Effect of Topical Programmed Death-Ligand1 on Corneal Epithelium in Dry Eye Mouse
Abstract
:1. Introduction
2. Methods
2.1. Animal Model
2.2. Lacrimal Gland Excision
2.3. DED Mouse Model
2.4. Tear Volume Measurement
2.5. Corneal Epithelial Fluorescein Staining
2.6. Immunofluorescence Staining
2.7. TUNEL Assay
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
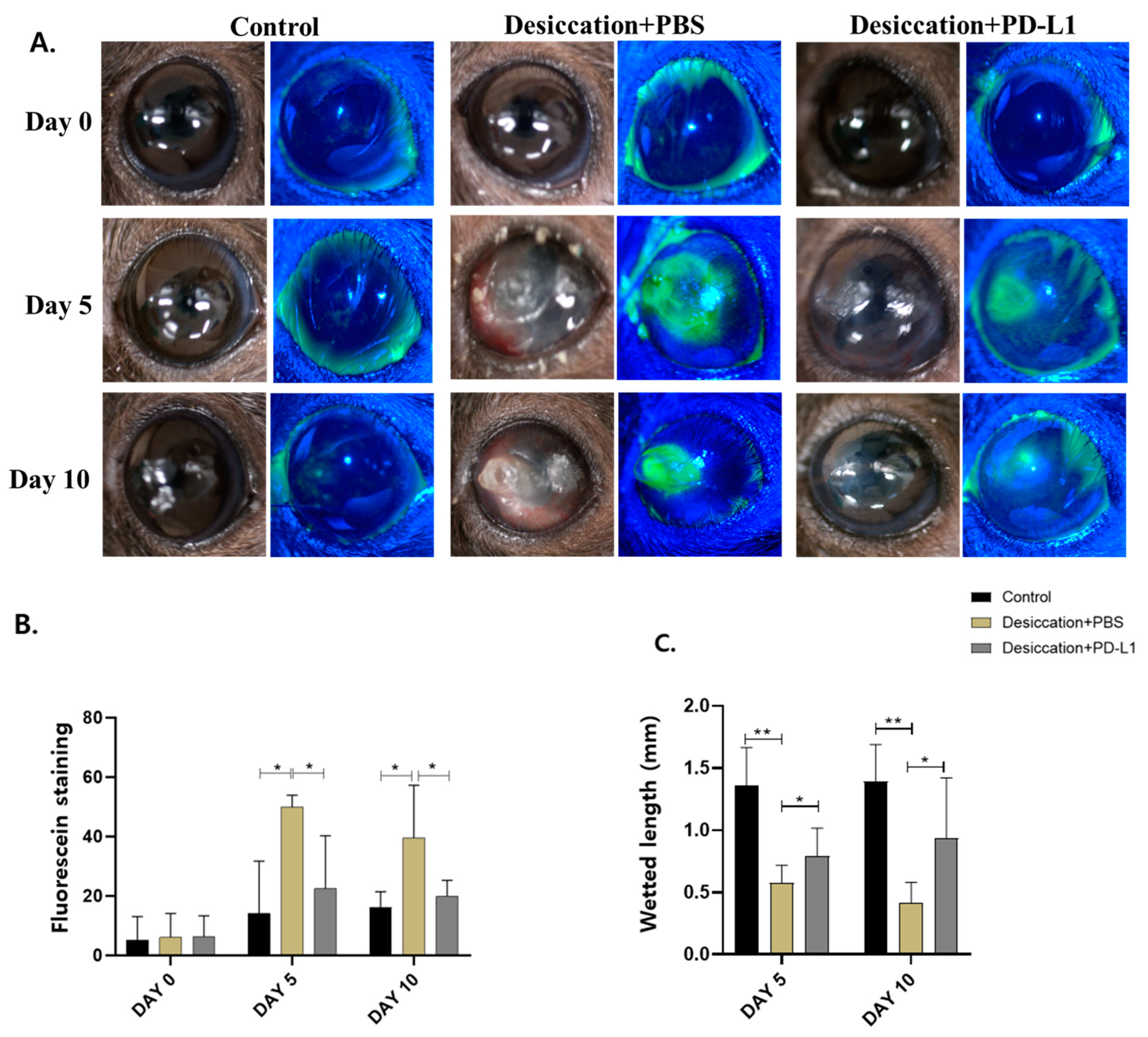
3.1. Effects of Topical PD-L1 on Corneal Epithelial Changes in Mice with Desiccation Stress-Induced DED
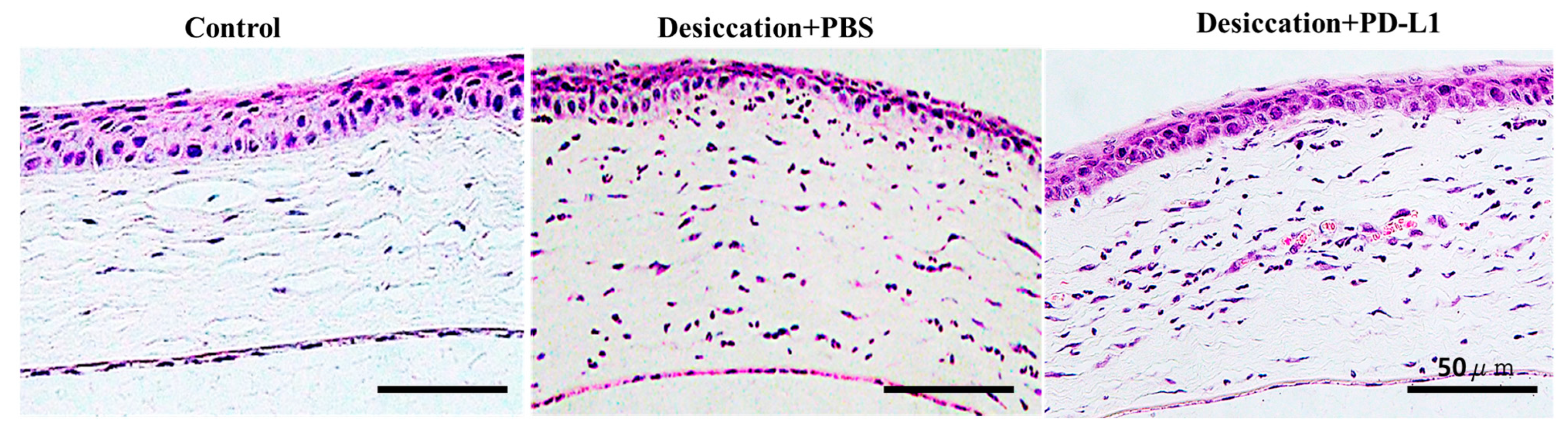
3.2. Effects of Topical PD-L1 on Corneal Histological Changes in Mice with Desiccation Stress-Induced DED
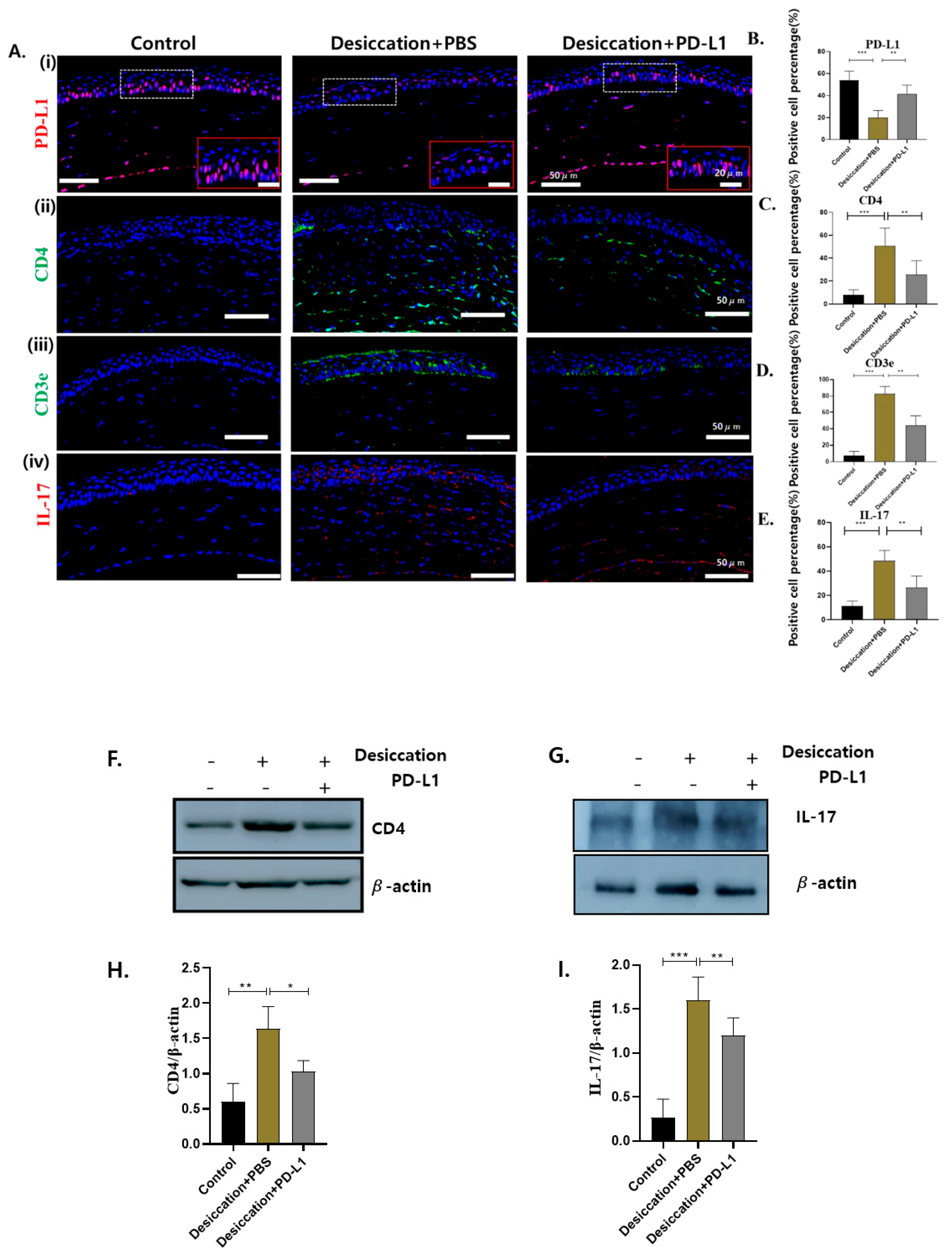
3.3. Expression of Corneal PD-L1 and T-Cell Markers in Mice with Desiccation Stress-Induced DED
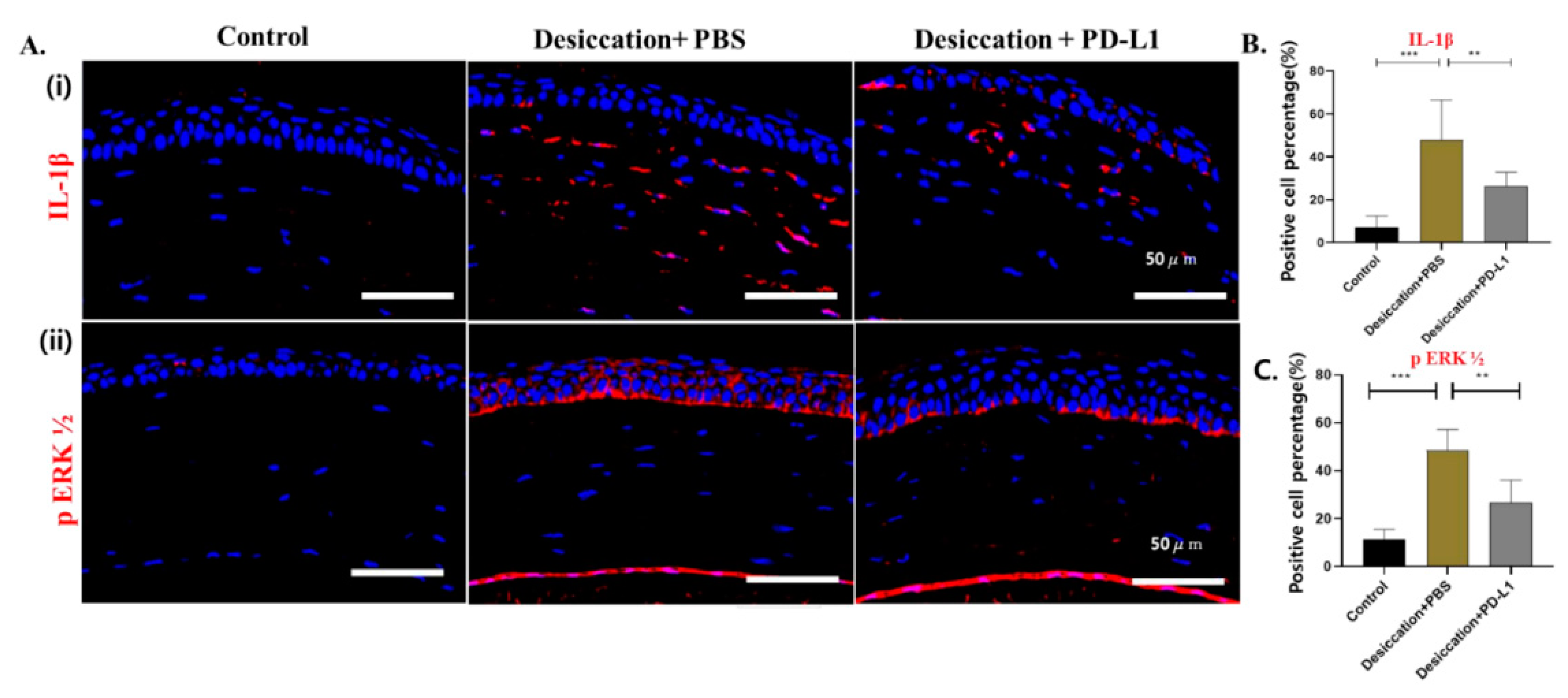
3.4. Effects of Topical PD-L1 on Corneal Inflammation in Mice with Desiccation Stress-Induced DED
3.5. Effects of Topical PD-L1 on Corneal Apoptosis in Mice with Desiccation Stress-Induced DED
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEWS II | Dry Eye Work Shop II |
| FDA | Food and Drug Administration |
| MMPs | Metalloproteinases |
| CECs | Corneal epithelial cells |
| DED | Dry eye disease |
| MGD | Meibomian gland dysfunction |
| PD-L1 | Programmed death-ligand 1 |
| MAPKs | Mitogen-activated protein kinases |
| ERK | Extracellular signal-regulated kinase |
| IL-17 | Interleukin-17 |
| IFN-γ | Interferon gamma |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT) deoxyuridine triphosphate (dUTP) nick-end labeling |
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; De Paiva, C.S.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.; Chotikavanich, S.; Pangelinan, S.; Pitcher Iii, J.; Fang, B.; Zheng, X.; Ma, P.; Farley, W.; Siemasko, K.; Niederkorn, J. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; El Annan, J.; Ecoiffier, T.; Goyal, S.; Zhang, Q.; Saban, D.R.; Dana, R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 2009, 182, 1247–1252. