Role of Natural Compounds Modulating Heme Catabolic Pathway in Gut, Liver, Cardiovascular, and Brain Diseases
Abstract
:1. Introduction
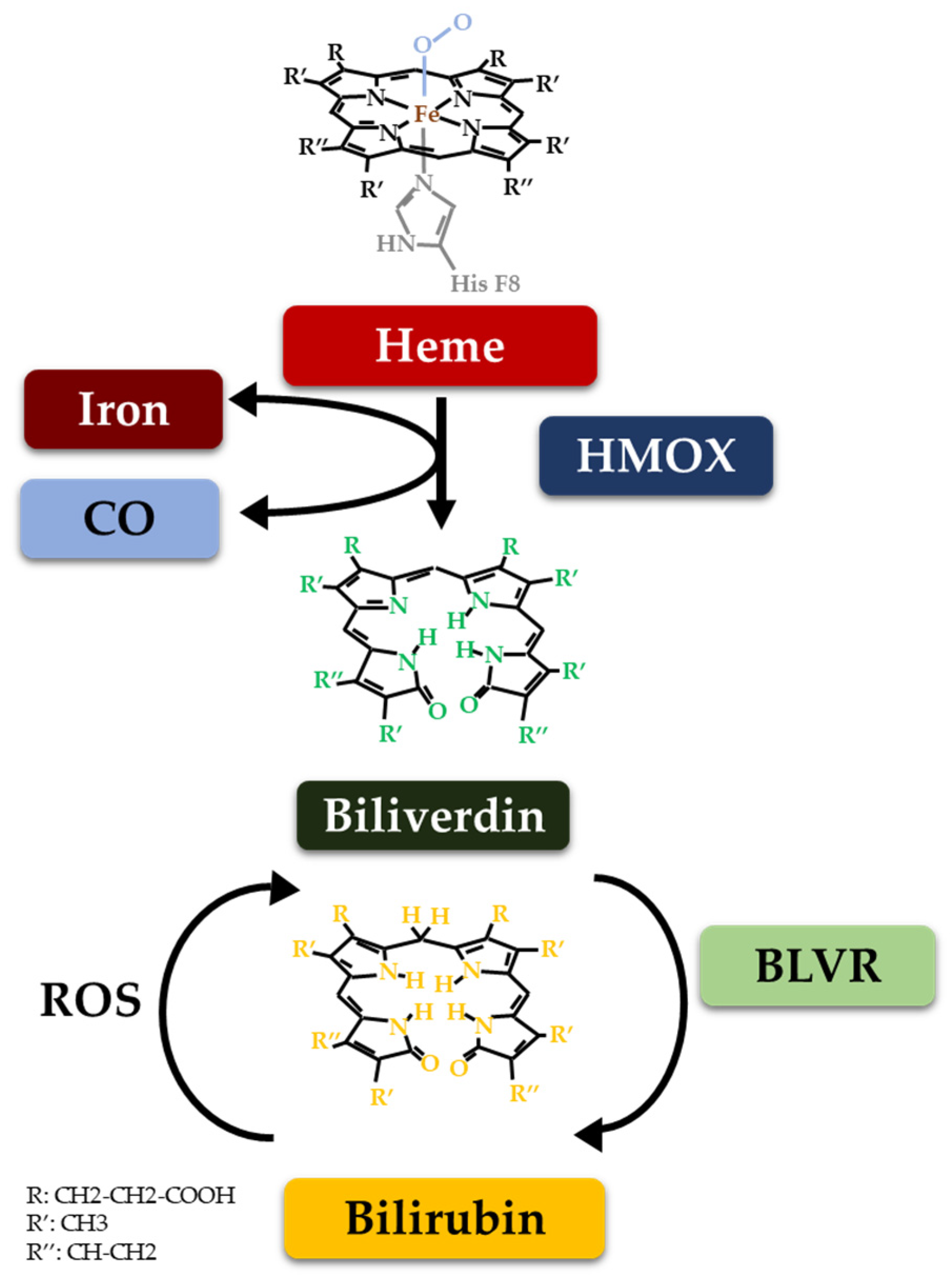
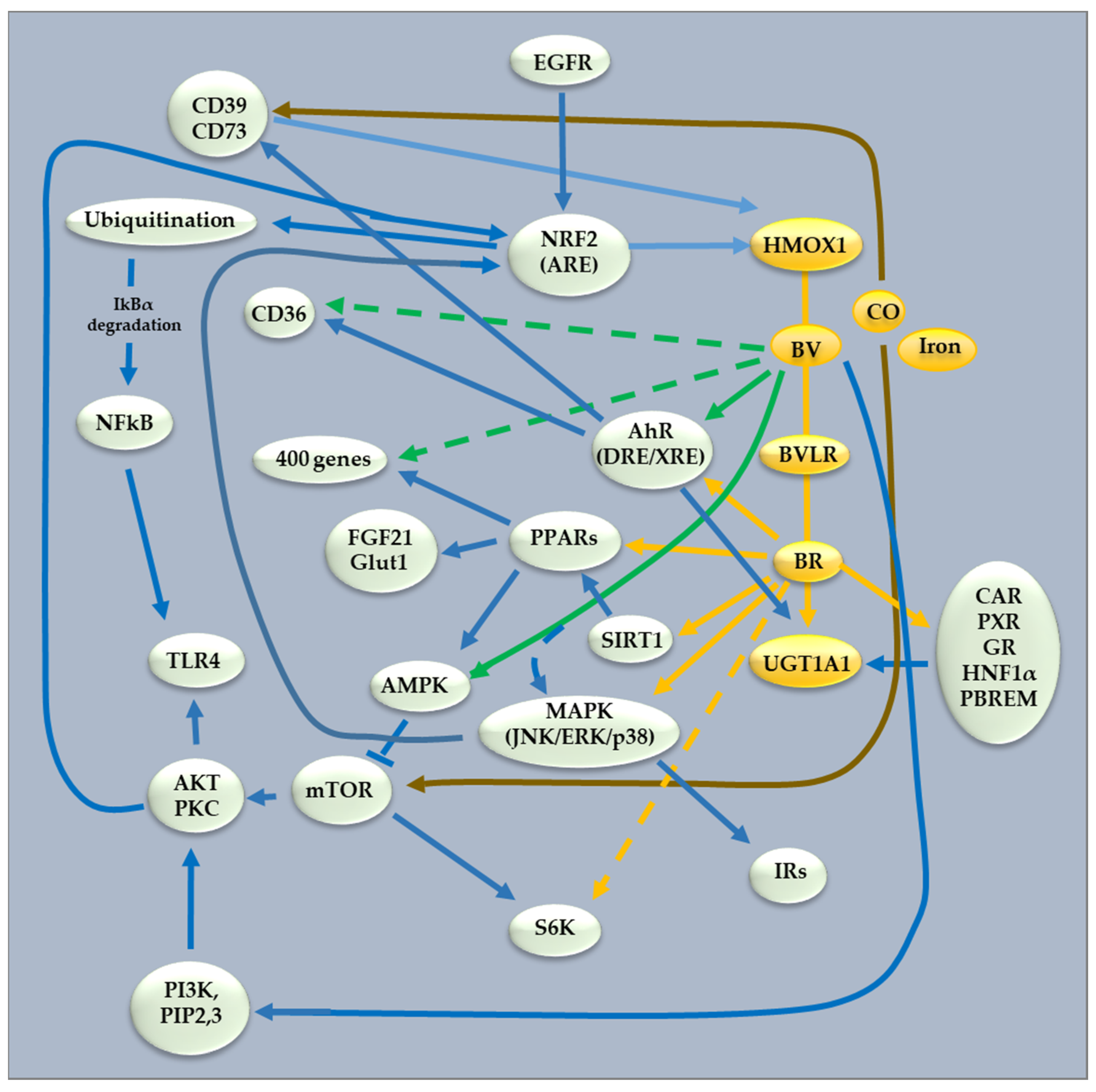
2. The Heme Catabolic Pathway and Metabolic Checkpoints
3. Natural Compounds with Demonstrated Effects on Bilirubin and Its Metabolic Enzymes
3.1. Natural Compounds
3.1.1. Flavonoids
3.1.2. Curcumin
3.1.3. Astragaloside
3.1.4. Vitamins
3.1.5. Madecassoside
3.1.6. Green Tea
3.1.7. S-Allyl Cysteine
3.1.8. 20C
3.1.9. Achyranthes bidentata
3.1.10. Coriolus versicolor and Hericium erinaceus
3.1.11. Hyperoside (Quercetin 3-O-galactoside)
3.1.12. Acerogin A
3.1.13. Kaempferol, Ginsenoside rh2
3.1.14. Mangiferin
3.1.15. Ellagic Acid
3.2. Natural Compounds Mimicking Products of the Heme Catabolic Pathway
3.2.1. Tetrapyrroles from Spirulina platensis, Phycocyanin, and Phycocyanobilin
3.2.2. Artificial Bezoar
3.2.3. Chlorophylls
4. Natural Compounds Targeting the Heme Catabolic Pathway
4.1. Modulation of HMOX1
4.2. Modulation of BLVRA
4.3. Modulation of the Hepatic Transport of Bilirubin
4.4. UGT1A1 Modulation
4.5. Gut Microbiome Modulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gazzin, S.; Vitek, L.; Watchko, J.; Shapiro, S.M.; Tiribelli, C. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends Mol. Med. 2016, 22, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Wallner, M.; Mölzer, C.; Gazzin, S.; Bulmer, A.C.; Tiribelli, C.; Vitek, L. Looking to the Horizon: The Role of Bilirubin in the Development and Prevention of Age-Related Chronic Diseases. Clin. Sci. 2015, 129, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Bellarosa, C.; Tiribelli, C. Induction of Mild Hyperbilirubinemia: Hype or Real Therapeutic Opportunity? Clin. Pharmacol. Ther. 2019, 106, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D. Bilirubin as a Metabolic Hormone: The Physiological Relevance of Low Levels. Am. J. Physiol.-Endocrinol. Metab. 2020, 320, E191–E207. [Google Scholar] [CrossRef]
- Jayanti, S.; Dalla Verde, C.; Tiribelli, C.; Gazzin, S. Inflammation, Dopaminergic Brain and Bilirubin. Int. J. Mol. Sci. 2023, 24, 11478. [Google Scholar] [CrossRef] [PubMed]
- Llido, J.P.; Jayanti, S.; Tiribelli, C.; Gazzin, S. Bilirubin and Redox Stress in Age-Related Brain Diseases. Antioxidants 2023, 12, 1525. [Google Scholar] [CrossRef] [PubMed]
- Ostrow, J.D.; Vitek, L. Bilirubin Chemistry and Metabolism; Harmful and Protective Aspects. Curr. Pharm. Design 2009, 15, 2869–2883. [Google Scholar]
- Ryter, S.W.; Otterbein, L.E. Carbon Monoxide in Biology and Medicine. BioEssays 2004, 26, 270–280. [Google Scholar] [CrossRef]
- Wegiel, B.; Otterbein, L. Go Green: The Anti-Inflammatory Effects of Biliverdin Reductase. Front. Pharmacol. 2012, 3, 47. [Google Scholar] [CrossRef]
- Gazzin, S.; Masutti, F.; Vítek, L.; Tiribelli, C. The Molecular Basis of Jaundice: An Old Symptom Revisited. Liver Int. 2016, 37, 1094–1102. [Google Scholar] [CrossRef]
- Vítek, L. Bilirubin and Atherosclerotic Diseases. Physiol. Res. 2017, 66, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L. Bilirubin as a Signaling Molecule. Med. Res. Rev. 2020, 40, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L. The Protective Role of the Heme Catabolic Pathway in Hepatic Disorders. Antioxid. Redox Signal. 2021, 35, 734–752. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L. Biliverdin and Bilirubin as Parallel Products of CO Formation. In Carbon Monoxide in Drug Discovery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 175–194. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Wilks, A.; Knör, G.; Wu, H.; Zheng, Y.; Liu, J.; Zhang, H.; Chen, H.; Buchberger, T.; Lamparter, T.; Estes, S.; et al. Heme Oxygenase: Evolution, Structure, and Mechanism. Antioxid. Redox Signal. 2002, 4, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. THE HEME OXYGENASE SYSTEM: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Choi, A.M.K. Heme Oxygenase-1. Am. J. Respir. Cell Mol. Biol. 2002, 27, 8–16. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally Derived Heme-Oxygenase 1 Inducers and Their Therapeutic Application to Immune-Mediated Diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a Potential Mediator of Cardiovascular Risk in Metabolic Diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Chapman, E. The Role of Natural Products in Revealing NRF2 Function. Nat. Prod. Rep. 2020, 37, 797–826. [Google Scholar] [CrossRef] [PubMed]
- Qader, M.; Xu, J.; Yang, Y.; Liu, Y.; Cao, S. Natural Nrf2 Activators from Juices, Wines, Coffee, and Cocoa. Beverages 2020, 6, 68. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.-X.; Zhu, J.-Q.; Dou, Y.-X.; Xian, Y.-F.; Lin, Z.-X. Natural Nrf2 Inhibitors: A Review of Their Potential for Cancer Treatment. Int. J. Biol. Sci. 2023, 19, 3029–3041. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nagalakshmi, D.; Sharma, K.K.; Ravichandiran, V. Natural Antioxidants for Neuroinflammatory Disorders and Possible Involvement of Nrf2 Pathway: A Review. Heliyon 2021, 7, e06216. [Google Scholar] [CrossRef] [PubMed]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Puentes-Pardo, J.D.; Moreno-SanJuan, S.; Carazo, Á.; León, J. Heme Oxygenase-1 in Gastrointestinal Tract Health and Disease. Antioxidants 2020, 9, 1214. [Google Scholar] [CrossRef]
- Song, W.; Zukor, H.; Lin, S.-H.; Hascalovici, J.; Liberman, A.; Tavitian, A.; Mui, J.; Vali, H.; Tong, X.-K.; Bhardwaj, S.K.; et al. Schizophrenia-Like Features in Transgenic Mice Overexpressing Human HO-1 in the Astrocytic Compartment. J. Neurosci. 2012, 32, 10841–10853. [Google Scholar] [CrossRef]
- Laskaris, L.E.; Di Biase, M.A.; Everall, I.; Chana, G.; Christopoulos, A.; Skafidas, E.; Cropley, V.L.; Pantelis, C. Microglial Activation and Progressive Brain Changes in Schizophrenia. Br. J. Pharmacol. 2016, 173, 666–680. [Google Scholar] [CrossRef]
- Zhuo, C.; Tian, H.; Song, X.; Jiang, D.; Chen, G.; Cai, Z.; Ping, J.; Cheng, L.; Zhou, C.; Chen, C. Microglia and Cognitive Impairment in Schizophrenia: Translating Scientific Progress into Novel Therapeutic Interventions. Schizophrenia 2023, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yuan, X.; Kang, Y.; Song, X. New Insight in the Cross-Talk between Microglia and Schizophrenia: From the Perspective of Neurodevelopment. Front. Psychiatry 2023, 14, 1126632. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, B.; Van Steenwinckel, J.; Bokobza, C.; Shearer, I.K.; Ross-Munro, E.; Gressens, P. Microglia-Mediated Neurodegeneration in Perinatal Brain Injuries. Biomolecules 2021, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The Sinister Face of Heme Oxygenase-1 in Brain Aging and Disease. Progress. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef] [PubMed]
