoren, G.; Lauritsen, L.; Shuttle, C.G.; Kazepidou, E.; Vonkova, I.; Zhang, Y.; Breuer, A.; Kennard, C.; Brunetti, R.M.; D’Este, E.; et al. Molecular mechanism of GPCR spatial organization at the plasma membrane. Nat. Chem. Biol. 2023, 1–9. [Google Scholar] [CrossRef]
- Kelly, M.J.; Levin, E.R. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2001, 12, 152–156. [Google Scholar] [CrossRef]
- Funakoshi, T.; Yanai, A.; Shinoda, K.; Kawano, M.M.; Mizukami, Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Commun. 2006, 346, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.; Quinn, J.; Pang, Y.; Graeber, C.; Shaw, S.; Dong, J.; Thomas, P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 2007, 148, 3236–3245. [Google Scholar] [CrossRef]
- Maiti, K.; Paul, J.W.; Read, M.; Chan, E.C.; Riley, S.C.; Nahar, P.; Smith, R. G-1-activated membrane estrogen receptors mediate increased contractility of the human myometrium. Endocrinology 2011, 152, 2448–2455. [Google Scholar] [CrossRef]
- Lindsey, S.H.; Yamaleyeva, L.M.; Brosnihan, K.B.; Gallagher, P.E.; Chappell, M.C. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 2011, 58, 665–671. [Google Scholar] [CrossRef]
- Akama, K.T.; Thompson, L.I.; Milner, T.A.; McEwen, B.S. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J. Biol. Chem. 2013, 288, 6438–6450. [Google Scholar] [CrossRef]
- Sakamoto, H.; Matsuda, K.; Hosokawa, K.; Nishi, M.; Morris, J.F.; Prossnitz, E.R.; Kawata, M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology 2007, 148, 5842–5850. [Google Scholar] [CrossRef]
- Otto, C.; Rohde-Schulz, B.; Schwarz, G.; Fuchs, I.; Klewer, M.; Brittain, D.; Langer, G.; Bader, B.; Prelle, K.; Nubbemeyer, R.; et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 2008, 149, 4846–4856. [Google Scholar] [CrossRef]
- Revankar, C.M.; Mitchell, H.D.; Field, A.S.; Burai, R.; Corona, C.; Ramesh, C.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem. Biol. 2007, 2, 536–544. [Google Scholar] [CrossRef]
- Deliu, E.; Brailoiu, G.C.; Arterburn, J.B.; Oprea, T.I.; Benamar, K.; Dun, N.J.; Brailoiu, E. Mechanisms of G protein-coupled estrogen receptor-mediated spinal nociception. J. Pain. 2012, 13, 742–754. [Google Scholar] [CrossRef]
- Warfvinge, K.; Krause, D.N.; Maddahi, A.; Edvinsson, J.C.A.; Edvinsson, L.; Haanes, K.A. Estrogen receptors alpha, beta and GPER in the CNS and trigeminal system–molecular and functional aspects. J. Headache Pain. 2020, 21, 131. [Google Scholar] [CrossRef]
- Pena-Gutierrez, K.M.; Hernandez-Ortega, K.; Bello-Alvarez, C.; Camacho-Arroyo, I. Expression and estrogen regulation of G protein-coupled estrogen receptor in human glioblastoma cells. Oncol. Lett. 2022, 24, 397. [Google Scholar] [CrossRef]
- Cheng, S.B.; Graeber, C.T.; Quinn, J.A.; Filardo, E.J. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids 2011, 76, 892–896. [Google Scholar] [CrossRef]
- Ramirez-Lopez, I.G.; Ramirez de Arellano, A.; Jave-Suarez, L.F.; Hernandez-Silva, C.D.; Garcia-Chagollan, M.; Hernandez-Bello, J.; Lopez-Pulido, E.I.; Macias-Barragan, J.; Montoya-Buelna, M.; Munoz-Valle, J.F.; et al. Interaction between 17beta-estradiol, prolactin and human papillomavirus induce E6/E7 transcript and modulate the expression and localization of hormonal receptors. Cancer Cell Int. 2019, 19, 227. [Google Scholar] [CrossRef]
- Caron, K.M.; James, L.R.; Kim, H.S.; Morham, S.G.; Sequeira Lopez, M.L.; Gomez, R.A.; Reudelhuber, T.L.; Smithies, O. A genetically clamped renin transgene for the induction of hypertension. Proc. Natl. Acad. Sci. USA 2002, 99, 8248–8252. [Google Scholar] [CrossRef]
- Caron, K.M.; James, L.R.; Kim, H.S.; Knowles, J.; Uhlir, R.; Mao, L.; Hagaman, J.R.; Cascio, W.; Rockman, H.; Smithies, O. Cardiac hypertrophy and sudden death in mice with a genetically clamped renin transgene. Proc. Natl. Acad. Sci. USA 2004, 101, 3106–3111. [Google Scholar] [CrossRef]
- Caron, K.M.; James, L.R.; Lee, G.; Kim, H.S.; Smithies, O. Lifelong genetic minipumps. Physiol. Genom. 2005, 20, 203–209. [Google Scholar] [CrossRef]
- Lenhart, P.M.; Broselid, S.; Barrick, C.J.; Leeb-Lundberg, L.M.; Caron, K.M. G-protein-coupled receptor 30 interacts with receptor activity-modifying protein 3 and confers sex-dependent cardioprotection. J. Mol. Endocrinol. 2013, 51, 191–202. [Google Scholar] [CrossRef]
- Cheng, S.B.; Quinn, J.A.; Graeber, C.T.; Filardo, E.J. Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J. Biol. Chem. 2011, 286, 22441–22455. [Google Scholar] [CrossRef]
- Lappano, R.; Rosano, C.; De Marco, P.; De Francesco, E.M.; Pezzi, V.; Maggiolini, M. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Mol. Cell. Endocrinol. 2010, 320, 162–170. [Google Scholar] [CrossRef]
- Hess, R.A. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 2003, 1, 52. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef]
- Martensson, U.E.; Salehi, S.A.; Windahl, S.; Gomez, M.F.; Sward, K.; Daszkiewicz-Nilsson, J.; Wendt, A.; Andersson, N.; Hellstrand, P.; Grande, P.O.; et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009, 150, 687–698. [Google Scholar] [CrossRef]
- Haas, E.; Bhattacharya, I.; Brailoiu, E.; Damjanovic, M.; Brailoiu, G.C.; Gao, X.; Mueller-Guerre, L.; Marjon, N.A.; Gut, A.; Minotti, R.; et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ. Res. 2009, 104, 288–291. [Google Scholar] [CrossRef]
- Meyer, M.R.; Amann, K.; Field, A.S.; Hu, C.; Hathaway, H.J.; Kanagy, N.L.; Walker, M.K.; Barton, M.; Prossnitz, E.R. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension 2012, 59, 507–512. [Google Scholar] [CrossRef]
- Delbeck, M.; Golz, S.; Vonk, R.; Janssen, W.; Hucho, T.; Isensee, J.; Schafer, S.; Otto, C. Impaired left-ventricular cardiac function in male GPR30-deficient mice. Mol. Med. Rep. 2011, 4, 37–40. [Google Scholar] [CrossRef]
