Are Adenomyosis and Endometriosis Phenotypes of the Same Disease Process?
Abstract
:1. Introduction
2. Materials and Methods
Search Strategy and Inclusion Criteria
3. Phenotypes of Endometriosis and Adenomyosis
- -
- A variant recognized today as adenomyosis, where the ectopic endometrial tissue raises by direct extension into the uterine wall.
- -
- A type resulting from retrograde menstruation, namely, the peritoneal and ovarian implantation of endometrial cells and stroma.
- -
- The transplantation of the ectopic tissue as a consequence of dissemination due to surgical wounds.
- -
- A “metastatic” variant as a consequence of lymphatic or hematogenous microembolization of endometrial cells.
- -
- A developmentally determined variant where the presence of ectopic endometrium is the consequence of embryonic remnants.
3.1. Phenotypes of Endometriosis
3.2. Phenotypes of Adenomyosis
4. Epidemiological Evidence of a Link
4.1. Prevalence of Endometriosis
4.2. Prevalence of Adenomyosis
4.3. Comparative Incidence and Prevalence
4.4. Coexistence of the Two Conditions
4.5. Comparison of Risk Factors
5. Common Features
5.1. Correlation between Phenotypes and Clinical Outcome
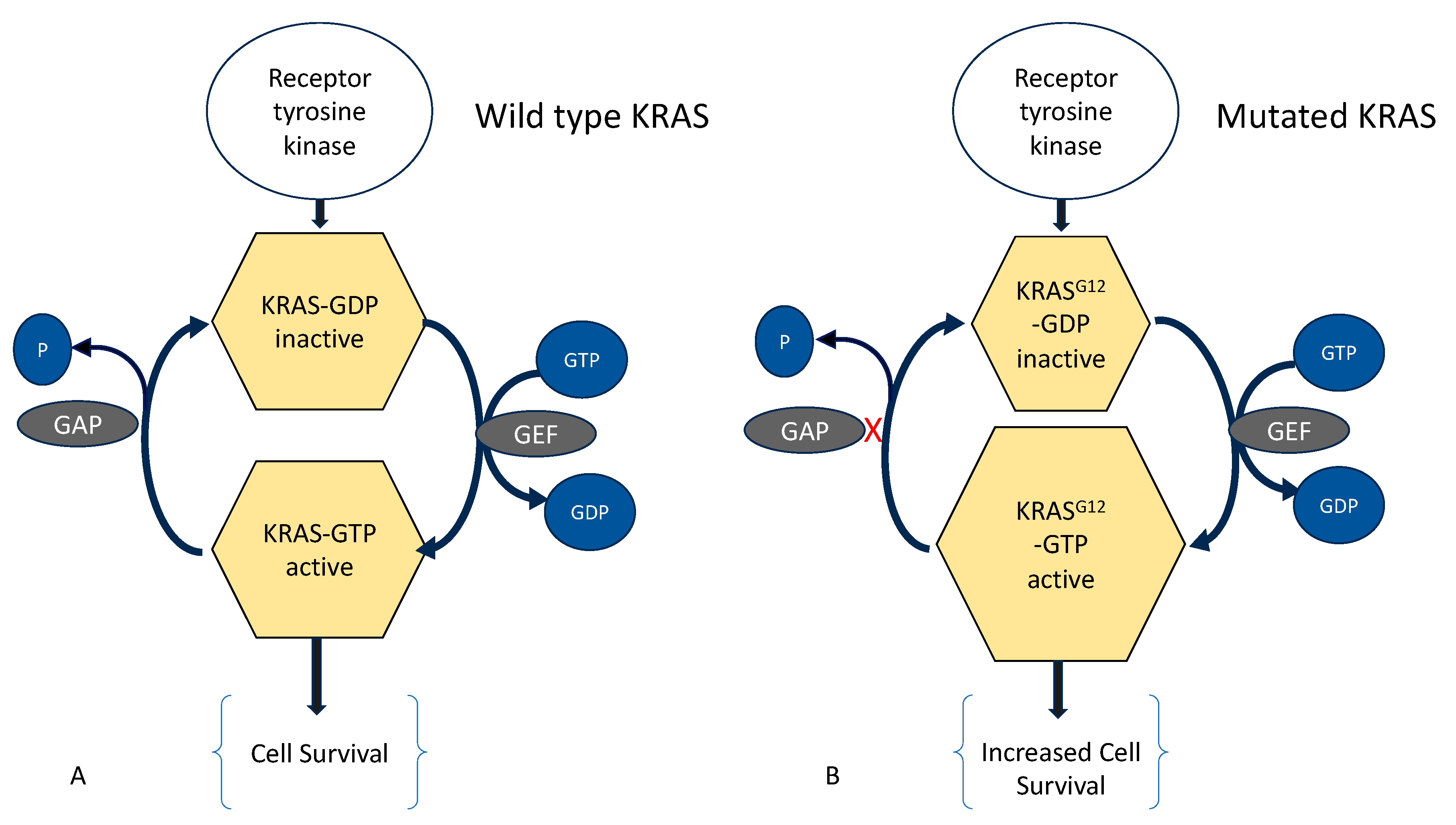
5.2. The Eutopic Endometrium in Adenomyosis and Endometriosis: Presence and Significance of Gene Mutations
5.3. Molecular Aberrations in Adenomyosis and Endometriosis
5.4. Effect of Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.W.; Habiba, M.; Benagiano, G. From Retrograde Menstruation to Endometrial Determinism and a Brave New World of “Root Treatment” of Endometriosis: Destiny or a Fanciful Utopia? Biomolecules 2023, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Benagiano, G.; Guo, S.W. An Appraisal of the Tissue Injury and Repair (TIAR) Theory on the Pathogenesis of Endometriosis and Adenomyosis. Biomolecules 