Novel Biomarkers and Imaging Indices for the “Vulnerable Patient” with Carotid Stenosis: A Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
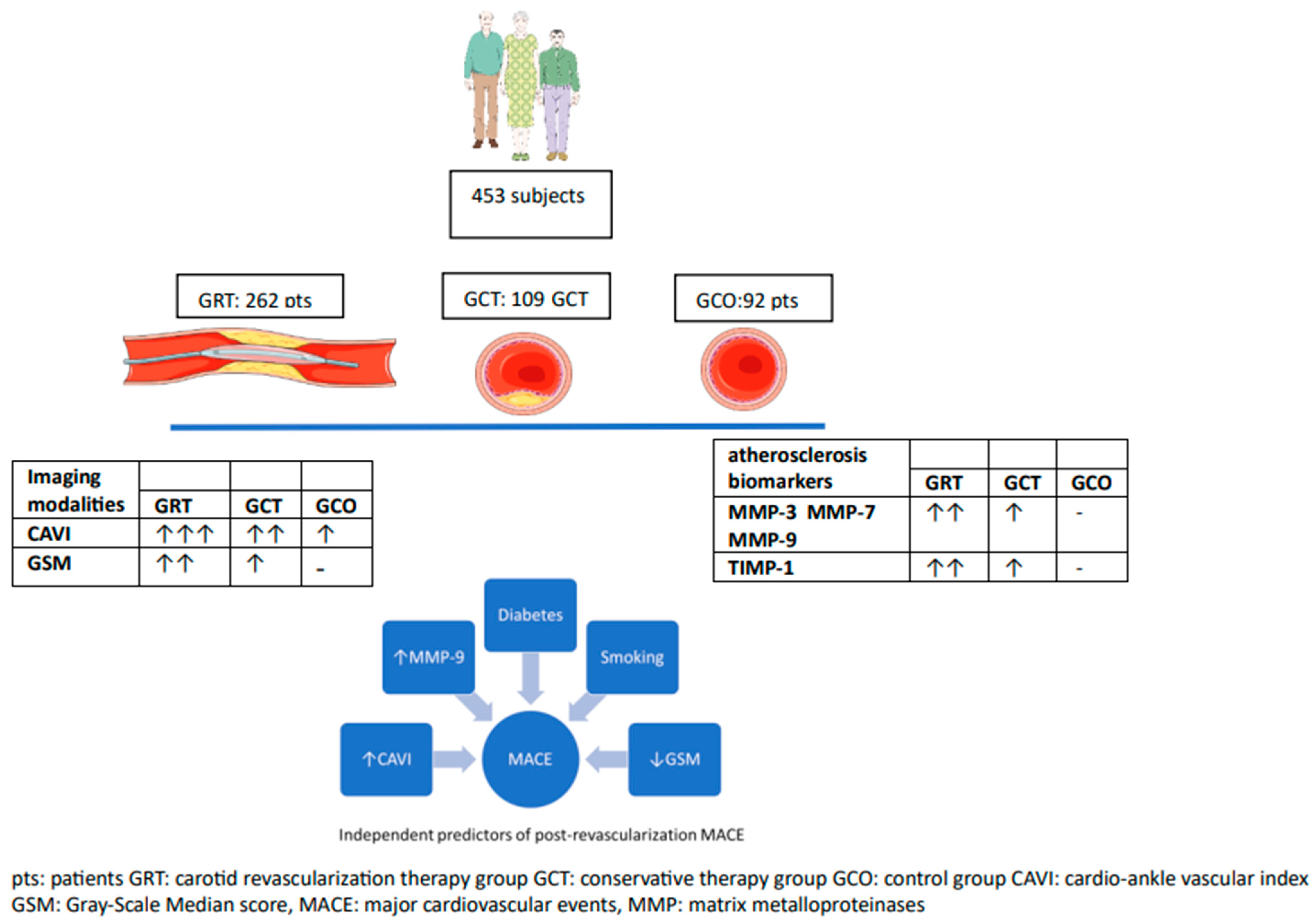
- Group of revascularization therapy of carotid stenosis (GRT, N = 288 patients): Patients fulfilling the criteria for carotid revascularization with either carotid endarterectomy or carotid artery stenting (CAS) [5]. This group was further subdivided into patients with symptomatic carotid artery stenosis ≥ 50% (n = 104), or asymptomatic carotid artery stenosis ≥ 70% (n = 184). The symptomatic subgroup had presented within the last 6 months with neurological symptoms and/or brain scan findings compatible with ipsilateral to the carotid stenosis transient ischemic attack (TIA), stroke, or amaurosis fugax. Asymptomatic patients were free of related neurological symptoms. In order to limit the possibility of patient misclassification, all patients had undergone brain computed tomography (CT) scan or brain magnetic resonance imaging (MRI) examination, when CT findings were questionable cerebral infarcts. The degree of carotid stenosis was calculated from carotid ultrasound or magnetic angiography of both carotids.