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Yang, Y.; Yuan, L.; Wang, P.; Zhao, H.; Ho, C.-T.; Lin, C.-C. Nobiletin and 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone ameliorate 12-O-tetradecanoylphorbol-13-acetate-Induced psoriasis-like mouse skin lesions by regulating the expression of Ki-67 and proliferating cell nuclear antigen and the differentiation of CD4+ T cells through mitogen-activated protein kinase signaling pathways. J. Agric. Food Chem. 2018, 66, 8299–8306. [Google Scholar]
- Chen, J.-Q.; Man, X.-Y.; Li, W.; Zhou, J.; Landeck, L.; Cai, S.-Q.; Zheng, M. Regulation of involucrin in psoriatic epidermal keratinocytes: The roles of ERK1/2 and GSK-3β. Cell Biochem. Biophys. 2013, 66, 523–528. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Hu, F.; Poorun, D.; Liu, X.; Wang, X.; Zhang, S.; Gan, L.; He, M.; Zhu, K. Resolvin D1 attenuates imiquimod-induced mice psoriasiform dermatitis through MAPKs and NF-κB pathways. J. Dermatol. Sci. 2018, 89, 127–135. [Google Scholar] [CrossRef]
- Duchnowska, R.; Pęksa, R.; Radecka, B.; Mandat, T.; Trojanowski, T.; Jarosz, B.; Czartoryska-Arłukowicz, B.; Olszewski, W.P.; Och, W.; Kalinka-Warzocha, E. Immune response in breast cancer brain metastases and their microenvironment: The role of the PD-1/PD-L axis. Breast Cancer Res. 2016, 18, 43. [Google Scholar] [CrossRef]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.A.; Xiao, N.; Overberg, P.; Rose, I.; Basu, G.D.; Vranic, S. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2965–2970. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Flies, D.B.; Van Deursen, J.M.; Chen, L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 2004, 20, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Kunishige, T.; Nakano, Y. Immune checkpoints contribute corneal immune privilege: Implications for dry eye associated with checkpoint inhibitors. Int. J. Mol. Sci. 2020, 21, 3962. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, M.; Cao, Y.; Zhang, Z.; Guo, F.; Li, X.; Zhang, Y. Exploring the role of programmed cell death protein 1 and its ligand 1 in eye diseases. Crit. Rev. Clin. Lab. Sci. 2019, 56, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Meng, Q.; Guo, H.; Wang, L.; Zhao, Z.; Zhang, L.; Kuang, J.; Cui, Y.; Mai, L.; Zhu, J. Programmed Death 1 (PD-1) is involved in the development of proliferative diabetic retinopathy by mediating activation-induced apoptosis. Mol. Vis. 2015, 21, 901. [Google Scholar]
- Meng, Q.; Yang, P.; Li, B.; Zhou, H.; Huang, X.; Zhu, L. Expression of programmed death 1 and its ligands in iris-ciliary body from mice with anterior chamber-associated immune deviation. Zhonghua Yan Ke Za Zhi Chin. J. Ophthalmol. 2008, 44, 558–562. [Google Scholar]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- El Annan, J.; Goyal, S.; Zhang, Q.; Freeman, G.J.; Sharpe, A.H.; Dana, R. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye–associated corneal inflammation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3418–3423. [Google Scholar] [CrossRef]
- Shen, L.; Jin, Y.; Freeman, G.J.; Sharpe, A.H.; Dana, M.R. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J. Immunol. 2007, 179, 3672–3679. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Chen, P.W.; Alizadeh, H.; He, Y.; Hogan, R.N.; Niederkorn, J.Y. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 273–280. [Google Scholar] [CrossRef]
- Hori, J.; Wang, M.; Miyashita, M.; Tanemoto, K.; Takahashi, H.; Takemori, T.; Okumura, K.; Yagita, H.; Azuma, M. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J. Immunol. 