Exploring PLGA-OH-CATH30 Microspheres for Oral Therapy of Escherichia coli-Induced Enteritis
Abstract
1. Introduction
2. Materials
2.1. Strains and Reagents
2.2. Mice
2.3. Modeling of Intestinal Bacterial Infection
2.4. Treatment Protocols and Sample Collection
2.5. Fecal Microbial Count
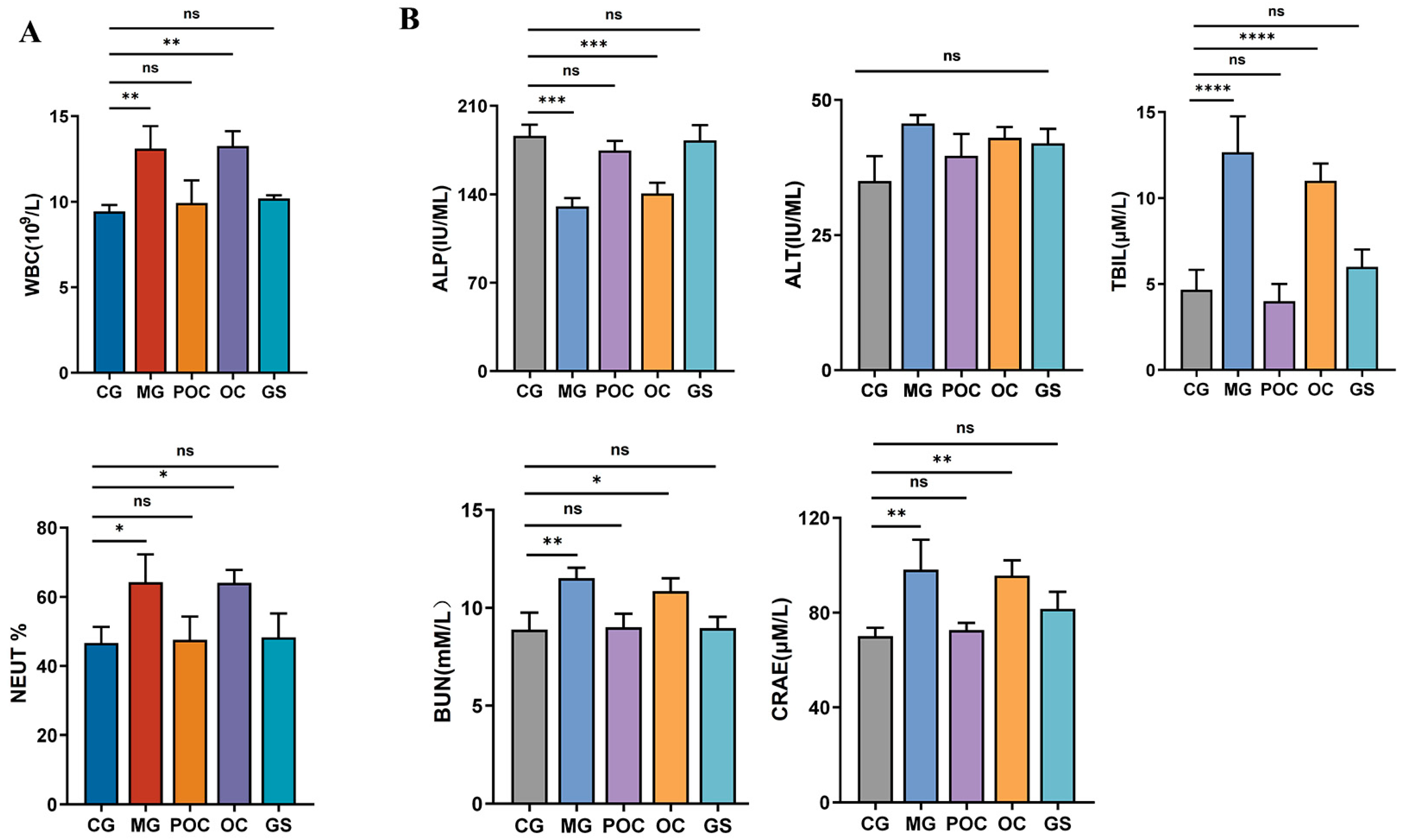
2.6. Blood Analysis of Mice
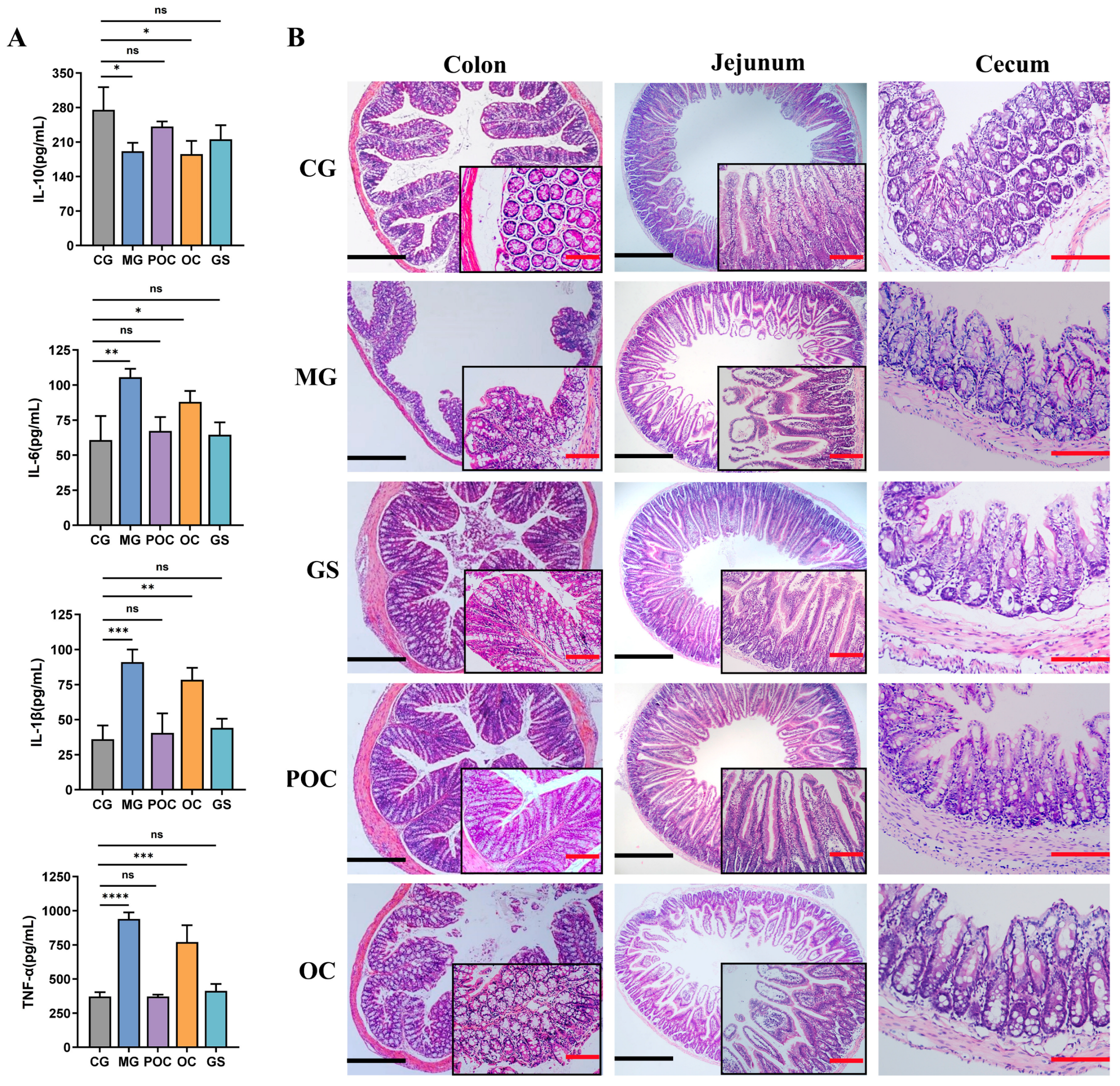
2.7. Histopathological Evaluation (HE)
2.8. Measurement of Cellular Inflammatory Factors
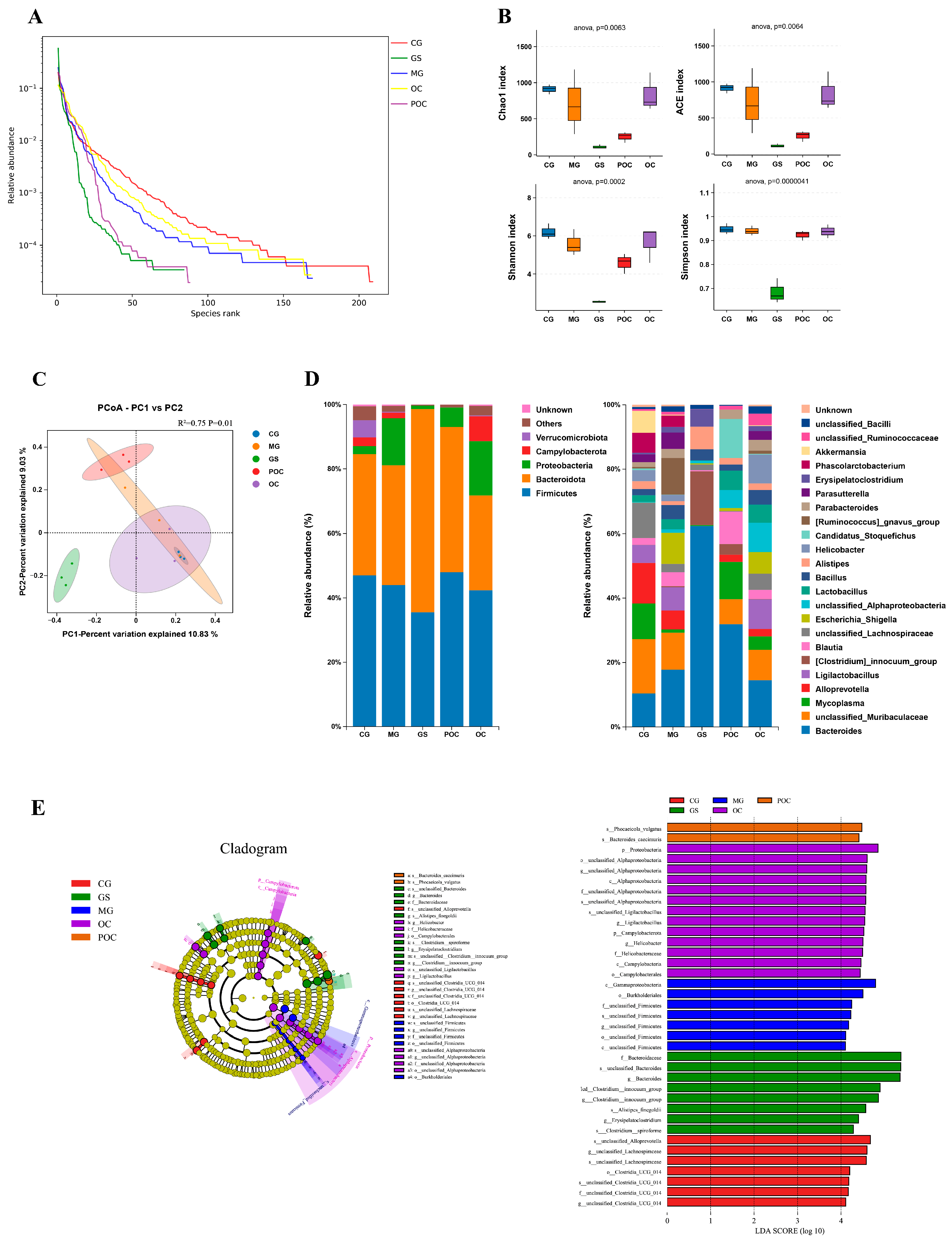
2.9. 16S rRNA Gene Sequencing and Analysis
2.10. Statistical Analyses
3. Results
3.1. Clinical Symptoms
3.2. Blood Analysis
3.3. Effect of MS on Inflammation and Intestinal Damage in Mice with Bacterial Enteritis
3.4. Effects of MS on Gut Microbiota Diversity and Community Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frazao, N.; Gordo, I. Shared Evolutionary Path in Social Microbiomes. Mol. Biol. Evol. 2023, 40, msad153. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Imran, A.; Malik, A.; Chaudhary, A.A.; Rub, A.; Jan, A.T.; Syed, J.B.; Rolfo, C. Bacterial imbalance and gut pathologies: Association and contribution of E. coli in inflammatory bowel disease. Crit. Rev. Clin. Lab. Sci. 2019, 56, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A.; Petersen, A.M. Escherichia coli Pathobionts Associated with Inflammatory Bowel Disease. Clin. Microbiol. Rev. 2019, 32, e00060-18. [Google Scholar] [CrossRef] [PubMed]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.; Bry, L.; Gordon, J.I.; Kahn, C.R. Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab. 2015, 22, 516–530. [Google Scholar] [CrossRef]
- Montgomery, T.L.; Künstner, A.; Kennedy, J.J.; Fang, Q.; Asarian, L.; Culp-Hill, R.; D’Alessandro, A.; Teuscher, C.; Busch, H.; Krementsov, D.N. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc. Natl. Acad. Sci. USA 2020, 117, 27516–27527. [Google Scholar] [CrossRef]
