Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review
Abstract
:1. Introduction
2. Potential Immune Cells Impacted by Exercise
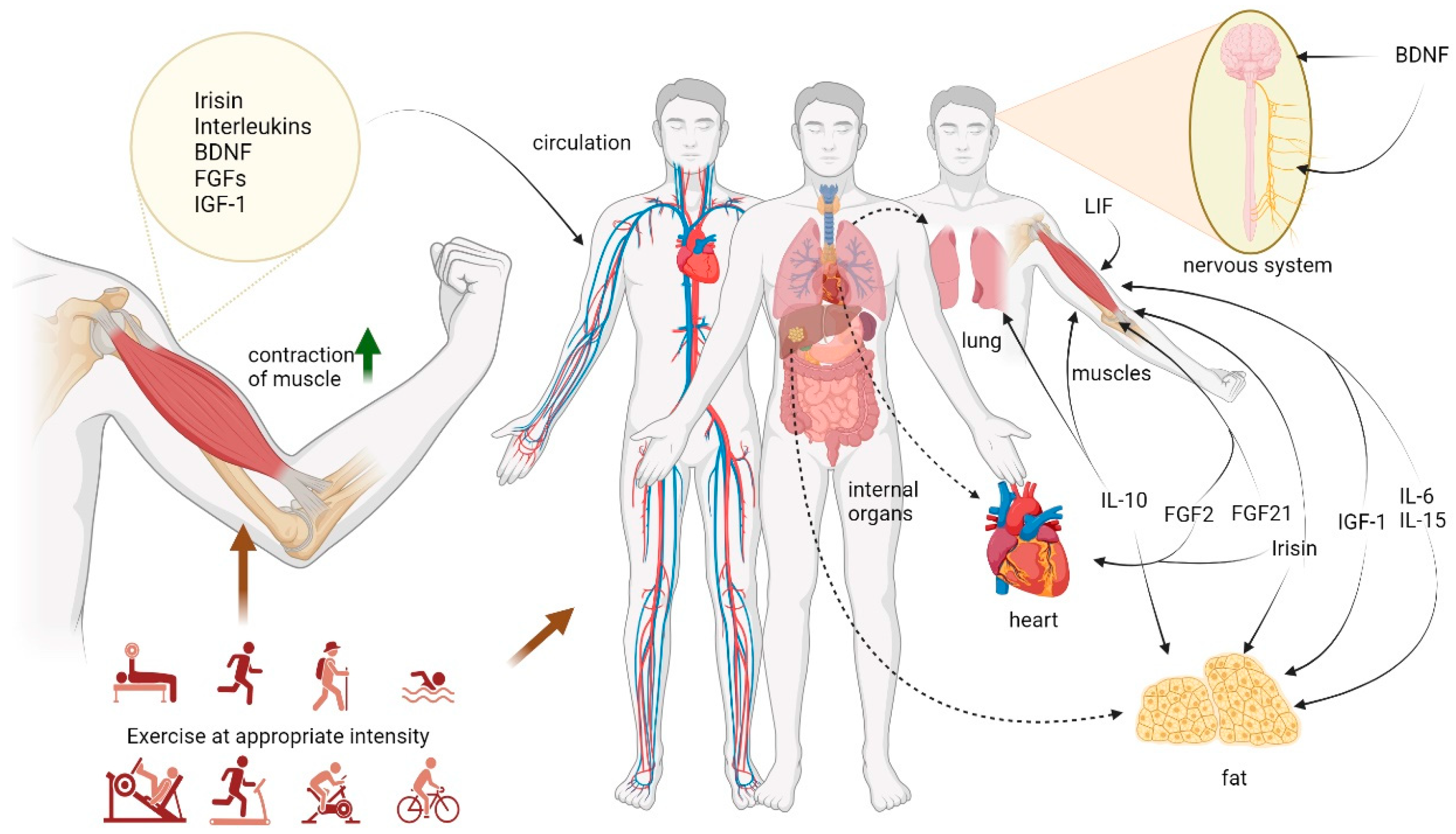
3. Exercise and Myokines
3.1. Irisin
3.2. Interleukins
3.3. Brain-Derived Neurotrophic Factor (BDNF)
3.4. Fibroblast Growth Factors (FGFs)
3.5. Insulin-like Growth Factor-1 (IGF-1)
4. Myokines and Immune Cells
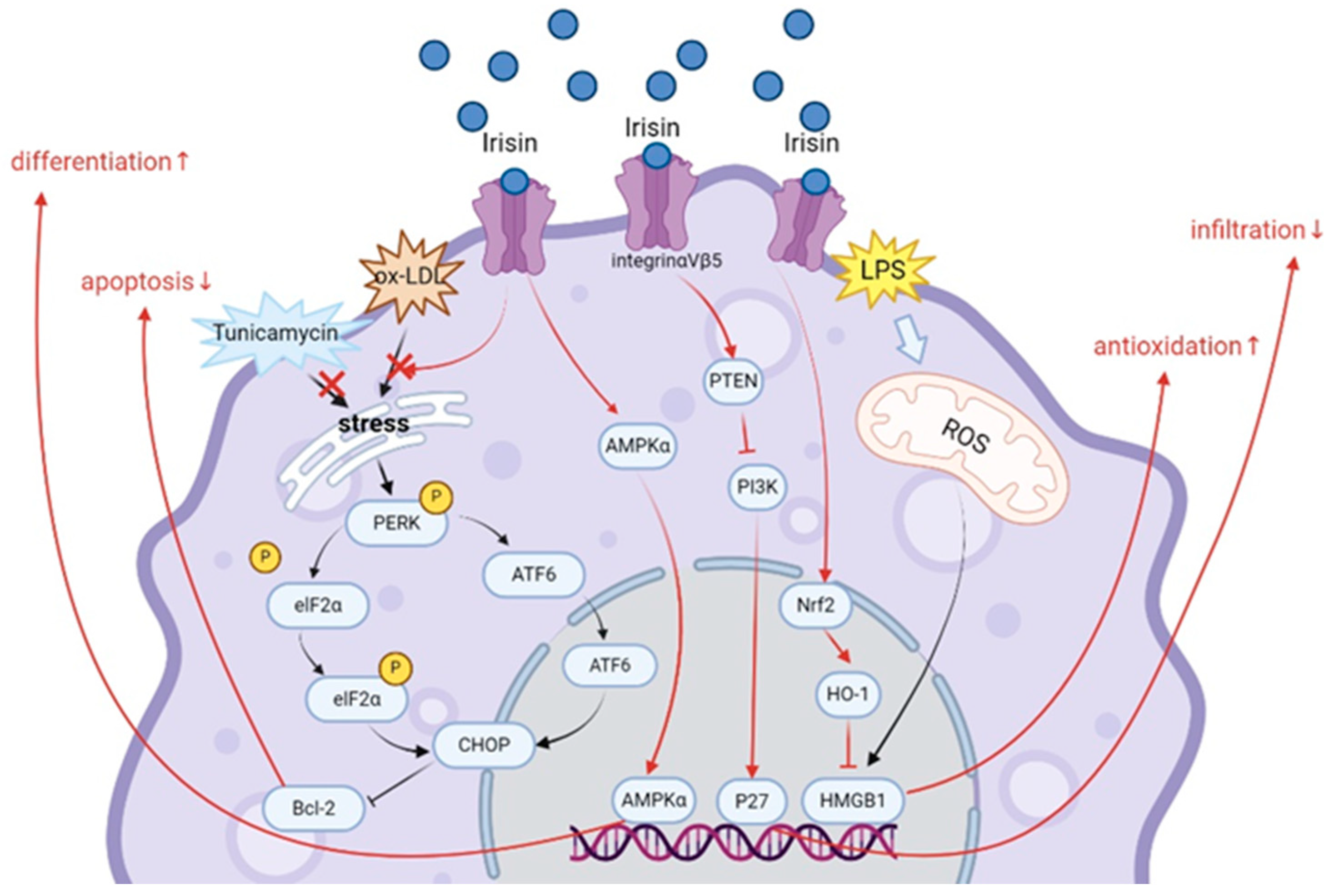
4.1. Irisin
4.2. Interleukins
4.2.1. IL-6 Family
4.2.2. IL-10
4.2.3. IL-15
4.3. BDNF
4.4. FGF Family
4.4.1. FGF2
4.4.2. FGF21
4.5. IGF-1
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Wang, J.; Liu, S.; Li, G.; Xiao, J. Exercise Regulates the Immune System. Adv. Exp. Med. Biol. 2020, 1228, 395–408. [Google Scholar] [PubMed]
- Crescioli, C. Vitamin D, exercise, and immune health in athletes: A narrative review. Front. Immunol. 2022, 13, 954994. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H. Exercise Therapy for People with Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front. Endocrinol. 2022, 13, 811751. [Google Scholar] [CrossRef]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Env. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar]
- Hojman, P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem. Soc. Trans. 2017, 45, 905–911. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Borkoles, E.; Polman, R.; Stojanovska, L. Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 2014, 6, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, G.; Rudensky, A.Y. Interactions between innate and adaptive lymphocytes. Nat. Rev. Immunol. 2014, 14, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 2016, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Murdoch, C.; Lewis, C.E. Macrophage migration and gene expression in response to tumor hypoxia. Int. J. Cancer 2005, 117, 701–708. [Google Scholar] [CrossRef]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Porta, C.; Riboldi, E.; Ippolito, A.; Sica, A. Molecular and epigenetic basis of macrophage polarized activation. Semin. Immunol. 2015, 27, 237–248. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011, 89, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; Muenchow, M.; Wallig, M.A.; Horn, P.L.; Woods, J.A. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J. Appl. Physiol. 2004, 96, 2249–2256. [Google Scholar] [CrossRef]
- Okutsu, M.; Suzuki, K.; Ishijima, T.; Peake, J.; Higuchi, M. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav. Immun. 2008, 22, 1066–1071. [Google Scholar] [CrossRef]
- Ortega, E.; Forner, M.A.; Barriga, C. Exercise-induced stimulation of murine macrophage chemotaxis: Role of corticosterone and prolactin as mediators. J. Physiol. 1997, 498 Pt 3, 729–734. [Google Scholar] [CrossRef]