2023, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Kunz, G.; Noe, M.; Herbertz, M.; Mall, G. Endometriosis: A dysfunction and disease of the archimetra. Hum. Reprod. Update 1998, 4, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Catalano, G.F.; Marana, R. Endometriosis externa and interna: Endoscopic diagnosis. Rays 1998, 23, 683–692. [Google Scholar] [PubMed]
- Donnez, J.; Dolmans, M.M.; Fellah, L. What if deep endometriotic nodules and uterine adenomyosis were actually two forms of the same disease? Fertil. Steril. 2019, 111, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Imanaka, S.; Nagayasu, M.; Kimura, M.; Kobayashi, H. Relationship between adenomyosis and endometriosis; Different phenotypes of a single disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 191–197. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I. Adenomyosis and Endometriosis have a common origin. J. Gynaecol. Obstet. India 2011, 61, 146–153. [Google Scholar] [CrossRef]
- Brosens, I.; Kunz, G.; Benagiano, G. Is adenomyosis the neglected phenotype of an endomyometrial dysfunction syndrome? Gynecol. Surg. 2012, 9, 131–137. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I.; Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 2014, 20, 386–402. [Google Scholar] [CrossRef]
- Guo, S.W. Cracking the enigma of adenomyosis: An update on its pathogenesis and pathophysiology. Reproduction 2022, 164, R101–R121. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Chakravarti, D.; Parker, J.B.; Milad, M.; Yang, L.; Chaudhari, A.; Tsai, S.; Wei, J.J.; et al. Endometriosis and adenomyosis: Shared pathophysiology. Fertil. Steril. 2023, 119, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T.; et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat. Commun. 2019, 10, 5785. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Endometriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/endometriosis (accessed on 28 April 2023).