- Merchel Piovesan Pereira, B.; Wang, X.; Tagkopoulos, I. Biocide-Induced Emergence of Antibiotic Resistance in Escherichia coli. Front. Microbiol. 2021, 12, 640923. [Google Scholar] [CrossRef]
- Spellberg, B.; Hansen, G.R.; Kar, A.; Cordova, C.D.; Price, L.B.; Johnson, J.R. Antibiotic Resistance in Humans and Animals; National Academy of Medicine: Washington, DC, USA, 2016; pp. 1–15. [Google Scholar]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, C.; Xiao, N.; Tan, Z. Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC Microbiol. 2020, 20, 313. [Google Scholar] [CrossRef]
- Theuretzbacher, U. Evaluating the innovative potential of the global antibacterial pipeline. Clin. Microbiol. Infect. 2023, in press. [CrossRef]
- Nurhannaá Riduan, S. Antibiotic resistance mitigation: The development of alternative general strategies. J. Mater. Chem. B 2020, 8, 6317–6321. [Google Scholar] [CrossRef]
- Nayab, S.; Aslam, M.A.; Rahman, S.u.; Sindhu, Z.U.D.; Sajid, S.; Zafar, N.; Razaq, M.; Kanwar, R.; Amanullah. A review of antimicrobial peptides: Its function, mode of action and therapeutic potential. Int. J. Pept. Res. Ther. 2022, 28, 46. [Google Scholar] [CrossRef]
- Shafique, B.; Ranjha, M.M.A.N.; Murtaza, M.A.; Walayat, N.; Nawaz, A.; Khalid, W.; Mahmood, S.; Nadeem, M.; Manzoor, M.F.; Ameer, K. Recent trends and applications of nanoencapsulated bacteriocins against microbes in food quality and safety. Microorganisms 2022, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, S.A.; Xiang, Y.; Wang, Y.J.; Liu, J.; Lee, W.H.; Zhang, Y. Naturally occurring antimicrobial peptide OH-CATH30 selectively regulates the innate immune response to protect against sepsis. J. Med. Chem. 2013, 56, 9136–9145. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Lee, W.H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Guo, X.; Wang, Y.; Huang, X.; Guo, L.; Zou, P.; Ge, D.; Wang, X.; Lee, W.; Sun, T.; et al. PEGylated Graphene Oxide Carried OH-CATH30 to Accelerate the Healing of Infected Skin Wounds. Int. J. Nanomed. 2021, 16, 4769–4780. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Dong, X.; Shan, H.; Qin, Z. Assessing the Efficacy of PLGA-Loaded Antimicrobial Peptide OH-CATH30 Microspheres for the Treatment of Bacterial Keratitis: A Promising Approach. Biomolecules 2023, 13, 1244. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Zhu, C.; Xia, X.; Zhang, S.; Wang, Y.; Zhang, H.; Xu, Y.; Chen, S.; Jiang, J. The antimicrobial peptide mastoparan X protects against enterohemorrhagic Escherichia coli O157: H7 infection, inhibits inflammation, and enhances the intestinal epithelial barrier. Front. Microbiol. 2021, 12, 644887. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-h. Current progress of research on intestinal bacterial translocation. Microb. Pathog. 2021, 152, 104652. [Google Scholar] [CrossRef] [PubMed]
- Ljuca, F.; Gegic, A.; Salkic, N.N.; Pavlovic-Calic, N. Circulating cytokines reflect mucosal inflammatory status in patients with Crohn’s disease. Dig. Dis. Sci. 2010, 55, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Včev, A.; Meštrović, T.; Pustijanac, E.; Jukić, M.; Škrlec, I. Homeostasis and Dysbiosis of the Intestinal Microbiota: Comparing Hallmarks of a Healthy State with Changes in Inflammatory Bowel Disease. Microorganisms 2022, 10, 2405. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Lin, S.F.; Chen, S.T.; Chang, P.Y.; Yeh, Y.M.; Lo, F.S.; Lu, J.J. Alterations of Gut Microbiota in Patients with Graves’ Disease. Front. Cell. Infect. Microbiol. 