- Meoli, L.; Isensee, J.; Zazzu, V.; Nabzdyk, C.S.; Soewarto, D.; Witt, H.; Foryst-Ludwig, A.; Kintscher, U.; Noppinger, P.R. Sex- and age-dependent effects of Gpr30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene 2014, 540, 210–216. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Chou, J.; Lin, M.; Ferrario, C.M.; Zapata-Sudo, G.; Groban, L. Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: A sex-specific gene profiling analysis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Lin, M.S.; Ferrario, C.M.; Van Remmen, H.; Groban, L. G protein-coupled estrogen receptor (GPER) deficiency induces cardiac remodeling through oxidative stress. Transl. Res. 2018, 199, 39–51. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Hodge, H.S.; Ferrario, C.M.; Groban, L. NLRP3 inhibition improves heart function in GPER knockout mice. Biochem. Biophys. Res. Commun. 2019, 514, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R., Jr.; Bland, K.I. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002, 16, 70–84. [Google Scholar] [CrossRef]
- Lindsey, S.H.; Liu, L.; Chappell, M.C. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 2014, 81, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Prossnitz, E.R. Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol. Metab. 2015, 26, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, G.H.; Jin, S.W.; Pham, T.H.; Thai, T.N.; Kim, J.Y.; Kim, C.Y.; Han, E.H.; Hwang, Y.P.; Choi, C.Y.; et al. G protein-coupled estrogen receptor regulates the KLF2-dependent eNOS expression by activating of Ca2+ and EGFR signaling pathway in human endothelial cells. Biochem. Pharmacol. 2021, 192, 114721. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, Y.; Zhang, L.; Liu, W. Involvement of estrogen receptor and GPER in bisphenol A induced proliferation of vascular smooth muscle cells. Toxicol. Vitr. 2019, 56, 156–162. [Google Scholar] [CrossRef]
- Kokai, D.; Stanic, B.; Tesic, B.; Samardzija Nenadov, D.; Pogrmic-Majkic, K.; Fa Nedeljkovic, S.; Andric, N. Dibutyl phthalate promotes angiogenesis in EA.hy926 cells through estrogen receptor-dependent activation of ERK1/2, PI3K-Akt, and NO signaling pathways. Chem. Biol. Interact. 2022, 366, 110174. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, A.M.; Murphy, E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1806–H1813. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; da Cruz Junho, C.V.; Bopassa, J.C. Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br. J. Pharmacol. 2017, 174, 4329–4344. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zaid, O.A.; Moawed, F.S.; Hassan, H.A.; Moustafa, E.M. Bisphenol-A/Radiation mediated inflammatory response activates EGFR/KRAS/ERK1/2 signaling pathway leads to lung carcinogenesis incidence. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221092918. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, S.; Luo, H.; Chen, C.; Zhang, X.; He, L.; Tu, G. GPR30-mediated HMGB1 upregulation in CAFs induces autophagy and tamoxifen resistance in ERalpha-positive breast cancer cells. Aging 2021, 13, 16178–16197. [Google Scholar] [CrossRef]
- Yin, J.; Tu, G.; Peng, M.; Zeng, H.; Wan, X.; Qiao, Y.; Qin, Y.; Liu, M.; Luo, H. GPER-regulated lncRNA-Glu promotes glutamate secretion to enhance cellular invasion and metastasis in triple-negative breast cancer. FASEB J. 2020, 34, 4557–4572. [Google Scholar] [CrossRef]
- Li, M.; Guo, J.; Gao, W.; Yu, J.; Han, X.; Zhang, J.; Shao, B. Bisphenol AF-induced endogenous transcription is mediated by ERalpha and ERK1/2 activation in human breast cancer cells. PLoS ONE 2014, 9, e94725. [Google Scholar] [CrossRef][Green Version]
- Li, F.; Yu, X.; Szynkarski, C.K.; Meng, C.; Zhou, B.; Barhoumi, R.; White, R.E.; Heaps, C.L.; Stallone, J.N.; Han, G. Activation of GPER Induces Differentiation and Inhibition of Coronary Artery Smooth Muscle Cell Proliferation. PLoS ONE 2013, 8, e64771. [Google Scholar] [CrossRef]
- Upadhayay, S.; Gupta, R.; Singh, S.; Mundkar, M.; Singh, G.; Kumar, P. Involvement of the G-Protein-Coupled Estrogen Receptor-1 (GPER) Signaling Pathway in Neurodegenerative Disorders: A Review. Cell Mol. Neurobiol. 2022, 43, 1833–1847. [Google Scholar] [CrossRef]
- Wang, X.W.; Yuan, L.J.; Yang, Y.; Zhang, M.; Chen, W.F. IGF-1 inhibits MPTP/MPP+-induced autophagy on dopaminergic neurons through the IGF-1R/PI3K-Akt-mTOR pathway and GPER. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E734–E743. [Google Scholar] [CrossRef]
- Ariyani, W.; Miyazaki, W.; Amano, I.; Hanamura, K.; Shirao, T.; Koibuchi, N. Soy Isoflavones Accelerate Glial Cell Migration via GPER-Mediated Signal Transduction Pathway. Front. Endocrinol. 2020, 11, 554941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Liu, N.; Weng, S.F.; Wang, H.S. Bisphenol A Increases the Migration and Invasion of Triple-Negative Breast Cancer Cells via Oestrogen-related Receptor Gamma. Basic Clin. Pharmacol. Toxicol. 2016, 119, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, F.; Klussmann, E.; Stallone, J.N.; Han, G. G protein-coupled estrogen receptor 1 mediates relaxation of coronary arteries via cAMP/PKA-dependent activation of MLCP. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E398–E407. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Q.; Zhao, Y.; Schwarz, B.J.; Stallone, J.N.; Heaps, C.L.; Han, G. Activation of G protein-coupled estrogen receptor 1 induces coronary artery relaxation via Epac/Rap1-mediated inhibition of RhoA/Rho kinase pathway in parallel with PKA. PLoS ONE 2017, 12, e0173085. [Google Scholar] [CrossRef]
