Insights into Pharmacological Activities of Nicotine and 6-Hydroxy-L-nicotine, a Bacterial Nicotine Derivative: A Systematic Review
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Nicotine
3.1. Chemical and Pharmacological Properties of Nicotine
3.2. The Cognitive Effects of Nicotine
3.3. Short-Term Cognitive Effects of Nicotine
3.4. Effects of Nicotine on Neuroinflammation
3.5. Effects of Nicotine on Apoptosis
3.6. Effects of Nicotine on Neurotrophic Factors
3.7. Effects of Nicotine on Amyloid-Beta Peptide
3.8. Effects of Nicotine on Oxidative Stress
3.9. Adverse Effects of Nicotine
4. 6-Hydroxy-L-nicotine
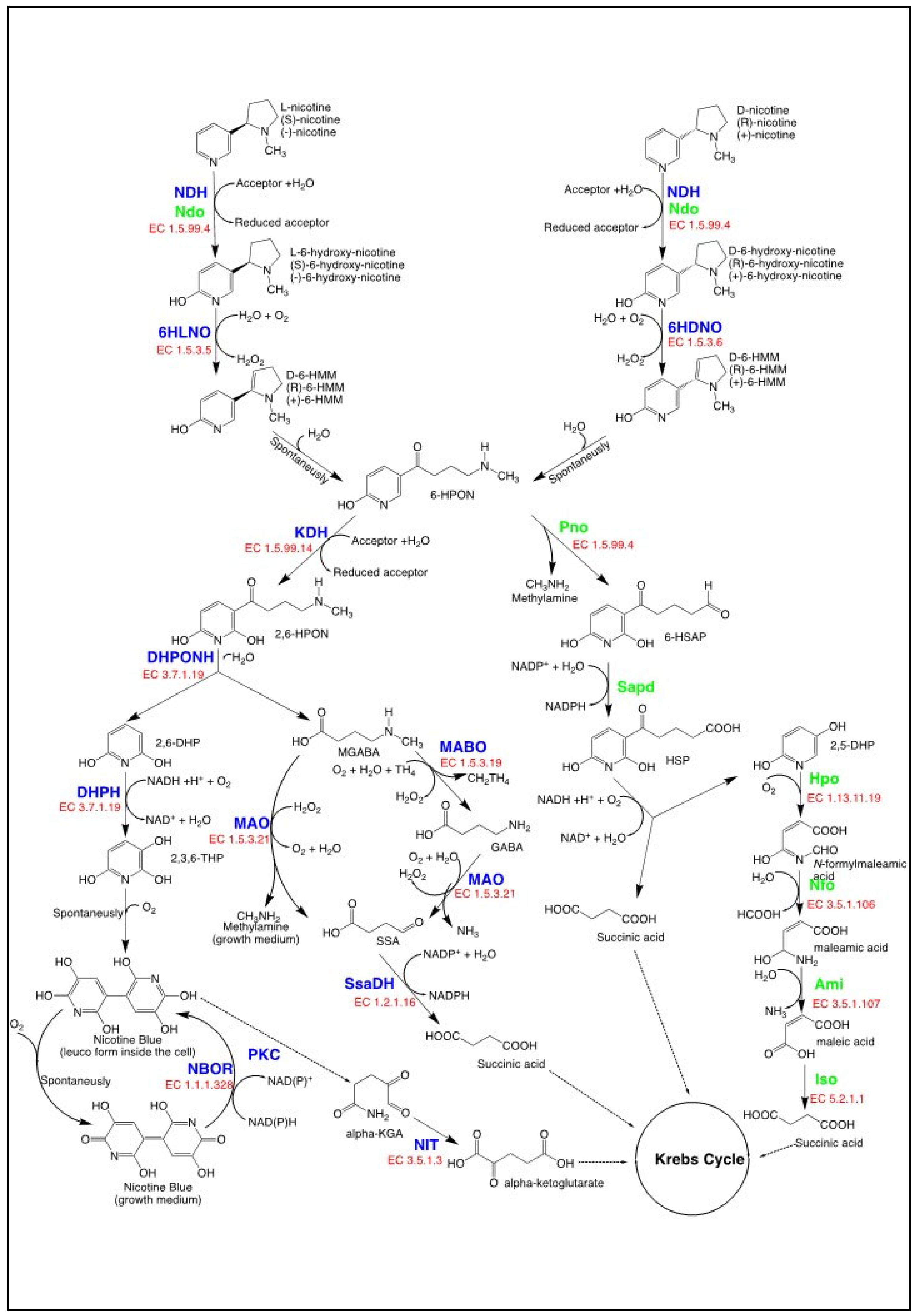
4.1. Nicotine Catabolism in Bacteria as a Source of 6-hydroxy-L-nicotine
4.1.1. The Pyridine Pathway for Nicotine Degradation
4.1.2. The VPP Pathway for Nicotine Degradation
4.2. Applications of NDB for 6-Hydroxy-L-nicotine Production from Nicotine-Containing Waste
4.3. The Behavioral Effects of 6-Hydroxy-L-nicotine
4.4. Effects of 6-Hydroxy-L-nicotine on Acetylcholinesterase Activity
4.5. Effects of 6-Hydroxy-L-nicotine on Oxidative Stress
4.6. The Proposed Mechanism of Action for 6-Hydroxy-L-nicotine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Picciotto, M.R.; Kenny, P.J. Mechanisms of Nicotine Addiction. Cold Spring Harb. Perspect. Med. 2021, 11, a039610. [Google Scholar] [CrossRef]
- Tutka, P.; Mosiewicz, J.; Wielosz, M. Pharmacokinetics and Metabolism of Nicotine. Pharmacol. Rep. 2005, 57, 143–153. [Google Scholar] [PubMed]
- Newhouse, P.; Kellar, K.; Aisen, P.; White, H.; Wesnes, K.; Coderre, E.; Pfaff, A.; Wilkins, H.; Howard, D.; Levin, E.D. Nicotine Treatment of Mild Cognitive Impairment: A 6-Month Double-Blind Pilot Clinical Trial. Neurology 2012, 78, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.B.; Alijevic, O.; Johne, S.; Overk, C.; Hashimoto, M.; Kondylis, A.; Adame, A.; Dulize, R.; Peric, D.; Nury, C.; et al. Nicotine-Mediated Effects in Neuronal and Mouse Models of Synucleinopathy. Front. Neurosci. 2023, 17, 1239009. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Xie, Z.; Liu, Q.; Zhang, L.; Han, X.; Liao, X.; Liu, J.; Gao, F. Nicotine and Menthol Independently Exert Neuroprotective Effects against Cisplatin- or Amyloid- Toxicity by Upregulating Bcl-Xl via JNK Activation in SH-SY5Y Cells. Biocell 2021, 45, 1059–1067. [Google Scholar] [CrossRef]
- Xue, M.Q.; Liu, X.X.; Zhang, Y.L.; Gao, F.G. Nicotine Exerts Neuroprotective Effects against β-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells through the Erk1/2-P38-JNK-Dependent Signaling Pathway. Int. J. Mol. Med. 2014, 33, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; He, X.-Y.; Ding, X.; Prabhu, S.; Hong, J.-Y. Metabolism of Nicotine and Cotinine by Human Cytochrome P450 2A13. Drug Metab. Dispos. 2005, 33, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Vatandoust, S.M.; Mahmoudi, J.; Rahigh Aghsan, S.; Majdi, A. Cotinine Ameliorates Memory and Learning Impairment in Senescent Mice. Brain Res. Bull. 2020, 164, 65–74. [Google Scholar] [CrossRef]
- Echeverria, V.; Zeitlin, R.; Burgess, S.; Patel, S.; Barman, A.; Thakur, G.; Mamcarz, M.; Wang, L.; Sattelle, D.B.; Kirschner, D.A.; et al. Cotinine Reduces Amyloid-β Aggregation and Improves Memory in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2011, 24, 817–835. [Google Scholar] [CrossRef]
- Echeverria, V.; Mendoza, C.; Iarkov, A. Nicotinic Acetylcholine Receptors and Learning and Memory Deficits in Neuroinflammatory Diseases. Front. Neurosci. 2023, 17, 1179611. [Google Scholar] [CrossRef]
- Gao, J.; Adam, B.-L.; Terry, A.V. Evaluation of Nicotine and Cotinine Analogs as Potential Neuroprotective Agents for Alzheimer’s Disease. Bioorg. Med. Chem. Lett. 2014, 24, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, V.; Zeitlin, R. Cotinine: A Potential New Therapeutic Agent against Alzheimer’s Disease. CNS Neurosci. Ther. 2012, 18, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. Handb. Exp. Pharmacol. 2009, 192, 29–60. [Google Scholar] [CrossRef]
- Gurusamy, R.; Natarajan, S. Current Status on Biochemistry and Molecular Biology of Microbial Degradation of Nicotine. Sci. World J. 2013, 2013, 125385. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.N.; Gregersen, P.K.; Malhotra, A.K. Metabolism and Biochemical Effects of Nicotine for Primary Care Providers. Med. Clin. N. Am. 2004, 88, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; McAfee, G.; Geldenhuys, W.J.; Van Der Schyf, C.J.; Abbruscato, T.J.; Allen, D.D. Brain Uptake Kinetics of Nicotine and Cotinine after Chronic Nicotine Exposure. J. Pharmacol. Exp. Ther. 2005, 314, 636–642. [Google Scholar] [CrossRef]
- Matta, S.G.; Balfour, D.J.; Benowitz, N.L.; Boyd, R.T.; Buccafusco, J.J.; Caggiula, A.R.; Craig, C.R.; Collins, A.C.; Damaj, M.I.; Donny, E.C.; et al. Guidelines on Nicotine Dose Selection for In Vivo Research. Psychopharmacology 2007, 190, 269–319. [Google Scholar] [CrossRef]
- Chi, L.; Mahbub, R.; Gao, B.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Nicotine Alters the Gut Microbiome and Metabolites of Gut-Brain Interactions in a Sex-Specific Manner. Chem. Res. Toxicol. 2017, 30, 2110–2119. [Google Scholar] [CrossRef]
- Lakosa, A.; Rahimian, A.; Tomasi, F.; Marti, F.; Reynolds, L.M.; Tochon, L.; David, V.; Danckaert, A.; Canonne, C.; Tahraoui, S.; et al. Impact of the Gut Microbiome on Nicotine’s Motivational Effects and Glial Cells in the Ventral Tegmental Area in Male Mice. Neuropsychopharmacology 2023, 48, 963–974. [Google Scholar] [CrossRef]
- Brandsch, R. Microbiology and Biochemistry of Nicotine Degradation. Appl. Microbiol. Biotechnol. 2006, 69, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Valentine, G.; Sofuoglu, M. Cognitive Effects of Nicotine: Recent Progress. Curr. Neuropharmacol. 2017, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, W.; Wang, H.; Geng, P.; Sun, Y.; Zhang, J.; Wang, W.; Jin, X. Nicotine’s Effect on Cognition, a Friend or Foe? Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 124, 110723. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Wang, M.; Song, M.; Sun, Y.; Shen, X.; Zhang, J.; Jin, X. Acute Nicotine Treatment Alleviates LPS-Induced Impairment of Fear Memory Reconsolidation through AMPK Activation and CRTC1 Upregulation in Hippocampus. Int. J. Neuropsychopharmacol. 2021, 23, 687–699. [Google Scholar] [CrossRef]
