Deciphering the Calcium Code: A Review of Calcium Activity Analysis Methods Employed to Identify Meaningful Activity in Early Neural Development
Abstract
1. Introduction
2. Background
3. Calcium Activity Detection Methods in Cleavage Stages
3.1. Early Cleavage Stages
3.2. Blastula and Gastrula Stages
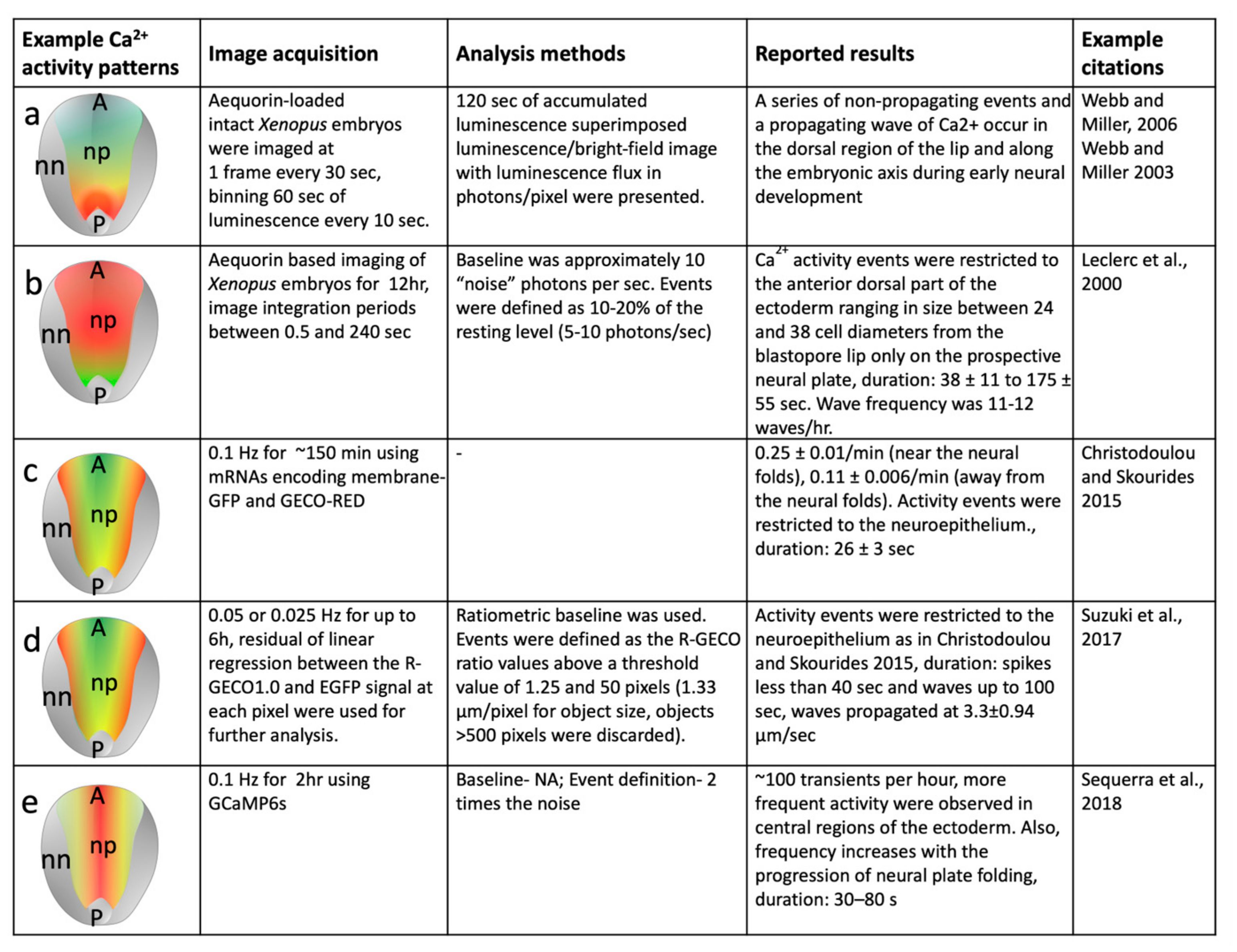
4. Calcium Activity Detection Methods during Neural Induction
5. Calcium Activity during Neuronal Subtype Formation and Neurotransmitter Phenotype Specification
6. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef]
- Noguchi, T.; Mabuchi, I. Localized calcium signals along the cleavage furrow of the Xenopus egg are not involved in cytokinesis. Mol. Biol. Cell 2002, 13, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Dreses-Werringloer, U.; Vingtdeux, V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; Kinet, J.P. Calcium signaling in immune cells. Nat. Immunol. 2009, 10, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, C.J.; Roderick, H.L.; Bootman, M.D. Calcium signaling in cardiac myocytes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004242. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef]
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018, 28, 258–273. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Subramaniam, T.; Fauzi, M.B.; Lokanathan, Y.; Law, J.X. The Role of Calcium in Wound Healing. Int. J. Mol. Sci. 2021, 22, 6486. [Google Scholar] [CrossRef]
- Inaba, H.; Li, H.; Kawatake-Kuno, A.; Dewa, K.I.; Nagai, J.; Oishi, N.; Murai, T.; Uchida, S. GPCR-mediated calcium and cAMP signaling determines psychosocial stress susceptibility and resiliency. Sci. Adv. 2023, 9, eade5397. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Zhu, K.; Czarnecka-Herok, J.; Vernier, M.; Bernard, D. Regulation and role of calcium in cellular senescence. Cell Calcium 2023, 110, 102701. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Sindelar, R.; Saha, M. Calcium Signaling in Vertebrate Development and Its Role in Disease. Int. J. Mol. Sci. 2018, 19, 3390. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M. Calcium at fertilization and in early development. Physiol. Rev. 2006, 86, 25–88. [Google Scholar] [CrossRef]
- Johnston, D. The calcium code. Biophys. J. 1996, 70, 1095. [Google Scholar] [CrossRef] [PubMed]
- Covelo, A.; Badoual, A.; Denizot, A. Reinforcing Interdisciplinary Collaborations to Unravel the Astrocyte “Calcium Code”. J. Mol. Neurosci. 2022, 72, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Spitzer, N.C. Breaking the code: Regulation of neuronal differentiation by spontaneous calcium transients. Dev. Neurosci. 1997, 19, 33–41. [Google Scholar] [CrossRef]
- Webb, S.E.; Miller, A.L. Calcium signalling during embryonic development. Nat. Rev. Mol. Cell Biol. 2003, 4, 539–551. [Google Scholar] [CrossRef]
- Rosenberg, S.S.; Spitzer, N.C. Calcium signaling in neuronal development. Cold Spring Harb. Perspect. Biol. 2011, 3, a004259. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Neant, I.; Moreau, M. The calcium: An early signal that initiates the formation of the nervous system during embryogenesis. Front. Mol. Neurosci. 2012, 5, 3. [Google Scholar] [CrossRef]
- Stewart, T.A.; Davis, F.M. An element for development: Calcium signaling in mammalian reproduction and development. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1230–1238. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Bultynck, G. Fundamentals of Cellular Calcium Signaling: A Primer. Cold Spring Harb. Perspect. Biol. 2020, 12, a038802. [Google Scholar] [CrossRef] [PubMed]