2006, 177, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Kezuka, T.; Usui, Y.; Okunuki, Y.; Takeuchi, M.; Maruyama, K.; Haneda, M.; Shirato, S.; Goto, H. Human iris pigment epithelial cells suppress T-cell activation via direct cell contact. Exp. Eye Res. 2009, 89, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Sun, D.; Jiang, G.; Kaplan, H.J.; Shao, H. PD-L1hi retinal pigment epithelium (RPE) cells elicited by inflammatory cytokines induce regulatory activity in uveitogenic T cells. J. Leukoc. Biol. 2010, 88, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.; Galor, A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: A review of the clinical evidence. Clin. Investig. 2015, 5, 267. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Kohler, N.G.; Joly, A. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-κB activation. FEBS Lett. 1997, 413, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Djalilian, A. Topical calcineurin inhibitors: Expanding indications for corneal and ocular surface inflammation. J. Ophthalmic Vis. Res. 2019, 14, 398. [Google Scholar] [CrossRef]
- Periman, L.M.; Mah, F.S.; Karpecki, P.M. A review of the mechanism of action of cyclosporine A: The role of cyclosporine A in dry eye disease and recent formulation developments. Clin. Ophthalmol. 2020, 14, 4187–4200. [Google Scholar] [CrossRef]
- Mecum, N.E.; Cyr, D.; Malon, J.; Demers, D.; Cao, L.; Meng, I.D. Evaluation of corneal damage after lacrimal gland excision in male and female mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3264–3274. [Google Scholar] [CrossRef]
- Kilic, S.; Kulualp, K. Tear Production Rate in a Mouse Model of Dry Eye According to the Phenol Red Thread and Endodontic Absorbent Paper Point Tear Tests. Comp. Med. 2016, 66, 367–372. [Google Scholar]
- Wang, H.-H.; Chen, W.-Y.; Huang, Y.-H.; Hsu, S.-M.; Tsao, Y.-P.; Hsu, Y.-H.; Chang, M.-S. Interleukin-20 is involved in dry eye disease and is a potential therapeutic target. J. Biomed. Sci. 2022, 29, 36. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wang, Y.; Reinach, P.S.; Ren, Y.; Li, J.; Hua, S.; Lu, H.; Chen, W. Dynamic ocular surface and lacrimal gland changes induced in experimental murine dry eye. PLoS ONE 2015, 10, e0115333. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3839. [Google Scholar]