- Group of conservative treatment of carotid stenosis (GCT, N = 114): Asymptomatic patients with significant carotid atherosclerotic plaques causing stenosis between 40% and 70%. All patients were evaluated by a vascular surgeon and neurologist and underwent at baseline anthropometrical/clinical assessment, carotid ultrasound, blood analyses, brain computed tomography (CT) scan, and/or brain magnetic resonance imaging (MRI), when CT findings were questionable.
- Group of controls (GCO group, N = 92): Age- and sex-matched individuals served as controls and were only assessed at baseline. Those subjects were selected after testing for exclusion criteria. All of them had visited our hospital for regular health check-ups. They had a maximum of two classical cardiovascular risk factors (such as hypertension, diabetes, dyslipidemia, smoking, or family history of premature coronary artery disease), but no evidence of overt cardiovascular disease. Moreover, their carotid and lower limb arteries were free from atherosclerotic plaques based on vascular ultrasound and they had a functional test negative for myocardial ischemia within the last year.
2.2. Study Design
2.3. Clinical Data Collection
2.4. Carotid Ultrasound Examination
2.5. Blood Assays
2.6. Statistical Analysis
3. Results
3.1. Baseline Results
3.2. Follow-Up Results and Prognosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Executive summary: Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation 2012, 125, 188–197. [Google Scholar] [PubMed]
- Nezu, T.; Hosomi, N. Usefulness of Carotid Ultrasonography for Risk Stratification of Cerebral and Cardiovascular Disease. J. Atheroscler Thromb. 2020, 27, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar]
- Golemati, S.; Gastounioti, A.; Nikita, K.S. Toward novel noninvasive and low-cost markers for predicting strokes in asymptomatic carotid atherosclerosis: The role of ultrasound image analysis. IEEE Trans. Biomed. Eng. Spec. Issue Grand Chall. Eng. Life Sci. Med. 2013, 60, 652–658. [Google Scholar] [CrossRef]
- Christodoulou, C.I.; Pattichis, C.S.; Pantziaris, M.; Nicolaides, A. Texture-based classification of atherosclerotic carotid plaques. IEEE Trans. Med. Imaging 2003, 22, 902–912. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Khattab, E.; Velidakis, N.; Patsourakos, N.; Lambadiari, V. A new approach of statin therapy in carotid atherosclerosis: Targeting indices of plaque vulnerability on the top of lipid-lowering. A narrative review. Kardiol. Pol. 2022, 80, 880–890. [Google Scholar] [CrossRef]
- Emfietzoglou, M.; Mavrogiannis, M.C.; García-García, H.M.; Stamatelopoulos, K.; Kanakakis, I.; Papafaklis, M.I. Current Toolset in Predicting Acute Coronary Thrombotic Events: The “Vulnerable Plaque” in a “Vulnerable Patient” Concept. Life 2023, 13, 696. [Google Scholar] [CrossRef]