- Soundarrajan, D.K.; Huizar, F.J.; Paravitorghabeh, R.; Robinett, T.; Zartman, J.J. From spikes to intercellular waves: Tuning intercellular calcium signaling dynamics modulates organ size control. PLoS Comput. Biol. 2021, 17, e1009543. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Olson, E.C.; Spitzer, N.C. Spontaneous neuronal calcium spikes and waves during early differentiation. J. Neurosci. 1994, 14, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Dupont, G.; Combettes, L.; Leybaert, L. Calcium dynamics: Spatio-temporal organization from the subcellular to the organ level. Int. Rev. Cytol. 2007, 261, 193–245. [Google Scholar] [CrossRef] [PubMed]
- Leybaert, L.; Sanderson, M.J. Intercellular Ca2+ waves: Mechanisms and function. Physiol. Rev. 2012, 92, 1359–1392. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Miyata, T.; Konno, D.; Ueda, H.R.; Kasukawa, T.; Hashimoto, M.; Matsuzaki, F.; Kawaguchi, A. Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells. Nat. Commun. 2016, 7, 11349. [Google Scholar] [CrossRef]
- Elias, L.A.; Kriegstein, A.R. Gap junctions: Multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008, 31, 243–250. [Google Scholar] [CrossRef]
- Chen, Y.; Song, X.; Ye, S.; Miao, L.; Zhu, Y.; Zhang, R.G.; Ji, G. Structural insight into enhanced calcium indicator GCaMP3 and GCaMPJ to promote further improvement. Protein Cell 2013, 4, 299–309. [Google Scholar] [CrossRef]
- Bootman, M.D.; Allman, S.; Rietdorf, K.; Bultynck, G. Deleterious effects of calcium indicators within cells; an inconvenient truth. Cell Calcium 2018, 73, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, J.; Chen, T.W.; Wardill, T.J.; Tian, L.; Marvin, J.S.; Mutlu, S.; Calderon, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012, 32, 13819–13840. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Akerboom, J.; Schreiter, E.R.; Looger, L.L. Neural activity imaging with genetically encoded calcium indicators. Prog. Brain Res. 2012, 196, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Deng, X.; Jiang, J.; Kirberger, M.; Yang, J.J. Design of Calcium-Binding Proteins to Sense Calcium. Molecules 2020, 25, 2148. [Google Scholar] [CrossRef] [PubMed]
- Li, E.S.; Saha, M.S. Optimizing Calcium Detection Methods in Animal Systems: A Sandbox for Synthetic Biology. Biomolecules 2021, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, P. Spectral analysis of calcium oscillations. Sci. STKE 2004, 2004, pl15. [Google Scholar] [CrossRef] [PubMed]
- Pnevmatikakis, E.A. Analysis pipelines for calcium imaging data. Curr. Opin. Neurobiol. 2019, 55, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.E.; Miller, A.L. Ca2+ signaling and early embryonic patterning during the blastula and gastrula periods of zebrafish and Xenopus development. Biochim. Biophys. Acta 2006, 1763, 1192–1208. [Google Scholar] [CrossRef]
- Leclerc, C.; Webb, S.E.; Daguzan, C.; Moreau, M.; Miller, A.L. Imaging patterns of calcium transients during neural induction in Xenopus laevis embryos. J. Cell Sci. 2000, 113 Pt. 19, 3519–3529. [Google Scholar] [CrossRef]