- Lau, K.M.; Ma, F.M.; Xia, J.T.; Chan, Q.K.Y.; Ng, C.F.; To, K.F. Activation of GPR30 stimulates GTP-binding of Galphai1 protein to sustain activation of Erk1/2 in inhibition of prostate cancer cell growth and modulates metastatic properties. Exp. Cell Res. 2017, 350, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, H.; Yang, Y.; Jiang, Z.; Ma, H. Dehydroepiandrosterone protects against oleic acid-triggered mitochondrial dysfunction to relieve oxidative stress and inflammation via activation of the AMPK-Nrf2 axis by targeting GPR30 in hepatocytes. Mol. Immunol. 2023, 155, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Su, G.; Zhang, L.; Liu, G.; Zhou, Y.; Fang, S.; Zhang, Q.; Wang, T.; Huang, C.; Huang, Z.; et al. Icaritin inhibits neuroinflammation in a rat cerebral ischemia model by regulating microglial polarization through the GPER-ERK-NF-kappaB signaling pathway. Mol. Med. 2022, 28, 142. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Suzuki, T.; Mizukami, Y.; Ikeda, T. The membrane-type estrogen receptor G-protein-coupled estrogen receptor suppresses lipopolysaccharide-induced interleukin 6 via inhibition of nuclear factor-kappa B pathway in murine macrophage cells. Anim. Sci. J. 2017, 88, 1870–1879. [Google Scholar] [CrossRef]
- Zhu, P.; Liao, L.Y.; Zhao, T.T.; Mo, X.M.; Chen, G.G.; Liu, Z.M. GPER/ERK&AKT/NF-kappaB pathway is involved in cadmium-induced proliferation, invasion and migration of GPER-positive thyroid cancer cells. Mol. Cell. Endocrinol. 2017, 442, 68–80. [Google Scholar] [CrossRef]
- Liang, S.; Chen, Z.; Jiang, G.; Zhou, Y.; Liu, Q.; Su, Q.; Wei, W.; Du, J.; Wang, H. Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-kappaB/IL-6 signals. Cancer Lett. 2017, 386, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Huang, Y.; Wu, C.; Wei, D.; Shi, Y. Activation of G-Protein-Coupled Estrogen Receptor Inhibits the Migration of Human Nonsmall Cell Lung Cancer Cells via IKK-beta/NF-kappaB Signals. DNA Cell Biol. 2016, 35, 434–442. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wei, W.; Jiang, G.M.; Liu, H.; Wei, W.D.; Yang, X.; Wu, Y.M.; Liu, H.; Wong, C.K.; Du, J.; et al. Activation of GPER suppresses epithelial mesenchymal transition of triple negative breast cancer cells via NF-kappaB signals. Mol. Oncol. 2016, 10, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W. Aldosterone and mineralocorticoid receptors in the cardiovascular system. Prog. Cardiovasc. Dis. 2010, 52, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.D.; Gros, R. Unraveling the mechanisms underlying the rapid vascular effects of steroids: Sorting out the receptors and the pathways. Br. J. Pharmacol. 2011, 163, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Wendler, A.; Albrecht, C.; Wehling, M. Nongenomic actions of aldosterone and progesterone revisited. Steroids 2012, 77, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Gros, R.; Ding, Q.; Sklar, L.A.; Prossnitz, E.E.; Arterburn, J.B.; Chorazyczewski, J.; Feldman, R.D. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension 2011, 57, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Gros, R.; Ding, Q.; Liu, B.; Chorazyczewski, J.; Feldman, R.D. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am. J. Physiol. Cell Physiol. 2013, 304, C532–C540. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Meyer, M.R. Permissive Role of GPER for Arterial Hypertension. Hypertension 2019, 73, e9–e10. [Google Scholar] [CrossRef] [PubMed]
- Waghulde, H.; Galla, S.; Chakraborty, S.; Joe, B. Response to Permissive Role of GPER for Arterial Hypertension. Hypertension 2019, 73, e11. [Google Scholar] [CrossRef]
- Wendler, A.; Wehling, M. Is GPR30 the membrane aldosterone receptor postulated 20 years ago? Hypertension 2011, 57, e16, author reply e17. [Google Scholar] [CrossRef]
- Ding, Q.; Chorazyczewski, J.; Gros, R.; Motulsky, H.J.; Limbird, L.E.; Feldman, R.D. Correlation of functional and radioligand binding characteristics of GPER ligands confirming aldosterone as a GPER agonist. Pharmacol. Res. Perspect. 2022, 10, e00995. [Google Scholar] [CrossRef]
- Ashton, A.W.; Le, T.Y.; Gomez-Sanchez, C.E.; Morel-Kopp, M.C.; McWhinney, B.; Hudson, A.; Mihailidou, A.S. Role of Nongenomic Signaling Pathways Activated by Aldosterone During Cardiac Reperfusion Injury. Mol. Endocrinol. 2015, 29, 1144–1155. [Google Scholar] [CrossRef]
- De Giusti, V.C.; Orlowski, A.; Ciancio, M.C.; Espejo, M.S.; Gonano, L.A.; Caldiz, C.I.; Vila Petroff, M.G.; Villa-Abrille, M.C.; Aiello, E.A. Aldosterone stimulates the cardiac sodium/bicarbonate cotransporter via activation of the g protein-coupled receptor gpr30. J. Mol. Cell. Cardiol. 2015, 89, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, A.; De Giusti, V.C.; Ciancio, M.C.; Espejo, M.S.; Aiello, E.A. The cardiac electrogenic sodium/bicarbonate cotransporter (NBCe1) is activated by aldosterone through the G protein-coupled receptor 30 (GPR 30). Channels 2016, 10, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, G.C.; Benamar, K.; Arterburn, J.B.; Gao, E.; Rabinowitz, J.E.; Koch, W.J.; Brailoiu, E. Aldosterone increases cardiac vagal tone via G protein-coupled oestrogen receptor activation. J. Physiol. 2013, 591, 4223–4235. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; D’Ambrosio, M.A.; Garvin, J.L.; Leung, P.; Kutskill, K.; Wang, H.; Peterson, E.L.; Carretero, O.A. Aldosterone sensitizes connecting tubule glomerular feedback via the aldosterone receptor GPR30. Am. J. Physiol. Ren. Physiol. 2014, 307, F427–F434. [Google Scholar] [CrossRef]
- Cheng, L.; Poulsen, S.B.; Wu, Q.; Esteva-Font, C.; Olesen, E.T.B.; Peng, L.; Olde, B.; Leeb-Lundberg, L.M.F.; Pisitkun, T.; Rieg, T.; et al. Rapid Aldosterone-Mediated Signaling in the DCT Increases Activity of the Thiazide-Sensitive NaCl Cotransporter. J. Am. Soc. Nephrol. 2019, 30, 1454–1470. [Google Scholar] [CrossRef] [PubMed]
- Caroccia, B.; Seccia, T.M.; Piazza, M.; Prisco, S.; Zanin, S.; Iacobone, M.; Lenzini, L.; Pallafacchina, G.; Domening, O.; Poglitsch, M.; et al. Aldosterone Stimulates Its Biosynthesis Via a Novel GPER-Mediated Mechanism. J. Clin. Endocrinol. Metab. 2019, 104, 6316–6324. [Google Scholar] [CrossRef] [PubMed]