- Woods, J.A.; Davis, J.M. Exercise, monocyte/macrophage function, and cancer. Med. Sci. Sports Exerc. 1994, 26, 147–156. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Myllymäki, T.; Tenhumäki, M.; Mutanen, N.; Puurtinen, R.; Paulsen, G.; Mero, A.A. Effects of resistance exercise and protein ingestion on blood leukocytes and platelets in young and older men. Eur. J. Appl. Physiol. 2010, 109, 343–353. [Google Scholar] [CrossRef]
- Grégoire, C.; Chasson, L.; Luci, C.; Tomasello, E.; Geissmann, F.; Vivier, E.; Walzer, T. The trafficking of natural killer cells. Immunol. Rev. 2007, 220, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8+ T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Imani, S.; Tao, L.; Deng, Y.; Cai, Y. Natural Killer Cells: Friend or Foe in Metabolic Diseases? Front. Immunol. 2021, 12, 614429. [Google Scholar] [CrossRef] [PubMed]
- Ramel, A.; Wagner, K.-H.; Elmadfa, I. Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J. Sports Sci. 2003, 21, 1001–1008. [Google Scholar] [CrossRef]
- Simonson, S.R.; Jackson, C.G.R. Leukocytosis occurs in response to resistance exercise in men. J. Strength. Cond. Res. 2004, 18, 266–271. [Google Scholar]
- Hussaarts, L.; Yazdanbakhsh, M.; Guigas, B. Priming dendritic cells for th2 polarization: Lessons learned from helminths and implications for metabolic disorders. Front. Immunol. 2014, 5, 499. [Google Scholar] [CrossRef]
- Chiang, L.M.; Chen, Y.J.; Chiang, J.; Lai, L.Y.; Chen, Y.Y.; Liao, H.F. Modulation of dendritic cells by endurance training. Int. J. Sports Med. 2007, 28, 798–803. [Google Scholar] [CrossRef]
- Bosteels, V.; Janssens, S. Striking a balance: New perspectives on homeostatic dendritic cell maturation. Nat. Rev. Immunol. 2024. [Google Scholar] [CrossRef]
- Eibel, H.; Kraus, H.; Sic, H.; Kienzler, A.-K.; Rizzi, M. B cell biology: An overview. Curr. Allergy Asthma Rep. 2014, 14, 434. [Google Scholar] [CrossRef]
- Dohi, K.; Mastro, A.M.; Miles, M.P.; Bush, J.A.; Grove, D.S.; Leach, S.K.; Volek, J.S.; Nindl, B.C.; Marx, J.O.; Gotshalk, L.A.; et al. Lymphocyte proliferation in response to acute heavy resistance exercise in women: Influence of muscle strength and total work. Eur. J. Appl. Physiol. 2001, 85, 367–373. [Google Scholar] [CrossRef]