- Grus, A.; Hromatko, I. Acute Administration of Nicotine Does Not Enhance Cognitive Functions. Arh. Hig. Rada Toksikol. 2019, 70, 273–282. [Google Scholar] [CrossRef]
- Belluzzi, J.D.; Wang, R.; Leslie, F.M. Acetaldehyde Enhances Acquisition of Nicotine Self-Administration in Adolescent Rats. Neuropsychopharmacology 2005, 30, 705–712. [Google Scholar] [CrossRef]
- Poltavski, D.V.; Petros, T.V.; Holm, J.E. Lower but Not Higher Doses of Transdermal Nicotine Facilitate Cognitive Performance in Smokers on Gender Non-Preferred Tasks. Pharmacol. Biochem. Behav. 2012, 102, 423–433. [Google Scholar] [CrossRef]
- Newhouse, P.A.; Potter, A.; Singh, A. Effects of Nicotinic Stimulation on Cognitive Performance. Curr. Opin. Pharmacol. 2004, 4, 36–46. [Google Scholar] [CrossRef]
- Grundey, J.; Amu, R.; Ambrus, G.G.; Batsikadze, G.; Paulus, W.; Nitsche, M.A. Double Dissociation of Working Memory and Attentional Processes in Smokers and Non-Smokers with and without Nicotine. Psychopharmacology 2015, 232, 2491–2501. [Google Scholar] [CrossRef]
- Potter, A.S.; Newhouse, P.A. Acute Nicotine Improves Cognitive Deficits in Young Adults with Attention-Deficit/Hyperactivity Disorder. Pharmacol. Biochem. Behav. 2008, 88, 407–417. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Age-Associated Changes in the Immune System and Blood–Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef] [PubMed]
- Shytle, R.D.; Mori, T.; Townsend, K.; Vendrame, M.; Sun, N.; Zeng, J.; Ehrhart, J.; Silver, A.A.; Sanberg, P.R.; Tan, J. Cholinergic Modulation of Microglial Activation by A7 Nicotinic Receptors. J. Neurochem. 2004, 89, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils Promote Alzheimer’s Disease-like Pathology and Cognitive Decline via LFA-1 Integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 413854. [Google Scholar] [CrossRef] [PubMed]
- Majdi, A.; Kamari, F.; Vafaee, M.S.; Sadigh-Eteghad, S. Revisiting Nicotine’s Role in the Ageing Brain and Cognitive Impairment. Rev. Neurosci. 2017, 28, 767–781. [Google Scholar] [CrossRef]
- Hao, J.; Simard, A.R.; Turner, G.H.; Wu, J.; Whiteaker, P.; Lukas, R.J.; Shi, F.D. Attenuation of CNS Inflammatory Responses by Nicotine Involves A7 and Non-A7 Nicotinic Receptors. Exp. Neurol. 2011, 227, 110–119. [Google Scholar] [CrossRef]
- Han, X.; Zhou, N.; Hu, H.; Li, X.; Liu, H. Nicotine Alleviates Cortical Neuronal Injury by Suppressing Neuroinflammation and Upregulating Neuronal PI3K-AKT Signaling in an Eclampsia-Like Seizure Model. Neurotox Res. 2020, 38, 665–681. [Google Scholar] [CrossRef]
- Wei, P.; Liu, Q.; Li, D.; Zheng, Q.; Zhou, J.; Li, J. Acute Nicotine Treatment Attenuates Lipopolysaccharide-Induced Cognitive Dysfunction by Increasing BDNF Expression and Inhibiting Neuroinflammation in the Rat Hippocampus. Neurosci. Lett. 2015, 604, 161–166. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Majdi, A.; Mahmoudi, J.; Golzari, S.E.J.; Talebi, M. Astrocytic and Microglial Nicotinic Acetylcholine Receptors: An Overlooked Issue in Alzheimer’s Disease. J. Neural. Transm. 2016, 123, 1359–1367. [Google Scholar] [CrossRef]
- Revathikumar, P.; Bergqvist, F.; Gopalakrishnan, S.; Korotkova, M.; Jakobsson, P.J.; Lampa, J.; Le Maître, E. Immunomodulatory Effects of Nicotine on Interleukin 1β Activated Human Astrocytes and the Role of Cyclooxygenase 2 in the Underlying Mechanism. J. Neuroinflamm. 2016, 13. [Google Scholar] [CrossRef]
- Han, Y.; Lau, Y.-L. Nicotine, an Anti-Inflammation Molecule. Inflamm. Cell Signal. 2014, 1, e155. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, H.; Zou, M.; Yuan, Q.; Huang, Z.; Pan, X.; Zhang, W. Nicotine in Inflammatory Diseases: Anti-Inflammatory and Pro-Inflammatory Effects. Front. Immunol. 2022, 13, 826889. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amelia, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, N.; Ulloa, L.; et al. Nicotinic Acetylcholine Receptor A7 Subunit Is an Essential Regulator of Inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Ajmone-Cat, M.A.; Carnevale, D.; Minghetti, L. Activation of A7 Nicotinic Acetylcholine Receptor by Nicotine Selectively Up-Regulates Cyclooxygenase-2 and Prostaglandin E2 in Rat Microglial Cultures. J. Neuroinflamm. 2005, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Razani-Boroujerdi, S.; Boyd, R.T.; Dávila-García, M.I.; Nandi, J.S.; Mishra, N.C.; Singh, S.P.; Pena-Philippides, J.C.; Langley, R.; Sopori, M.L. T Cells Express A7-Nicotinic Acetylcholine Receptor Subunits That Require a Functional TCR and Leukocyte-Specific Protein Tyrosine Kinase for Nicotine-Induced Ca2+ Response. J. Immunol. 2007, 179, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the Cholinergic Anti-Inflammatory System by Nicotine Attenuates Neuroinflammation via Suppression of Th1 and Th17 Responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, E.; Agrawal, R.; Nath, C.; Shukla, R. Cholinergic Protection via A7 Nicotinic Acetylcholine Receptors and PI3K-Akt Pathway in LPS-Induced Neuroinflammation. Neurochem. Int. 2010, 56, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T. Apoptosis and Its Functional Significance in Molluscs. Apoptosis 2010, 15, 313–321. [Google Scholar] [CrossRef]
- Majdi, A.; Mahmoudi, J.; Sadigh-Eteghad, S.; Golzari, S.E.J.; Sabermarouf, B.; Reyhani-Rad, S. Permissive Role of Cytosolic PH Acidification in Neurodegeneration: A Closer Look at Its Causes and Consequences. J. Neurosci. Res. 2016, 94, 879–887. [Google Scholar] [CrossRef]
- Tizabi, Y.; Manaye, K.F.; Taylor, R.E. Nicotine Blocks Ethanol-Induced Apoptosis in Primary Cultures of Rat Cerebral Cortical and Cerebellar Granule Cells. Neurotox Res. 2005, 7, 319–322. [Google Scholar] [CrossRef]