- Bereczki, D.; Balla, J.; Bereczki, D. Heme Oxygenase-1: Clinical Relevance in Ischemic Stroke. Curr. Pharm. Des. 2018, 24, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.; Sureda, A.; Belwal, T.; Sanches Silva, A.; Vacca, R.A.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Shirooie, S.; Dehpour, A.R.; et al. Natural Products, PGC-1 α, and Duchenne Muscular Dystrophy. Acta Pharm. Sin. B 2020, 10, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Bottari, N.B.; Reichert, K.P.; Fracasso, M.; Dutra, A.; Assmann, C.E.; Ulrich, H.; Schetinger, M.R.C.; Morsch, V.M.; Da Silva, A.S. Neuroprotective Role of Resveratrol Mediated by Purinergic Signalling in Cerebral Cortex of Mice Infected by Toxoplasma Gondii. Parasitol. Res. 2020, 119, 2897–2905. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, J.; Baldissarelli, J.; Mann, T.; Abdalla, F.; Fiorenza, A.; Rosa, M.; Carvalho, F.; Gutierres, J.; Andrade, C.; et al. Curcumin Attenuates Memory Deficits and the Impairment of Cholinergic and Purinergic Signaling in Rats Chronically Exposed to Cadmium. Environ. Toxicol. 2015, 32, 70–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Jin, X.; Zhou, X.; Dong, X.; Yu, W.; Gao, W. The Role of Astragaloside IV against Cerebral Ischemia/Reperfusion Injury: Suppression of Apoptosis via Promotion of P62-LC3-Autophagy. Molecules 2019, 24, 1838. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Brondolo, L.; Marinari, U.M.; Pronzato, M.A.; Furfaro, A.L. Heme Oxygenase 1 in the Nervous System: Does It Favor Neuronal Cell Survival or Induce Neurodegeneration? Int. J. Mol. Sci. 2018, 19, 2260. [Google Scholar] [CrossRef]
- Dwyer, B.E.; Nishimura, R.N.; Lu, S.Y. Differential Expression of Heme Oxygenase-1 in Cultured Cortical Neurons and Astrocytes Determined by the Aid of a New Heme Oxygenase Antibody. Response to Oxidative Stress. Brain Res. Mol. Brain Res. 1995, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Shen, Y.; Cheng, Z.; Shao, Q.; Wang, C.; Sun, H.; Zhang, Q. Achyranthes Bidentata Polypeptide k Suppresses Neuroinflammation in BV2 Microglia through Nrf2-Dependent Mechanism. Ann. Transl. Med. 2019, 7, 575. [Google Scholar] [CrossRef]
- Jayanti, S.; Vítek, L.; Tiribelli, C.; Gazzin, S. The Role of Bilirubin and the Other “Yellow Players” in Neurodegenerative Diseases. Antioxidants 2020, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Sahebnasagh, A.; Eghbali, S.; Saghafi, F.; Sureda, A.; Avan, R. Neurohormetic Phytochemicals in the Pathogenesis of Neurodegenerative Diseases. Immun. Ageing 2022, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Yuan, Y.-H.; Yan, J.-Q.; Wang, Y.-N.; Chu, S.-F.; Zhu, C.-G.; Guo, Q.-L.; Shi, J.-G.; Chen, N.-H. 20C, a Bibenzyl Compound Isolated from Gastrodia Elata, Protects PC12 Cells against Rotenone-Induced Apoptosis via Activation of the Nrf2/ARE/HO-1 Signaling Pathway. Acta Pharmacol. Sin. 2016, 37, 731–740. [Google Scholar] [CrossRef]
- Cordaro, M.; Modafferi, S.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; et al. Natural Compounds Such as Hericium erinaceus and Coriolus versicolor Modulate Neuroinflammation, Oxidative Stress and Lipoxin A4 Expression in Rotenone-Induced Parkinson’s Disease in Mice. Biomedicines 2022, 10, 2505. [Google Scholar] [CrossRef]
- Duan, C.; Wang, H.; Jiao, D.; Geng, Y.; Wu, Q.; Yan, H.; Li, C. Curcumin Restrains Oxidative Stress of After Intracerebral Hemorrhage in Rat by Activating the Nrf2/HO-1 Pathway. Front. Pharmacol. 2022, 13, 889226. [Google Scholar] [CrossRef]
- Trock, B.; Lanza, E.; Greenwald, P. Dietary Fiber, Vegetables, and Colon Cancer: Critical Review and Meta-Analyses of the Epidemiologic Evidence. J. Natl. Cancer Inst. 1990, 82, 650–661. [Google Scholar] [CrossRef]
- Qaisiya, M.; Coda Zabetta, C.D.; Bellarosa, C.; Tiribelli, C. Bilirubin Mediated Oxidative Stress Involves Antioxidant Response Activation via Nrf2 Pathway. Cell. Signal. 2014, 26, 512–520. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Lee, S.R.; Park, Y.J.; Ra, M.; Lee, Y.; Pang, C.; Kim, K.H. Suppression of 6-Hydroxydopamine-Induced Oxidative Stress by Hyperoside via Activation of Nrf2/HO-1 Signaling in Dopaminergic Neurons. Int. J. Mol. Sci. 2019, 20, 5832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M.; et al. Berberine Protects against 6-OHDA-Induced Neurotoxicity in PC12 Cells and Zebrafish through Hormetic Mechanisms Involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 Pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Zhang, Z.; Gao, P.; Zhang, X. Breviscapine Provides a Neuroprotective Effect after Traumatic Brain Injury by Modulating the Nrf2 Signaling Pathway. J. Cell Biochem. 2019, 120, 14899–14907. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Cha, B.-Y.; Woo, J.-T.; Kim, Y.-C.; Jang, J.-H. Acerogenin A from Acer nikoense Maxim Prevents Oxidative Stress-Induced Neuronal Cell Death through Nrf2-Mediated Heme Oxygenase-1 Expression in Mouse Hippocampal HT22 Cell Line. Molecules 2015, 20, 12545–12557. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.-M.; Lu, P.-H.; Zhang, K.; Wang, X.; Sun, M.; Chen, G.-Q.; Wang, Q. EGFR Mediates Astragaloside IV-Induced Nrf2 Activation to Protect Cortical Neurons against in vitro Ischemia/Reperfusion Damages. Biochem. Biophys. Res. Commun. 2015, 457, 391–397. [Google Scholar] [CrossRef]
- Li, C.; Yang, F.; Liu, F.; Li, D.; Yang, T. NRF2/HO-1 Activation via ERK Pathway Involved in the Anti-Neuroinflammatory Effect of Astragaloside IV in LPS Induced Microglial Cells. Neurosci. Lett. 2018, 666, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Ghasemi, Z.; Roghani, M. S-Allyl Cysteine Ameliorates Cognitive Deficits in Streptozotocin-Diabetic Rats via Suppression of Oxidative Stress, Inflammation, and Acetylcholinesterase. Eur. J. Pharmacol. 2017, 794, 69–76. [Google Scholar] [CrossRef]
- Feng, S.-T.; Wang, Z.-Z.; Yuan, Y.-H.; Sun, H.-M.; Chen, N.-H.; Zhang, Y. Mangiferin: A Multipotent Natural Product Preventing Neurodegeneration in Alzheimer’s and Parkinson’s Disease Models. Pharmacol. Res. 2019, 146, 104336. [Google Scholar] [CrossRef]
- Siddique, Y.H. Role of Luteolin in Overcoming Parkinson’s Disease. Biofactors 2021, 47, 198–206. [Google Scholar] [CrossRef]
- Jin, M.; Park, S.Y.; Shen, Q.; Lai, Y.; Ou, X.; Mao, Z.; Lin, D.; Yu, Y.; Zhang, W. Anti-Neuroinflammatory Effect of Curcumin on Pam3CSK4-Stimulated Microglial Cells. Int. J. Mol. Med. 2018, 41, 521–530. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin Provides Neuroprotection in Model of Traumatic Brain Injury via the Nrf2-ARE Signaling Pathway. Brain Res. Bull. 2018, 140, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yang, B.; Wang, L.; Li, B.; Guo, X.; Zhang, M.; Jiang, Z.; Fu, J.; Pi, J.; Guan, D.; et al. Curcumin Plays Neuroprotective Roles against Traumatic Brain Injury Partly via Nrf2 Signaling. Toxicol. Appl. Pharmacol. 2018, 346, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, C.; Zhou, Y.; Wang, Q.; Jiang, Y.; Bai, Y.; Qian, H.; Xing, G.; Wang, S.; Li, F.; et al. Curcumin Protects against Methylmercury-Induced Cytotoxicity in Primary Rat Astrocytes by Activating the Nrf2/ARE Pathway Independently of PKCδ. Toxicology 2019, 425, 152248. [Google Scholar] [CrossRef]
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behaviors via Restoring Changes in Oxidative Stress and the Activation of Nrf2 Signaling Pathway in Rats. Oxidative Med. Cell. Longev. 2020, 2020, e9268083. [Google Scholar] [CrossRef] [PubMed]