- Miles, M.P.; Kraemer, W.J.; Nindl, B.C.; Grove, D.S.; Leach, S.K.; Dohi, K.; Marx, J.O.; Volek, J.S.; Mastro, A.M. Strength, workload, anaerobic intensity and the immune response to resistance exercise in women. Acta Physiol. Scand. 2003, 178, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.M.; Brenner, I.K.; Moldoveanu, A.I.; Vasiliou, P.; Shek, P.; Shephard, R.J. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med. J. Rev. Paul. De. Med. 2003, 121, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Szlezak, A.M.; Szlezak, S.L.; Keane, J.; Tajouri, L.; Minahan, C. Establishing a dose-response relationship between acute resistance-exercise and the immune system: Protocol for a systematic review. Immunol. Lett. 2016, 180, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. T cells and their immunometabolism: A novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur. J. Cell Biol. 2018, 97, 379–392. [Google Scholar] [CrossRef]
- Mathur, N.; Pedersen, B.K. Exercise as a mean to control low-grade systemic inflammation. Mediat. Inflamm. 2008, 2008, 109502. [Google Scholar] [CrossRef]
- Kawao, N.; Iemura, S.; Kawaguchi, M.; Mizukami, Y.; Takafuji, Y.; Kaji, H. Role of irisin in effects of chronic exercise on muscle and bone in ovariectomized mice. J. Bone Miner. Metab. 2021, 39, 547–557. [Google Scholar] [CrossRef]
- Tsourdi, E.; Anastasilakis, A.D.; Hofbauer, L.C.; Rauner, M.; Lademann, F. Irisin and Bone in Sickness and in Health: A Narrative Review of the Literature. J. Clin. Med. 2022, 11, 6863. [Google Scholar] [CrossRef]
- Arıkan, S.; Alaca, N.; Özbeyli, D.; Elmas, M.A.; Arbak, S.; Suyen, G. Effects of moderate aerobic exercise, low-level laser therapy, or their combination on muscles pathology, oxidative stress and irisin levels in the mdx mouse model of Duchenne muscular dystrophy. Lasers Med. Sci. 2022, 37, 2925–2936. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Kim, J.-C.; Kim, J.-S.; Kim, S.H. Effects of Swimming Exercise on Serum Irisin and Bone FNDC5 in Rat Models of High-Fat Diet-Induced Osteoporosis. J. Sports Sci. Med. 2019, 18, 596–603. [Google Scholar]
- Li, H.; Qin, S.; Liang, Q.; Xi, Y.; Bo, W.; Cai, M.; Tian, Z. Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice. Biomedicines 2021, 9, 701. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.J.; King, K.E.; Notley, S.R.; Fujii, N.; Boulay, P.; Sigal, R.J.; Kenny, G.P. Exercise in the heat induces similar elevations in serum irisin in young and older men despite lower resting irisin concentrations in older adults. J. Therm. Biol. 2022, 104, 103189. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin-mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; McFarlin, B.; Bois, C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int. J. Sports Med. 2003, 24, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Kim, K. Effects of Aerobic and Resistance Exercise on Myokines in High Fat Diet-Induced Middle-Aged Obese Rats. Int. J. Environ. Res. Public Health 2020, 17, 2685. [Google Scholar] [CrossRef]