- Mitsis, A.; Kadoglou, N.P.E.; Lambadiari, V.; Alexiou, S.; Theodoropoulos, K.C.; Avraamides, P.; Kassimis, G. Prognostic role of inflammatory cytokines and novel adipokines in acute myocardial infarction: An updated and comprehensive review. Cytokine 2022, 153, 155848. [Google Scholar] [CrossRef]
- Weng, Z.; Zhao, C.; Qin, Y.; Liu, C.; Pan, W.; Hu, S.; He, L.; Xu, Y.; Zeng, M.; Feng, X.; et al. Peripheral atherosclerosis in acute coronary syndrome patients with plaque rupture vs plaque erosion: A prospective coronary optical coherence tomography and peripheral ultrasound study. Am. Heart J. 2023, 263, 159–168. [Google Scholar] [CrossRef]
- Achim, A.; Kákonyi, K.; Nagy, F.; Jambrik, Z.; Varga, A.; Nemes, A.; Chan, J.S.K.; Toth, G.G.; Ruzsa, Z. Radial Artery Calcification in Predicting Coronary Calcification and Atherosclerosis Burden. Cardiol. Res. Pract. 2022, 2022, 5108389. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Mengozzi, A.; Dentali, F.; Pomero, F.; Virdis, A.; Camerota, A.; Muselli, M.; Necozione, S.; Bocale, R.; Ferri, C.; et al. Enhanced Carotid Plaque Echolucency Is Associated with Reduced Cognitive Performance in Elderly Patients with Atherosclerotic Disease Independently on Metabolic Profile. Metabolites 2023, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Watanabe, S.; Ishizu, T.; Moriyama, N.; Takeyasu, N.; Maeda, H.; Ishimitsu, T.; Aonuma, K.; Yamaguchi, I. Echolucent carotid plaques as a feature in patients with acute coronary syndrome. Circ. J. 2006, 70, 1629–1634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irie, Y.; Katakami, N.; Kaneto, H.; Takahara, M.; Nishio, M.; Kasami, R.; Sakamoto, K.; Umayahara, Y.; Sumitsuji, S.; Ueda, Y.; et al. The utility of ultrasonic tissue characterization of carotid plaque in the prediction of cardiovascular events in diabetic patients. Atherosclerosis 2013, 230, 399–405. [Google Scholar] [CrossRef]
- Joo, H.J.; Cho, S.A.; Cho, J.Y.; Lee, S.; Park, J.H.; Hwang, S.H.; Hong, S.J.; Yu, C.W.; Lim, D.-S. Brachial-ankle pulse wave velocity is associated with composite carotid and coronary atherosclerosis in a middle-aged asymptomatic population. J. Atheroscler. Thromb. 2016, 23, 1033–1046. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef]
- Matsumoto, M.; Inoue, K.; Moriki, A. Associations of brachial-ankle pulse wave velocity and carotid atherosclerotic lesions with silent cerebral lesions. Hypertens. Res. 2007, 30, 767–773. [Google Scholar] [CrossRef]
- Inoue, K.; Matsumoto, M.; Shono, T.; Toyokawa, S.; Moriki, A. Increased intima media thickness and atherosclerotic plaques in the carotid artery as risk factors for silent brain infarcts. J. Stroke Cerebrovasc. Dis. 2007, 16, 14–20. [Google Scholar] [CrossRef]
- Puig, N.; Jiménez-Xarrié, E.; Camps-Renom, P.; Benitez, S. Search for Reliable Circulating Biomarkers to Predict Carotid Plaque Vulnerability. Int. J. Mol. Sci. 2020, 21, 8236. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Kassimis, G.; Patsourakos, N.; Kanonidis, I.; Valsami, G. Omentin-1 and vaspin serum levels in patients with pre-clinical carotid atherosclerosis and the effect of statin therapy on them. Cytokine 2021, 138, 155364. [Google Scholar] [CrossRef]