- Santana-Martínez, R.A.; Silva-Islas, C.A.; Fernández-Orihuela, Y.Y.; Barrera-Oviedo, D.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Maldonado, P.D. The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats Is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes. Antioxidants 2019, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, J.; Guo, D.; Pang, H.; Zhao, Y.; Song, J. Curcumin Mitigates Axonal Injury and Neuronal Cell Apoptosis through the PERK/Nrf2 Signaling Pathway Following Diffuse Axonal Injury. NeuroReport 2018, 29, 661. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Saeed, K.; Khan, A.; Muhammad, T.; Khan, M.S.; Jo, M.G.; Rehman, S.U.; Kim, M.O. Natural Dietary Supplementation of Curcumin Protects Mice Brains against Ethanol-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment via Nrf2/TLR4/RAGE Signaling. Nutrients 2019, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yan, Y.; Jiang, Y.; Meng, X. Ellagic Acid and Its Anti-Aging Effects on Central Nervous System. Int. J. Mol. Sci. 2022, 23, 10937. [Google Scholar] [CrossRef]
- Park, J.-Y.; Sohn, H.-Y.; Koh, Y.H.; Jo, C. Curcumin Activates Nrf2 through PKCδ-Mediated P62 Phosphorylation at Ser351. Sci. Rep. 2021, 11, 8430. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, C.; Jing, R.; Mu, L.; Liu, P.; Hu, Y. Antidepressant Mechanism of Kaixinsan and Its Active Compounds Based on Upregulation of Antioxidant Thioredoxin. Evid. -Based Complement. Altern. Med. 2022, 2022, e7302442. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; McCarty, M.F. C-Phycocyanin-Derived Phycocyanobilin as a Potential Nutraceutical Approach for Major Neurodegenerative Disorders and COVID-19-Induced Damage to the Nervous System. Curr. Neuropharmacol. 2021, 19, 2250–2275. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Hinds, T.D.; Stec, D.E.; Tiribelli, C. The Physiology of Bilirubin: Health and Disease Equilibrium. Trends Mol. Med. 2023, 29, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural Product Agonists of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ): A Review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Sirignano, C.; Taglialatela-Scafati, O. The Potential of Natural Products for Targeting PPARα. Acta Pharm. Sin. B 2017, 7, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mölzer, C.; Wallner, M.; Kern, C.; Tosevska, A.; Schwarz, U.; Zadnikar, R.; Doberer, D.; Marculescu, R.; Wagner, K.-H. Features of an Altered AMPK Metabolic Pathway in Gilbert’s Syndrome, and Its Role in Metabolic Health. Sci. Rep. 2016, 6, 30051. [Google Scholar] [CrossRef]
- Longhi, M.S.; Vuerich, M.; Kalbasi, A.; Kenison, J.E.; Yeste, A.; Csizmadia, E.; Vaughn, B.; Feldbrugge, L.; Mitsuhashi, S.; Wegiel, B.; et al. Bilirubin Suppresses Th17 Immunity in Colitis by Upregulating CD39. JCI Insight 2017, 2, e92791. [Google Scholar] [CrossRef]
- Correa-Costa, M.; Gallo, D.; Csizmadia, E.; Gomperts, E.; Lieberum, J.-L.; Hauser, C.J.; Ji, X.; Wang, B.; Câmara, N.O.S.; Robson, S.C.; et al. Carbon Monoxide Protects the Kidney through the Central Circadian Clock and CD39. Proc. Natl. Acad. Sci. USA 2018, 115, E2302–E2310. [Google Scholar] [CrossRef]
- McCarty, M.F. Practical Prospects for Boosting Hepatic Production of the “pro-Longevity” Hormone FGF21. Horm. Mol. Biol. Clin. Investig. 2017, 30, 20150057. [Google Scholar] [CrossRef]
- Hinds, T.D.; Stec, D.E. Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension 2018, 72, 788–795. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Activating Effects of the Bioactive Compounds From Coffee By-Products on FGF21 Signaling Modulate Hepatic Mitochondrial Bioenergetics and Energy Metabolism in vitro. Front. Nutr. 2022, 9, 866233. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phytochemicals from the Cocoa Shell Modulate Mitochondrial Function, Lipid and Glucose Metabolism in Hepatocytes via Activation of FGF21/ERK, AKT, and mTOR Pathways. Antioxidants 2022, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, C.; Zuliani, I.; Vasavda, C.; Snyder, S.H.; Paul, B.D.; Perluigi, M.; Di Domenico, F.D.; Barone, E. BVR-A Deficiency Leads to Autophagy Impairment through the Dysregulation of AMPK/mTOR Axis in the Brain-Implications for Neurodegeneration. Antioxidants 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Joe, Y.; Kim, S.-K.; Park, S.-U.; Park, J.; Chen, Y.; Kim, J.; Ryu, J.; Cho, G.J.; Surh, Y.-J.; et al. Carbon Monoxide Protects against Hepatic Steatosis in Mice by Inducing Sestrin-2 via the PERK-eIF2α-ATF4 Pathway. Free Radic. Biol. Med. 2017, 110, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Inhibition of PI3K/Akt/mTOR Signaling by Natural Products. Anticancer. Agents Med. Chem. 2013, 13, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Vakili, O.; Borji, M.; Saffari-Chaleshtori, J.; Shafiee, S.M. Ameliorative Effects of Bilirubin on Cell Culture Model of Non-Alcoholic Fatty Liver Disease. Mol. Biol. Rep. 2023, 50, 4411–4422. [Google Scholar] [CrossRef] [PubMed]
- Alcaín, F.J.; Villalba, J.M. Sirtuin Activators. Expert. Opin. Ther. Pat. 2009, 19, 403–414. [Google Scholar] [CrossRef]
- Zhang, Z.; Amorosa, L.F.; Petrova, A.; Coyle, S.; Macor, M.; Nair, M.; Lee, L.Y.; Haimovich, B. TLR4 Counteracts BVRA Signaling in Human Leukocytes via Differential Regulation of AMPK, mTORC1 and mTORC2. Sci. Rep. 2019, 9, 7020. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Asgary, S.; Mohammadi, P.; Hozeifi, S.; Hoseinzadeh-Chahkandak, F.; Xu, S.; Farzaei, M.H. Natural AMPK Activators in Cardiovascular Disease Prevention. Front. Pharmacol. 2021, 12, 738420. [Google Scholar] [CrossRef]
- Hinds, T.D.; Burns, K.A.; Hosick, P.A.; McBeth, L.; Nestor-Kalinoski, A.; Drummond, H.A.; AlAmodi, A.A.; Hankins, M.W.; Vanden Heuvel, J.P.; Stec, D.E. Biliverdin Reductase A Attenuates Hepatic Steatosis by Inhibition of Glycogen Synthase Kinase (GSK) 3β Phosphorylation of Serine 73 of Peroxisome Proliferator-Activated Receptor (PPAR) α. J. Biol. Chem. 2016, 291, 25179–25191. [Google Scholar] [CrossRef]
- Duda, P.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Martelli, A.M.; Cocco, L.; Ratti, S.; Candido, S.; Libra, M.; Montalto, G.; et al. Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells 2020, 9, 1110. [Google Scholar] [CrossRef]
- Song, X.; Long, D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R. The Pathophysiology of Heme in the Brain. Curr. Alzheimer Res. 2016, 13, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M. Brain Iron Deposition and the Free Radical-Mitochondrial Theory of Ageing. Ageing Res. Rev. 2004, 3, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Kitase, Y.; Vasan, V.; Burkhardt, C.; Ramachandra, S.; Robinson, S.; Jantzie, L.L. Chorioamnionitis Precipitates Perinatal Alterations of Heme-Oxygenase-1 (HO-1) Homeostasis in the Developing Rat Brain. Int. J. Mol. Sci. 2021, 22, 5773. [Google Scholar] [CrossRef]
- Kram, H.; Prokop, G.; Haller, B.; Gempt, J.; Wu, Y.; Schmidt-Graf, F.; Schlegel, J.; Conrad, M.; Liesche-Starnecker, F. Glioblastoma Relapses Show Increased Markers of Vulnerability to Ferroptosis. Front. Oncol. 2022, 12, 841418. [Google Scholar] [CrossRef]
- Hara, E.; Takahashi, K.; Tominaga, T.; Kumabe, T.; Kayama, T.; Suzuki, H.; Fujita, H.; Yoshimoto, T.; Shirato, K.; Shibahara, S. Expression of Heme Oxygenase and Inducible Nitric Oxide Synthase mRNA in Human Brain Tumors. Biochem. Biophys. Res. Commun. 1996, 224, 153–158. [Google Scholar] [CrossRef]
- Vandenbark, A.A.; Offner, H.; Matejuk, S.; Matejuk, A. Microglia and Astrocyte Involvement in Neurodegeneration and Brain Cancer. J. Neuroinflammation 2021, 18, 298. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Abels, E.R.; Van De Haar, L.L.; Zhang, X.; Morsett, L.; Sil, S.; Guedes, J.; Sen, P.; Prabhakar, S.; Hickman, S.E.; et al. Glioblastoma Hijacks Microglial Gene Expression to Support Tumor Growth. J. Neuroinflammation 2020, 17, 120. [Google Scholar] [CrossRef]