2021, 11, 663131. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, C.; Arafath, M.N.; Apaire-Marchais, V.; Roger, E. Drug delivery systems for the oral administration of antimicrobial peptides: Promising tools to treat infectious diseases. Front. Med. Technol. 2022, 3, 778645. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J. Control Release 1998, 52, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, S.A.; Lee, W.H.; Zhang, Y. Efficacy of OH-CATH30 and its analogs against drug-resistant bacteria in vitro and in mouse models. Antimicrob. Agents Chemother. 2012, 56, 3309–3317. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yin, Z.; Li, L.; Chen, B.; Dai, H.; Wu, D.; Cong, J.; Ye, L.; Liao, C.; Li, L.; et al. Food antigens exacerbate intestinal damage and inflammation following the disruption of the mucosal barrier. Int. Immunopharmacol. 2021, 96, 107670. [Google Scholar] [CrossRef]
- Tóth, Š.; Fagová, Z.; Holodová, M.; Zeidan, D.; Hartel, P.; Čurgali, K.; Mechírová, E.; Maretta, M.; Nemcová, R.; Gancarčíková, S.; et al. Influence of Escherichia coli infection on intestinal mucosal barrier integrity of germ-free piglets. Life Sci. 2023, 331, 122036. [Google Scholar] [CrossRef]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shao, D.; Shi, J. Lactobacillus rhamnosus from human breast milk ameliorates ulcerative colitis in mice via gut microbiota modulation. Food Funct. 2021, 12, 5171–5186. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Wang, P.; Liu, Y.; Wang, G.; Shan, Y.; Yi, Y.; Zhou, Y.; Liu, B.; Wang, X.; et al. Lactobacillus coryniformis MXJ32 administration ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated colorectal cancer via reshaping intestinal microenvironment and alleviating inflammatory response. Eur. J. Nutr. 2022, 61, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N.; Richmond, A. The role of chemokines in intestinal inflammation and cancer. Curr. Opin. Pharmacol. 