- Sequerra, E.B.; Goyal, R.; Castro, P.A.; Levin, J.B.; Borodinsky, L.N. NMDA Receptor Signaling Is Important for Neural Tube Formation and for Preventing Antiepileptic Drug-Induced Neural Tube Defects. J. Neurosci. 2018, 38, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, N.; Skourides, P.A. Cell-Autonomous Ca2+ Flashes Elicit Pulsed Contractions of an Apical Actin Network to Drive Apical Constriction during Neural Tube Closure. Cell Rep. 2015, 13, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sato, M.; Koyama, H.; Hara, Y.; Hayashi, K.; Yasue, N.; Imamura, H.; Fujimori, T.; Nagai, T.; Campbell, R.E.; et al. Distinct intracellular Ca2+ dynamics regulate apical constriction and differentially contribute to neural tube closure. Development 2017, 144, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Fluck, R.A.; Miller, A.L.; Jaffe, L.F. Slow calcium waves accompany cytokinesis in medaka fish eggs. J. Cell Biol. 1991, 115, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Fluck, R.A.; Miller, A.L.; Jaffe, L.F. Calcium Waves Accompany Contraction Waves in the Oryzias latipes (Medaka) Blastoderm. Biol. Bull. 1991, 181, 352. [Google Scholar] [CrossRef] [PubMed]
- Blinks, J.R. Use of photoproteins as intracellular calcium indicators. Environ. Health Perspect. 1990, 84, 75–81. [Google Scholar] [CrossRef]
- Webb, S.E.; Lee, K.W.; Karplus, E.; Miller, A.L. Localized calcium transients accompany furrow positioning, propagation, and deepening during the early cleavage period of zebrafish embryos. Dev. Biol. 1997, 192, 78–92. [Google Scholar] [CrossRef]
- Muto, A.; Kume, S.; Inoue, T.; Okano, H.; Mikoshiba, K. Calcium waves along the cleavage furrows in cleavage-stage Xenopus embryos and its inhibition by heparin. J. Cell Biol. 1996, 135, 181–190. [Google Scholar] [CrossRef]
- Mizuno, H.; Sassa, T.; Higashijima, S.; Okamoto, H.; Miyawaki, A. Transgenic zebrafish for ratiometric imaging of cytosolic and mitochondrial Ca2+ response in teleost embryo. Cell Calcium 2013, 54, 236–245. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Sakagami, H. Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. Int. J. Mol. Sci. 2022, 23, 1025. [Google Scholar] [CrossRef]
- Stith, B.J. Phospholipase C and D regulation of Src, calcium release and membrane fusion during Xenopus laevis development. Dev. Biol. 2015, 401, 188–205. [Google Scholar] [CrossRef]
- Westfall, T.A.; Brimeyer, R.; Twedt, J.; Gladon, J.; Olberding, A.; Furutani-Seiki, M.; Slusarski, D.C. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J. Cell Biol. 2003, 162, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Lyman Gingerich, J.; Westfall, T.A.; Slusarski, D.C.; Pelegri, F. hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev. Biol. 2005, 286, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Slusarski, D.C.; Yang-Snyder, J.; Busa, W.B.; Moon, R.T. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 1997, 182, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Westfall, T.A.; Hjertos, B.; Slusarski, D.C. Requirement for intracellular calcium modulation in zebrafish dorsal-ventral patterning. Dev. Biol. 2003, 259, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Slusarski, D.C.; Corces, V.G.; Moon, R.T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 1997, 390, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Eno, C.; Gomez, T.; Slusarski, D.C.; Pelegri, F. Slow calcium waves mediate furrow microtubule reorganization and germ plasm compaction in the early zebrafish embryo. Development 2018, 145, dev156604. [Google Scholar] [CrossRef]
- Ma, L.H.; Webb, S.E.; Chan, C.M.; Zhang, J.; Miller, A.L. Establishment of a transitory dorsal-biased window of localized Ca2+ signaling in the superficial epithelium following the mid-blastula transition in zebrafish embryos. Dev. Biol. 2009, 327, 143–157. [Google Scholar] [CrossRef]