- Catalano, M.; Serpe, C.; Limatola, C. Microglial Extracellular Vesicles as Modulators of Brain Microenvironment in Glioma. Int. J. Mol. Sci. 2022, 23, 13165. [Google Scholar] [CrossRef]
- Lanza, M.; Casili, G.; Campolo, M.; Paterniti, I.; Colarossi, C.; Mare, M.; Giuffrida, R.; Caffo, M.; Esposito, E.; Cuzzocrea, S. Immunomodulatory Effect of Microglia-Released Cytokines in Gliomas. Brain Sci. 2021, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, M.R.; Saffarian, A.; Khademolhosseini, A.; Dehghanian, A.; Ghaderi, A.; Sotoodeh Jahromi, A. Simultaneous Increase in Serum Levels of IL-37 and IL-18 Binding Protein In Low-Grade and High-Grade Brain Tumors. Asian Pac. J. Cancer Prev. 2022, 23, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D. Bilirubin Binding to PPARα Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Lee, J.H.; Wada, T.; Febbraio, M.; He, J.; Matsubara, T.; Lee, M.J.; Gonzalez, F.J.; Xie, W. A Novel Role for the Dioxin Receptor in Fatty Acid Metabolism and Hepatic Steatosis. Gastroenterology 2010, 139, 653–663. [Google Scholar] [CrossRef]
- Phelan, D.; Winter, G.M.; Rogers, W.J.; Lam, J.C.; Denison, M.S. Activation of the Ah Receptor Signal Transduction Pathway by Bilirubin and Biliverdin. Arch. Biochem. Biophys. 1998, 357, 155–163. [Google Scholar] [CrossRef]
- Gordon, D.M.; Blomquist, T.M.; Miruzzi, S.A.; McCullumsmith, R.; Stec, D.E.; Hinds, T.D. RNA Sequencing in Human HepG2 Hepatocytes Reveals PPAR-α Mediates Transcriptome Responsiveness of Bilirubin. Physiol. Genom. 2019, 51, 234–240. [Google Scholar] [CrossRef]
- Nakao, A.; Murase, N.; Ho, C.; Toyokawa, H.; Billiar, T.R.; Kanno, S. Biliverdin Administration Prevents the Formation of Intimal Hyperplasia Induced by Vascular Injury. Circulation 2005, 112, 587–591. [Google Scholar] [CrossRef]
- Deguchi, K.; Hayashi, T.; Nagotani, S.; Sehara, Y.; Zhang, H.; Tsuchiya, A.; Ohta, Y.; Tomiyama, K.; Morimoto, N.; Miyazaki, M.; et al. Reduction of Cerebral Infarction in Rats by Biliverdin Associated with Amelioration of Oxidative Stress. Brain Res. 2008, 1188, 1–8. [Google Scholar] [CrossRef]
- Zou, Z.-Y.; Liu, J.; Chang, C.; Li, J.-J.; Luo, J.; Jin, Y.; Ma, Z.; Wang, T.-H.; Shao, J.-L. Biliverdin Administration Regulates the microRNA-mRNA Expressional Network Associated with Neuroprotection in Cerebral Ischemia Reperfusion Injury in Rats. Int. J. Mol. Med. 2019, 43, 1356–1372. [Google Scholar] [CrossRef]
- Triani, F.; Tramutola, A.; Di Domenico, F.; Sharma, N.; Butterfield, D.A.; Head, E.; Perluigi, M.; Barone, E. Biliverdin Reductase-A Impairment Links Brain Insulin Resistance with Increased Aβ Production in an Animal Model of Aging: Implications for Alzheimer Disease. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 3181–3194. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Mancuso, C.; Di Domenico, F.; Sultana, R.; Murphy, M.P.; Head, E.; Butterfield, D.A. Biliverdin Reductase-A: A Novel Drug Target for Atorvastatin in a Dog Pre-Clinical Model of Alzheimer Disease. J. Neurochem. 2012, 120, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.E.M.; Maines, M.D. Biliverdin Inhibits Activation of NF-κB: Reversal of Inhibition by Human Biliverdin Reductase. Int. J. Cancer 2007, 121, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Atukeren, P.; Oner, S.; Baran, O.; Kemerdere, R.; Eren, B.; Cakatay, U.; Tanriverdi, T. Oxidant and Anti-Oxidant Status in Common Brain Tumors: Correlation to TP53 and Human Biliverdin Reductase. Clin. Neurol. Neurosurg. 2017, 158, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, N.; Kurepa, D.; Bader, M.Y.; Nagy, N.; Ahmed, M.N. Prophylactic Inhibition of NF-κB Expression in Microglia Leads to Attenuation of Hypoxic Ischemic Injury of the Immature Brain. J. Neuroinflammation 2020, 17, 365. [Google Scholar] [CrossRef] [PubMed]
- Costa-De-Santana, B.J.R.; Manhães-De-Castro, R.; Gouveia, H.J.C.B.; Silva, E.R.; Araújo, M.A.d.S.; Lacerda, D.C.; Guzmán-Quevedo, O.; Torner, L.; Toscano, A.E. Motor Deficits Are Associated with Increased Glial Cell Activation in the Hypothalamus and Cerebellum of Young Rats Subjected to Cerebral Palsy. Brain Res. 2023, 1814, 148447. [Google Scholar] [CrossRef] [PubMed]
- Mallard, C.; Davidson, J.O.; Tan, S.; Green, C.R.; Bennet, L.; Robertson, N.J.; Gunn, A.J. Astrocytes and Microglia in Acute Cerebral Injury Underlying Cerebral Palsy Associated with Preterm Birth. Pediatr. Res. 2014, 75, 234–240. [Google Scholar] [CrossRef]
- Hu, C.; Li, H.; Li, J.; Luo, X.; Hao, Y. Microglia: Synaptic Modulator in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 958661. [Google Scholar] [CrossRef]
- Brégère, C.; Schwendele, B.; Radanovic, B.; Guzman, R. Microglia and Stem-Cell Mediated Neuroprotection after Neonatal Hypoxia-Ischemia. Stem Cell Rev. Rep. 2022, 18, 474–522. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Cheng, Z.; Zhang, Y.; Wang, W.; Guo, B.; Wu, S. Mechanism of Action and Therapeutic Targeting of Microglia in Autism Spectrum Disorder. Adv. Neurol. 2022, 1, 167. [Google Scholar] [CrossRef]
- Zhang, F.; Nance, E.; Alnasser, Y.; Kannan, R.; Kannan, S. Microglial Migration and Interactions with Dendrimer Nanoparticles Are Altered in the Presence of Neuroinflammation. J. Neuroinflammation 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Davoli-Ferreira, M.; Thomson, C.A.; McCoy, K.D. Microbiota and Microglia Interactions in ASD. Front. Immunol. 2021, 12, 676255. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Ikegaya, Y. Microglia in the Pathogenesis of Autism Spectrum Disorders. Neurosci. Res. 2015, 100, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Patel, A.B.; Pantazopoulos, H.; Berretta, S.; Conti, P.; Leeman, S.E.; Theoharides, T.C. IL-37 Is Increased in Brains of Children with Autism Spectrum Disorder and Inhibits Human Microglia Stimulated by Neurotensin. Proc. Natl. Acad. Sci. USA 2019, 116, 21659–21665. [Google Scholar] [CrossRef]
- Vítek, L.; Tiribelli, C. Gilbert’s Syndrome Revisited. J. Hepatol. 2023, 79, 1049–1055. [Google Scholar] [CrossRef]
- Sugatani, J.; Mizushima, K.; Osabe, M.; Yamakawa, K.; Kakizaki, S.; Takagi, H.; Mori, M.; Ikari, A.; Miwa, M. Transcriptional Regulation of Human UGT1A1 Gene Expression through Distal and Proximal Promoter Motifs: Implication of Defects in the UGT1A1 Gene Promoter. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W.; Köhle, C. Contributions of the Ah Receptor to Bilirubin Homeostasis and Its Antioxidative and Atheroprotective Functions. Biol. Chem. 2010, 391, 645–653. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Z.; Luo, X. Roles of Xenobiotic Receptors in Vascular Pathophysiology. Circ. J. 2014, 78, 1520–1530. [Google Scholar] [CrossRef]
- Jayanti, S.; Moretti, R.; Tiribelli, C.; Gazzin, S. Bilirubin Prevents the TH+ Dopaminergic Neuron Loss in a Parkinson’s Disease Model by Acting on TNF-α. Int. J. Mol. Sci. 2022, 23, 14276. [Google Scholar] [CrossRef]
- Hernandez, J.P.; Mota, L.C.; Baldwin, W.S. Activation of CAR and PXR by Dietary, Environmental and Occupational Chemicals Alters Drug Metabolism, Intermediary Metabolism, and Cell Proliferation. Curr. Pharmacogenomics Person. Med. 2009, 7, 81–105. [Google Scholar] [CrossRef]