- Zhang, R.; Park, M.; Richardson, A.; Tedla, N.; Pandzic, E.; de Paiva, C.S.; Watson, S.; Wakefield, D.; Di Girolamo, N. Dose-dependent benzalkonium chloride toxicity imparts ocular surface epithelial changes with features of dry eye disease. Ocul. Surf. 2020, 18, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Jiang, Y.; Zhou, J.; Zhang, J. Rebamipide ophthalmic solution modulates the ratio of T helper cell 17/regulatory T cells in dry eye disease mice. Mol. Med. Rep. 2019, 19, 4011–4018. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Gao, J.; Wang, L.; Hao, P.; Chen, X.; Wang, Y.; Jiang, Z.; Jiang, L.; Wang, T.; Zhu, L. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci. Rep. 2023, 13, 11192. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.T.; Xiao, Y.; Pflugfelder, S.C.; de Paiva, C.S. Inflammatory response to lipopolysaccharide on the ocular surface in a murine dry eye model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2443–2451. [Google Scholar] [CrossRef]
- Ma, B.; Pang, L.; Huang, P.; Bai, J.; Zhang, Z.; Wu, H.; Cai, M.; Yang, J.; Xu, Y.; Yin, X. Topical delivery of levocarnitine to the cornea and anterior eye by thermosensitive in-situ gel for dry eye disease. Drug Des. Dev. Ther. 2021, 15, 2357–2373. [Google Scholar] [CrossRef]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W. miR-204–containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef]
- Ansari, M.J.I.; Salama, A.D.; Chitnis, T.; Smith, R.N.; Yagita, H.; Akiba, H.; Yamazaki, T.; Azuma, M.; Iwai, H.; Khoury, S.J. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003, 198, 63–69. [Google Scholar] [CrossRef]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chauhan, S.K.; Soo Lee, H.; Saban, D.R.; Dana, R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014, 7, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, D.-Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Gulati, A.; Sacchetti, M.; Bonini, S.; Dana, R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch. Ophthalmol. 2006, 124, 710–716. [Google Scholar] [CrossRef]
- Hemady, R.; Chu, W.; Foster, C.S. Keratoconjunctivitis sicca and corneal ulcers. Cornea 1990, 9, 170–173. [Google Scholar] [CrossRef]
- McCulley, J.P.; Dougherty, J.M.; Deneau, D.G. Classification of chronic blepharitis. Ophthalmology 1982, 89, 1173–1180. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Farley, W.; Luo, L.; Chen, L.Z.; de Paiva, C.S.; Olmos, L.C.; Li, D.-Q.; Fini, M.E. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am. J. Pathol. 2005, 166, 61–71. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Y.; Chi, H. Regulation of TH17 cell differentiation by innate immune signals. Cell. Mol. Immunol. 2012, 9, 287–295. [Google Scholar] [CrossRef]
- Mele, F.; Basso, C.; Leoni, C.; Aschenbrenner, D.; Becattini, S.; Latorre, D.; Lanzavecchia, A.; Sallusto, F.; Monticelli, S. ERK phosphorylation and miR-181a expression modulate activation of human memory TH17 cells. Nat. Commun. 2015, 6, 6431. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Villarreal, A.L.; Corrales, R.M.; Rahman, H.T.; Chang, V.Y.; Farley, W.J.; Stern, M.E.; Niederkorn, J.Y.; Li, D.-Q.; Pflugfelder, S.C. Dry eye–induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2553–2560. [Google Scholar] [CrossRef]
- Stern, M.E.; Gao, J.; Schwalb, T.A.; Ngo, M.; Tieu, D.D.; Chan, C.-C.; Reis, B.L.; Whitcup, S.M.; Thompson, D.; Smith, J.A. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2609–2614. [Google Scholar]
- Pflugfelder, S.C.; de Paiva, C.S.; Li, D.-Q.; Stern, M.E. Epithelial–immune cell interaction in dry eye. Cornea 2008, 27, S9. [Google Scholar] [CrossRef]