- Yu, W.; Mechawar, N.; Krantic, S.; Quirion, R. A7 Nicotinic Receptor Activation Reduces β-Amyloid-Induced Apoptosis by Inhibiting Caspase-Independent Death through Phosphatidylinositol 3-Kinase Signaling. J. Neurochem. 2011, 119, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, B. Nicotine Attenuates Beta-Amyloid Peptide-Induced Neurotoxicity, Free Radical and Calcium Accumulation in Hippocampal Neuronal Cultures. Br. J. Pharmacol. 2004, 141, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Hejmadi, M.V.; Dajas-Bailador, F.; Barns, S.M.; Jones, B.; Wonnacott, S. Neuroprotection by Nicotine against Hypoxia-Induced Apoptosis in Cortical Cultures Involves Activation of Multiple Nicotinic Acetylcholine Receptor Subtypes. Mol. Cell. Neurosci. 2003, 24, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Ciobica, A.; Gorgan, L. Nicotine-Induced Memory Impairment by Increasing Brain Oxidative Stress. Cent. Eur. J. Biol. 2009, 4, 335–342. [Google Scholar] [CrossRef]
- Jang, M.H.; Shin, M.C.; Jung, S.B.; Lee, T.H.; Bahn, G.H.; Kwon, Y.K.; Kim, E.H.; Kim, C.J. Alcohol and Nicotine Reduce Cell Proliferation and Enhance Apoptosis in Dentate Gyrus. Neuroreport 2002, 13, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Garrido, R.; Mattson, M.P.; Hennig, B.; Toborek, M. Nicotine Protects against Arachidonic-Acid-Induced Caspase Activation, Cytochrome c Release and Apoptosis of Cultured Spinal Cord Neurons. J. Neurochem. 2001, 76, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Marrero, M.B.; Bencherif, M. Convergence of Alpha 7 Nicotinic Acetylcholine Receptor-Activated Pathways for Anti-Apoptosis and Anti-Inflammation: Central Role for JAK2 Activation of STAT3 and NF-ΚB. Brain Res. 2009, 1256, 1–7. [Google Scholar] [CrossRef]
- De Azevedo Cardoso, T.; Mondin, T.C.; Wiener, C.D.; Marques, M.B.; Fucolo, B.D.Á.; Pinheiro, R.T.; De Souza, L.D.M.; Da Silva, R.A.; Jansen, K.; Oses, J.P. Neurotrophic Factors, Clinical Features and Gender Differences in Depression. Neurochem. Res. 2014, 39, 1571–1578. [Google Scholar] [CrossRef]
- Erraji-Benchekroun, L.; Underwood, M.D.; Arango, V.; Galfalvy, H.; Pavlidis, P.; Smyrniotopoulos, P.; Mann, J.J.; Sibille, E. Molecular Aging in Human Prefrontal Cortex Is Selective and Continuous throughout Adult Life. Biol. Psychiatry 2005, 57, 549–558. [Google Scholar] [CrossRef]
- Nordvall, G.; Forsell, P.; Sandin, J. Neurotrophin-Targeted Therapeutics: A Gateway to Cognition and More? Drug Discov. Today 2022, 27, 103318. [Google Scholar] [CrossRef]
- Pradhan, J.; Noakes, P.G.; Bellingham, M.C. The Role of Altered BDNF/TrkB Signaling in Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2019, 13, 473534. [Google Scholar] [CrossRef] [PubMed]
- Ferrea, S.; Winterer, C. Neuroprotective and Neurotoxic Effects of Nicotine. Pharmacopsychiatry 2009, 42, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakool, C.; Grooms, K.; Bijli, K.M.; Crothers, K.; Fitzpatrick, A.M.; Hart, C.M. Nicotine Stimulates Nerve Growth Factor in Lung Fibroblasts through an NFκB-Dependent Mechanism. PLoS ONE 2014, 9, e109602. [Google Scholar] [CrossRef] [PubMed]
- Garrido, R.; King-Pospisil, K.; Son, K.W.; Hennig, B.; Toborek, M. Nicotine Upregulates Nerve Growth Factor Expression and Prevents Apoptosis of Cultured Spinal Cord Neurons. Neurosci. Res. 2003, 47, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, R.; Toledano, A.; Álvarez, M.I.; Turégano, L.; Colman, O.; Rosés, P.; Gómez de Segura, I.; De Miguel, E. Chronic Nicotine Administration Increases NGF-like Immunoreactivity in Frontoparietal Cerebral Cortex. J. Neurosci. Res. 2003, 73, 708–716. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, R.; Garcia, A.A.; Braschi, C.; Capsoni, S.; Maffei, L.; Berardi, N.; Cattaneo, A. Intranasal Administration of Nerve Growth Factor (NGF) Rescues Recognition Memory Deficits in AD11 Anti-NGF Transgenic Mice. Proc. Natl. Acad. Sci. USA 2005, 102, 3811–3816. [Google Scholar] [CrossRef]
- Czubak, A.; Nowakowska, E.; Kus, K.; Burda, K.; Metelska, J.; Baer-Dubowska, W.; Cichocki, M. Influences of Chronic Venlafaxine, Olanzapine and Nicotine on the Hippocampal and Cortical Concentrations of Brain-Derived Neurotrophic Factor (BDNF). Pharmacol. Rep. 2009, 61, 1017–1023. [Google Scholar] [CrossRef]
- Freedman, R.; Wetmore, C.; Stromberg, I.; Leonard, S.; Olson, L. α-Bungarotoxin Binding to Hippocampal Interneurons: Immunocytochemical Characterization and Effects on Growth Factor Expression. J. Neurosci. 1993, 13, 1965–1975. [Google Scholar] [CrossRef]
- Yamada, K.; Mizuno, M.; Nabeshima, T. Role for Brain-Derived Neurotrophic Factor in Learning and Memory. Life Sci. 2002, 70, 735–744. [Google Scholar] [CrossRef]
- Rodrigue, K.M.; Kennedy, K.M.; Devous, M.D.; Rieck, J.R.; Hebrank, A.C.; Diaz-Arrastia, R.; Mathews, D.; Park, D.C. β-Amyloid Burden in Healthy Aging: Regional Distribution and Cognitive Consequences. Neurology 2012, 78, 387–395. [Google Scholar] [CrossRef]
- Zahs, K.R.; Ashe, K.H. β-Amyloid Oligomers in Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2013, 5, 51139. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kumar, V.B.; Farr, S.A.; Nakaoke, R.; Robinson, S.M.; Morley, J.E. Impairments in Brain-to-Blood Transport of Amyloid-β and Reabsorption of Cerebrospinal Fluid in an Animal Model of Alzheimer’s Disease Are Reversed by Antisense Directed against Amyloid-β Protein Precursor. J. Alzheimer’s Dis. 2011, 23, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Boche, D.; Wilkinson, D.; Yadegarfar, G.; Hopkins, V.; Bayer, A.; Jones, R.W.; Bull-ock, R.; Love, S.; Neal, J.W.; et al. Long-Term Effects of Aβ42 Immunisation in Alzheimer’s Disease: Follow-up of a Randomised, Placebo-Controlled Phase I Trial. Lancet 2008, 372, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hellström-Lindahl, E.; Court, J.; Keverne, J.; Svedberg, M.; Lee, M.; Marutle, A.; Thomas, A.; Perry, E.; Bednar, I.; Nordberg, A. Nicotine Reduces Aβ in the Brain and Cerebral Vessels of APPsw Mice. Eur. J. Neurosci. 2004, 19, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, A.; Hellström-Lindahl, E.; Lee, M.; Johnson, M.; Mousavi, M.; Hall, R.; Perry, E.; Bednar, I.; Court, J. Chronic Nicotine Treatment Reduces Beta-Amyloidosis in the Brain of a Mouse Model of Alzheimer’s Disease (APPsw). J. Neurochem. 2002, 81, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, Y.; Li, H.; Jin, Y.; Zhao, L.; Wang, X. Nicotine Prevents in Vivo Aβ Toxicity in Caenorhabditis Elegans via SKN-1. Neurosci. Lett. 2021, 761, 136114. [Google Scholar] [CrossRef] [PubMed]