- Zhang, J.; Webb, S.E.; Ma, L.H.; Chan, C.M.; Miller, A.L. Necessary role for intracellular Ca2+ transients in initiating the apical-basolateral thinning of enveloping layer cells during the early blastula period of zebrafish development. Dev. Growth Differ. 2011, 53, 679–696. [Google Scholar] [CrossRef]
- Gilland, E.; Miller, A.L.; Karplus, E.; Baker, R.; Webb, S.E. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. Proc. Natl. Acad. Sci. USA 1999, 96, 157–161. [Google Scholar] [CrossRef]
- Reinhard, E.; Yokoe, H.; Niebling, K.R.; Allbritton, N.L.; Kuhn, M.A.; Meyer, T. Localized calcium signals in early zebrafish development. Dev. Biol. 1995, 170, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.Y.; Webb, S.E.; Chan, C.M.; Thisse, B.; Thisse, C.; Miller, A.L. Characterization of Ca2+ signaling in the external yolk syncytial layer during the late blastula and early gastrula periods of zebrafish development. Biochim. Biophys. Acta 2013, 1833, 1641–1656. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, L.; Bruchas, M.R.; Solnica-Krezel, L. Imaging early embryonic calcium activity with GCaMP6s transgenic zebrafish. Dev. Biol. 2017, 430, 385–396. [Google Scholar] [CrossRef]
- Créton, R.; Kreiling, J.A.; Jaffe, L.F. Presence and roles of calcium gradients along the dorsal-ventral axis in Drosophila embryos. Dev. Biol. 2000, 217, 375–385. [Google Scholar] [CrossRef]
- Creton, R.; Speksnijder, J.E.; Jaffe, L.F. Patterns of free calcium in zebrafish embryos. J. Cell Sci. 1998, 111 Pt. 12, 1613–1622. [Google Scholar] [CrossRef]
- Markova, O.; Senatore, S.; Chardes, C.; Lenne, P.F. Calcium Spikes in Epithelium: Study on Drosophila early embryos. Sci. Rep. 2015, 5, 11379. [Google Scholar] [CrossRef]
- Mikhaleva, Y.; Tolstenkov, O.; Glover, J.C. Gap junction-dependent coordination of intercellular calcium signalling in the developing appendicularian tunicate Oikopleura dioica. Dev. Biol. 2019, 450, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wilde, J.J.; Petersen, J.R.; Niswander, L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu. Rev. Genet. 2014, 48, 583–611. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.; Copp, A.J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef]
- Webb, S.E.; Moreau, M.; Leclerc, C.; Miller, A.L. Calcium transients and neural induction in vertebrates. Cell Calcium 2005, 37, 375–385. [Google Scholar] [CrossRef]
- Levine, A.J.; Brivanlou, A.H. Proposal of a model of mammalian neural induction. Dev. Biol. 2007, 308, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Neant, I.; Webb, S.E.; Miller, A.L.; Leclerc, C. Calcium signalling during neural induction in Xenopus laevis embryos. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Ozair, M.Z.; Kintner, C.; Brivanlou, A.H. Neural induction and early patterning in vertebrates. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Smedley, M.J.; Stanisstreet, M. Calcium and neurulation in mammalian embryos. II. Effects of cytoskeletal inhibitors and calcium antagonists on the neural folds of rat embryos. J. Embryol. Exp. Morphol. 1986, 93, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C.; Goldstein, B. Apical constriction: Themes and variations on a cellular mechanism driving morphogenesis. Development 2014, 141, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Leclerc, C. The choice between epidermal and neural fate: A matter of calcium. Int. J. Dev. Biol. 2004, 48, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Tang, Y.; Davila, J.; Deng, S.; Chen, L.; Miller, E.; Wernig, M.; Graef, I.A. Calcineurin signaling regulates neural induction through antagonizing the BMP pathway. Neuron 2014, 82, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Batut, J.; Vandel, L.; Leclerc, C.; Daguzan, C.; Moreau, M.; Neant, I. The Ca2+-induced methyltransferase xPRMT1b controls neural fate in amphibian embryo. Proc. Natl. Acad. Sci. USA 2005, 102, 15128–15133. [Google Scholar] [CrossRef] [PubMed]