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrao, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Koga, K.; Osuga, Y. Extra-pelvic endometriosis: A review. Reprod. Med. Biol. 2020, 19, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Brosens, I.A. Is mild endometriosis a progressive disease? Hum. Reprod. 1994, 9, 2209–2211. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Martin, D.C. Deep endometriosis: A consequence of infiltration or retraction or possibly adenomyosis externa? Fertil. Steril. 1992, 58, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Togashi, K.; Nishimura, K.; Itoh, K.; Fujisawa, I.; Noma, S.; Kanaoka, M.; Nakano, Y.; Itoh, H.; Ozasa, H.; Fujii, S.; et al. Adenomyosis: Diagnosis with MR imaging. Radiology 1988, 166 Pt 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Darai, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Spada, F.; Squifflet, J.; Nisolle, M. Bladder endometriosis must be considered as bladder adenomyosis. Fertil. Steril. 2000, 74, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e17. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Oliveira, J.; Marcellin, L.; Santulli, P.; Bordonne, C.; Maitrot Mantelet, L.; Millischer, A.E.; Plu Bureau, G.; Chapron, C. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum. Reprod. 2021, 36, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Critchley, H.; Fu, Z.; Guo, S.-W. How does the extent of fibrosis in adenomyosis lesions contribute to heavy menstrual bleeding? Reprod. Med. Biol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Liu, X.; Guo, S.W. Decreased Glycolysis at Menstruation is Associated with Increased Menstrual Blood Loss. Reprod. Sci. 2023, 30, 928–951. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Guo, S.W. Reduced endometrial expression of histone deacetylase 3 in women with adenomyosis who complained of heavy menstrual bleeding. Reprod. Biomed. Online 2023, 47, 103288. [Google Scholar] [CrossRef]
- Kim, T.H.; Yoo, J.Y.; Choi, K.C.; Shin, J.H.; Leach, R.E.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.G.; Jeong, J.W. Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci. Transl. Med. 2019, 11, eaaf7533. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Koshiba, A.; Kuroboshi, H.; Mori, T.; Ogi, H.; Itoh, K.; Nakashima, M.; Kitawaki, J. Biological differences between intrinsic and extrinsic adenomyosis with coexisting deep infiltrating endometriosis. Reprod. Biomed. Online 2019, 39, 343–353. [Google Scholar] [CrossRef]
- Rees, C.O.; Nederend, J.; Mischi, M.; van Vliet, H.; Schoot, B.C. Objective measures of adenomyosis on MRI and their diagnostic accuracy-a systematic review & meta-analysis. Acta Obstet. Gynecol. Scand. 2021, 100, 1377–1391. [Google Scholar] [CrossRef]
- Koepsell, T.D.; Weiss, N.S. Epidemiologic Methods: Studying the Occurrence of Illness; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- El-Toukhy, T. Prevalence of endometriosis: How close are we to the truth? BJOG 2021, 128, 666. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Houston, D.E.; Noller, K.L.; Melton, L.J., 3rd; Selwyn, B.J.; Hardy, R.J. Incidence of pelvic endometriosis in Rochester, Minnesota, 1970–1979. Am. J. Epidemiol. 1987, 125, 959–969. [Google Scholar] [CrossRef]
- Leibson, C.L.; Good, A.E.; Hass, S.L.; Ransom, J.; Yawn, B.P.; O’Fallon, W.M.; Melton, L.J., 3rd. Incidence and characterization of diagnosed endometriosis in a geographically defined population. Fertil. Steril. 2004, 82, 314–321. [Google Scholar] [CrossRef]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Marshall, L.M.; Hunter, D.J. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 2004, 160, 784–796. [Google Scholar] [CrossRef]