- Paulson, O.B.; Vigdis, I. Cigarette Smoking and Cerebral Blood Flow in a Cohort of Middle-Aged Adults. J. Cereb. Blood Flow Metab. 2020, 40, 904–905. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Yamada, M.; Naiki, H. Nicotine Breaks down Preformed Alzheimer’s β-Amyloid Fibrils In Vitro. Biol. Psychiatry 2002, 52, 880–886. [Google Scholar] [CrossRef]
- Dineley, K.T.; Westerman, M.; Bui, D.; Bell, K.; Ashe, K.H.; Sweatt, J.D. β-Amyloid Activates the Mitogen-Activated Protein Kinase Cascade via Hippocampal A7 Nicotinic Acetylcholine Receptors: In Vitro and In Vivo Mechanisms Related to Alzheimer’s Disease. J. Neurosci. 2001, 21, 4125–4133. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, M.; Guan, Z.; Yu, W. Astrocytic A7 Nicotinic Receptor Activation Inhibits Amyloid-β Aggregation by Upregulating Endogenous AB-Crystallin through the PI3K/Akt Signaling Pathway. Curr. Alzheimer Res. 2018, 16, 39–48. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Jones, A.K.; Brown, L.A.; Sattelle, D.B. Nicotinic Acetylcholine Receptor Signalling: Roles in Alzheimer’s Disease and Amyloid Neuroprotection. Pharmacol. Rev. 2009, 61, 39. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Godoy, J.A.; Vargas, J.Y.; Arrazola, M.S.; Rios, J.A.; Carvajal, F.J.; Serrano, F.G.; Farias, G.G. Nicotine Prevents Synaptic Impairment Induced by Amyloid-β Oligomers through A7-Nicotinic Acetylcholine Receptor Activation. Neuromol. Med. 2013, 15, 549–569. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Utsuki, T.; Chen, D.; Farlow, M.R.; Shoaib, M.; Ingram, D.K.; Greig, N.H. Nicotine Reduces the Secretion of Alzheimer’s Beta-Amyloid Precursor Protein Containing Beta-Amyloid Peptide in the Rat without Altering Synaptic Proteins. Ann. N. Y. Acad. Sci. 2002, 965, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The Role of Iron in Brain Ageing and Neurodegenerative Disorders. Lancet Neurol. 2014, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.M.; Raz, N. Appraising the Role of Iron in Brain Aging and Cognition: Promises and Limitations of MRI Methods. Neuropsychol. Rev. 2015, 25, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, M.; Jahromi, S.R.; Sagar, B.K.C.; Patil, R.K.; Shivanandappa, T.; Ramesh, S.R. Brain Aging, Memory Impairment and Oxidative Stress: A Study in Drosophila Melanogaster. Behav. Brain Res. 2014, 259, 60–69. [Google Scholar] [CrossRef]
- Guan, Z.Z.; Yu, W.F.; Nordberg, A.; Guan, Z.-Z.; Yu, W.-F.; Nordberg, A. Dual Effects of Nicotine on Oxidative Stress and Neuroprotection in PC12 Cells. Neurochem. Int. 2003, 43, 243–249. [Google Scholar] [CrossRef]
- Pachauri, V.; Flora, S.J.S. Effect of Nicotine Pretreatment on Arsenic-Induced Oxidative Stress in Male Wistar Rats. Hum. Exp. Toxicol. 2013, 32, 972–982. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Méndez-Alvarez, E.; Hermida-Ameijeiras, A.; López-Real, A.M.; Labandeira-García, J.L. Effects of (−)-Nicotine and (−)-Cotinine on 6-Hydroxydopamine-Induced Oxidative Stress and Neurotoxicity: Relevance for Parkinson’s Disease. Biochem. Pharmacol. 2002, 64, 125–135. [Google Scholar] [CrossRef]
- Goerig, M.; Ullrich, V.; Schettler, G.; Foltis, C.; Habenicht, A. A New Role for Nicotine: Selective Inhibition of Thromboxane Formation by Direct Interaction with Thromboxane Synthase in Human Promyelocytic Leukaemia Cells Differentiating into Macrophages. Clin. Investig. 1992, 70, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Linert, W.; Bridge, M.H.; Huber, M.; Bjugstad, K.B.; Grossman, S.; Arendash, G.W. In Vitro and In Vivo Studies Investigating Possible Antioxidant Actions of Nicotine: Relevance to Parkinson’s and Alzheimer’s Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 1999, 1454, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, D.; Ercal, N.; Armstrong, D.W. Nicotine Enantiomers and Oxidative Stress. Toxicology 1998, 130, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Nesil, T.; Cao, J.; Yang, Z.; Chang, S.L.; Li, M.D. Nicotine Mediates Expression of Genes Related to Antioxidant Capacity and Oxidative Stress Response in HIV-1 Transgenic Rat Brain. J. Neurovirol. 2016, 22, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Crowley-Weber, C.L.; Dvorakova, K.; Crowley, C.; Bernstein, H.; Bernstein, C.; Garewal, H.; Payne, C.M. Nicotine Increases Oxidative Stress, Activates NF-KappaB and GRP78, Induces Apoptosis and Sensitizes Cells to Genotoxic/Xenobiotic Stresses by a Multiple Stress Inducer, Deoxycholate: Relevance to Colon Carcinogenesis. Chem. Biol. Interact. 2003, 145, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular Toxicity of Nicotine: Implications for Electronic Cigarette Use. Trends. Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef]
- Schuller, H.M. The Impact of Smoking and the Influence of Other Factors on Lung Cancer. Expert. Rev. Respir. Med. 2019, 13, 761–769. [Google Scholar] [CrossRef]
- Chu, K.-M.; Cho, C.H.; Shin, V.Y. Nicotine and Gastrointestinal Disorders: Its Role in Ulceration and Cancer Development. Curr. Pharm. Des. 2013, 19, 5–10. [Google Scholar] [CrossRef]
- Georgiou, A.N.; Ntritsos, G.; Papadimitriou, N.; Dimou, N.; Evangelou, E. Cigarette Smoking, Coffee Consumption, Alcohol Intake, and Risk of Crohn’s Disease and Ulcerative Colitis: A Mendelian Randomization Study. Inflamm. Bowel. Dis. 2021, 27, 162–168. [Google Scholar] [CrossRef]
- Khademi, F.; Totonchi, H.; Mohammadi, N.; Zare, R.; Zal, F. Nicotine-Induced Oxidative Stress in Human Primary Endometrial Cells. Int. J. Toxicol. 2019, 38, 202–208. [Google Scholar] [CrossRef]
- Zanetti, F.; Giacomello, M.; Donati, Y.; Carnesecchi, S.; Frieden, M.; Barazzone-Argiroffo, C. Nicotine Mediates Oxidative Stress and Apoptosis through Cross Talk between NOX1 and Bcl-2 in Lung Epithelial Cells. Free Radic. Biol. Med. 2014, 76, 173–184. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.M.; Zhang, L.; Wilkes, B.J.; Vaillancourt, D.E.; Biederman, J.; Bhide, P.G. Nicotine and the Developing Brain: Insights from Preclinical Models. Pharmacol. Biochem. Behav. 2022, 214, 173355. [Google Scholar] [CrossRef] [PubMed]
- Igloi, G.; Brandsch, R. Arthrobacter Nicotinovorans PAO1 Megaplasmid Sequence, Strain ATCC 49919. GenBank 2002. Available online: https://www.ncbi.nlm.nih.gov/nuccore/25169022 (accessed on 12 July 2023).