- Giagtzidis, I.; Karkos, C.; Kadoglou, N.P.E.; Spathis, A.; Papazoglou, K. Serum Levels of Matrix Metalloproteinases in Patients Undergoing Endovascular Intervention for Peripheral Arterial Disease. Ann. Vasc. Surg. 2023, 94, 154–164. [Google Scholar] [CrossRef]
- Gaubatz, J.W.; Ballantyne, C.M.; Wasserman, B.A.; He, M.; Chambless, L.E.; Boerwinkle, E.; Hoogeveen, R.C. Association of circulating matrix metalloproteinases with carotid artery characteristics: The Atherosclerosis Risk in Communities Carotid MRI Study. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Lahdentausta, L.; Leskelä, J.; Winkelmann, A.; Tervahartiala, T.; Sorsa, T.; Pesonen, E.; Pussinen, P.J. Serum MMP-9 Diagnostics, Prognostics, and Activation in Acute Coronary Syndrome and Its Recurrence. J. Cardiovasc. Transl. Res. 2018, 11, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Gerasimidis, T.; Moumtzouoglou, A.; Kapelouzou, A.; Sailer, N.; Fotiadis, G.; Vitta, I.; Katinios, A.; Kougias, P.; Bandios, S.; et al. Intensive lipid-lowering therapy ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Parkkonen, O.; Nieminen, M.T.; Vesterinen, P.; Tervahartiala, T.; Perola, M.; Salomaa, V.; Jousilahti, P.; Sorsa, T.; Pussinen, P.J.; Sinisalo, J. Low MMP-8/TIMP-1 reflects left ventricle impairment in takotsubo cardiomyopathy and high TIMP-1 may help to differentiate it from acute coronary syndrome. PLoS ONE 2017, 12, e0173371. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Hashmi, S.; Mao, X.; Zeng, Q.T. Relationships of adiponectin and matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio with coronary plaque morphology in patients with acute coronary syndrome. Can. J. Cardiol. 2008, 24, 385–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beaudeux, J.L.; Giral, P.; Bruckert, E.; Bernard, M.; Foglietti, M.J.; Chapman, M.J. Serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 as potential markers of carotid atherosclerosis in infraclinical hyperlipidemia. Atherosclerosis 2003, 169, 139–146. [Google Scholar] [CrossRef]
- Liapis, C.D.; Bell, P.R.; Mikhailidis, D.; Sivenius, J.; Nicolaides, A.; Fernandes e Fernandes, J.; Biasi, G.; Norgren, L.; ESVS Guidelines Collaborators. ESVS guidelines. Invasive treatment for carotid stenosis: Indications, techniques. Eur. J. Vasc. Endovasc. Surg. 2009, 37 (Suppl. S4), 1–19. [Google Scholar] [CrossRef]
- Gastounioti, A.; Kolias, V.; Golemati, S.; Tsiaparas, N.N.; Matsakou, A.; Stoitsis, J.S.; Kadoglou, N.P.; Gkekas, C.; Kakisis, J.D.; Liapis, C.D.; et al. CAROTID—A web-based platform for optimal personalized management of atherosclerotic patients. Comput. Methods Programs Biomed. 2014, 114, 183–193. [Google Scholar] [CrossRef]
- Naylor, A.R.; Sillesen, H.; Schroeder, T.V. Clinical and imaging features associated with an increased risk of early and late stroke in patients with symptomatic carotid disease. Eur J Vasc Endovasc Surg. 2015, 49, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Simões, G.; Pereira, T.; Caseiro, A. Matrix metaloproteinases in vascular pathology. Microvasc. Res. 2022, 143, 104398. [Google Scholar] [CrossRef]