- El Annan, J.; Chauhan, S.K.; Ecoiffier, T.; Zhang, Q.; Saban, D.R.; Dana, R. Characterization of effector T cells in dry eye disease. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3802–3807. [Google Scholar] [CrossRef]
- Li, B.; Sheng, M.; Li, J.; Yan, G.; Lin, A.; Li, M.; Wang, W.; Chen, Y. Tear proteomic analysis of Sjögren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci. Rep. 2014, 4, 5772. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gelber-Schwalb, T.A.; Addeo, J.V.; Stern, M.E. Apoptosis in the lacrimal gland and conjunctiva of dry eye dogs. Adv. Exp. Med. Biol. 1998, 438, 453–460. [Google Scholar]
- Zhang, X.; Chen, W.; De Paiva, C.S.; Volpe, E.A.; Gandhi, N.B.; Farley, W.J.; Li, D.-Q.; Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C. Desiccating stress induces CD4+ T-cell–mediated Sjögren’s syndrome–like corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway by interferon-γ. Am. J. Pathol. 2011, 179, 1807–1814. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.E.; Oh, S.; Bhujel, B.; Kim, C.M.; Lee, H.; Park, J.H.; Kim, J.Y. Effect of Topical Programmed Death-Ligand1 on Corneal Epithelium in Dry Eye Mouse. Biomolecules 2024, 14, 68. https://doi.org/10.3390/biom14010068
Lee KE, Oh S, Bhujel B, Kim CM, Lee H, Park JH, Kim JY. Effect of Topical Programmed Death-Ligand1 on Corneal Epithelium in Dry Eye Mouse. Biomolecules. 2024; 14(1):68. https://doi.org/10.3390/biom14010068
Chicago/Turabian StyleLee, Ko Eun, Seheon Oh, Basanta Bhujel, Chang Min Kim, Hun Lee, Jin Hyoung Park, and Jae Yong Kim. 2024. "Effect of Topical Programmed Death-Ligand1 on Corneal Epithelium in Dry Eye Mouse" Biomolecules 14, no. 1: 68. https://doi.org/10.3390/biom14010068
APA StyleLee, K. E., Oh, S., Bhujel, B., Kim, C. M., Lee, H., Park, J. H., & Kim, J. Y. (2024). Effect of Topical Programmed Death-Ligand1 on Corneal Epithelium in Dry Eye Mouse. Biomolecules, 14(1), 68. https://doi.org/10.3390/biom14010068