- Caroccia, B.; Seccia, T.M.; Campos, A.G.; Gioco, F.; Kuppusamy, M.; Ceolotto, G.; Guerzoni, E.; Simonato, F.; Mareso, S.; Lenzini, L.; et al. GPER-1 and estrogen receptor-beta ligands modulate aldosterone synthesis. Endocrinology 2014, 155, 4296–4304. [Google Scholar] [CrossRef]
- Rossi, G.P.; Caroccia, B.; Seccia, T.M. Role of estrogen receptors in modulating aldosterone biosynthesis and blood pressure. Steroids 2019, 152, 108486. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Haggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Bologa, C.G.; Revankar, C.M.; Young, S.M.; Edwards, B.S.; Arterburn, J.B.; Kiselyov, A.S.; Parker, M.A.; Tkachenko, S.E.; Savchuck, N.P.; Sklar, L.A.; et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006, 2, 207–212. [Google Scholar] [CrossRef]
- Dennis, M.K.; Burai, R.; Ramesh, C.; Petrie, W.K.; Alcon, S.N.; Nayak, T.K.; Bologa, C.G.; Leitao, A.; Brailoiu, E.; Deliu, E.; et al. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 2009, 5, 421–427. [Google Scholar] [CrossRef]
- Dennis, M.K.; Field, A.S.; Burai, R.; Ramesh, C.; Petrie, W.K.; Bologa, C.G.; Oprea, T.I.; Yamaguchi, Y.; Hayashi, S.; Sklar, L.A.; et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J. Steroid Biochem. Mol. Biol. 2011, 127, 358–366. [Google Scholar] [CrossRef]
- Prossnitz, E.R. GPER modulators: Opportunity Nox on the heels of a class Akt. J. Steroid Biochem. Mol. Biol. 2018, 176, 73–81. [Google Scholar] [CrossRef]
- Gui, Y.; Shi, Z.; Wang, Z.; Li, J.J.; Xu, C.; Tian, R.; Song, X.; Walsh, M.P.; Li, D.; Gao, J.; et al. The GPER agonist G-1 induces mitotic arrest and apoptosis in human vascular smooth muscle cells independent of GPER. J. Cell. Physiol. 2015, 230, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Grande, P.O.; Luduena, R.F.; Olde, B.; Prasad, V.; Leeb-Lundberg, L.M.; Nilsson, B.O. The G protein-coupled oestrogen receptor 1 agonist G-1 disrupts endothelial cell microtubule structure in a receptor-independent manner. Mol. Cell. Biochem. 2012, 366, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Baldetorp, B.; Olde, B.; Leeb-Lundberg, L.M.; Nilsson, B.O. The GPER1 agonist G-1 attenuates endothelial cell proliferation by inhibiting DNA synthesis and accumulating cells in the S and G2 phases of the cell cycle. J. Vasc. Res. 2011, 48, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ito, F.; Matsushima, H.; Takaoka, O.; Tanaka, Y.; Koshiba, A.; Kusuki, I.; Kitawaki, J. G protein-coupled estrogen receptor 1 agonist G-1 induces cell cycle arrest in the mitotic phase, leading to apoptosis in endometriosis. Fertil. Steril. 2015, 103, 1228–1235.e1. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, J.B.; Oprea, T.I.; Prossnitz, E.R.; Edwards, B.S.; Sklar, L.A. Discovery of selective probes and antagonists for G-protein-coupled receptors FPR/FPRL1 and GPR30. Curr. Top. Med. Chem. 2009, 9, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Rosano, C.; Santolla, M.F.; Pupo, M.; De Francesco, E.M.; De Marco, P.; Ponassi, M.; Spallarossa, A.; Ranise, A.; Maggiolini, M. Two novel GPER agonists induce gene expression changes and growth effects in cancer cells. Curr. Cancer Drug Targets 2012, 12, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Babiloni-Chust, I.; Dos Santos, R.S.; Medina-Gali, R.M.; Perez-Serna, A.A.; Encinar, J.A.; Martinez-Pinna, J.; Gustafsson, J.A.; Marroqui, L.; Nadal, A. G protein-coupled estrogen receptor activation by bisphenol-A disrupts the protection from apoptosis conferred by the estrogen receptors ERalpha and ERbeta in pancreatic beta cells. Environ. Int. 2022, 164, 107250. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Gao, Z.; Liu, S.; Tan, L.; Wu, Y.; Liu, J.; Zheng, Z.; Fan, W.; Luo, Y.; Chen, Z.; et al. G protein-coupled estrogen receptor activation by bisphenol-A disrupts lipid metabolism and induces ferroptosis in the liver. Environ. Pollut. 2023, 334, 122211. [Google Scholar] [CrossRef] [PubMed]
- Pupo, M.; Pisano, A.; Lappano, R.; Santolla, M.F.; De Francesco, E.M.; Abonante, S.; Rosano, C.; Maggiolini, M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2012, 120, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Gu, Z.W.; Hao, L.Y. The environmental hormone nonylphenol interferes with the therapeutic effects of G protein-coupled estrogen receptor specific agonist G-1 on murine allergic rhinitis. Int. Immunopharmacol. 2020, 78, 106058. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, S.; Chen, Z.; Sun, X.; Gao, Q.; Lei, M.; Hao, L. Exploration of interaction property between nonylphenol and G protein-coupled receptor 30 based on molecular simulation and biological experiments. Steroids 2022, 188, 109114. [Google Scholar] [CrossRef] [PubMed]
- Albanito, L.; Lappano, R.; Madeo, A.; Chimento, A.; Prossnitz, E.R.; Cappello, A.R.; Dolce, V.; Abonante, S.; Pezzi, V.; Maggiolini, M. Effects of atrazine on estrogen receptor alpha- and G protein-coupled receptor 30-mediated signaling and proliferation in cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2015, 123, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Albanito, L.; Lappano, R.; Madeo, A.; Chimento, A.; Prossnitz, E.R.; Cappello, A.R.; Dolce, V.; Abonante, S.; Pezzi, V.; Maggiolini, M. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ. Health Perspect. 2008, 116, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Reyes, S.; Vargas-Castillo, A.; Noriega, L.G.; Velazquez-Villegas, L.A.; Perez, B.; Sanchez-Tapia, M.; Ordaz, G.; Suarez-Monroy, R.; Ulloa-Aguirre, A.; Offner, H.; et al. Genistein Stimulation of White Adipose Tissue Thermogenesis Is Partially Dependent on GPR30 in Mice. Mol. Nutr. Food Res. 2022, 66, e2100838. [Google Scholar] [CrossRef] [PubMed]