- Lyngbakken, M.N.; Myhre, P.L.; Røsjø, H.; Omland, T. Novel biomarkers of cardiovascular disease: Applications in clinical practice. Crit. Rev. Clin. Lab. Sci. 2019, 56, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Pleskovič, A.; Letonja, M.Š.; Vujkovac, A.C.; Starčević, J.N.; Caprnda, M.; Curilla, E.; Mozos, I.; Kruzliak, P.; Prosecky, R.; Petrovič, D. Matrix metalloproteinase-3 gene polymorphism (rs3025058) affects markers atherosclerosis in type 2 diabetes mellitus. Vasa 2017, 46, 363–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giagtzidis, I.T.; Kadoglou, N.P.; Mantas, G.; Spathis, A.; Papazoglou, K.O.; Karakitsos, P.; Liapis, C.D.; Karkos, C.D. The Profile of Circulating Matrix Metalloproteinases in Patients Undergoing Lower Limb Endovascular Interventions for Peripheral Arterial Disease. Ann. Vasc. Surg. 2017, 43, 188–196. [Google Scholar] [CrossRef]
- Wu, T.C.; Leu, H.B.; Lin, W.T.; Lin, C.P.; Lin, S.J.; Chen, J.W. Plasma matrix metalloproteinase-3 level is an independent prognostic factor in stable coronary artery disease. Eur. J. Clin. Investig. 2005, 35, 537–545. [Google Scholar] [CrossRef]
- Nilsson, L.; Jonasson, L.; Nijm, J.; Hamsten, A.; Eriksson, P. Increased plasma concentration of matrix metalloproteinase-7 in patients with coronary artery disease. Clin. Chem. 2006, 52, 1522–1527. [Google Scholar] [CrossRef]
- Santiago-Raber, M.L.; Montecucco, F.; Vuilleumier, N.; Miteva, K.; Baptista, D.; Carbone, F.; Pagano, S.; Roth, A.; Burger, F.; Mach, F.; et al. Atherosclerotic plaque vulnerability is increased in mouse model of lupus. Sci. Rep. 2020, 10, 18324. [Google Scholar] [CrossRef]
- Sbrana, S.; Cecchettini, A.; Bastiani, L.; Mazzone, A.; Vozzi, F.; Caselli, C.; Neglia, D.; Clemente, A.; Scholte, A.J.H.A.; Parodi, O.; et al. Association of Circulating Neutrophils with Relative Volume of Lipid-Rich Necrotic Core of Coronary Plaques in Stable Patients: A Substudy of SMARTool European Project. Life 2023, 13, 428. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Chen, Y.; Yuan, F.; Xu, F.; Zhang, M.; Tan, K.; Yang, X.; Yu, X.; Lv, S. The Long-Term Influence of Tissue Inhibitor of Matrix Metalloproteinase-1 in Patients with Mild to Moderate Coronary Artery Lesions in a Chinese Population: A 7-Year Follow-Up Study. Cardiology 2015, 132, 151–158. [Google Scholar] [CrossRef]
- Mérei, Á.; Nagy, B.; Woth, G.; Lantos, J.; Kövér, F.; Bogár, L.; Mühl, D. Comparison of the perioperative time courses of matrix metalloproteinase-9 (MMP-9) and its inhibitor (TIMP-1) during carotid artery stenting (CAS) and carotid endarterectomy (CEA). BMC Neurol. 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Ben Braiek, A.; Chahed, H.; Dumont, F.; Abdelhak, F.; Hichem, D.; Gamra, H.; Baudin, B. Identification of biomarker panels as predictors of severity in coronary artery disease. J. Cell Mol. Med. 2021, 25, 1518–1530. [Google Scholar] [CrossRef]