- Borodinsky, L.N.; Root, C.M.; Cronin, J.A.; Sann, S.B.; Gu, X.; Spitzer, N.C. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 2004, 429, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, J.B.; Ewald, A.J.; Harland, R.M.; Fraser, S.E. Calcium signaling during convergent extension in Xenopus. Curr. Biol. 2001, 11, 652–661. [Google Scholar] [CrossRef]
- Akahoshi, T.; Hotta, K.; Oka, K. Characterization of calcium transients during early embryogenesis in ascidians Ciona robusta (Ciona intestinalis type A) and Ciona savignyi. Dev. Biol. 2017, 431, 205–214. [Google Scholar] [CrossRef]
- Shindo, A.; Hara, Y.; Yamamoto, T.S.; Ohkura, M.; Nakai, J.; Ueno, N. Tissue-tissue interaction-triggered calcium elevation is required for cell polarization during Xenopus gastrulation. PLoS ONE 2010, 5, e8897. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamamoto, T.S.; Ueno, N. Intracellular calcium signal at the leading edge regulates mesodermal sheet migration during Xenopus gastrulation. Sci. Rep. 2018, 8, 2433. [Google Scholar] [CrossRef] [PubMed]
- Kreiling, J.A.; Balantac, Z.L.; Crawford, A.R.; Ren, Y.; Toure, J.; Zchut, S.; Kochilas, L.; Creton, R. Suppression of the endoplasmic reticulum calcium pump during zebrafish gastrulation affects left-right asymmetry of the heart and brain. Mech. Dev. 2008, 125, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Papanayotou, C.; De Almeida, I.; Liao, P.; Oliveira, N.M.; Lu, S.Q.; Kougioumtzidou, E.; Zhu, L.; Shaw, A.; Sheng, G.; Streit, A.; et al. Calfacilitin is a calcium channel modulator essential for initiation of neural plate development. Nat. Commun. 2013, 4, 1837. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.; Solon, J. Drosophila dorsal closure: An orchestra of forces to zip shut the embryo. Mech. Dev. 2017, 144, 2–10. [Google Scholar] [CrossRef]
- Hunter, G.L.; Crawford, J.M.; Genkins, J.Z.; Kiehart, D.P. Ion channels contribute to the regulation of cell sheet forces during Drosophila dorsal closure. Development 2014, 141, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Garcia-Garcia, M.J. Secretory pathway calcium ATPase 1 (SPCA1) controls mouse neural tube closure by regulating cytoskeletal dynamics. Development 2018, 145. [Google Scholar] [CrossRef]
- Sakurada, S.; Takuwa, N.; Sugimoto, N.; Wang, Y.; Seto, M.; Sasaki, Y.; Takuwa, Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ. Res. 2003, 93, 548–556. [Google Scholar] [CrossRef]
- Antunes, M.; Pereira, T.; Cordeiro, J.V.; Almeida, L.; Jacinto, A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J. Cell Biol. 2013, 202, 365–379. [Google Scholar] [CrossRef]
- Borodinsky, L.N.; Spitzer, N.C. Second messenger pas de deux: The coordinated dance between calcium and cAMP. Sci. STKE 2006, 2006, pe22. [Google Scholar] [CrossRef] [PubMed]
- Borodinsky, L.N.; Spitzer, N.C. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2007, 104, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Belgacem, Y.H.; Borodinsky, L.N. Inversion of Sonic hedgehog action on its canonical pathway by electrical activity. Proc. Natl. Acad. Sci. USA 2015, 112, 4140–4145. [Google Scholar] [CrossRef] [PubMed]
- Belgacem, Y.H.; Borodinsky, L.N. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc. Natl. Acad. Sci. USA 2011, 108, 4482–4487. [Google Scholar] [CrossRef] [PubMed]