- Busbee, P.B.; Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. Use of Natural AhR Ligands as Potential Therapeutic Modalities against Inflammatory Disorders. Nutr. Rev. 2013, 71, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef] [PubMed]
- Bragt, M.C.E.; Popeijus, H.E. Peroxisome Proliferator-Activated Receptors and the Metabolic Syndrome. Physiol. Behav. 2008, 94, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Duszka, K.; Gregor, A.; Guillou, H.; König, J.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Caloric Restriction—Common Pathways Affecting Metabolism, Health, and Longevity. Cells 2020, 9, 1708. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Kliewer, S.A.; Mangelsdorf, D.J. Endocrine Fibroblast Growth Factors 15/19 and 21: From Feast to Famine. Genes. Dev. 2012, 26, 312–324. [Google Scholar] [CrossRef]
- Liu, J.; Dong, H.; Zhang, Y.; Cao, M.; Song, L.; Pan, Q.; Bulmer, A.; Adams, D.B.; Dong, X.; Wang, H. Bilirubin Increases Insulin Sensitivity by Regulating Cholesterol Metabolism, Adipokines and PPARγ Levels. Sci. Rep. 2015, 5, 9886. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Huang, H.; Yun, X.; Kim, D.; Yue, Y.; Wu, H.; Sutter, A.; Chavin, K.D.; Otterbein, L.E.; Adams, D.B.; et al. Bilirubin Increases Insulin Sensitivity in Leptin-Receptor Deficient and Diet-Induced Obese Mice through Suppression of ER Stress and Chronic Inflammation. Endocrinology 2014, 155, 818–828. [Google Scholar] [CrossRef]
- Zhang, F.; Guan, W.; Fu, Z.; Zhou, L.; Guo, W.; Ma, Y.; Gong, Y.; Jiang, W.; Liang, H.; Zhou, H. Relationship between Serum Indirect Bilirubin Level and Insulin Sensitivity: Results from Two Independent Cohorts of Obese Patients with Impaired Glucose Regulation and Type 2 Diabetes Mellitus in China. Int. J. Endocrinol. 2020, 2020, 5681296. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Kuo, H.-K.; Hwang, J.-J.; Lai, L.-P.; Chiang, F.-T.; Tseng, C.-D.; Lin, J.-L. Serum Bilirubin Is Inversely Associated with Insulin Resistance and Metabolic Syndrome among Children and Adolescents. Atherosclerosis 2009, 203, 563–568. [Google Scholar] [CrossRef]
- Shao, X.; Wang, M.; Wei, X.; Deng, S.; Fu, N.; Peng, Q.; Jiang, Y.; Ye, L.; Xie, J.; Lin, Y. Peroxisome Proliferator-Activated Receptor-γ: Master Regulator of Adipogenesis and Obesity. Curr. Stem Cell Res. Ther. 2016, 11, 282–289. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Hou, G.; Kang, J.; Wang, Q. Peroxisome Proliferator-Activated Receptor (PPARγ) Plays a Protective Role in Cigarette Smoking-Induced Inflammation via AMP-Activated Protein Kinase (AMPK) Signaling. Med. Sci. Monit. 2018, 24, 5168–5177. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Sung, Y.M.; Digiovanni, J.; Fischer, S.M. Thiazolidinediones Inhibit Insulin-like Growth Factor-i-Induced Activation of p70S6 Kinase and Suppress Insulin-like Growth Factor-I Tumor-Promoting Activity. Cancer Res. 2006, 66, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.; Hosick, P.A.; Chen, S.; Tukey, R.H.; Hankins, M.W.; Nestor-Kalinoski, A.; Stec, D.E.; Creeden, J.F.; Gordon, D.M.; Hipp, J.A.; et al. Mice with Hyperbilirubinemia Due to Gilbert’s Syndrome Polymorphism Are Resistant to Hepatic Steatosis by Decreased Serine 73 Phosphorylation of PPARα. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E244–E252. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hébrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-Activated Protein Kinase in the Regulation of Hepatic Energy Metabolism: From Physiology to Therapeutic Perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Hardie, D.G. AMPK: Sensing Glucose as Well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Stallone, G.; Infante, B.; Prisciandaro, C.; Grandaliano, G. mTOR and Aging: An Old Fashioned Dress. Int. J. Mol. Sci. 2019, 20, 2774. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Zelenka, J.; Dvořák, A.; Alán, L.; Zadinová, M.; Haluzík, M.; Vítek, L. Hyperbilirubinemia Protects against Aging-Associated Inflammation and Metabolic Deterioration. Oxid. Med. Cell Longev. 2016, 2016, 6190609. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F.; Shute, R.J.; Heesch, M.W.; Zak, R.B.; Kreiling, J.L.; Slivka, D.R.; Sun, S.; Li, H.; Chen, J.; et al. PGC-1alpha: A Key Regulator of Energy Metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Jiang, W. Sirtuins: Novel Targets for Metabolic Disease in Drug Development. Biochem. Biophys. Res. Commun. 2008, 373, 341–344. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an Energy Sensing Network That Controls Energy Expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Zhang, X.-L.; Ying, P.-J.; Wu, Z.-Q.; Lin, L.-L.; Chen, W.; Zheng, G.-Q.; Zhu, W.-Z. Neuroprotective Effect of Astragaloside IV on Cerebral Ischemia/Reperfusion Injury Rats Through Sirt1/Mapt Pathway. Front. Pharmacol. 2021, 12, 639898. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in Immunity and Inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R.; Shaefi, S.; Otterbein, L.E. HO-1 and CD39: It Takes Two to Protect the Realm. Front. Immunol. 2019, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Enjyoji, K.; Kotani, K.; Thukral, C.; Blumel, B.; Sun, X.; Wu, Y.; Imai, M.; Friedman, D.; Csizmadia, E.; Bleibel, W.; et al. Deletion of Cd39/Entpd1 Results in Hepatic Insulin Resistance. Diabetes 2008, 57, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.G.; Jarzyna, R.; Specht, A.; Kaczmarek, E. Extracellular Nucleotides and Adenosine Independently Activate AMP-Activated Protein Kinase in Endothelial Cells. Circ. Res. 2006, 98, e39–e47. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jia, J.; Zhang, D. Purinergic Signalling in Liver Diseases: Pathological Functions and Therapeutic Opportunities. JHEP Rep. 2020, 2, 100165. [Google Scholar] [CrossRef]
- Wang, S.; Gao, S.; Zhou, D.; Qian, X.; Luan, J.; Lv, X. The Role of the CD39-CD73-Adenosine Pathway in Liver Disease. J. Cell Physiol. 2021, 236, 851–862. [Google Scholar] [CrossRef]
- Andersson, C.; Weeke, P.; Fosbøl, E.L.; Brendorp, B.; Køber, L.; Coutinho, W.; Sharma, A.M.; Van Gaal, L.; Finer, N.; James, W.P.T.; et al. Acute Effect of Weight Loss on Levels of Total Bilirubin in Obese, Cardiovascular High-Risk Patients: An Analysis from the Lead-in Period of the Sibutramine Cardiovascular Outcome Trial. Metabolism 2009, 58, 1109–1115. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Y.; Wei, H.; Ning, H.; Bi, C.; Zhao, Y.; Qin, Y.; Li, Y. Acetyl-CoA Carboxylase (ACC) as a Therapeutic Target for Metabolic Syndrome and Recent Developments in ACC1/2 Inhibitors. Expert. Opin. Investig. Drugs 2019, 28, 917–930. [Google Scholar] [CrossRef]