- Jones, G.T.; Tarr, G.P.; Phillips, L.V.; Wilkins, G.T.; van Rij, A.M.; Williams, M.J. Active matrix metalloproteinases 3 and 9 are independently associated with coronary artery in-stent restenosis. Atherosclerosis 2009, 207, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.; Lackó, D.; Hüttl, A.; Csobay-Novák, C.; Csavajda, Á.; Sótonyi, P.; Merkely, B.; Nemes, B.; Ruzsa, Z. Impact of Diabetes Mellitus on Early Clinical Outcome and Stent Restenosis after Carotid Artery Stenting. J. Diabetes Res. 2022, 2022, 4196195. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Moulakakis, K.G.; Mantas, G.; Kakisis, J.D.; Mylonas, S.N.; Valsami, G.; Liapis, C.D. The Association of Arterial Stiffness With Significant Carotid Atherosclerosis and Carotid Plaque Vulnerability. Angiology 2022, 73, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Guizani, I.; Zidi, W.; Zayani, Y.; Boudiche, S.; Hadj-Taieb, S.; Sanhaji, H.; Zaroui, A.; Mechmeche, R.; Mourali, M.S.; Feki, M.; et al. Matrix metalloproteinase-3 predicts clinical cardiovascular outcomes in patients with coronary artery disease: A 5 years cohort study. Mol. Biol. Rep. 2019, 46, 4699–4707. [Google Scholar] [CrossRef]
- Abbas, A.; Aukrust, P.; Russell, D.; Krohg-Sørensen, K.; Almås, T.; Bundgaard, D.; Bjerkeli, V.; Sagen, E.L.; Michelsen, A.E.; Dahl, T.B.; et al. Matrix metalloproteinase 7 is associated with symptomatic lesions and adverse events in patients with carotid atherosclerosis. PLoS ONE 2014, 9, e84935. [Google Scholar] [CrossRef]
- Silvello, D.; Narvaes, L.B.; Albuquerque, L.C.; Forgiarini, L.F.; Meurer, L.; Martinelli, N.C.; Andrades, M.E.; Clausell, N.; dos Santos, K.G.; Rohde, L.E. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: Higher MMP-9 levels are associated with plaque vulnerability. Biomarkers 2014, 19, 49–55. [Google Scholar] [CrossRef]
- Pelisek, J.; Rudelius, M.; Zepper, P.; Poppert, H.; Reeps, C.; Schuster, T.; Eckstein, H.H. Multiple biological predictors for vulnerable carotid lesions. Cerebrovasc. Dis. 2009, 28, 601–610. [Google Scholar] [CrossRef]
- Ezhov, M.V.; Tmoyan, N.A.; Afanasieva, O.I.; Afanasieva, M.I.; Pokrovsky, S.N. Lipoprotein(a) and Cardiovascular Outcomes after Revascularization of Carotid and Lower Limbs Arteries. Biomolecules 2021, 11, 257. [Google Scholar] [CrossRef]
- Jovanovic, K.; Trailovic, R.; Jonsson, M.; Capoccia, L.; Grego, F.; Stankovic, S.; Stevanovic, P.; Koncar, I. The Value of Troponin Measurement in Carotid Revascularization: A Scoping Review. J. Endovasc. Ther. 2023, online ahead of print, 15266028231179874. [Google Scholar] [CrossRef]