2009, 9, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Kolls, J.K.; Chen, H.; McAllister, F.; Oliver, P.D.; Zhang, Z. Chemokines orchestrate leukocyte trafficking in inflammatory bowel disease. Front. Biosci. 2008, 13, 1654–1664. [Google Scholar] [CrossRef]
- Castillo, P.; Kolls, J.K. IL-10: A Paradigm for Counterregulatory Cytokines. J. Immunol. 2016, 197, 1529–1530. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Schultz, B.M.; Nieto, P.A.; Salazar, G.A.; Suazo, I.; Gonzalez, P.A.; Riedel, C.A.; Alvarez-Lobos, M.M.; Kalergis, A.M.; Bueno, S.M. Opposing roles of IL-10 in acute bacterial infection. Cytokine Growth Factor Rev. 2016, 32, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Burcelin, R. Gut microbiota and immune crosstalk in metabolic disease. Mol. Metab. 2016, 5, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Kumar, P.; Mohan, H.; Goyal, S.; Sharma, K.K. Inflammatory bowel disease: Tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Stecher, B.; Maier, L.; Hardt, W.D. ‘Blooming’ in the gut: How dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 2013, 11, 277–284. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Wang, M.; Sun, P.; Li, Z.; Li, J.; Lv, X.; Chen, S.; Zhu, X.; Chai, X.; Zhao, S. Eucommiae cortex polysaccharides attenuate gut microbiota dysbiosis and neuroinflammation in mice exposed to chronic unpredictable mild stress: Beneficial in ameliorating depressive-like behaviors. J. Affect. Disord. 2023, 334, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, D.; Sun, H. Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. Biomed. Pharmacother. 2023, 161, 114409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, H.; Huang, H.; Xiao, Y.; Wu, X.; Li, M.