- El-Sabeh, A.; Honceriu, I.; Kallabi, F.; Boiangiu, R.-S.; Mihasan, M. Complete Genome Sequences of Two Closely Related Paenarthrobacter Nicotinovorans Strains. Microbiol. Resour. Announc. 2022, 11, e0013322. [Google Scholar] [CrossRef] [PubMed]
- Ruan, A.; Min, H.; Zhu, W. Studies on Biodegradation of Nicotine by Arthrobacter Sp. Strain HF-2. J. Environ. Sci. Health B 2006, 41, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tang, H.; Ren, H.; Yu, H.; Wang, L.; Xu, P. Genome Sequence of a Nicotine-Degrading Strain of Arthrobacter. J. Bacteriol. 2012, 194, 5714–5715. [Google Scholar] [CrossRef] [PubMed]
- Ruan, A.; Gao, Y.; Fang, C.; Xu, Y. Isolation and Characterization of a Novel Nicotinophilic Bacterium, Arthrobacter Sp. ARF-1 and Its Metabolic Pathway. Biotechnol. Appl. Biochem. 2018, 65, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yu, H.; Tai, C.; Huang, K.; Liu, Y.; Wang, L.; Yao, Y.; Wu, G.; Xu, P. Genome Sequence of a Novel Nicotine-Degrading Strain, Pseudomonas Geniculata N1. J. Bacteriol. 2012, 194, 3553–3554. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Huang, K.; Wang, W.; Nie, X.; Jiang, Y.; Li, P.; Liu, S.; Xu, P.; Tang, H. Physiological and Biochemical Characterization of a Novel Nicotine-Degrading Bacterium Pseudomonas Geniculata N1. PLoS ONE 2014, 9, e84399. [Google Scholar] [CrossRef]
- Wei, H.; Lei, L.; Xia, Z.; Liu, S.; Liu, P.; Liu, X. Characterisation of a Novel Aerobic Nicotine-Biodegrading Strain of Pseudomonas Putida. Ann. Microbiol. 2008, 58, 41–45. [Google Scholar] [CrossRef]
- Pan, D.; Sun, M.; Wang, Y.; Lv, P.; Wu, X.; Li, Q.X.; Cao, H.; Hua, R. Characterization of Nicotine Catabolism through a Novel Pyrrolidine Pathway in Pseudomonas Sp. S-1. J. Agric. Food Chem. 2018, 66, 7393–7401. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Yu, Q.; Lei, L.P.; Wu, Y.P.; Ren, K.; Li, Y.; Zou, C.M. A Novel Nicotine-Degrading Bacterium Pseudomonas Fluorescens Strain 1206. Appl. Biochem. Microbiol. 2019, 55, 123–128. [Google Scholar] [CrossRef]
- Ruan, A.; Min, H.; Peng, X.; Huang, Z. Isolation and Characterization of Pseudomonas Sp. Strain HF-1, Capable of Degrading Nicotine. Res. Microbiol. 2005, 156, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Mohan, K.N.; Manohar, V.; Sakthivel, N. Biodegradation of Nicotine by a Novel Nicotine-Degrading Bacterium, Pseudomonas Plecoglossicida TND35 and Its New Biotransformation Intermediates. Biodegradation 2014, 25, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Ma, Y.; Zhang, J.; Wen, Y.; Liu, W. Cloning of a Novel Nicotine Oxidase Gene from Pseudomonas Sp. Strain HZN6 Whose Product Nonenantioselectively Degrades Nicotine to Pseudooxynicotine. Appl. Environ. Microbiol. 2013, 79, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Yin, B.; Peng, X.X.; Wang, J.Y.; Xie, Z.H.; Gao, J.; Tang, X.K. Biodegradation of Nicotine by Newly Isolated Pseudomonas Sp. CS3 and Its Metabolites. J. Appl. Microbiol. 2012, 112, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, X.; Yang, J.; Gong, X.; Li, B.; Zhang, K.Q. Isolation of Nicotine-Degrading Bacterium Pseudomonas Sp. Nic22, and Its Potential Application in Tobacco Processing. Int. Biodeterior. Biodegrad. 2008, 62, 226–231. [Google Scholar] [CrossRef]
- Wang, S.N.; Liu, Z.; Xu, P. Biodegradation of Nicotine by a Newly Isolated Agrobacterium Sp. Strain S33. J. Appl. Microbiol. 2009, 107, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wang, X.; Zhang, M.; Li, L.; Wang, R.; Huang, H.; Wang, S. An NAD-Specific 6-Hydroxy-3-Succinoyl-Semialdehyde-Pyridine Dehydrogenase from Nicotine-Degrading Agrobacterium Tumefaciens Strain S33. Microbiol. Spectr. 2021, 9, e0092421. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, Y.; Zhao, L.; He, Q.; Jiang, J.; Hong, Q.; He, J. Isolation and Characterization of the Cotinine-Degrading Bacterium Nocardioides Sp. Strain JQ2195. J. Hazard Mater. 2018, 353, 158–165. [Google Scholar] [CrossRef]
- Cobzaru, C.; Ganas, P.; Mihasan, M.; Schleberger, P.; Brandsch, R. Homologous Gene Clusters of Nicotine Catabolism, Including a New ω-Amidase for α-Ketoglutaramate, in Species of Three Genera of Gram-Positive Bacteria. Res. Microbiol. 2011, 162, 285–291. [Google Scholar] [CrossRef]
- Yu, M.F.; Xia, Z.Z.; Yao, J.C.; Feng, Z.; Li, D.H.; Liu, T.; Cheng, G.J.; He, D.L.; Li, X.H. Functional Analysis of the Ocne Gene Involved in Nicotine-Degradation Pathways in Ochrobactrum Intermedium SCUEC4 and Its Enzymatic Properties. Can. J. Microbiol. 2021, 67, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, H.; Zhu, X.; Li, Y.; Xu, P. Molecular Mechanism of Nicotine Degradation by a Newly Isolated Strain, Ochrobactrum Sp. Strain SJY1. Appl. Environ. Microbiol. 2015, 81, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Lu, Z.X.; Wu, N.; Huang, L.J.; Lü, F.X.; Bie, X.M. Isolation and Preliminary Characterization of a Novel Nicotine-Degrading Bacterium, Ochrobactrum Intermedium DN2. Int. Biodeterior. Biodegrad. 2005, 56, 45–50. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, Y.; Zhang, J.; Wang, H.; Ma, Y.; He, J.; Lu, Z. The Complete Genome Sequence of the Nicotine-Degrading Bacterium Shinella Sp. HZN7. Front. Microbiol. 2016, 7, 1348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.J.; Ma, Y.; Qiu, G.J.; Wu, F.L.; Chen, S.L. Biodegradation of Nicotine by a Novel Strain Shinella Sp. HZN1 Isolated from Activated Sludge. J. Environ. Sci. Health B 2011, 46, 703–708. [Google Scholar] [PubMed]
- Wang, M.; Yang, G.; Wang, X.; Yao, Y.; Min, H.; Lu, Z. Nicotine Degradation by Two Novel Bacterial Isolates of Acinetobacter Sp. TW and Sphingomonas Sp. TY and Their Responses in the Presence of Neonicotinoid Insecticides. World J. Microbiol. Biotechnol. 2011, 27, 1633–1670. [Google Scholar] [CrossRef]
- Wang, J.H.; He, H.Z.; Wang, M.Z.; Wang, S.; Zhang, J.; Wei, W.; Xu, H.X.; Lv, Z.M.; Shen, D.S. Bioaugmentation of Activated Sludge with Acinetobacter Sp. TW Enhances Nicotine Degradation in a Synthetic Tobacco Wastewater Treatment System. Bioresour. Technol. 2013, 142, 445–453. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, J.; Chen, Y.; Hu, B.; Sun, J.; Zhu, Y.; Xia, Z.; Zou, C. Biodegradation of Nicotine and TSNAs by Bacterium sp. Strain J54. Iran. J. Biotechnol. 2021, 19, 20–27. [Google Scholar] [CrossRef]
- Zhang, K.; Yin, M.; Lei, S.; Zhang, H.; Yin, X.; Niu, Q. Bacillus Sp. YC7 from Intestines of Lasioderma Serricorne Degrades Nicotine Due to Nicotine Dehydrogenase. AMB Express 2023, 13, 87. [Google Scholar] [CrossRef]