- Trucco, E.; Tolosana, J.M.; Castel, M.Á.; Batlle, M.; Borràs, R.; Sitges, M.; Guash, E.; Matas, M.; Arbelo, E.; Berruezo, A.; et al. Plasma tissue inhibitor of matrix metalloproteinase-1 a predictor of long-term mortality in patients treated with cardiac resynchronization therapy. Europace 2016, 18, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Geronimo, F.R.B.; Barter, P.J.; Rye, K.A.; Heather, A.K.; Shearston, K.D.; Rodgers, K.J. Plaque stabilizing effects of apolipoprotein A-IV. Atherosclerosis 2016, 251, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Feng, Y.; Dong, G.; Wang, Y.; Yang, J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediat. Inflamm. 2020, 2020, 3872367. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Huston, J., 3rd; Rabinstein, A.A.; Kim, G.M.; Lerman, A.; Lanzino, G. Contemporary carotid imaging: From degree of stenosis to plaque vulnerability. J. Neurosurg. 2016, 124, 27–42. [Google Scholar] [CrossRef]
| GRT (N = 262) | GCT (N = 109) | GCO (N = 92) | p | |
|---|---|---|---|---|
| Age, years | 74 ± 11 | 70 ± 12 | 65 ± 13 | 0.111 |
| Males/females | 201/61 | 88/21 | 70/22 | 0.775 |
| CAD, n | 104 (41%) | 36 (33%) | 0 (0%) | - |
| Diabetes, n | 79 (30.1%) | 37 (33.9%) | 11 (12%) | <0.001 |
| Smoking, n | 45 (17.2%) | 27 (25%) | 30 (32.6%) * | 0.015 |
| Statins, n | 254 (97%) | 92 (84.4%) | 43 (46.7%) *# | <0.001 |
| Anti-hypertensive medications, n | 231 (88.2%) | 92 (84.4%) | 57 (62%) *# | <0.001 |
| BMI, kg/m2 | 29.8 ± 4.2 | 29.4 ± 3.8 | 27.5 ± 2.9 | 0.099 |
| SBP, mmHg | 128 ± 14 | 125 ± 12 | 131 ± 15 | 0.641 |
| DBP, mmHg | 80 ± 9 | 80 ± 10 | 82 ± 10 | 0.956 |
| FPG, mg/dL | 121 ± 23 | 117 ± 14 | 94 ± 15 *# | <0.001 |
| hsCRP (mg/L) | 5.6 ± 1.1 | 3.5 ± 0.9 | 1.7 ± 0.6 *# | <0.001 |
| WBC, cells/μL | 10,051 ± 3114 | 8234 ± 2995 | 7580 ± 1816 * | 0.031 |
| TChol, mg/dL | 159 ± 35 | 151 ± 28 | 191 ± 39 *# | <0.001 |
| HDL-C, mg/dL | 41 ± 11 | 44 ± 10 | 48 ± 11 * | 0.022 |
| LDL-C, mg/dL | 92 ± 25 | 85 ± 22 | 123 ± 22 *# | <0.001 |
| TG, mg/dL | 128 ± 49 | 111 ± 49 | 101 ± 31 | 0.276 |
| CAVI, m/s | 10.45 ± 2.08 | 9.42 ± 1.55 | 7.58 ± 1.03 *# | <0.001 |
| GSM score | 65 ± 18 | 91 ± 22 | - | - |
| MMP-3 (ng/mL) | 68.03 ± 36.12 | 49.3 ± 24.23 | 22.67 ± 6.88 *# | <0.001 |
| MMP-7 (ng/mL) | 39.77 ± 8.08 | 30.55 ± 7.49 | 11.45 ± 2.91 *# | <0.001 |
| MMP-9 (ng/mL) | 555.59 ± 71.13 | 375 ± 51.93 | 185.32 ± 39.14 *# | <0.001 |
| TIMP-1 (ng/mL) | 118.58 ± 19.09 | 236.65 ± 31.98 | 368.47 ± 66.82 *# | <0.001 |
| MMP-9/TIMP-1 ratio | 4.68 ± 0.82 | 1.59 ± 0.30 | 0.5 ± 0.09 *# | <0.001 |
| Carotid Atherosclerosis | |||
|---|---|---|---|
| Variables | OR | p | 95% CI |
| Age | 1.34 | 0.591 | 1.101–1.505 |
| CAD | 1.56 | <0.001 | 1.331–1.723 |
| Diabetes | 1.72 | 0.023 | 1.371–2.202 |
| CAVI | 1.29 | 0.012 | 1.201–1.429 |
| MMP-7 | 1.09 | 0.156 | −0.01–0.175 |
| MMP-9 | 1.38 | <0.001 | 1.212–1.504 |
| TIMP-1 | 0.87 | 0.349 | 0.77–0.99 |
| Event-Free (N = 211) | MACE (N = 51) | p | |
|---|---|---|---|
| Age, years | 76 ± 12 | 73 ± 12 | 0.798 |
| Males/females, n | 167/54 | 44/7 | 0.775 |
| CAD, n | 78 (37%) | 26 (51%) | 0.081 |
| Diabetes, n | 57 (27%) | 22 (43.1%) | <0.001 |
| Smoking, n | 25 (11.8%) | 20 (39.2%) | <0.001 |
| Statins, n | 206 (97.6%) | 48 (94.2%) | 0.923 |