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; et al. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. 2021, 12, 3142–3158. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.O.; Ridgway, K.; Scovell, L.; Kemsley, E.K.; Lund, E.K.; Jamieson, C.; Johnson, I.T.; Narbad, A. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Lück, R.; Deppenmeier, U. Genetic tools for the redirection of the central carbon flow towards the production of lactate in the human gut bacterium Phocaeicola (Bacteroides) vulgatus. Appl. Microbiol. Biotechnol. 2022, 106, 1211–1225. [Google Scholar] [CrossRef]
- Liu, X.; Guo, W.; Cui, S.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. A Comprehensive Assessment of the Safety of Blautia producta DSM 2950. Microorganisms 2021, 9, 908. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, X.; Liu, B.; Dong, X.; Wang, S.; Cai, X.; Zhang, H.; Qin, Z. Exploring PLGA-OH-CATH30 Microspheres for Oral Therapy of Escherichia coli-Induced Enteritis. Biomolecules 2024, 14, 86. https://doi.org/10.3390/biom14010086
Jiao X, Liu B, Dong X, Wang S, Cai X, Zhang H, Qin Z. Exploring PLGA-OH-CATH30 Microspheres for Oral Therapy of Escherichia coli-Induced Enteritis. Biomolecules. 2024; 14(1):86. https://doi.org/10.3390/biom14010086
Chicago/Turabian StyleJiao, Xiaoqian, Bin Liu, Xufeng Dong, Shubai Wang, Xiulei Cai, Hongliang Zhang, and Zhihua Qin. 2024. "Exploring PLGA-OH-CATH30 Microspheres for Oral Therapy of Escherichia coli-Induced Enteritis" Biomolecules 14, no. 1: 86. https://doi.org/10.3390/biom14010086
APA StyleJiao, X., Liu, B., Dong, X., Wang, S., Cai, X., Zhang, H., & Qin, Z. (2024). Exploring PLGA-OH-CATH30 Microspheres for Oral Therapy of Escherichia coli-Induced Enteritis. Biomolecules, 14(1), 86. https://doi.org/10.3390/biom14010086