- Swapna, I.; Borodinsky, L.N. Interplay between electrical activity and bone morphogenetic protein signaling regulates spinal neuron differentiation. Proc. Natl. Acad. Sci. USA 2012, 109, 16336–16341. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.K.; Borodinsky, L.N. Spontaneous calcium transients manifest in the regenerating muscle and are necessary for skeletal muscle replenishment. Cell Calcium 2014, 56, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wajid, S.; Morales-Diaz, H.; Khairallah, S.M.; Smith, W.C. T-type Calcium Channel Regulation of Neural Tube Closure and EphrinA/EPHA Expression. Cell Rep. 2015, 13, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, N.C.; Borodinsky, L.N.; Root, C.M. Imaging and manipulating calcium transients in developing Xenopus spinal neurons. Cold Spring Harb. Protoc. 2013, 2013, 653–664. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, L.; Hernandez-Ochoa, E.; Schneider, M.F.; Reece, E.A. Disturbed intracellular calcium homeostasis in neural tube defects in diabetic embryopathy. Biochem. Biophys. Res. Commun. 2019, 514, 960–966. [Google Scholar] [CrossRef] [PubMed]
- McMillen, P.; Novak, R.; Levin, M. Toward Decoding Bioelectric Events in Xenopus Embryogenesis: New Methodology for Tracking Interplay Between Calcium and Resting Potentials In Vivo. J. Mol. Biol. 2020, 432, 605–620. [Google Scholar] [CrossRef]
- Spitzer, N.C. Developmental Neuroscience. Neurotransmitter-Tailored Dendritic Trees. Science 2015, 350, 510–511. [Google Scholar] [CrossRef]
- Spitzer, N.C. Neurotransmitter Switching? No Surprise. Neuron 2015, 86, 1131–1144. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, L.; Spitzer, N.C. Target-dependent regulation of neurotransmitter specification and embryonic neuronal calcium spike activity. J. Neurosci. 2010, 30, 5792–5801. [Google Scholar] [CrossRef] [PubMed]
- Demarque, M.; Spitzer, N.C. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron 2010, 67, 321–334. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.C.; Kulesa, P.M. In vivo calcium dynamics during neural crest cell migration and patterning using GCaMP3. Dev. Biol. 2011, 358, 309–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velazquez-Ulloa, N.A.; Spitzer, N.C.; Dulcis, D. Contexts for dopamine specification by calcium spike activity in the CNS. J. Neurosci. 2011, 31, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.U.; Visetsouk, M.R.; Garde, R.J.; Hennes, L.; Kwas, C.; Gutzman, J.H. Calcium signals drive cell shape changes during zebrafish midbrain-hindbrain boundary formation. Mol. Biol. Cell 2017, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Han, S.; Kim, G.; Eom, M.; Lee, K.H.; Kim, C.H.; Yoon, Y.G. In vivo whole-brain imaging of zebrafish larvae using three-dimensional fluorescence microscopy. J. Vis. Exp. 2023, 194, e65218. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wei, Z.; Looger, L.L.; Koyama, M.; Druckmann, S.; Keller, P.J. Single-Cell Reconstruction of Emerging Population Activity in an Entire Developing Circuit. Cell 2019, 179, 355–372.e23. [Google Scholar] [CrossRef]
- Warp, E.; Agarwal, G.; Wyart, C.; Friedmann, D.; Oldfield, C.S.; Conner, A.; Del Bene, F.; Arrenberg, A.B.; Baier, H.; Isacoff, E.Y. Emergence of patterned activity in the developing zebrafish spinal cord. Curr. Biol. 2012, 22, 93–102. [Google Scholar] [CrossRef]