- Jia, D.; Cai, M.; Xi, Y.; Du, S.; Tian, Z. Interval exercise training increases LIF expression and prevents myocardial infarction-induced skeletal muscle atrophy in rats. Life Sci. 2018, 193, 77–86. [Google Scholar] [CrossRef]
- Broholm, C.; Laye, M.J.; Brandt, C.; Vadalasetty, R.; Pilegaard, H.; Pedersen, B.K.; Scheele, C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 2011, 111, 251–259. [Google Scholar] [CrossRef]
- Saberi, S.; Askaripour, M.; Khaksari, M.; Amin Rajizadeh, M.; Abbas Bejeshk, M.; Akhbari, M.; Jafari, E.; Khoramipour, K. Exercise training improves diabetic renal injury by reducing fetuin-A, oxidative stress and inflammation in type 2 diabetic rats. Heliyon 2024, 10, e27749. [Google Scholar] [CrossRef]
- Lira, F.S.; Dos Santos, T.; Caldeira, R.S.; Inoue, D.S.; Panissa, V.L.G.; Cabral-Santos, C.; Campos, E.Z.; Rodrigues, B.; Monteiro, P.A. Short-Term High- and Moderate-Intensity Training Modifies Inflammatory and Metabolic Factors in Response to Acute Exercise. Front. Physiol. 2017, 8, 856. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.A.d.S.; Lira, F.S.; Pimentel, G.D.; Oliveira de Souza, C.; Batatinha, H.; Biondo, L.A.; Yamashita, A.S.; Junior, E.A.L.; Neto, J.C.R. Aerobic Exercise Modulates the Free Fatty Acids and Inflammatory Response During Obesity and Cancer Cachexia. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, A.; McKendry, J.; Martin-Rincon, M.; Morales-Alamo, D.; Pérez-Köhler, B.; Valadés, D.; Buján, J.; Calbet, J.A.L.; Breen, L. Skeletal muscle IL-15/IL-15Rα and myofibrillar protein synthesis after resistance exercise. Scand. J. Med. Sci. Sports 2018, 28, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Micielska, K.; Gmiat, A.; Zychowska, M.; Kozlowska, M.; Walentukiewicz, A.; Lysak-Radomska, A.; Jaworska, J.; Rodziewicz, E.; Duda-Biernacka, B.; Ziemann, E. The beneficial effects of 15 units of high-intensity circuit training in women is modified by age, baseline insulin resistance and physical capacity. Diabetes Res. Clin. Pract. 2019, 152, 156–165. [Google Scholar] [CrossRef]
- Wang, L.; Bian, X.; Liu, L.; He, Q.; Xu, J.; Chen, X.; Ye, H.; Yang, J.; Jiang, L. Association between cognitive function and skeletal muscle in patients undergoing maintenance hemodialysis. Front. Endocrinol. 2024, 15, 1324867. [Google Scholar] [CrossRef]
- Cheng, S.-M.; Lee, S.-D. Exercise Training Enhances BDNF/TrkB Signaling Pathway and Inhibits Apoptosis in Diabetic Cerebral Cortex. Int. J. Mol. Sci. 2022, 23, 6740. [Google Scholar] [CrossRef]
- Bastioli, G.; Arnold, J.C.; Mancini, M.; Mar, A.C.; Gamallo-Lana, B.; Saadipour, K.; Chao, M.V.; Rice, M.E. Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF. J. Neurosci. 2022, 42, 4725–4736. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef]