- Illum, L.R.H.; Forman, A.; Melgaard, A.; Hansen, K.E.; Hansen, S.N.; Nyegaard, M.; Hummelshoj, L.; Rytter, D. Temporal and regional differences in the incidence of hospital-diagnosed endometriosis: A Danish population-based study. Acta Obstet. Gynecol. Scand. 2022, 101, 737–746. [Google Scholar] [CrossRef]
- Christ, J.P.; Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Reed, S.D. Incidence, prevalence, and trends in endometriosis diagnosis: A United States population-based study from 2006 to 2015. Am. J. Obstet. Gynecol. 2021, 225, 500.e1–500.e9. [Google Scholar] [CrossRef]
- Rowlands, I.J.; Abbott, J.A.; Montgomery, G.W.; Hockey, R.; Rogers, P.; Mishra, G.D. Prevalence and incidence of endometriosis in Australian women: A data linkage cohort study. BJOG 2021, 128, 657–665. [Google Scholar] [CrossRef]
- Rawson, J.M. Prevalence of endometriosis in asymptomatic women. J. Reprod. Med. 1991, 36, 513–515. [Google Scholar]
- Gylfason, J.T.; Kristjansson, K.A.; Sverrisdottir, G.; Jonsdottir, K.; Rafnsson, V.; Geirsson, R.T. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am. J. Epidemiol. 2010, 172, 237–243. [Google Scholar] [CrossRef]
- Kristjansdottir, A.; Rafnsson, V.; Geirsson, R.T. Comprehensive evaluation of the incidence and prevalence of surgically diagnosed pelvic endometriosis in a complete population. Acta Obstet. Gynecol. Scand. 2023, 102, 1329–1337. [Google Scholar] [CrossRef]
- Williams, T.J.; Pratt, J.H. Endometriosis in 1,000 consecutive celiotomies: Incidence and management. Am. J. Obstet. Gynecol. 1977, 129, 245–250. [Google Scholar] [CrossRef]
- Eggert, J.; Li, X.; Sundquist, K. Country of birth and hospitalization for pelvic inflammatory disease, ectopic pregnancy, endometriosis, and infertility: A nationwide study of 2 million women in Sweden. Fertil. Steril. 2008, 90, 1019–1025. [Google Scholar] [CrossRef]
- Moen, M.H.; Schei, B. Epidemiology of endometriosis in a Norwegian county. Acta Obstet. Gynecol. Scand. 1997, 76, 559–562. [Google Scholar] [CrossRef]
- Vessey, M.P.; Villard-Mackintosh, L.; Painter, R. Epidemiology of endometriosis in women attending family planning clinics. BMJ 1993, 306, 182–184. [Google Scholar] [CrossRef]
- Vercellini, P.; Parazzini, F.; Oldani, S.; Panazza, S.; Bramante, T.; Crosignani, P.G. Adenomyosis at hysterectomy: A study on frequency distribution and patient characteristics. Hum. Reprod. 1995, 10, 1160–1162. [Google Scholar] [CrossRef]
- Parazzini, F.; Vercellini, P.; Panazza, S.; Chatenoud, L.; Oldani, S.; Crosignani, P.G. Risk factors for adenomyosis. Hum. Reprod. 1997, 12, 1275–1279. [Google Scholar] [CrossRef]
- Vavilis, D.; Agorastos, T.; Tzafetas, J.; Loufopoulos, A.; Vakiani, M.; Constantinidis, T.; Patsiaoura, K.; Bontis, J. Adenomyosis at hysterectomy: Prevalence and relationship to operative findings and reproductive and menstrual factors. Clin. Exp. Obstet. Gynecol. 1997, 24, 36–38. [Google Scholar] [PubMed]
- Krentel, H.; De Wilde, R.L. Prevalence of adenomyosis in women undergoing hysterectomy for abnormal uterine bleeding, pelvic pain or uterine prolapse—A retrospective cohort study. Ann. Med. Surg. 2022, 78, 103809. [Google Scholar] [CrossRef]
- Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Scholes, D.; Reed, S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020, 223, 94.e1–94.e10. [Google Scholar] [CrossRef]