- Wang, H.; Zhi, X.Y.; Qiu, J.; Shi, L.; Lu, Z. Characterization of a Novel Nicotine Degradation Gene Cluster Ndp in Sphingomonas Melonis TY and Its Evolutionary Analysis. Front. Microbiol. 2017, 8, 337. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Tang, Q.; Wang, L.; Mei, C.; Shao, Y.; Xu, Y.; Lu, Z.; Zhong, W. Regulation Mechanism of Nicotine Catabolism in Sphingomonas Melonis TY by a Dual Role Transcriptional Regulator NdpR. Appl. Environ. Microbiol. 2023, 89, e00324-23. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wen, R.; Qiu, J.; Hong, J.; Liu, M.; Zhang, D. Biodegradation of Nicotine by a Novel Strain Pusillimonas. Res. Microbiol. 2015, 166, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.W.; Yang, J.K.; Duan, Y.Q.; Dong, J.Y.; Zhe, W.; Wang, L.; Li, Q.H.; Zhang, K.Q. Isolation and Characterization of Rhodococcus Sp. Y22 and Its Potential Application to Tobacco Processing. Res. Microbiol. 2009, 160, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Ganas, P.; Sachelaru, P.; Mihasan, M.; Igloi, G.L.; Brandsch, R. Two Closely Related Pathways of Nicotine Catabolism in Arthrobacter Nicotinovorans and Nocardioides sp. Strain JS614. Arch. Microbiol. 2008, 189, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, W.; Wei, H.; Xia, Z.; Liu, X. Characterization of a Novel Nicotine-Degrading Ensifer Sp. Strain N7 Isolated from Tobacco Rhizosphere. Ann. Microbiol. 2009, 59, 247–252. [Google Scholar] [CrossRef]
- Liu, G.; Wang, W.; He, F.; Zhang, P.; Xu, P.; Tang, H. Structural Insights into 6-Hydroxypseudooxynicotine Amine Oxidase from Pseudomonas Geniculata N1, the Key Enzyme Involved in Nicotine Degradation. Appl. Environ. Microbiol. 2020, 86, e01559-20. [Google Scholar] [CrossRef]
- Zhang, Z.; Mei, X.; He, Z.; Xie, X.; Yang, Y.; Mei, C.; Xue, D.; Hu, T.; Shu, M.; Zhong, W. Nicotine Metabolism Pathway in Bacteria: Mechanism, Modification, and Application. Appl. Microbiol. Biotechnol. 2022, 106, 889–904. [Google Scholar] [CrossRef]
- Mu, Y.; Chen, Q.; Parales, R.E.; Lu, Z.; Hong, Q.; He, J.; Qiu, J.; Jiang, J. Bacterial Catabolism of Nicotine: Catabolic Strains, Pathways and Modules. Environ. Res. 2020, 183, 109258. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Igloi, G.L.; Pust, S.; Schilz, E.; Decker, K.; Brandsch, R. Structural Analysis and Molybdenum-dependent Expression of the PAO1-encoded Nicotine Dehydrogenase Genes of Arthrobacter Nicotinovorans. Mol. Microbiol. 1994, 13, 929–936. [Google Scholar] [CrossRef]
- Li, A.; Qiu, J.; Chen, D.; Ye, J.; Wang, Y.; Tong, L.; Jiang, J.; Chen, J. Characterization and Genome Analysis of a Nicotine and Nicotinic Acid-Degrading Strain Pseudomonas Putida JQ581 Isolated from Marine. Mar. Drugs 2017, 15, 156. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, W.; Lei, L.; Liu, X.; Wei, H.L. Genome-Wide Investigation of the Genes Involved in Nicotine Metabolism in Pseudomonas Putida J5 by Tn5 Transposon Mutagenesis. Appl. Microbiol. Biotechnol. 2015, 99, 6503–6514. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, X.; Liu, X.; Wu, W.; Xu, P.; Tang, H. Cloning and Characterization the Nicotine Degradation Enzymes 6-Hydroxypseudooxynicotine Amine Oxidase and 6-Hydroxy-3-Succinoylpyridine Hydroxylase in Pseudomonas Geniculata N1. Int. Biodeterior. Biodegrad. 2019, 142, 83–90. [Google Scholar] [CrossRef]
- Li, H.; Xie, K.; Yu, W.; Hu, L.; Huang, H.; Xie, H.; Wang, S. Nicotine Dehydrogenase Complexed with 6-Hydroxypseudooxynicotine Oxidase Involved in the Hybrid Nicotine-Degrading Pathway in Agrobacterium Tumefaciens S33. Appl. Environ. Microbiol. 2016, 82, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, R.; Mihasan, M. A Soil Bacterial Catabolic Pathway on the Move: Transfer of Nicotine Catabolic Genes between Arthrobacter Genus Megaplasmids and Invasion by Mobile Elements. J. Biosci. 2020, 45, 58. [Google Scholar] [CrossRef]
- El-Sabeh, A.; Mlesnita, A.M.; Munteanu, I.T.; Honceriu, I.; Kallabi, F.; Boiangiu, R.S.; Mihasan, M. Characterisation of the Paenarthrobacter Nicotinovorans ATCC 49919 Genome and Identification of Several Strains Harbouring a Highly Syntenic Nic-Genes Cluster. BMC Genom. 2023, 24, 536. [Google Scholar] [CrossRef] [PubMed]
- Igloi, G.L.; Brandsch, R. Sequence of the 165-Kilobase Catabolic Plasmid PAO1 from Arthrobacter Nicotinovorans and Identification of a PAO1-Dependent Nicotine Uptake System. J. Bacteriol. 2003, 185, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Mihǎşan, M.; Boiangiu, R.Ş.; Guzun, D.; Babii, C.; Aslebagh, R.; Channaveerappa, D.; Dupree, E.; Darie, C.C.; Mihǎşan, M. Time-Dependent Analysis of Paenarthrobacter Nicotinovorans PAO1 Nicotine-Related Proteome. ACS Omega 2021, 6, 14242–14251. [Google Scholar] [CrossRef]
- Mihăşan, M.; Babii, C.; Aslebagh, R.; Channaveerappa, D.; Dupree, E.J.; Darie, C.C. Exploration of Nicotine Metabolism in Paenarthrobacter Nicotinovorans PAO1 by Microbial Proteomics. Adv. Exp. Med. Biol. 2019, 1140, 515–529. [Google Scholar] [CrossRef]
- Mihăşan, M.; Babii, C.; Aslebagh, R.; Channaveerappa, D.; Dupree, E.; Darie, C.C. Proteomics Based Analysis of the Nicotine Catabolism in Paenarthrobacter Nicotinovorans PAO1. Sci. Rep. 2018, 8, 16239. [Google Scholar] [CrossRef]
- Chiribau, C.B.; Mihasan, M.; Ganas, P.; Igloi, G.L.; Artenie, V.; Brandsch, R. Final Steps in the Catabolism of Nicotine Deamination versus Demethylation of γ-N-Methylaminobutyrate. FEBS J. 2006, 273, 1528–1536. [Google Scholar] [CrossRef]
- Mihasan, M.; Brandsch, R. PAO1 of Arthrobacter Nicotinovorans and the Spread of Catabolic Traits by Horizontal Gene Transfer in Gram-Positive Soil Bacteria. J. Mol. Evol. 2013, 77, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ganas, P.; Brandsch, R. Uptake of L-Nicotine and of 6-Hydroxy-L-Nicotine by Arthrobacter Nicotinovorans and by Escherichia Coli Is Mediated by Facilitated Diffusion and Not by Passive Diffusion or Active Transport. Microbiology 2009, 155, 1866–1867. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, W.; König, K.; Andreesen, J.R. Nicotine Dehydrogenase from Arthrobacter Oxidans: A Molybdenum-Containing Hydroxylase. FEMS Microbiol. Lett. 1988, 52, 13–17. [Google Scholar] [CrossRef]
- Fitzpatrick, P.F.; Chadegani, F.; Zhang, S.; Roberts, K.M.; Hinck, C.S. Mechanism of the Flavoprotein l -Hydroxynicotine Oxidase: Kinetic Mechanism, Substrate Specificity, Reaction Product, and Roles of Active-Site Residues. Biochemistry 2016, 55, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, I.; Yildiz, B.S. Mechanistic Study of L-6-Hydroxynicotine Oxidase by DFT and ONIOM Methods. J. Mol. Model 2021, 27, 53. [Google Scholar] [CrossRef] [PubMed]
- Koetter, J.W.A.; Schulz, G.E. Crystal Structure of 6-Hydroxy-D-Nicotine Oxidase from Arthrobacter Nicotinovorans. J. Mol. Biol. 2005, 352, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Sachelaru, P.; Schiltz, E.; Brandsch, R. A Functional MobA Gene for Molybdopterin Cytosine Dinucleotide Cofactor Biosynthesis Is Required for Activity and Holoenzyme Assembly of the Heterotrimeric Nicotine Dehydrogenases of Arthrobacter Nicotinovorans. Appl. Environ. Microbiol. 2006, 72, 5126–5131. [Google Scholar] [CrossRef]
- Menéndez, C.; Otto, A.; Igloi, G.; Nick, P.; Brandsch, R.; Schubach, B.; Böttcher, B.; Brandsch, R. Molybdate-Uptake Genes and Molybdopterin-Biosynthesis Genes on a Bacterial Plasmid. Characterization of MoeA as a Filament-Forming Protein with Adenosinetriphosphatase Activity. Eur. J. Biochem. 1997, 250, 524–531. [Google Scholar] [CrossRef]
- Schleberger, C.; Sachelaru, P.; Brandsch, R.; Schulz, G.E. Structure and Action of a C–C Bond Cleaving α/β-Hydrolase Involved in Nicotine Degration. J. Mol. Biol. 2007, 367, 409–418. [Google Scholar] [CrossRef]
- Ganas, P.; Mihasan, M.; Igloi, G.L.; Brandsch, R. A Two-Component Small Multidrug Resistance Pump Functions as a Metabolic Valve during Nicotine Catabolism by Arthrobacter Nicotinovorans. Microbiology 2007, 153, 1546–1555. [Google Scholar] [CrossRef]