- Rahmani, F.; Saghazadeh, A.; Rahmani, M.; Teixeira, A.L.; Rezaei, N.; Aghamollaii, V.; Ardebili, H.E. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: A systematic review and meta-analysis. Brain Res. 2019, 1704, 127–136. [Google Scholar] [CrossRef]
- Eliakim, A.; Oh, Y.; Cooper, D.M. Effect of single wrist exercise on fibroblast growth factor-2, insulin-like growth factor, and growth hormone. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2000, 279, R548–R553. [Google Scholar] [CrossRef]
- Soori, R.; Amini, A.A.; Choobineh, S.; Eskandari, A.; Behjat, A.; Ghram, A.; Voltarelli, F.A. Exercise attenuates myocardial fibrosis and increases angiogenesis-related molecules in the myocardium of aged rats. Arch. Physiol. Biochem. 2022, 128, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, W.; Zeng, L.-Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.-Y.; Fang, C.-W.; Wang, F.; Qin, X.-J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Song, W. Resistance training increases fibroblast growth factor-21 and irisin levels in the skeletal muscle of Zucker diabetic fatty rats. J. Exerc. Nutr. Biochem. 2017, 21, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Alamdari, K.A.; Symonds, M.E.; Nobari, H.; Carlos-Vivas, J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Hormones 2021, 20, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ren, H.; Gao, S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: Roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010, 167, 344–351. [Google Scholar] [CrossRef]
- Matheny, R.W.; Merritt, E.; Zannikos, S.V.; Farrar, R.P.; Adamo, M.L. Serum IGF-I-deficiency does not prevent compensatory skeletal muscle hypertrophy in resistance exercise. Exp. Biol. Med. 2009, 234, 164–170. [Google Scholar] [CrossRef]
- Ning, K.; Wang, Z.; Zhang, X.-A. Exercise-induced modulation of myokine irisin in bone and cartilage tissue-Positive effects on osteoarthritis: A narrative review. Front. Aging Neurosci. 2022, 14, 934406. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Leger, C.; Quirié, A.; Méloux, A.; Fontanier, E.; Chaney, R.; Basset, C.; Lemaire, S.; Garnier, P.; Prigent-Tessier, A. Impact of Exercise Intensity on Cerebral BDNF Levels: Role of FNDC5/Irisin. Int. J. Mol. Sci. 2024, 25, 1213. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of Adipocyte-Fndc5/Irisin Expression and Secretion Reduces Thermogenesis and Enhances Adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Ding, D.; Ji, C.; Wu, Q.; Xia, Y.; Zhou, L.; Yang, L.; Ba, G.; Chang, Q.; Fu, Q.; et al. Relationships Between Circulating Irisin Response to Ice Swimming and Body Composition in People with Regular Exercise Experience. Front. Physiol. 