- Ahmad, F.; Woodgett, J.R. Emerging Roles of GSK-3α in Pathophysiology: Emphasis on Cardio-Metabolic Disorders. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118616. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen Synthase Kinase-3 (GSK3): Regulation, Actions, and Diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Bösch, F.; Thomas, M.; Kogler, P.; Oberhuber, R.; Sucher, R.; Aigner, F.; Semsroth, S.; Wiedemann, D.; Yamashita, K.; Troppmair, J.; et al. Bilirubin Rinse of the Graft Ameliorates Ischemia Reperfusion Injury in Heart Transplantation. Transpl. Int. 2014, 27, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, W. P38 Mitogen-Activated Protein Kinase: A Critical Node Linking Insulin Resistance and Cardiovascular Diseases in Type 2 Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Šuk, J.; Jašprová, J.; Biedermann, D.; Petrásková, L.; Valentová, K.; Křen, V.; Muchová, L.; Vítek, L. Isolated Silymarin Flavonoids Increase Systemic and Hepatic Bilirubin Concentrations and Lower Lipoperoxidation in Mice. Oxidative Med. Cell. Longev. 2019, 2019, e6026902. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Gustafson, D.L.; Su, L.-J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glodé, L.M. A Phase I and Pharmacokinetic Study of Silybin-Phytosome in Prostate Cancer Patients. Investig. New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mariño, Z.; Crespo, G.; D’Amato, M.; Brambilla, N.; Giacovelli, G.; Rovati, L.; Costa, J.; Navasa, M.; Forns, X. Intravenous Silibinin Monotherapy Shows Significant Antiviral Activity in HCV-Infected Patients in the Peri-Transplantation Period. J. Hepatol. 2013, 58, 415–420. [Google Scholar] [CrossRef]
- Maher, P. The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3056. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant hSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef]
- Li, L.; Li, W.-J.; Zheng, X.-R.; Liu, Q.-L.; Du, Q.; Lai, Y.-J.; Liu, S.-Q. Eriodictyol Ameliorates Cognitive Dysfunction in APP/PS1 Mice by Inhibiting Ferroptosis via Vitamin D Receptor-Mediated Nrf2 Activation. Mol. Med. 2022, 28, 11. [Google Scholar] [CrossRef]
- Mhillaj, E.; Tarozzi, A.; Pruccoli, L.; Cuomo, V.; Trabace, L.; Mancuso, C. Curcumin and Heme Oxygenase: Neuroprotection and Beyond. Int. J. Mol. Sci. 2019, 20, 2419. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-on Therapy to Riluzole in Patients with Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Salazar, V.; Ruiz-Gabarre, D.; García-Escudero, V. Alzheimer’s Disease and Green Tea: Epigallocatechin-3-Gallate as a Modulator of Inflammation and Oxidative Stress. Antioxidants 2023, 12, 1460. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, R.; Mir, R.H.; Shah, A.J.; Sabreen, S.; Wani, T.U.; Masoodi, M.H.; Akkol, E.K.; Bhat, Z.A.; Khan, H. Plant-Derived Natural Compounds for the Treatment of Amyotrophic Lateral Sclerosis: An Update. Curr. Neuropharmacol. 2022, 20, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tian, X.; Guo, Y.; Duan, W.; Bu, H.; Li, C. Activation of Nuclear Factor Erythroid 2-Related Factor 2 Cytoprotective Signaling by Curcumin Protect Primary Spinal Cord Astrocytes against Oxidative Toxicity. Biol. Pharm. Bull. 2011, 34, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.-H.; Xu, Y.; Li, Y.-B.; Wu, H.-L.; Guo, H.; Zhang, J.-Z.; Zhang, J.-J.; Pan, X.-Y.; Li, X.-J. Curcumin Produces Neuroprotective Effects via Activating Brain-Derived Neurotrophic Factor/TrkB-Dependent MAPK and PI-3K Cascades in Rodent Cortical Neurons. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Xu, J.; Zhang, N.; Chen, J.; Wang, G.; Zhao, Y. Curcumin Induces Ferroptosis in Follicular Thyroid Cancer by Upregulating HO-1 Expression. Oxidative Med. Cell. Longev. 2023, 2023, e6896790. [Google Scholar] [CrossRef]
- Meng, P.; Yang, R.; Jiang, F.; Guo, J.; Lu, X.; Yang, T.; He, Q. Molecular Mechanism of Astragaloside IV in Improving Endothelial Dysfunction of Cardiovascular Diseases Mediated by Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, e1481236. [Google Scholar] [CrossRef]
- Huang, X.-P.; Qiu, Y.-Y.; Wang, B.; Ding, H.; Tang, Y.-H.; Zeng, R.; Deng, C.-Q. Effects of Astragaloside IV Combined with the Active Components of Panax Notoginseng on Oxidative Stress Injury and Nuclear Factor-Erythroid 2-Related Factor 2/Heme Oxygenase-1 Signaling Pathway after Cerebral Ischemia-Reperfusion in Mice. Pharmacogn. Mag. 2014, 10, 402–409. [Google Scholar] [CrossRef]
- Kontush, A.; Mann, U.; Arlt, S.; Ujeyl, A.; Lührs, C.; Müller-Thomsen, T.; Beisiegel, U. Influence of Vitamin E and C Supplementation on Lipoprotein Oxidation in Patients with Alzheimer’s Disease. Free Radic. Biol. Med. 2001, 31, 345–354. [Google Scholar] [CrossRef]
- Mudgal, R.; Sharma, S.; Singh, S.; Ravichandiran, V. The Neuroprotective Effect of Ascorbic Acid against Imidacloprid-Induced Neurotoxicity and the Role of HO-1 in Mice. Front. Neurol. 2023, 14, 1130575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, W.; Hu, Z.-J.; Ge, S.-M.; Huo, Y.; Liu, L.-X.; Gao, B.-L. Protective Effects and Mechanisms of High-Dose Vitamin C on Sepsis-Associated Cognitive Impairment in Rats. Sci. Rep. 2021, 11, 14511. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.M.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the Risk of Dementia and Alzheimer Disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Saad El-Din, S.; Rashed, L.; Medhat, E.; Emad Aboulhoda, B.; Desoky Badawy, A.; Mohammed ShamsEldeen, A.; Abdelgwad, M. Active Form of Vitamin D Analogue Mitigates Neurodegenerative Changes in Alzheimer’s Disease in Rats by Targeting Keap1/Nrf2 and MAPK-38p/ERK Signaling Pathways. Steroids 2020, 156, 108586. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, G.; Tang, H.; Pan, R.; Wang, H.; Jin, F.; Yan, X.; Xing, Y.; Chen, G.; Fu, Y.; et al. Madecassoside Ameliorates Lipopolysaccharide-Induced Neurotoxicity in Rats by Activating the Nrf2-HO-1 Pathway. Neurosci. Lett. 2019, 709, 134386. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.; Mahjoub, S.; Hajian-Tilaki, K.; Moghadasi, M. The Effect of Green Tea Consumption on Oxidative Stress Markers and Cognitive Function in Patients with Alzheimer’s Disease: A Prospective Intervention Study. Casp. J. Intern. Med. 2016, 7, 188–194. [Google Scholar]
- Na, H.-K.; Kim, E.-H.; Jung, J.-H.; Lee, H.-H.; Hyun, J.-W.; Surh, Y.-J. (−)-Epigallocatechin Gallate Induces Nrf2-Mediated Antioxidant Enzyme Expression via Activation of PI3K and ERK in Human Mammary Epithelial Cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar] [CrossRef]
- Zhou, H.; Mao, Z.; Zhang, X.; Li, R.; Yin, J.; Xu, Y. Neuroprotective Effect of Mangiferin against Parkinson’s Disease through G-Protein-Coupled Receptor-Interacting Protein 1 (GIT1)-Mediated Antioxidant Defense. ACS Chem. Neurosci. 2023, 14, 1379–1387. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and Cancer: Mechanisms of Action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef]
- Dillon, J.C.; Phuc, A.P.; Dubacq, J.P. Nutritional Value of the Alga Spirulina. World Rev. Nutr. Diet. 1995, 77, 32–46. [Google Scholar] [CrossRef]
- Gershwin, M.E.; Amha, B. (Eds.) Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Padyana, A.K.; Bhat, V.B.; Madyastha, K.M.; Rajashankar, K.R.; Ramakumar, S. Crystal Structure of a Light-Harvesting Protein C-Phycocyanin from Spirulina platensis. Biochem. Biophys. Res. Commun. 2001, 282, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.J.; Maines, M.D.; Lagarias, J.C. Inactivation of Phytochrome- and Phycobiliprotein-Chromophore Precursors by Rat Liver Biliverdin Reductase. J. Biol. Chem. 1993, 268, 26099–26106. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Clinical Potential of Spirulina as a Source of Phycocyanobilin. J. Med. Food 2007, 10, 566–570. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Hendler, S.S.; Rorvik, D.M.; Inoguchi, T. Compositions for Inhibiting NADPH Oxidase Activity. US20100172971A1, 8 July 2010. Available online: https://patents.google.com/patent/US20100172971A1/en (accessed on 15 November 2023).