- Thent, Z.C.; Froemming, G.R.A.; Ismail, A.B.M.; Fuad, S.; Muid, S. Phytoestrogens by inhibiting the non-classical oestrogen receptor, overcome the adverse effect of bisphenol A on hFOB 1.19 cells. Iran. J. Basic Med. Sci. 2020, 23, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Y.L.; Xu, M.L.; Jin, Y.; Wu, Q.; Dong, T.T.X.; Tsim, K.W.K. Genistein, a Phytoestrogen in Soybean, Induces the Expression of Acetylcholinesterase via G Protein-Coupled Receptor 30 in PC12 Cells. Front. Mol. Neurosci. 2018, 11, 59. [Google Scholar] [CrossRef]
- Kajta, M.; Rzemieniec, J.; Litwa, E.; Lason, W.; Lenartowicz, M.; Krzeptowski, W.; Wojtowicz, A.K. The key involvement of estrogen receptor beta and G-protein-coupled receptor 30 in the neuroprotective action of daidzein. Neuroscience 2013, 238, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.J.; Chapple, S.; Siow, R.C.; Mann, G.E. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: Roles for F-actin and GPR30. Hypertension 2011, 57, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Santolla, M.F.; Maggiolini, M.; Pezzi, V.; et al. Oleuropein and hydroxytyrosol activate GPER/ GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wang, X.; Feng, C.; Liu, D.; Liu, Y.; Liu, Y.; Li, J.; Zhang, J.; Li, N.; Deng, Y.; et al. Study of the Sensing Kinetics of G Protein-Coupled Estrogen Receptor Sensors for Common Estrogens and Estrogen Analogs. Molecules 2023, 28, 3286. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Gianquinto, E.; Rossetti, G.; Cruciani, G.; Lorenzetti, S.; Spyrakis, F. Binding of Androgen- and Estrogen-Like Flavonoids to Their Cognate (Non)Nuclear Receptors: A Comparison by Computational Prediction. Molecules 2021, 26, 1613. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.H.; Chen, J.C.; He, Y.L.; Xu, J.J.; Mei, Y.A. Resveratrol inhibits K(v)2.2 currents through the estrogen receptor GPR30-mediated PKC pathway. Am. J. Physiol. Cell Physiol. 2013, 305, C547–C557. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Vivacqua, A.; Fasanella, G.; Recchia, A.G.; Sisci, D.; Pezzi, V.; Montanaro, D.; Musti, A.M.; Picard, D.; Ando, S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J. Biol. Chem. 2004, 279, 27008–27016. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, S.B.; Jung, S.H.; Cha, K.H.; Park, W.D.; Sohn, Y.C.; Nho, C.W. Tectoridin, a poor ligand of estrogen receptor alpha, exerts its estrogenic effects via an ERK-dependent pathway. Mol. Cells 2009, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wei, C.; Li, Y.; Liu, Y.; Wang, Y.; Pan, J.; Liu, J.; Wu, Y.; Cui, S. Zearalenone and alpha-zearalenol inhibit the synthesis and secretion of pig follicle stimulating hormone via the non-classical estrogen membrane receptor GPR30. Mol. Cell. Endocrinol. 2018, 461, 43–54. [Google Scholar] [CrossRef]
- Nakamura, U.; Kadokawa, H. The nonsteroidal mycoestrogen zearalenone and its five metabolites suppress LH secretion from the bovine anterior pituitary cells via the estradiol receptor GPR30 in vitro. Theriogenology 2015, 84, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Perego, P.; Palombo, D. Apigenin inhibits the TNFalpha-induced expression of eNOS and MMP-9 via modulating Akt signalling through oestrogen receptor engagement. Mol. Cell. Biochem. 2012, 371, 129–136. [Google Scholar] [CrossRef]
- Koganti, S.; Snyder, R.; Gumaste, U.; Karamyan, V.T.; Thekkumkara, T. 2-methoxyestradiol binding of GPR30 down-regulates angiotensin AT(1) receptor. Eur. J. Pharmacol. 2014, 723, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, T.K.; Pang, Y.; Thomas, P. The catecholestrogen, 2-hydroxyestradiol-17beta, acts as a G protein-coupled estrogen receptor 1 (GPER/GPR30) antagonist to promote the resumption of meiosis in zebrafish oocytes. Biol. Reprod. 2015, 92, 69. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Dong, J.; Pang, Y.; LaRocca, J.; Hixon, M.; Thomas, P.; Filardo, E.J. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol. Cell. Endocrinol. 2014, 382, 950–959. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Jamal, S.F. Essential Hypertension. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Curfman, G.; Bauchner, H.; Greenland, P. Treatment and Control of Hypertension in 2020: The Need for Substantial Improvement. JAMA 2020, 324, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Tahvanainen, A.; Taurio, J.; Maki-Jouppi, J.; Koobi, P.; Mustonen, J.; Kahonen, M.; Sand, J.; Nordback, I.; Porsti, I. Increased wall tension in response to vasoconstrictors in isolated mesenteric arterial rings from patients with high blood pressure. Basic Clin. Pharmacol. Toxicol. 2006, 99, 440–449. [Google Scholar] [CrossRef]
- O’Dowd, B.F.; Nguyen, T.; Marchese, A.; Cheng, R.; Lynch, K.R.; Heng, H.H.; Kolakowski, L.F., Jr.; George, S.R. Discovery of three novel G-protein-coupled receptor genes. Genomics 1998, 47, 310–313. [Google Scholar] [CrossRef]
- Stowasser, M.; Gordon, R.D. Aldosterone excess, hypertension, and chromosome 7p22: Evidence continues to mount. Hypertension 2007, 49, 761–762. [Google Scholar] [CrossRef]
- Liu, S.; Ding, T.; Liu, H.; Jian, L. GPER was Associated with Hypertension in Post-Menopausal Women. Open Med. 2018, 13, 338–343. [Google Scholar] [CrossRef]
- Feldman, R.D.; Gros, R.; Ding, Q.; Hussain, Y.; Ban, M.R.; McIntyre, A.D.; Hegele, R.A. A common hypofunctional genetic variant of GPER is associated with increased blood pressure in women. Br. J. Clin. Pharmacol. 2014, 78, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Vinh, A.; Kim, H.A.; Saini, N.; Broughton, B.R.S.; Chrissobolis, S.; Diep, H.; Judkins, C.P.; Drummond, G.R.; Sobey, C.G. Aldosterone-induced hypertension is sex-dependent, mediated by T cells and sensitive to GPER activation. Cardiovasc. Res. 2021, 117, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kashyap, S.; Murphy, B.; Hutson, D.D.; Budish, R.A.; Trimmer, E.H.; Zimmerman, M.A.; Trask, A.J.; Miller, K.S.; Chappell, M.C.; et al. GPER activation ameliorates aortic remodeling induced by salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H953–H961. [Google Scholar] [CrossRef]