- Morassutto, C.; Monasta, L.; Ricci, G.; Barbone, F.; Ronfani, L. Incidence and Estimated Prevalence of Endometriosis and Adenomyosis in Northeast Italy: A Data Linkage Study. PLoS ONE 2016, 11, e0154227. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Guo, S.W. Clinical profiles of 710 premenopausal women with adenomyosis who underwent hysterectomy. J. Obstet. Gynaecol. Res. 2014, 40, 485–494. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.W.; Wang, S.; Fan, Q.B.; Shi, H.H.; Leng, J.H.; Sun, D.W.; Lang, J.H.; Zhu, L. Clinical Manifestations of Adenomyosis Patients With or Without Pain Symptoms. J. Pain Res. 2019, 12, 3127–3133. [Google Scholar] [CrossRef]
- Naphatthalung, W.; Cheewadhanaraks, S. Prevalence of endometriosis among patients with adenomyosis and/or myoma uteri scheduled for a hysterectomy. J. Med. Assoc. Thai. 2012, 95, 1136–1140. [Google Scholar]
- Leyendecker, G.; Kunz, G. [Endometriosis and adenomyosis]. Zentralbl. Gynakol. 2005, 127, 288–294. [Google Scholar] [CrossRef]
- Di Donato, N.; Montanari, G.; Benfenati, A.; Leonardi, D.; Bertoldo, V.; Monti, G.; Raimondo, D.; Seracchioli, R. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 289–293. [Google Scholar] [CrossRef]
- Scala, C.; Leone Roberti Maggiore, U.; Racca, A.; Barra, F.; Vellone, V.G.; Venturini, P.L.; Ferrero, S. Influence of adenomyosis on pregnancy and perinatal outcomes in women with endometriosis. Ultrasound. Obstet. Gynecol. 2018, 52, 666–671. [Google Scholar] [CrossRef]
- Matalliotaki, C.; Matalliotakis, M.; Ieromonachou, P.; Goulielmos, G.N.; Zervou, M.I.; Laliotis, A.; Spandidos, D.A.; Arici, A.; Matalliotakis, I. Co-existence of benign gynecological tumors with endometriosis in a group of 1,000 women. Oncol. Lett. 2018, 15, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Crestani, A.; Arfi, A.; Ploteau, S.; Breban, M.; Boudy, A.S.; Bendifallah, S.; Ferrier, C.; Darai, E. Anogenital distance in adult women is a strong marker of endometriosis: Results of a prospective study with laparoscopic and histological findings. Hum. Reprod. Open. 2020, 2020, hoaa023. [Google Scholar] [CrossRef]
- Hernandez-Penalver, A.I.; Sanchez-Ferrer, M.L.; Mendiola, J.; Adoamnei, E.; Prieto-Sanchez, M.T.; Corbalan-Biyang, S.; Carmona-Barnosi, A.; Nieto, A.; Torres-Cantero, A.M. Assessment of anogenital distance as a diagnostic tool in polycystic ovary syndrome. Reprod. Biomed. Online 2018, 37, 741–749. [Google Scholar] [CrossRef]
- Mendiola, J.; Sanchez-Ferrer, M.L.; Jimenez-Velazquez, R.; Canovas-Lopez, L.; Hernandez-Penalver, A.I.; Corbalan-Biyang, S.; Carmona-Barnosi, A.; Prieto-Sanchez, M.T.; Nieto, A.; Torres-Cantero, A.M. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum. Reprod. 2016, 31, 2377–2383. [Google Scholar] [CrossRef]
- Peters, H.E.; Laeven, C.H.C.; Trimbos, C.; van de Ven, P.M.; Verhoeven, M.O.; Schats, R.; Mijatovic, V.; Lambalk, C.B. Anthropometric biomarkers for abnormal prenatal reproductive hormone exposure in women with Mayer-Rokitanksy-Kuster-Hauser syndrome, polycystic ovary syndrome, and endometriosis. Fertil. Steril. 2020, 114, 1297–1305. [Google Scholar] [CrossRef]
- Liu, X.; Ding, D.; Shen, M.; Yan, D.; Guo, S.-W. Shorter Anogenital Distance in Women with Ovarian Endometriomas and Adenomyosis, but Not Uterine Leiomyomas. Biomedicines 2023, 11, 2618. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Ye, S.; Zhang, B.; Li, X.; Yuan, J.; Du, Y.; Wang, J.; Yang, Y. The effects of coagulation factors on the risk of endometriosis: A Mendelian randomization study. BMC Med. 2023, 21, 195. [Google Scholar] [CrossRef]
- Parazzini, F.; Vigano, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef]