- Li, Y. The Bioactivities of Phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, e4008991. [Google Scholar] [CrossRef] [PubMed]
- Strasky, Z.; Zemankova, L.; Nemeckova, I.; Rathouska, J.; Wong, R.J.; Muchova, L.; Subhanova, I.; Vanikova, J.; Vanova, K.; Vitek, L.; et al. Spirulina platensis and Phycocyanobilin Activate Atheroprotective Heme Oxygenase-1: A Possible Implication for Atherogenesis. Food Funct. 2013, 4, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and Phycocyanobilin from Spirulina platensis Protect against Diabetic Nephropathy by Inhibiting Oxidative Stress. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2013, 304, R110–R120. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.M.; Hikal, M.S.; Abo El-Khair, B.E.; El-Ghobashy, R.E.; El-Assar, A.M. Hypoglycemic and Hypolipidemic Effects of Spirulina platensis, Phycocyanin, Phycocyanopeptide and Phycocyanobilin on Male Diabetic Rats. Arab. Univ. J. Agric. Sci. 2018, 26 (Suppl. S2), 1121–1134. [Google Scholar] [CrossRef]
- Koníčková, R.; Vaňková, K.; Vaníková, J.; Vánová, K.; Muchová, L.; Subhanová, I.; Zadinová, M.; Zelenka, J.; Dvořák, A.; Kolář, M.; et al. Anti-Cancer Effects of Blue-Green Alga Spirulina platensis, a Natural Source of Bilirubin-like Tetrapyrrolic Compounds. Ann. Hepatol. 2014, 13, 273–283. [Google Scholar] [CrossRef]
- Hussein, N.; Ebied, S.; Saleh, M. Evaluation of the Anticancer Effect of Violacein, Phycocyanin and Phycocyanobilin on Apoptotic Genes Expression and Glycan Profiles in Breast Cancer Cells. Int. J. Cancer Biomed. Res. 2021, 5, 81–97. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.-Y.; Yu, L.-M.; Liu, B.; Li, M.-Y.; Zhu, R.-Z. Phycocyanobilin Accelerates Liver Regeneration and Reduces Mortality Rate in Carbon Tetrachloride-Induced Liver Injury Mice. World J. Gastroenterol. 2015, 21, 5465–5472. [Google Scholar] [CrossRef]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Marín-Prida, J.; Pavón-Fuentes, N.; Falcon-Cama, V.; Piniella-Matamoros, B.; Camacho-Rodríguez, H.; Fernández-Massó, J.R.; Valenzuela-Silva, C.; Raíces-Cruz, I.; et al. Beneficial Effects of Oral Administration of C-Phycocyanin and Phycocyanobilin in Rodent Models of Experimental Autoimmune Encephalomyelitis. Life Sci. 2018, 194, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Pavón-Fuentes, N.; Marín-Prida, J.; Llópiz-Arzuaga, A.; Falcón-Cama, V.; Campos-Mojena, R.; Cervantes-Llanos, M.; Piniella-Matamoros, B.; Pentón-Arias, E.; Pentón-Rol, G. Phycocyanobilin Reduces Brain Injury after Endothelin-1-Induced Focal Cerebral Ischaemia. Clin. Exp. Pharmacol. Physiol. 2020, 47, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; Falcón-Cama, V. C-Phycocyanin and Phycocyanobilin as Remyelination Therapies for Enhancing Recovery in Multiple Sclerosis and Ischemic Stroke: A Preclinical Perspective. Behav. Sci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. Oral Phycocyanobilin May Diminish the Pathogenicity of Activated Brain Microglia in Neurodegenerative Disorders. Med. Hypotheses 2010, 74, 601–605. [Google Scholar] [CrossRef]
- Chamorro, G.; Pérez-Albiter, M.; Serrano-García, N.; Mares-Sámano, J.J.; Rojas, P. Spirulina Maxima Pretreatment Partially Protects against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Neurotoxicity. Nutr. Neurosci. 2006, 9, 207–212. [Google Scholar] [CrossRef]
- Marín-Prida, J.; Pavón-Fuentes, N.; Lagumersindez-Denis, N.; Camacho-Rodríguez, H.; García-Soca, A.M.; Sarduy-Chávez, R.d.l.C.; Vieira, L.M.; Carvalho-Tavares, J.; Falcón-Cama, V.; Fernández-Massó, J.R.; et al. Anti-Inflammatory Mechanisms and Pharmacological Actions of Phycocyanobilin in a Mouse Model of Experimental Autoimmune Encephalomyelitis: A Therapeutic Promise for Multiple Sclerosis. Front. Immunol. 2022, 13, 1036200. [Google Scholar] [CrossRef]
- Gardón, D.P.; Cervantes-Llanos, M.; Matamoros, B.P.; Rodríguez, H.C.; Tan, C.-Y.; Marín-Prida, J.; Falcón-Cama, V.; Pavón-Fuentes, N.; Lemus, J.G.; Ruiz, L.d.l.C.B.; et al. Positive Effects of Phycocyanobilin on Gene Expression in Glutamate-Induced Excitotoxicity in SH-SY5Y Cells and Animal Models of Multiple Sclerosis and Cerebral Ischemia. Heliyon 2022, 8, e09769. [Google Scholar] [CrossRef]
- Pendyala, B.; Patras, A.; Dash, C. Phycobilins as Potent Food Bioactive Broad-Spectrum Inhibitors Against Proteases of SARS-CoV-2 and Other Coronaviruses: A Preliminary Study. Front. Microbiol. 2021, 12, 645713. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chang, G.-K.; Kuo, S.-M.; Huang, S.-Y.; Hu, I.-C.; Lo, Y.-L.; Shih, S.-R. Well-Tolerated Spirulina Extract Inhibits Influenza Virus Replication and Reduces Virus-Induced Mortality. Sci. Rep. 2016, 6, 24253. [Google Scholar] [CrossRef]
- Teas, J.; Hebert, J.R.; Fitton, J.H.; Zimba, P.V. Algae—A Poor Man’s HAART? Med. Hypotheses 2004, 62, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Matip, M.-E.; Pieme, C.A.; Azabji-Kenfack, M.; Moukette, B.M.; Korosky, E.; Stefanini, P.; Ngogang, J.Y.; Mbofung, C.M. Impact of Daily Supplementation of Spirulina platensis on the Immune System of Naïve HIV-1 Patients in Cameroon: A 12-Months Single Blind, Randomized, Multicenter Trial. Nutr. J. 2015, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Teas, J.; Irhimeh, M.R. Dietary Algae and HIV/AIDS: Proof of Concept Clinical Data. J. Appl. Phycol. 2012, 24, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Nihal, B.; Gupta, N.V.; Gowda, D.V.; Manohar, M. Formulation and Development of Topical Anti Acne Formulation of Spirulina Extract. Int. J. Appl. Pharm. 2018, 10, 229–233. [Google Scholar] [CrossRef]
- Mapoung, S.; Arjsri, P.; Thippraphan, P.; Semmarath, W.; Yodkeeree, S.; Chiewchanvit, S.; Piyamongkol, W.; Limtrakul, P. Photochemoprotective Effects of Spirulina platensis Extract against UVB Irradiated Human Skin Fibroblasts. South. Afr. J. Bot. 2020, 130, 198–207. [Google Scholar] [CrossRef]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Reportlinker. Global Market Study on Spirulina: Powder Product form Segment Anticipated to Dominate the Global Market in Terms of both Value and Volume during 2016–2026. Available online: https://www.prnewswire.com/news-releases/global-market-study-on-spirulina-powder-product-form-segment-anticipated-to-dominate-the-global-market-in-terms-of-both-value-and-volume-during-2016---2026-300443004.html (accessed on 15 November 2023).
- Qin, X. Bilirubin Would Be the Indispensable Component for Some of the Most Important Therapeutic Effects of Calculus Bovis (Niuhuang). Chin. Med. J. 2008, 121, 480. [Google Scholar] [CrossRef]
- Yu, Z.-J.; Xu, Y.; Peng, W.; Liu, Y.-J.; Zhang, J.-M.; Li, J.-S.; Sun, T.; Wang, P. Calculus Bovis: A Review of the Traditional Usages, Origin, Chemistry, Pharmacological Activities and Toxicology. J. Ethnopharmacol. 2020, 254, 112649. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Wang, Y.; Lin, K.; Zhang, Z.; Zhang, Z.; Zhu, P.; Ng, M.L.; Qu, S.; Sze, S.C.W.; et al. Protective Effect of An-Gong-Niu-Huang Wan Pre-Treatment Against Experimental Cerebral Ischemia Injury via Regulating GSK-3β/HO-1 Pathway. Front. Pharmacol. 2021, 12, 640297. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Wudtiwai, B.; Khawon, P. Induction of Human Hepatocellular Carcinoma HepG2 Cell Apoptosis by Naringin. Asian Pac. J. Cancer Prev. 2016, 17, 3289–3294. [Google Scholar]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Bachheti, R.; Husen, A. Medicinal Uses of Chlorophyll: A Critical Overview. In Chlorophyll: Structure, Production and Medicinal Uses; Nova Biomedical: Waltham, MA, USA, 2011; pp. 177–196. [Google Scholar]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, Absorption, and Cancer Preventative Activity of Dietary Chlorophyll Derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Vaňková, K.; Marková, I.; Jašprová, J.; Dvořák, A.; Subhanová, I.; Zelenka, J.; Novosádová, I.; Rasl, J.; Vomastek, T.; Sobotka, R.; et al. Chlorophyll-Mediated Changes in the Redox Status of Pancreatic Cancer Cells Are Associated with Its Anticancer Effects. Oxid. Med. Cell Longev. 2018, 2018, 4069167. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Vanella, L.; Salerno, L.; Di Giacomo, C.; Acquaviva, R.; Raffaele, M.; Romeo, G.; Modica, M.N.; Prezzavento, O.; Sorrenti, V. Novel Caffeic Acid Phenethyl Ester (Cape) Analogues as Inducers of Heme Oxygenase-1. Curr. Pharm. Des. 2017, 23, 2657–2664. [Google Scholar] [CrossRef]