- Delgado, N.T.B.; Rouver, W.D.N.; Freitas-Lima, L.C.; Vieira-Alves, I.; Lemos, V.S.; Dos Santos, R.L. Sex Differences in the Vasodilation Mediated by G Protein-Coupled Estrogen Receptor (GPER) in Hypertensive Rats. Front. Physiol. 2021, 12, 659291. [Google Scholar] [CrossRef]
- Meyer, M.R.; Prossnitz, E.R.; Barton, M. GPER/GPR30 and Regulation of Vascular Tone and Blood Pressure. Immunol. Endocr. Metab. Agents Med. Chem. 2011, 11, 255–261. [Google Scholar] [CrossRef]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. 2012, 14, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Liu, J.C.; Liu, S.B.; Li, X.Q.; Yi, D.H.; Zhao, M.G. Improvement of vascular function by acute and chronic treatment with the GPR30 agonist G1 in experimental diabetes mellitus. PLoS ONE 2012, 7, e38787. [Google Scholar] [CrossRef]
- Jang, E.J.; Seok, Y.M.; Arterburn, J.B.; Olatunji, L.A.; Kim, I.K. GPER-1 agonist G1 induces vasorelaxation through activation of epidermal growth factor receptor-dependent signalling pathway. J. Pharm. Pharmacol. 2013, 65, 1488–1499. [Google Scholar] [CrossRef]
- Bai, J.; Qi, Q.R.; Li, Y.; Day, R.; Makhoul, J.; Magness, R.R.; Chen, D.B. Estrogen Receptors and Estrogen-Induced Uterine Vasodilation in Pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef]
- Tropea, T.; De Francesco, E.M.; Rigiracciolo, D.; Maggiolini, M.; Wareing, M.; Osol, G.; Mandala, M. Pregnancy Augments G Protein Estrogen Receptor (GPER) Induced Vasodilation in Rat Uterine Arteries via the Nitric Oxide–cGMP Signaling Pathway. PLoS ONE 2015, 10, e0141997. [Google Scholar] [CrossRef]
- Batenburg, W.W.; Jansen, P.M.; van den Bogaerdt, A.J.; AH, J.D. Angiotensin II-aldosterone interaction in human coronary microarteries involves GPR30, EGFR, and endothelial NO synthase. Cardiovasc. Res. 2012, 94, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Young, B.D.; Bender, J.R. Endothelial estrogen receptor isoforms and cardiovascular disease. Mol. Cell. Endocrinol. 2014, 389, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.; Aires, R.D.; Lemos, V.S.; Bissoli, N.S.; Santos, R.L.D. GPER agonist dilates mesenteric arteries via PI3K-Akt-eNOS and potassium channels in both sexes. Life Sci. 2017, 183, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.; Vieira-Alves, I.; Couto, G.K.; Lemos, V.S.; Rossoni, L.V.; Bissoli, N.S.; Santos, R.L.D. Sex differences in the participation of endothelial mediators and signaling pathways involved in the vasodilator effect of a selective GPER agonist in resistance arteries of gonadectomized Wistar rats. Life Sci. 2022, 308, 120917. [Google Scholar] [CrossRef] [PubMed]
- Browner, N.C.; Dey, N.B.; Bloch, K.D.; Lincoln, T.M. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J. Biol. Chem. 2004, 279, 46631–46636. [Google Scholar] [CrossRef]
- Yu, X.; Ma, H.; Barman, S.A.; Liu, A.T.; Sellers, M.; Stallone, J.N.; Prossnitz, E.R.; White, R.E.; Han, G. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E882–E888. [Google Scholar] [CrossRef]
- Yu, X.; Stallone, J.N.; Heaps, C.L.; Han, G. The activation of G protein-coupled estrogen receptor induces relaxation via cAMP as well as potentiates contraction via EGFR transactivation in porcine coronary arteries. PLoS ONE 2018, 13, e0191418. [Google Scholar] [CrossRef]
- Keung, W.; Vanhoutte, P.M.; Man, R.Y. Acute impairment of contractile responses by 17beta-estradiol is cAMP and protein kinase G dependent in vascular smooth muscle cells of the porcine coronary arteries. Br. J. Pharmacol. 2005, 144, 71–79. [Google Scholar] [CrossRef][Green Version]
- Nadadur, R.D.; Umar, S.; Wong, G.; Eghbali, M.; Iorga, A.; Matori, H.; Partow-Navid, R.; Eghbali, M. Reverse right ventricular structural and extracellular matrix remodeling by estrogen in severe pulmonary hypertension. J. Appl. Physiol. 2012, 113, 149–158. [Google Scholar] [CrossRef]
- Frost, A.E.; Badesch, D.B.; Barst, R.J.; Benza, R.L.; Elliott, C.G.; Farber, H.W.; Krichman, A.; Liou, T.G.; Raskob, G.E.; Wason, P.; et al. The changing picture of patients with pulmonary arterial hypertension in the United States: How REVEAL differs from historic and non-US Contemporary Registries. Chest 2011, 139, 128–137. [Google Scholar] [CrossRef]
- Resta, T.C.; Kanagy, N.L.; Walker, B.R. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L88–L97. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Rabinovitch, M.; Eghbali, M. Estrogen paradox in pulmonary hypertension: Current controversies and future perspectives. Am. J. Respir. Crit. Care Med. 2012, 186, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sangam, S.; Guo, Q.; Wang, J.; Tang, H.; Black, S.M.; Desai, A.A. Sex Differences, Estrogen Metabolism and Signaling in the Development of Pulmonary Arterial Hypertension. Front. Cardiovasc. Med. 2021, 8, 719058. [Google Scholar] [CrossRef] [PubMed]
- Alencar, A.K.; Montes, G.C.; Montagnoli, T.; Silva, A.M.; Martinez, S.T.; Fraga, A.G.; Wang, H.; Groban, L.; Sudo, R.T.; Zapata-Sudo, G. Activation of GPER ameliorates experimental pulmonary hypertension in male rats. Eur. J. Pharm. Sci. 2017, 97, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Alencar, A.K.N.; Montes, G.C.; Costa, D.G.; Mendes, L.V.P.; Silva, A.M.S.; Martinez, S.T.; Trachez, M.M.; Cunha, V.; Montagnoli, T.L.; Fraga, A.G.M.; et al. Cardioprotection Induced by Activation of GPER in Ovariectomized Rats With Pulmonary Hypertension. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1158–1166. [Google Scholar] [CrossRef]
- Insull, W., Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef]