- Treiber, N.; Schulz, G.E. Structure of 2,6-Dihydroxypyridine 3-Hydroxylase from a Nicotine-Degrading Pathway. J. Mol. Biol. 2008, 379, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Chiribau, C.B.; Sandu, C.; Igloi, G.L.; Brandsch, R. Characterization of PmfR, the Transcriptional Activator of the PAO1-Borne PurU-MabO-FolD Operon of Arthrobacter Nicotinovorans. J. Bacteriol. 2005, 187, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Mihasan, M.; Chiribau, C.-B.; Friedrich, T.; Artenie, V.; Brandsch, R. An NAD(P)H-Nicotine Blue Oxidoreductase Is Part of the Nicotine Regulon and May Protect Arthrobacter Nicotinovorans from Oxidative Stress during Nicotine Catabolism. Appl. Environ. Microbiol. 2007, 73, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Sandu, C.; Chiribau, C.B.; Brandsch, R. Characterization of HdnoR, the Transcriptional Repressor of the 6-Hydroxy-D-Nicotine Oxidase Gene of Arthrobacter Nicotinovorans PAO1, and Its DNA-Binding Activity in Response to L- and D-Nicotine Derivatives. J. Biol. Chem. 2003, 278, 51307–51315. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, Y.; Qiu, J.; Wen, R.; Hong, J.; Liu, W. Isolation, Transposon Mutagenesis, and Characterization of the Novel Nicotine-Degrading Strain Shinella Sp. HZN7. Appl. Microbiol. Biotechnol. 2014, 98, 2625–2636. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, W.; Wang, R.; Li, H.; Xie, H.; Wang, S. Genomic and Transcriptomic Analyses of Agrobacterium Tumefaciens S33 Reveal the Molecular Mechanism of a Novel Hybrid Nicotine-Degrading Pathway. Sci. Rep. 2017, 7, 4813. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yi, J.; Shang, J.; Yu, W.; Li, Z.; Huang, H.; Xie, H.; Wang, S. 6-Hydroxypseudooxynicotine Dehydrogenase Delivers Electrons to Electron Transfer Flavoprotein during Nicotine Degradation by Agrobacterium Tumefaciens S33. Appl. Environ. Microbiol. 2019, 85, e00454-19. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Xie, K.; Xu, P. Identification of Nicotine Biotransformation Intermediates by Agrobacterium Tumefaciens Strain S33 Suggests a Novel Nicotine Degradation Pathway. Appl. Microbiol. Biotechnol. 2012, 95, 1567–1578. [Google Scholar] [CrossRef]
- Yu, W.; Wang, R.; Li, H.; Liang, J.; Wang, Y.; Huang, H.; Xie, H.; Wang, S. Green Route to Synthesis of Valuable Chemical 6-Hydroxynicotine from Nicotine in Tobacco Wastes Using Genetically Engineered Agrobacterium Tumefaciens S33. Biotechnol. Biofuels 2017, 10, 288. [Google Scholar] [CrossRef]
- Qiu, J.; Wei, Y.; Ma, Y.; Wen, R.; Wen, Y.; Liu, W. A Novel (S)-6-Hydroxynicotine Oxidase Gene from Shinella Sp. Strain HZN7. Appl. Environ. Microbiol. 2014, 80, 5552–5560. [Google Scholar] [CrossRef]
- Qiu, J.; Ma, Y.; Wen, Y.; Chen, L.; Wu, L.; Liu, W. Functional Identification of Two Novel Genes from Pseudomonas Sp. Strain HZN6 Involved in the Catabolism of Nicotine. Appl. Environ. Microbiol. 2012, 78, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Novotny, T.E.; Zhao, F. Consumption and Production Waste: Another Externality of Tobacco Use. Tob. Control 1999, 8, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Hendlin, Y.H.; Bialous, S.A. The Environmental Externalities of Tobacco Manufacturing: A Review of Tobacco Industry Reporting. Ambio 2020, 49, 17. [Google Scholar] [CrossRef] [PubMed]
- Civilini, M. Nicotine Decontamination of Tobacco Agro-Industrial Waste and Its Degradation by Micro-Organisms. Waste Manag. Res. 1997, 15, 349–358. [Google Scholar] [CrossRef]
- Tang, H.; Wang, L.; Meng, X.; Lanying, M.; Wang, S.; He, X.; Wu, G.; Xu, P. Novel Nicotine Oxidoreductase-Encoding Gene Involved in Nicotine Degradation by Pseudomonas Putida Strain S16. Appl. Environ. Microbiol. 2009, 75, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Lenkey, A.A.; Hills, S. Nicotine Removal Process and Product Produced Thereby. U.S. Patent 4,848,373, 18 July 1987. [Google Scholar]
- Wang, M.; Yang, G.; Min, H.; Lv, Z.; Jia, X. Bioaugmentation with the Nicotine-Degrading Bacterium Pseudomonas sp. HF-1 in a Sequencing Batch Reactor Treating Tobacco Wastewater: Degradation Study and Analysis of Its Mechanisms. Water Res. 2009, 43, 4187–4196. [Google Scholar] [CrossRef]
- Zhong, W.; Zhu, C.; Shu, M.; Sun, K.; Zhao, L.; Wang, C.; Ye, Z.; Chen, J. Degradation of Nicotine in Tobacco Waste Extract by Newly Isolated Pseudomonas Sp. ZUTSKD. Bioresour. Technol. 2010, 101, 6935–6941. [Google Scholar] [CrossRef]
- Shen, D.S.; Wang, L.J.; He, H.Z.; Wang, M.Z. Effect of Transient Nicotine Load Shock on the Performance of Pseudomonas Sp. HF-1 Bioaugmented Sequencing Batch Reactors. J. Chem. 2016, 2016, 4982395. [Google Scholar] [CrossRef]
- Wang, X.; Tang, L.; Yao, Y.; Wang, H.; Min, H.; Lu, Z. Bioremediation of the Tobacco Waste-Contaminated Soil by Pseudomonas Sp. HF-1: Nicotine Degradation and Microbial Community Analysis. Appl. Microbiol. Biotechnol. 2013, 97, 6077–6088. [Google Scholar] [CrossRef]
- Wang, M.Z.; He, H.Z.; Zheng, X.; Feng, H.J.; Lv, Z.M.; Shen, D.S. Effect of Pseudomonas Sp. HF-1 Inoculum on Construction of a Bioaugmented System for Tobacco Wastewater Treatment: Analysis from Quorum Sensing. Environ. Sci. Pollut. Res. 2014, 21, 7945–7955. [Google Scholar] [CrossRef]
- Briški, F.; Kopčić, N.; Ćosić, I.; Kučić, D.; Vuković, M. Biodegradation of Tobacco Waste by Composting: Genetic Identification of Nicotine-Degrading Bacteria and Kinetic Analysis of Transformations in Leachate. Chem. Pap. 2012, 66, 1103–1110. [Google Scholar] [CrossRef]
- Mandić, N.; Lalević, B.; Raičević, V.; Radojičić, V. Impact of Composting Conditions on the Nicotine Degradation Rate Using Nicotinophilic Bacteria from Tobacco Waste. Int. J. Environ. Sci. Technol. 2023, 20, 7787–7798. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Chu, P.; Shi, H.; Wang, R.; Li, J.; Zheng, S. Cultivation and Application of Nicotine-Degrading Bacteria and Environmental Functioning in Tobacco Planting Soil. Bioresour. Bioprocess 2023, 10, 10. [Google Scholar] [CrossRef]
- Wang, S.N.; Xu, P.; Tang, H.Z.; Meng, J.; Liu, X.L.; Qing, C. “Green” Route to 6-Hydroxy-3-Succinoyl-Pyridine from (S)-Nicotine of Tobacco Waste by Whole Cells of a Pseudomonas sp. Environ. Sci. Technol. 2005, 39, 6877–6880. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, H.; Xu, P. Green Strategy from Waste to Value-Added-Chemical Production: Efficient Biosynthesis of 6-Hydroxy-3-Succinoyl-Pyridine by an Engineered Biocatalyst. Sci. Rep. 2014, 4, 5397. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, P.; Tang, H. Sustainable Production of Valuable Compound 3-Succinoyl-Pyridine by Genetically Engineering Pseudomonas Putida Using the Tobacco Waste. Sci. Rep. 2015, 5, 16411. [Google Scholar] [CrossRef]
- Mihalache, G.; Babii, C.; Stefan, M.; Motei, D.; Marius, M. Steps towards an Arthrobacter Nicotinovorans Based Biotechnology for Production of 6-Hidroxy-Nicotine. In Proceedings of the 16th International Multidisciplinary Scientific Geoconference, Albena, Bulgaria, 30 June–6 July 2016; pp. 341–346. [Google Scholar]