- Hemmert, R.; Schliep, K.C.; Willis, S.; Peterson, C.M.; Louis, G.B.; Allen-Brady, K.; Simonsen, S.E.; Stanford, J.B.; Byun, J.; Smith, K.R. Modifiable life style factors and risk for incident endometriosis. Paediatr. Perinat. Epidemiol. 2019, 33, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Bravi, F.; Cipriani, S.; Parazzini, F.; Ricci, E.; Vigano, P.; La Vecchia, C. Coffee and caffeine intake and risk of endometriosis: A meta-analysis. Eur. J. Nutr. 2014, 53, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Parazzini, F.; Cipriani, S.; Chiaffarino, F.; Ricci, E.; Chiantera, V.; Vigano, P.; La Vecchia, C. Tobacco smoking and risk of endometriosis: A systematic review and meta-analysis. BMJ Open 2014, 4, e006325. [Google Scholar] [CrossRef] [PubMed]
- Taran, F.A.; Weaver, A.L.; Coddington, C.C.; Stewart, E.A. Understanding adenomyosis: A case control study. Fertil. Steril. 2010, 94, 1223–1228. [Google Scholar] [CrossRef]
- Mangtani, P.; Booth, M. Epidemiology of endometriosis. J. Epidemiol. Community Health 1993, 47, 84–88. [Google Scholar] [CrossRef]
- Hao, M.; Liu, X.; Guo, S.W. Adenomyosis Resulting from Mechanically or Thermally Induced Endometrial-Myometrial Interface Disruption in Mouse and Its Possible Prevention. Reprod. Biomed. Online 2020. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Guo, S.W. Perioperative Suppression of Schwann Cell Dedifferentiation Reduces the Risk of Adenomyosis Resulting from Endometrial-Myometrial Interface Disruption in Mice. Biomedicines 2022, 10, 1218. [Google Scholar] [CrossRef]
- Guo, S.W. Fibrogenesis resulting from cyclic bleeding: The Holy Grail of the natural history of ectopic endometrium. Hum. Reprod. 2018, 33, 353–356. [Google Scholar] [CrossRef]
- Tellum, T.; Matic, G.V.; Dormagen, J.B.; Nygaard, S.; Viktil, E.; Qvigstad, E.; Lieng, M. Diagnosing adenomyosis with MRI: A prospective study revisiting the junctional zone thickness cutoff of 12 mm as a diagnostic marker. Eur. Radiol. 2019, 29, 6971–6981. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrao, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.W.; Just, P.A.; Noel, J.C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef]
- Nie, J.; Zhao, C.; Lagana, A.S.; Liu, X.; Guo, S.W. Identification of lesional attributes of dysmenorrhea severity and the serum antimullerian hormone levels in women with ovarian endometriomas. Fertil. Steril. 2022, 118, 191–202. [Google Scholar] [CrossRef]
- May, K.E.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Endometrial alterations in endometriosis: A systematic review of putative biomarkers. Hum. Reprod. Update 2011, 17, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Benagiano, G.; Habiba, M.; Brosens, I. The pathophysiology of uterine adenomyosis: An update. Fertil. Steril. 2012, 98, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, C.; Ye, M.; Zhang, Z.; Han, W.; Fan, W.; Meng, Y. Identification of global transcriptome abnormalities and potential biomarkers in eutopic endometria of women with endometriosis: A preliminary study. Biomed. Rep. 2017, 6, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Herndon, C.N.; Aghajanova, L.; Balayan, S.; Erikson, D.; Barragan, F.; Goldfien, G.; Vo, K.C.; Hawkins, S.; Giudice, L.C. Global Transcriptome Abnormalities of the Eutopic Endometrium From Women With Adenomyosis. Reprod. Sci. 2016, 23, 1289–1303. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.J. Adenomyosis pathogenesis: Insights from next-generation sequencing. Hum. Reprod. Update 2021, 27, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Sun, Y.; Yang, B.; Yang, Y.; Zhang, Y.; Yu, T.; Huang, H.; Zhang, J.; Xu, H. Transcriptome sequencing of adenomyosis eutopic endometrium: A new insight into its pathophysiology. J. Cell Mol. Med. 2019, 23, 8381–8391. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Li, Y.; Chen, Y.; Huang, M.; Cao, J.; Cao, M.; Wang, Z.; Wan, G.; Gui, T. Transcriptome analysis of eutopic endometrial stromal cells in women with adenomyosis by RNA-sequencing. Bioengineered 2022, 13, 12637–12649. [Google Scholar] [CrossRef]