- Joakimsen, O.; Bonaa, K.H.; Stensland-Bugge, E.; Jacobsen, B.K. Population-based study of age at menopause and ultrasound assessed carotid atherosclerosis: The Tromso Study. J. Clin. Epidemiol. 2000, 53, 525–530. [Google Scholar] [CrossRef]
- Sharma, G.; Hu, C.; Brigman, J.L.; Zhu, G.; Hathaway, H.J.; Prossnitz, E.R. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology 2013, 154, 4136–4145. [Google Scholar] [CrossRef]
- Hussain, Y.; Ding, Q.; Connelly, P.W.; Brunt, J.H.; Ban, M.R.; McIntyre, A.D.; Huff, M.W.; Gros, R.; Hegele, R.A.; Feldman, R.D. G-protein estrogen receptor as a regulator of low-density lipoprotein cholesterol metabolism: Cellular and population genetic studies. Arter. Thromb. Vasc. Biol. 2015, 35, 213–221. [Google Scholar] [CrossRef]
- Ghaffari, S.; Naderi Nabi, F.; Sugiyama, M.G.; Lee, W.L. Estrogen Inhibits LDL (Low-Density Lipoprotein) Transcytosis by Human Coronary Artery Endothelial Cells via GPER (G-Protein-Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arter. Thromb. Vasc. Biol. 2018, 38, 2283–2294. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Davidge, S.T. G-protein coupled receptor 30 (GPR30): A novel regulator of endothelial inflammation. PLoS ONE 2012, 7, e52357. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wei, W.; Ning, H.; Sun, C. G protein-coupled estrogen receptor increases vascular endothelial inflammatory response after ovariectomized mice. Wei Sheng Yan Jiu 2021, 50, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Gros, R.; Hussain, Y.; Chorazyczewski, J.; Pickering, J.G.; Ding, Q.; Feldman, R.D. Extent of Vascular Remodeling Is Dependent on the Balance Between Estrogen Receptor alpha and G-Protein-Coupled Estrogen Receptor. Hypertension 2016, 68, 1225–1235. [Google Scholar] [CrossRef]
- Bowling, M.R.; Xing, D.; Kapadia, A.; Chen, Y.F.; Szalai, A.J.; Oparil, S.; Hage, F.G. Estrogen effects on vascular inflammation are age dependent: Role of estrogen receptors. Arter. Thromb. Vasc. Biol. 2014, 34, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Blasko, E.; Haskell, C.A.; Leung, S.; Gualtieri, G.; Halks-Miller, M.; Mahmoudi, M.; Dennis, M.K.; Prossnitz, E.R.; Karpus, W.J.; Horuk, R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J. Neuroimmunol. 2009, 214, 67–77. [Google Scholar] [CrossRef]
- Yates, M.A.; Li, Y.; Chlebeck, P.J.; Offner, H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010, 11, 20. [Google Scholar] [CrossRef]
- Tamaki, M.; Konno, Y.; Kobayashi, Y.; Takeda, M.; Itoga, M.; Moritoki, Y.; Oyamada, H.; Kayaba, H.; Chihara, J.; Ueki, S. Expression and functional roles of G-protein-coupled estrogen receptor (GPER) in human eosinophils. Immunol. Lett. 2014, 160, 72–78. [Google Scholar] [CrossRef]
- Rettew, J.A.; McCall, S.H.t.; Marriott, I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol. Cell. Endocrinol. 2010, 328, 87–92. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Ni, W.; Liu, T. Upregulation of Nuclear Factor IA Suppresses Oxidized Low-Density Lipoprotein-Induced Endoplasmic Reticulum Stress and Apoptosis in Human Umbilical Vein Endothelial Cells. Med. Sci. Monit. 2019, 25, 1009–1016. [Google Scholar] [CrossRef]
- Yang, G.; Zhuo, J.; Lin, Y.; Zhang, M.; Liu, L.; Chen, X.; Gao, R. Ginsenoside Rb1 Prevents Dysfunction of Endothelial Cells by Suppressing Inflammatory Response and Apoptosis in the High-Fat Diet Plus Balloon Catheter-Injured Rabbit Model via the G Protein-Coupled Estrogen Receptor-Mediated Phosphatidylinositol 3-Kinases (PI3K)/Akt Pathway. Med. Sci. Monit. 2019, 25, 7407–7417. [Google Scholar] [CrossRef]
- Jing, Y.; Cai, D.; Chen, Q.; Xiong, Q.; Hu, T.; Yao, Y.; Lin, C.; Sun, X.; Lu, Y.; Kong, X.; et al. Liuwei Dihuang soft capsules attenuates endothelial cell apoptosis to prevent atherosclerosis through GPR30-mediated regulation in ovariectomized ApoE-deficient mice. J. Ethnopharmacol. 2017, 208, 185–198. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, C.; Jin, Y.; Meng, Q.; Wu, J.; Sun, H. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm. Biol. 2021, 59, 1106–1116. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhou, C.; He, X.; Liu, C.; Wang, G.; Sun, X. The relationship between estrogen-induced phenotypic transformation and proliferation of vascular smooth muscle and hypertensive intracerebral hemorrhage. Ann. Transl. Med. 2020, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Ye, S.; Zhang, Y.; Li, P.; Wang, L.; Wang, T.H. Oestrogen Inhibits VEGF Expression And Angiogenesis In Triple-Negative Breast Cancer By Activating GPER-1. J. Cancer 2018, 9, 3802–3811. [Google Scholar] [CrossRef]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, T.H.; Choi, K.C. Functions and physiological roles of two types of estrogen receptors, ERalpha and ERbeta, identified by estrogen receptor knockout mouse. Lab. Anim. Res. 2012, 28, 71–76. [Google Scholar] [CrossRef]
- Nilsson, S.; Gustafsson, J.A. Estrogen receptors: Therapies targeted to receptor subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar] [CrossRef]
- Shook, L.L. An update on hormone replacement therapy: Health and medicine for women: A multidisciplinary, evidence-based review of mid-life health concerns. Yale J. Biol. Med. 2011, 84, 39–42. [Google Scholar]
- Wassertheil-Smoller, S.; Hendrix, S.L.; Limacher, M.; Heiss, G.; Kooperberg, C.; Baird, A.; Kotchen, T.; Curb, J.D.; Black, H.; Rossouw, J.E.; et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized trial. JAMA 2003, 289, 2673–2684. [Google Scholar] [CrossRef] [PubMed]
- Gillum, L.A.; Mamidipudi, S.K.; Johnston, S.C. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA 2000, 284, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Venous thromboembolic disease and combined oral contraceptives: Results of international multicentre case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1995, 346, 1575–1582. [CrossRef]