- Hrițcu, L.; Ștefan, M.; Brandsch, R.; Mihășan, M. 6-Hydroxy-l-Nicotine from Arthrobacter Nicotinovorans Sustain Spatial Memory Formation by Decreasing Brain Oxidative Stress in Rats. J. Physiol. Biochem. 2013, 69, 25–34. [Google Scholar] [CrossRef]
- Hritcu, L.; Stefan, M.; Brandsch, R.; Mihasan, M. Enhanced Behavioral Response by Decreasing Brain Oxidative Stress to 6-Hydroxy-l-Nicotine in Alzheimer’s Disease Rat Model. Neurosci. Lett. 2015, 591, 41–47. [Google Scholar] [CrossRef]
- Hritcu, L.; Ionita, R.; Motei, D.E.; Babii, C.; Stefan, M.; Mihasan, M. Nicotine versus 6-Hydroxy-l-Nicotine against Chlorisondamine Induced Memory Impairment and Oxidative Stress in the Rat Hippocampus. Biomed. Pharmacother. 2017, 86, 102–108. [Google Scholar] [CrossRef]
- Ioniță, R.; Valu, V.M.; Postu, P.A.; Cioancă, O.; Hrițcu, L.; Mihasan, M. 6-Hydroxy-l-Nicotine Effects on Anxiety and Depression in a Rat Model of Chlorisondamine. Farmacia 2017, 65, 237–240. [Google Scholar]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Petre, B.A.; Hritcu, L. Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease. Antioxidants 2020, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Hritcu, L. Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio Rerio) Model of Alzheimer’s Disease. Antioxidants 2021, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Lv, M.; Xu, H. Acetylcholinesterase: A Primary Target for Drugs and Insecticides. Mini-Rev. Med. Chem. 2017, 17, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Girgis, A.S.; Prakash, A.; Khanna, L.; Khanna, P.; Shalaby, E.M.; Fawzy, N.G.; Jain, S.C. Protective Effects of Aporosa Octandra Bark Extract against D-Galactose Induced Cognitive Impairment and Oxidative Stress in Mice. Heliyon 2018, 4, e00951. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liao, C.; Ge, F.; Ao, J.; Liu, T. Acetylcholine Bidirectionally Regulates Learning and Memory. J. Neurorestoratol. 2022, 10, 100002. [Google Scholar] [CrossRef]
- Sam, C.; Bordoni, B. Physiology, Acetylcholine; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315. [Google Scholar] [CrossRef]
- Mihășan, M.; Căpățînă, L.; Neagu, E.; Ștefan, M.; Hrițcu, L. In-Silico Identification of 6-Hydroxy-L-Nicotine as a Novel Neuroprotective Drug. Rom. Biotechnol. Lett. 2013, 18, 8333–8340. [Google Scholar]
- Mocanu, E.M.; Mazarachi, A.L.; Mihasan, M. In Vitro Stability and Antioxidant Potential of the Neuprotective Metabolite 6-Hydroxy-Nicotine. J. Exp. Mol. Biol. 2018, 19, 53–58. [Google Scholar]
- Yamazaki, H.; Tanji, K.; Wakabayashi, K.; Matsuura, S.; Itoh, K. Role of the Keap1/Nrf2 Pathway in Neurodegenerative Diseases. Pathol. Int. 2015, 65, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Taly, A.; Corringer, P.-J.; Guedin, D.; Lestage, P.; Changeux, J.-P. Nicotinic Receptors: Allosteric Transitions and Therapeutic Targets in the Nervous System. Nat. Rev. Drug. Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Maskos, U. Role of the Nicotinic Acetylcholine Receptor in Alzheimer’s Disease Pathology and Treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef]
- Posadas, I.; López-Hernández, B.; Ceña, V. Nicotinic Receptors in Neurodegeneration. Curr. Neuropharmacol. 2013, 11, 298–314. [Google Scholar] [CrossRef]
- Rezvani, A.H.; Levin, E.D. Cognitive Effects of Nicotine. Biol. Psychiatry 2001, 49, 258–267. [Google Scholar] [CrossRef]

| Model | Model Inducer (Dose, Route of Administration, and Time of Exposure) | Dose of 6HLN, Route of Administration, and Time of Exposure | Behavioral Task | Phenotype | References |
|---|---|---|---|---|---|
| Ratus norvegicus | |||||
| Normal | - | 0.3 mg/kg, b.w., i.p., for 7 consecutive days | Y-maze | Improves short-term memory acquisition and increases locomotor activity | [190] |
| Normal | - | 0.3 mg/kg, b.w., i.p., for 7 consecutive days | Radial-arm maze | Improves working memory; no effect on long-term memory | [190] |
| AD | SCOP (0.7 mg/kg, b.w., i.p., 24 h before testing) | 0.3 mg/kg, b.w., i.p., 30 min before testing | Y-maze | Improves spatial working memory; no effect on locomotor activity | [191] |
| AD | SCOP (0.7 mg/kg, b.w., i.p., 24 h before testing) | 0.3 mg/kg, b.w., i.p., 30 min before testing | Radial-arm maze | Improves spatial memory formation | [191] |
| AD | CHL (10 mg/kg, b.w., i.p., 24 h before testing) | 0.3 mg/kg, b.w., i.p., 30 min before testing | Y-maze | Enhances spatial memory formation | [192] |
| AD | CHL (10 mg/kg, b.w., i.p., 24 h before testing) | 0.3 mg/kg, b.w., i.p., 30 min before testing | Radial-arm maze | Improves short- and long-term memory | [192] |
| AD | CHL (10 mg/kg, b.w., i.p., 24 h before testing) | 0.3 mg/kg, b.w., i.p., 30 min before testing | Elevated plus maze | Anxiolytic properties (increased time and entries in open arms) | [193] |
| AD | CHL (10 mg/kg, b.w., i.p., 24 h before testing) | 0.3 mg/kg, b.w., i.p., 30 min before testing | Forced swimming | Anti-depressant effect (reduced the immobility period) | [193] |
| AD | Aβ25–35 peptide fragment (0.5 mg/mL, 4 µL, i.c.v.) | 0.3 and 0.7 mg/kg, b.w., i.p., for 33 days | Y-maze | Improves spatial recognition memory; restored normal locomotor activity | [194] |
| AD | Aβ25–35 peptide fragment (0.5 mg/mL, 4 µL, i.c.v.) | 0.3 and 0.7 mg/kg, b.w., i.p., for 33 days | Radial-arm maze | Rescues short- and long-term memory | [194] |
| AD | Aβ25–35 peptide fragment (0.5 mg/mL, 4 µL, i.c.v.) | 0.3 and 0.7 mg/kg, b.w., i.p., for 33 days | Novel object recognition | Improves recognition memory | [194] |
| Danio rerio | |||||
| AD | SCOP (100 µM, immersion, 30 before testing) | 1 and 2 mg/L, immersion, for 3 min | Y-maze | Rescues spatial recognition memory; enhances locomotor activity | [195] |
| AD | SCOP (100 µM, immersion, 30 before testing) | 1 and 2 mg/L, immersion, for 3 min | Novel object recognition | Improves recognition memory | [195] |
| AD | SCOP (100 µM, immersion, 30 before testing) | 1 and 2 mg/L, immersion, for 3 min | Novel tank diving test | Reduces anxiety-like behavior; induces hyperactivity | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiangiu, R.S.; Brinza, I.; Honceriu, I.; Mihasan, M.; Hritcu, L. Insights into Pharmacological Activities of Nicotine and 6-Hydroxy-L-nicotine, a Bacterial Nicotine Derivative: A Systematic Review. Biomolecules 2024, 14, 23. https://doi.org/10.3390/biom14010023
Boiangiu RS, Brinza I, Honceriu I, Mihasan M, Hritcu L. Insights into Pharmacological Activities of Nicotine and 6-Hydroxy-L-nicotine, a Bacterial Nicotine Derivative: A Systematic Review. Biomolecules. 2024; 14(1):23. https://doi.org/10.3390/biom14010023
Chicago/Turabian StyleBoiangiu, Razvan Stefan, Ion Brinza, Iasmina Honceriu, Marius Mihasan, and Lucian Hritcu. 2024. "Insights into Pharmacological Activities of Nicotine and 6-Hydroxy-L-nicotine, a Bacterial Nicotine Derivative: A Systematic Review" Biomolecules 14, no. 1: 23. https://doi.org/10.3390/biom14010023
APA StyleBoiangiu, R. S., Brinza, I., Honceriu, I., Mihasan, M., & Hritcu, L. (2024). Insights into Pharmacological Activities of Nicotine and 6-Hydroxy-L-nicotine, a Bacterial Nicotine Derivative: A Systematic Review. Biomolecules, 14(1), 23. https://doi.org/10.3390/biom14010023