- Šmíd, V.; Šuk, J.; Kachamakova-Trojanowska, N.; Jašprová, J.; Valášková, P.; Józkowicz, A.; Dulak, J.; Šmíd, F.; Vítek, L.; Muchová, L. Heme Oxygenase-1 May Affect Cell Signalling via Modulation of Ganglioside Composition. Oxid. Med. Cell Longev. 2018, 2018, 3845027. [Google Scholar] [CrossRef]
- Moon, S.; Kim, C.-H.; Park, J.; Kim, M.; Jeon, H.S.; Kim, Y.-M.; Choi, Y.K. Induction of BVR-A Expression by Korean Red Ginseng in Murine Hippocampal Astrocytes: Role of Bilirubin in Mitochondrial Function via the LKB1–SIRT1–ERRα Axis. Antioxidants 2022, 11, 1742. [Google Scholar] [CrossRef]
- Wu, L.-X.; Guo, C.-X.; Qu, Q.; Yu, J.; Chen, W.-Q.; Wang, G.; Fan, L.; Li, Q.; Zhang, W.; Zhou, H.-H. Effects of Natural Products on the Function of Human Organic Anion Transporting Polypeptide 1B1. Xenobiotica 2012, 42, 339–348. [Google Scholar] [CrossRef]
- Vítek, L.; Carey, M.C. Enterohepatic Cycling of Bilirubin as a Cause of “black” Pigment Gallstones in Adult Life. Eur. J. Clin. Investig. 2003, 33, 799–810. [Google Scholar] [CrossRef]
- Vítek, L.; Zelenka, J.; Zadinová, M.; Malina, J. The Impact of Intestinal Microflora on Serum Bilirubin Levels. J. Hepatol. 2005, 42, 238–243. [Google Scholar] [CrossRef]
- Hall, B.; Levy, S.; Dufault-Thompson, K.; Ndjite, G.M.; Weiss, A.; Braccia, D.; Jenkins, C.; Yang, Y.; Arp, G.; Abeysinghe, S.; et al. Discovery of the Gut Microbial Enzyme Responsible for Bilirubin Reduction to Urobilinogen. bioRxiv 2023, Preprint. [Google Scholar] [CrossRef]
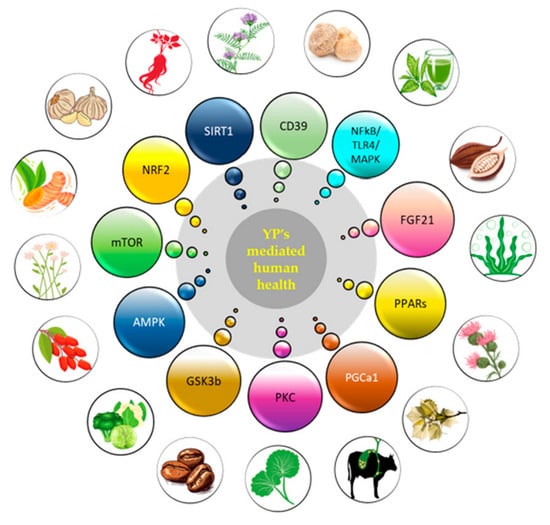
| Metabolic Checkpoint | Heme Catabolic Pathway Modulator | Natural Compound (Some Examples) | Possible Clinical Impact |
|---|---|---|---|
| NRF2 | NRF2 activates HMOX1 [29,49,50] Bilirubin activates NRF2 [51] | Sulphoraphan, curcumin, bixin, apigenin, cinnamaldehyde, withaferin A, luteolin, wogonin, chrysin… [23,24,25,26,27,28] | Regulator of cellular resistance to oxidants, inflammatory stimuli and toxic xenobiotics, modulator of longevity and cardiovascular and metabolic diseases. |
| HMOX1 | 20C (bibenzyl compound isolated from Gastrodia elvata) [47] | Suppresses the pro-apoptotic effect of Rot by inhibition of Bax and suppress the accumulation of intracellular ROS and the collapse of the mitochondrial membrane potential. | |
| HMOX1 | (ABPK) achyrantes bidentata polypeptide K [43] | Neuroprotective agent inhibiting the neuroinflammation on BV2 microglia cell culture. | |
| HMOX1 | Coriolus versicolor, Hericium erinaceus [48] | Anti-inflammatory modulating the lipoxin A4 levels (LXA4), resolving neuroinflammation and limiting the motor and non-motor symptoms, typical of PD. | |
| HMOX1 | Hyperoside (quercetin 3-O-galactoside) [52] | Protects cultured dopaminergic neurons from death via ROS-dependent mechanisms. | |
| HMOX1 | Berberine (BBR) [53] | Binds specific DNA sequences triggering DNA repair process. | |
| HMOX1 | Breviscapine [54] | HMOX1 and NQO1 increases. | |
| HMOX1 via PI3K/AKT | Acerogin A [55] | Prevent glutamate-induced oxidative damage. | |
| HMOX1 via EGFR/ERK | Astragaloside IV+/− Panax notoginseng [56,57] | Reduction of the oxidative stress markers, inhibition inflammatory mediators (NO, TNFα, IL6) and increase of SOD and GSH level. | |
| HMOX1 and NFkB/TLR4 signaling cascade | S-allyl cysteine (SAC) from aged garlic extract [58] | Improve cognitive deficits by attenuation of oxidative stress and neuroinflammation. | |
| HMOX1 | Mangiferin [59] | Protects neurons and glia from the oxidative damage by increasing HMOX1 in AD. | |
| HMOX1 | Luteolin [60] | Increases cells’ survival by preventing apoptosis and oxidative stress. | |
| - | Curcumin [61] | Inhibits the secretion of pro-neuroinflammatory mediators by increasing HMOX. | |
| - | Curcumin [62,63,64,65] | Protects neurons by ameliorating brain water content, oxidative stress, inflammation, and apoptosis, as well as reversal of depressive-like behaviors. | |
| - | Quercetin, anthocyanins, tea polyphenols, kaempferol, hesperetin, icariin, and various forms of terpenoids [28] | Protect from glutamate neurotoxicity and rescue of impaired cognitive function by increasing antioxidant responses, improving cell viability, and decreasing pro-inflammatory mediators. | |
| - | Curcumin [66] | Improves motor deficits and morphological alterations through antioxidant activity in an in vivo model of quinolinic acid neurotoxicity. | |
| NRF2 and PERK pathway | Curcumin [67] | Improves motor, sensory, reflex, and balance through inhibition of oxidative stress and apoptotic process. | |
| NFkB/TLR4 | NRF2 and NFkB | Curcumin [68] | Improves memory and behavior. |
| NFkB/STAT3/Ap-1 | Luteolin [60] | Reduces neuroinflammation induced by astrocytes. | |
| NFkB/MAPKs | NFkB/MAPK pathways | Curcumin [61] | Inhibits the secretion of pro-neuroinflammatory mediators by increasing Hmox. |
| NFkB | Ellagic acid [69] | Promotes anti-inflammatory and anti-antioxidant effects in AD and PD. | |
| PKC | PKC activates NRF2 | Curcumin [70] | Neurons are stimulated to increase antioxidant gene expression (GST-mu1, NQO1, and Hmox1), as well as p62, resulting in a positive feedback loop. |
| ERK | ERK modulate NRF2 anti-oxidant signaling | Curcumin [66] | Improve motor deficits and morphological alterations through antioxidant activity. |
| AKT2/NRF2 pathways | Kaempferol Ginsenoside rh2 [71] | Upregulation of the antioxidant enzyme thioredoxin linked to antidepressant mechanism. | |
| AKT/NRF2 | Curcumin [72] | Protects neurons and reduces infarct size in in vitro (oxygen and glucose deprivation/reoxygenation) and in vivo (middle cerebral artery occlusion) models of ischemic injury. | |
| NADPH oxidase | Bilirubin, biliverdin | C-phycocyanin (C- PC) [73] | Protective in many neurodegenerative diseases and in COVID-19-induced neurologic damage. |
| PPARs | Bilirubin [74] | Resveratrol [75,76] | Beneficiary effects on glucose and adipose tissue metabolism. |
| PGC1a | Bilirubin [77] | Resveratrol, quercetin, curcumin, saponins, epigallocatechin-3-gallate (EGCG) [37] | Regulation of cellular energy metabolism with beneficiary effects on civilization diseases. |
| CD39 | Bilirubin [78] CO [79] | Resveratrol [38] Curcumin [39] | Control of inflammatory processes via purinergic signaling. |
| FGF21 | Bilirubin [80,81] | Coffee phytochemicals (chlorogenic/protocatechuic acid) [82] Cocoa phytochemicals (theobromine/protocatechuic acid) [83] | Energy homeostasis, via FGF21 signaling, a late-acting fed and fasting-state hormone. |
| mTOR | Bilirubin [10,14] biliverdin [84] CO [85] | Curcumin, quercetin, apigenin [86] | Modulation of nutrient-sensing with impact on intermediary metabolism, aging processes, and overall life span. |
| SIRT1 | Bilirubin [87] | Resveratrol, butein, quercetin [88], astragaloside IV [40] | Control of fat and glucose metabolism, and energy expenditure. Anti-inflammatory and antioxidant. Reducing infarct size in ischemic stroke. |
| AMPK | Bilirubin [77] biliverdin [89] CO [85] | Resveratrol Berberine Quercetin [90] | Prevention of cardiovascular and metabolic diseases (T2DM), energy homeostasis. |
| GSK3b | BLVRA/bilirubin [91] | Resveratrol, curcumin, berberine [92] | Modulation of cellular kinase, with >100 known targets affecting lipid and glucose metabolism, and cell proliferation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayanti, S.; Vitek, L.; Verde, C.D.; Llido, J.P.; Sukowati, C.; Tiribelli, C.; Gazzin, S. Role of Natural Compounds Modulating Heme Catabolic Pathway in Gut, Liver, Cardiovascular, and Brain Diseases. Biomolecules 2024, 14, 63. https://doi.org/10.3390/biom14010063
Jayanti S, Vitek L, Verde CD, Llido JP, Sukowati C, Tiribelli C, Gazzin S. Role of Natural Compounds Modulating Heme Catabolic Pathway in Gut, Liver, Cardiovascular, and Brain Diseases. Biomolecules. 2024; 14(1):63. https://doi.org/10.3390/biom14010063
Chicago/Turabian StyleJayanti, Sri, Libor Vitek, Camilla Dalla Verde, John Paul Llido, Caecilia Sukowati, Claudio Tiribelli, and Silvia Gazzin. 2024. "Role of Natural Compounds Modulating Heme Catabolic Pathway in Gut, Liver, Cardiovascular, and Brain Diseases" Biomolecules 14, no. 1: 63. https://doi.org/10.3390/biom14010063
APA StyleJayanti, S., Vitek, L., Verde, C. D., Llido, J. P., Sukowati, C., Tiribelli, C., & Gazzin, S. (2024). Role of Natural Compounds Modulating Heme Catabolic Pathway in Gut, Liver, Cardiovascular, and Brain Diseases. Biomolecules, 14(1), 63. https://doi.org/10.3390/biom14010063