2020, 11, 596896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The Role of Interleukins in Colorectal Cancer. Int. J. Biol. Sci. 2020, 16, 2323–2339. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Northoff, H.; Berg, A. Immunologic mediators as parameters of the reaction to strenuous exercise. Int. J. Sports Med. 1991, 12 (Suppl. S1), S9–S15. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508 Pt 3, 949–953. [Google Scholar] [CrossRef]
- Steensberg, A.; Keller, C.; Starkie, R.L.; Osada, T.; Febbraio, M.A.; Pedersen, B.K. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1272–E1278. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.L.; Farnfield, M.M.; Garnham, A.P.; Caldow, M.K.; Cameron-Smith, D.; Peake, J.M. Early inflammatory and myogenic responses to resistance exercise in the elderly. Muscle Nerve 2012, 46, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Mancilla, J.; Endres, S.; Ghorbani, R.; Clark, S.C.; Dinarello, C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, H.S.; Kim, K.-S.; Choi, W.H.; Ahn, C.W.; Kim, B.T.; Kim, S.M.; Lee, S.Y.; Ahn, S.M.; Kim, Y.K.; et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008, 19, 371–375. [Google Scholar] [CrossRef]
- Oliver, J.M.; Jenke, S.C.; Mata, J.D.; Kreutzer, A.; Jones, M.T. Acute Effect of Cluster and Traditional Set Configurations on Myokines Associated with Hypertrophy. Int. J. Sports Med. 2016, 37, 1019–1024. [Google Scholar] [CrossRef]
- Park, K.M.; Park, S.C.; Kang, S. Effects of resistance exercise on adipokine factors and body composition in pre- and postmenopausal women. J. Exerc. Rehabil. 2019, 15, 676–682. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Foltran, R.B.; Diaz, S.L. BDNF isoforms: A round trip ticket between neurogenesis and serotonin? J. Neurochem. 2016, 138, 204–221. [Google Scholar] [CrossRef]
- Tsimpolis, A.; Kalafatakis, K.; Charalampopoulos, I. Recent advances in the crosstalk between the brain-derived neurotrophic factor and glucocorticoids. Front. Endocrinol. 2024, 15, 1362573. [Google Scholar] [CrossRef]
- Sakuma, K.; Watanabe, K.; Sano, M.; Uramoto, I.; Nakano, H.; Li, Y.J.; Kaneda, S.; Sorimachi, Y.; Yoshimoto, K.; Yasuhara, M.; et al. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res. 2001, 907, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Yan, J.; Cheng, F.; Ma, X.; Chen, C.; Wang, W.; Wang, Q. Cholic Acid Protects In Vitro Neurovascular Units against Oxygen and Glucose Deprivation-Induced Injury through the BDNF-TrkB Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 1201624. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Sartorius, A.; Hellweg, R.; Litzke, J.; Vogt, M.; Dormann, C.; Vollmayr, B.; Danker-Hopfe, H.; Gass, P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 2009, 42, 270–276. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Edman, S.; Horwath, O.; Van der Stede, T.; Blackwood, S.J.; Moberg, I.; Strömlind, H.; Nordström, F.; Ekblom, M.; Katz, A.; Apró, W.; et al. Pro-Brain-Derived Neurotrophic Factor (BDNF), but Not Mature BDNF, Is Expressed in Human Skeletal Muscle: Implications for Exercise-Induced Neuroplasticity. Function 2024, 5, zqae005. [Google Scholar] [CrossRef]
- Håkansson, K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.-C.; Mohammed, A.K.H. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J. Alzheimer’s Dis. 2017, 55, 645–657. [Google Scholar] [CrossRef]
- Azevedo, L.V.D.S.; Pereira, J.R.; Silva Santos, R.M.; Rocha, N.P.; Teixeira, A.L.; Christo, P.P.; Santos, V.R.; Scalzo, P.L. Acute exercise increases BDNF serum levels in patients with Parkinson’s disease regardless of depression or fatigue. Eur. J. Sport. Sci. 2022, 22, 1296–1303. [Google Scholar] [CrossRef]
- Wagner, G.; Herbsleb, M.; de la Cruz, F.; Schumann, A.; Brünner, F.; Schachtzabel, C.; Gussew, A.; Puta, C.; Smesny, S.; Gabriel, H.W.; et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: A controlled trial. J. Cereb. Blood Flow. Metab. 2015, 35, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef] [PubMed]
- Oulion, S.; Bertrand, S.; Escriva, H. Evolution of the FGF Gene Family. Int. J. Evol. Biol. 2012, 2012, 298147. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McNeil, P.L.; Patterson, S.L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64–70. [Google Scholar] [PubMed]
- Brenner, D.R.; Ruan, Y.; Adams, S.C.; Courneya, K.S.; Friedenreich, C.M. The impact of exercise on growth factors (VEGF and FGF2): Results from a 12-month randomized intervention trial. Eur. Rev. Aging Phys. Act. 2019, 16, 8. [Google Scholar] [CrossRef]
- Romanello, V. The Interplay between Mitochondrial Morphology and Myomitokines in Aging Sarcopenia. Int. J. Mol. Sci. 2020, 22, 91. [Google Scholar] [CrossRef]
- Garneau, L.; Aguer, C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef]
- Ji, M.; Cho, C.; Lee, S. Acute effect of exercise intensity on circulating FGF-21, FSTL-1, cathepsin B, and BDNF in young men. J. Exerc. Sci. Fit. 2024, 22, 51–58. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Higuchi, M. Endurance Exercise Reduces Hepatic Fat Content and Serum Fibroblast Growth Factor 21 Levels in Elderly Men. J. Clin. Endocrinol. Metab. 2016, 101, 191–198. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chung, Y.-C.; Chen, Y.-J.; Ho, S.-Y.; Wu, H.-J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef]