- Praetorius, T.H.; Leonova, A.; Lac, V.; Senz, J.; Tessier-Cloutier, B.; Nazeran, T.M.; Kobel, M.; Grube, M.; Kraemer, B.; Yong, P.J.; et al. Molecular analysis suggests oligoclonality and metastasis of endometriosis lesions across anatomically defined subtypes. Fertil. Steril. 2022, 118, 524–534. [Google Scholar] [CrossRef]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef]
- Guo, S.W. Cancer driver mutations in endometriosis: Variations on the major theme of fibrogenesis. Reprod. Med. Biol. 2018, 17, 369–397. [Google Scholar] [CrossRef]
| Database: Ovid MEDLINE(R) ALL <1946 to 13 October 2023> | ||
|---|---|---|
| Search Strategy: | No. of Articles | |
| 1 | adenomyosis.mp. or Adenomyosis | 3775 |
| 2 | endometriosis.mp. or Endometriosis | 33,643 |
| 3 | etiology.mp. | 2,939,921 |
| 4 | pathophysiology.mp. | 179,252 |
| 5 | 3 or 4 | 3,080,247 |
| 6 | 1 and 5 | 877 |
| 7 | 2 and 5 | 8862 |
| 8 | 7 and 8 | 496 |
| 9 | from 8 keep 2, 12, 26, 27, 34, 77, 122, 151, 166, 227, 233, 320, 324, 430 | 14 |
| 10 | Prevalence/or prevalence.mp. | 896,010 |
| 11 | incidence.mp. or Incidence | 1,039,284 |
| 12 | 10 or 11 | 1,830,071 |
| 13 | 1 and 12 | 436 |
| 14 | 2 and 12 | 2663 |
| 15 | 13 and 14 | 247 |
| 16 | 1 and 2 | 1972 |
| 17 | 12 and 16 | 247 |
| 18 | from 17 keep 17, 38, 51, 62, 66, 78, 79, 85, 86, 91, 101, 102, 104, 106, 113, 125, 145, 147, 149, 162, 166, 204 | 22 |
| Affection of the Abdominal Wall | Primary Abdominal Wall Endometriosis (No Previous Scar) |
|---|---|
| Scar endometriosis | |
| Umbilical endometriosis | |
| Inguinal endometriosis | |
| Perineal endometriosis | Primary (no previous surgery) |
| Following scarring | |
| Visceral endometriosis | Kidney |
| Liver | |
| Pancreas | |
| Biliary tract | |
| Diaphragm | |
| Thoracic endometriosis | Diaphragm |
| Pleura pneumothorax | |
| Lung parenchyma | |
| Nose | |
| Central nervous system | Brain |
| Lumbar vertebrae | |
| Conus medullaris | |
| Extra-pelvic muscles | |
| Peripheral nerves |
| Subtype | Description |
|---|---|
| I | Adenomyosis that occurs in the uterine inner layer without affecting the outer structures. |
| II | Adenomyosis that occurs in the uterine outer layer without affecting the inner structure |
| III | Adenomyosis that occurs solitarily without relationship to structural components |
| IV | Adenomyosis that did not satisfy the above criteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habiba, M.; Guo, S.-W.; Benagiano, G. Are Adenomyosis and Endometriosis Phenotypes of the Same Disease Process? Biomolecules 2024, 14, 32. https://doi.org/10.3390/biom14010032
Habiba M, Guo S-W, Benagiano G. Are Adenomyosis and Endometriosis Phenotypes of the Same Disease Process? Biomolecules. 2024; 14(1):32. https://doi.org/10.3390/biom14010032
Chicago/Turabian StyleHabiba, Marwan, Sun-Wei Guo, and Giuseppe Benagiano. 2024. "Are Adenomyosis and Endometriosis Phenotypes of the Same Disease Process?" Biomolecules 14, no. 1: 32. https://doi.org/10.3390/biom14010032
APA StyleHabiba, M., Guo, S.-W., & Benagiano, G. (2024). Are Adenomyosis and Endometriosis Phenotypes of the Same Disease Process? Biomolecules, 14(1), 32. https://doi.org/10.3390/biom14010032