| Anti-hypertensive medications, n | 185 (87.7%) | 46 (90.5%) | 0.906 |
| BMI, kg/m2 | 29.6 ± 4 | 30.3 ± 4.3 | 0.519 |
| SBP, mmHg | 131 ± 15 | 125 ± 13 | 0.763 |
| DBP, mmHg | 80 ± 8 | 80 ± 10 | 0.977 |
| FPG, mg/dL | 118 ± 21 | 127 ± 20 | 0.102 |
| hsCRP (mg/L) | 5.1 ± 1.2 | 6.9 ± 1.5 | 0.025 |
| WBC, cells/μL | 9807 ± 2456 | 10,889 ± 3225 | 0.045 |
| TChol, mg/dL | 156 ± 37 | 164 ± 32 | 0.765 |
| HDL-C, mg/dL | 43 ± 10 | 38 ± 9 | 0.081 |
| LDL-C, mg/dL | 89 ± 25 | 100 ± 28 | 0.228 |
| TG, mg/dL | 120 ± 48 | 131 ± 54 | 0.276 |
| CAVI, m/s | 10.45 ± 1.08 | 9.42 ± 1.55 | 0.010 |
| GSM score | 65 ± 18 | 91 ± 22 | <0.001 |
| MMP-3 (ng/mL) | 65.03 ± 36.12 | 79.55 ± 29.32 | 0.040 |
| MMP-7 (ng/mL) | 36.74 ± 8.08 | 51.01 ± 8.23 | <0.001 |
| MMP-9 (ng/mL) | 490.91 ± 71.13 | 826.39 ± 91 | <0.001 |
| TIMP-1 (ng/mL) | 128.59 ± 12.11 | 81.13 ± 5.89 | <0.001 |
| MMP-9/TIMP-1 ratio | 3.82 ± 0.79 | 10.18 ± 2.1 | <0.001 |
| Variable | Univariable HR (95% CI) | p | Multivariable HR (95% CI) | p |
|---|---|---|---|---|
| Age (years) | 1.13 (1.01–1.18) | 0.031 | 1.05 (0.98–1.10) | 0.451 |
| Male gender | 1.05 (0.98–1.13) | 0.045 | 1.01 (0.95–1.10) | 0.881 |
| Smoking | 2.28 (1.69–2.92) | <0.001 | 1.67 (1.35–1.95) | <0.001 |
| Diabetes mellitus | 2.82 (2.28–3.55) | <0.001 | 2.07 (1.55–2.78) | <0.001 |
| GSM | 1.88 (1.29–2.24) | <0.001 | 1.40 (1.16–2.12) | 0.002 |
| CAVI | 1.40 (1.10–1.95) | <0.001 | 1.22 (1.09–1.43) | 0.023 |
| MMP-7 | 1.22 (1.02–1.88) | 0.025 | 1.11 (1.01–1.54) | 0.125 |
| MMP-9 | 1.59 (1.24–2.22) | <0.001 | 1.44 (1.29–2.15) | 0.005 |
| TIMP-1 | 0.72 (0.51–0.97) | 0.043 | 0.92 (0.70–0.99) | 0.346 |
| MMP9/TIMP-1 ratio | 1.72 (1.32–2.59) | <0.001 | 1.39 (1.11–2.22) | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadoglou, N.; Moulakakis, K.G.; Mantas, G.; Spathis, A.; Gkougkoudi, E.; Mylonas, S.N.; Kakisis, J.; Liapis, C. Novel Biomarkers and Imaging Indices for the “Vulnerable Patient” with Carotid Stenosis: A Single-Center Study. Biomolecules 2023, 13, 1427. https://doi.org/10.3390/biom13091427
Kadoglou N, Moulakakis KG, Mantas G, Spathis A, Gkougkoudi E, Mylonas SN, Kakisis J, Liapis C. Novel Biomarkers and Imaging Indices for the “Vulnerable Patient” with Carotid Stenosis: A Single-Center Study. Biomolecules. 2023; 13(9):1427. https://doi.org/10.3390/biom13091427
Chicago/Turabian StyleKadoglou, Nikolaos, Konstantinos G. Moulakakis, George Mantas, Aris Spathis, Evangelia Gkougkoudi, Spyridon N. Mylonas, John Kakisis, and Christos Liapis. 2023. "Novel Biomarkers and Imaging Indices for the “Vulnerable Patient” with Carotid Stenosis: A Single-Center Study" Biomolecules 13, no. 9: 1427. https://doi.org/10.3390/biom13091427
APA StyleKadoglou, N., Moulakakis, K. G., Mantas, G., Spathis, A., Gkougkoudi, E., Mylonas, S. N., Kakisis, J., & Liapis, C. (2023). Novel Biomarkers and Imaging Indices for the “Vulnerable Patient” with Carotid Stenosis: A Single-Center Study. Biomolecules, 13(9), 1427. https://doi.org/10.3390/biom13091427