- Barton, M. Cholesterol and atherosclerosis: Modulation by oestrogen. Curr. Opin. Lipidol. 2013, 24, 214–220. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef] [PubMed]

| Compound | Structure | Agonist/ Antagonist | Affinity/Efficacy Kd/IC50/EC50 | Reference(s) |
|---|---|---|---|---|
| Endogenous Ligand | ||||
| Estrogen | ||||
| Estrone (E1) |  | Unknown | >>10 μM | [17] |
| Estradiol (E2) | ||||
| 17β-Estradiol |  | Agonist | 3–6 nM | [17,18] |
| 17α-Estradiol |  | Unknown | >10 μM | [17] |
| 2-Methoxy-E2 |  | Agonist | 10 nM | [143] |
| 2-Hydroxy-E2 |  | Antagonist | 0.1–1 μM | [144] |
| Estriol (E3) |  | Antagonist | >1 μM | [17,54] |
| Aldosterone |  | Agonist | None detected | [95,104,145] |
| Synthetic Ligands | ||||
| G1 |  | Agonist | 7–11 nM | [111,112] |
| G15 |  | Antagonist | 20 nM | [112,119] |
| G36 |  | Antagonist | ~ 20 nM | [113] |
| GPER-L1 |  | Agonist | 100 nM | [120] |
| GPER-L2 |  | Agonist | 100 nM | [120] |
| Bisphenol A |  | Agonist | 0.6 μM | [121,122,123,124] |
| Nonylphenol |  | Agonist | 0.8 μM | [124,125,126] |
| Atrazine |  | Agonist | >10 μM | [124,127,128] |
| Kepone |  | Agonist | 1.4 μM | [124] |
| Methoxychlor |  | Unknown | ~10 μM | [124] |
| p,p′-DDT |  | Unknown | 2.8 μM | [124] |
| o,p′-DDT |  | Agonist | 7.1 μM | [17,124] |
| p,p′-DDE |  | Unknown | ~10 μM | [124] |
| 2,2′5′-PCB-4-OH |  | Unknown | 3.8 μM | [124] |
| Phytoestrogens | ||||
| Genistein |  | Agonist | 133 nM | [124,129,130,131] |
| Daidzein |  | Agonist | <1 nM | [132] |
| Equol |  | Agonist | 100 nM | [133] |
| Oleuropein |  | Agonist | 200 nM | [134] |
| Hydroxytyrosol |  | Agonist | 100 nM | [133] |
| Resveratrol |  | Agonist | 300 nM | [135,136,137] |
| Quercetin |  | Agonist | 1 μM | [136,138] |
| Tectoridin |  | Agonist | 10 μM | [139] |
| Apigenin |  | Agonist | 20–50 μM | [136,142] |
| Zearalenone |  | Unknown | 0.8 μM | [124,140,141] |
| Model | Intervention | Outcome | Reference |
|---|---|---|---|
| Hypertension | |||
| Human endothelial cells | GPER agonist | GPER↑→eNOS↑→Src/EGFR/PI3K/ERK↑→NO↑→Vasodilation↑→HTN↓ | [13] |
| Human endothelial cells | GPER antagonist | GPER↓→NO↓→Vasodilation↓→HTN↑ | [13] |
| Human endothelial EA.hy2 cells | GPER agonist | GPER↑→CaMKKβ, AMPK, CaMKIIα phosphorylation↑→HDAC5, KLF2 →eNOS↑→NO↑→Vasodilation↑ | [68] |
| Pig coronary artery | GPER agonist | GPER↑→cAMP/PKA↑→RhoA↓→MLCP↑→Vasodilation↑ | [82] |
| Pig coronary artery | GPER agonist | GPER↑→Epac/Rap1↑→RhoA/Rho↓→Vasodilation↑ | [83] |
| Rat aortic smooth muscle cells | GPER mutation | ERK↓, Apoptosis↓→ HTN↑ | [153] |
| Aldosterone-induced hypertension in mice | GPER KO or antagonist | GPER↓→HTN↑ | [154] |
| mRen2 female hypertensive rat | GPER agonist | GPER↑→Oxidative stress↓→Aortic remodeling↓ | [155] |
| Mesenteric artery of rat | GPER agonist | GPER↑→NO↑, H2O2↑→Vasodilation↑ | [156] |
| Mesenteric artery of rat | GPER agonist | GPER↑→PI3K/Akt/eNOS↑→NO↑→Vasodilation↑ | [165] |
| Mesenteric artery of male rat | GPER agonist | GPER↑→PI3K/Akt/eNOS↑→NO↑→Vasodilation↑ | [166] |
| Mesenteric artery of female rat | GPER agonist | GPER↑→MEK/ERK/eNOS↑→NO↑→Vasodilation↑ | [166] |
| Human or pig coronary smooth muscle cells | GPER agonist | GPER↑→BK (Ca)↑→Potassium efflux↑→Vasodilation↑ | [168] |
| Pulmonary arterial hypertension | |||
| Male rats with MCT-induced PH | GPER agonist | GPER↑→eNOS↑, Collagen deposition in pulmonary and cardiac fibroblasts↓, Ca2+↑, Inflammation in cardiomyocytes↓→Pulmonary flow↑, RV hypertrophy↑, LV dysfunction↑ | [176] |
| OVX female rats with MCT-induced PH | GPER agonist | GPER↑→Pulmonary artery dysfunction↓, RV overload↓, RV dilation↓, Wall hypertrophy↓, Collagen deposition↓, Normalizes LV dysfunction | [177] |
| Atherosclerosis | |||
| Mice fed high-fat diet | GPER KO or OVX | GPER↓→TC↑, LDL↑, Inflammation↑, NO bioactivity↓→AS↑ | [32] |
| Mice fed high-fat diet | GPER agonist | GPER↑→Inflammation↓→AS↓ | [32] |
| Male and female mice | GPER deletion | GPER↓→ endothelium-dependent vasoconstriction↑, visceral obesity↑, LDL↑, inflammation↑ | [67] |
| Male mice | GPER KO | GPER KO→Insulin resistance, Dyslipidemia, Inflammatory effects↑ | [180] |
| Female mice | GPER KO | GPER KO→HDL↓, TG↑ | [180] |
| Human coronary artery ECs | GPER knockdown | GPER↑→EGFR↑→endothelial scavenger receptor class B type I↓→LDL transcytosis↓ | [182] |
| Female mice | GPER agonist | Ca↑→GPER↓→TRPC1/ERK1/2↓→AS↑ | [184] |
| Rat aortic SMCs | GPER agonist, ERα knockdown | Hypertrophy and inflammation of medial vessels after carotid ligation↓ | [185] |
| Rabbit atherosclerotic ECs | GPER agonist | GPER↑→PI3K/Akt↑→Inflammation↓, Apoptosis↓→Dysfunction of ECs↓→TC↓, TG↓, LDL↓, HDL↑→AS↓ | [192] |
| ApoE mice | GPER agonist | GPER↑→Apoptosis of ECs↓→Plasma lipid↓→AS↓ | [193] |
| ApoE mice | GPER agonist | GPER↑→PI3K/Akt/Nrf2↑→Inflammation↓, Apoptosis↓→AS↓ | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Ma, J.; Wang, X.; Wang, X.; Fang, W.; Sun, J.; Li, Z.; Liu, J. The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology. Biomolecules 2023, 13, 1410. https://doi.org/10.3390/biom13091410
Xu F, Ma J, Wang X, Wang X, Fang W, Sun J, Li Z, Liu J. The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology. Biomolecules. 2023; 13(9):1410. https://doi.org/10.3390/biom13091410
Chicago/Turabian StyleXu, Fujie, Jipeng Ma, Xiaowu Wang, Xiaoya Wang, Weiyi Fang, Jingwei Sun, Zilin Li, and Jincheng Liu. 2023. "The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology" Biomolecules 13, no. 9: 1410. https://doi.org/10.3390/biom13091410
APA StyleXu, F., Ma, J., Wang, X., Wang, X., Fang, W., Sun, J., Li, Z., & Liu, J. (2023). The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology. Biomolecules, 13(9), 1410. https://doi.org/10.3390/biom13091410