- Chang, L.W.; Spitzer, N.C. Spontaneous calcium spike activity in embryonic spinal neurons is regulated by developmental expression of the Na+, K+-ATPase beta3 subunit. J. Neurosci. 2009, 29, 7877–7885. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.W.; Kurtz, L.M.; Spitzer, N.C. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat. Neurosci. 2010, 13, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.M.; Spitzer, N.C. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 1999, 397, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Guemez-Gamboa, A.; Xu, L.; Meng, D.; Spitzer, N.C. Non-cell-autonomous mechanism of activity-dependent neurotransmitter switching. Neuron 2014, 82, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Spitzer, N.C. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature 2008, 456, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Root, C.M.; Velazquez-Ulloa, N.A.; Monsalve, G.C.; Minakova, E.; Spitzer, N.C. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J. Neurosci. 2008, 28, 4777–4784. [Google Scholar] [CrossRef] [PubMed]
- Bataille, S.; Jalaber, H.; Colin, I.; Remy, D.; Affaticati, P.; Froc, C.; Levraud, J.P.; Vernier, P.; Demarque, M. Plasticity of Dopaminergic Phenotype and Locomotion in Larval Zebrafish Induced by Brain Excitability Changes during the Embryonic Period. eNeuro 2023, 10. [Google Scholar] [CrossRef]
- Munz, M.; Bharioke, A.; Kosche, G.; Moreno-Juan, V.; Brignall, A.; Rodrigues, T.M.; Graff-Meyer, A.; Ulmer, T.; Haeuselmann, S.; Pavlinic, D.; et al. Pyramidal neurons form active, transient, multilayered circuits perturbed by autism-associated mutations at the inception of neocortex. Cell 2023, 186, 1930–1949.e31. [Google Scholar] [CrossRef]
- Eom, D.S.; Amarnath, S.; Agarwala, S. Apicobasal polarity and neural tube closure. Dev. Growth Differ. 2013, 55, 164–172. [Google Scholar] [CrossRef]
- Leclerc, C.; Guerrier, P.; Moreau, M. Role of dihydropyridine-sensitive calcium channels in meiosis and fertilization in the bivalve molluscs Ruditapes philippinarum and Crassostrea gigas. Biol. Cell 2000, 92, 285–299. [Google Scholar] [CrossRef]
- Gomez, T.M.; Robles, E.; Poo, M.; Spitzer, N.C. Filopodial calcium transients promote substrate-dependent growth cone turning. Science 2001, 291, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.A.; Belgacem, Y.H.; Visina, O.; Shim, S.; Genus, H.; Borodinsky, L.N. Growth at Cold Temperature Increases the Number of Motor Neurons to Optimize Locomotor Function. Curr. Biol. 2019, 29, 1787–1799.e5. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, S.; Yue, M.; Nalamalapu, R.; Saha, M.S. Deciphering the Calcium Code: A Review of Calcium Activity Analysis Methods Employed to Identify Meaningful Activity in Early Neural Development. Biomolecules 2024, 14, 138. https://doi.org/10.3390/biom14010138
Paudel S, Yue M, Nalamalapu R, Saha MS. Deciphering the Calcium Code: A Review of Calcium Activity Analysis Methods Employed to Identify Meaningful Activity in Early Neural Development. Biomolecules. 2024; 14(1):138. https://doi.org/10.3390/biom14010138
Chicago/Turabian StylePaudel, Sudip, Michelle Yue, Rithvik Nalamalapu, and Margaret S. Saha. 2024. "Deciphering the Calcium Code: A Review of Calcium Activity Analysis Methods Employed to Identify Meaningful Activity in Early Neural Development" Biomolecules 14, no. 1: 138. https://doi.org/10.3390/biom14010138
APA StylePaudel, S., Yue, M., Nalamalapu, R., & Saha, M. S. (2024). Deciphering the Calcium Code: A Review of Calcium Activity Analysis Methods Employed to Identify Meaningful Activity in Early Neural Development. Biomolecules, 14(1), 138. https://doi.org/10.3390/biom14010138