- Bozinovski, S.; Seow, H.J.; Crack, P.J.; Anderson, G.P.; Vlahos, R. Glutathione peroxidase-1 primes pro-inflammatory cytokine production after LPS challenge in vivo. PLoS ONE 2012, 7, e33172. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I. Irisin acts as a regulator of macrophages host defense. Life Sci. 2017, 176, 21–25. [Google Scholar] [CrossRef]
- Wang, J.-S.; Ho, F.-M.; Kang, H.-C.; Lin, W.-W.; Huang, K.-C. Celecoxib induces heme oxygenase-1 expression in macrophages and vascular smooth muscle cells via ROS-dependent signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 159–168. [Google Scholar] [CrossRef]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Thirupathi, A.; Gu, Y.; Pinho, R.A. Exercise Cuts Both Ways with ROS in Remodifying Innate and Adaptive Responses: Rewiring the Redox Mechanism of the Immune System during Exercise. Antioxidants 2021, 10, 1846. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J. Physiol. Pharmacol. 2018, 69, 117–125. [Google Scholar]
- Mazur-Bialy, A.I.; Pocheć, E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxidants 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Lin, D.; Ding, Z. The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int. J. Biol. Macromol. 2020, 146, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, H.; Zhang, T.; Yang, L.; Yao, S.; Chen, S.; Zheng, M.; Zhao, Q.; Tian, H. Irisin protects macrophages from oxidized low density lipoprotein-induced apoptosis by inhibiting the endoplasmic reticulum stress pathway. Saudi J. Biol. Sci. 2018, 25, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, J.; Wang, C.; Shao, J.; Lai, Z.; Yu, X.; Du, F.; Gao, R.; Wang, J.; Liu, B. Irisin ameliorates nicotine-mediated atherosclerosis via inhibition of the PI3K pathway. Ann. Transl. Med. 2021, 9, 805. [Google Scholar] [CrossRef]
- Bustamante, M.; Fernández-Verdejo, R.; Jaimovich, E.; Buvinic, S. Electrical stimulation induces IL-6 in skeletal muscle through extracellular ATP by activating Ca(2+) signals and an IL-6 autocrine loop. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E869–E882. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem. Biophys. Rep. 2016, 5, 146–151. [Google Scholar] [CrossRef]
- Tominaga, T.; Ma, S.; Saitou, K.; Suzuki, K. Glucose Ingestion Inhibits Endurance Exercise-Induced IL-6 Producing Macrophage Infiltration in Mice Muscle. Nutrients 2019, 11, 1496. [Google Scholar] [CrossRef]
- Carson, J.A.; Baltgalvis, K.A. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc. Sport. Sci. Rev. 2010, 38, 168–176. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334 Pt 2, 297–314. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Peng, L.; Zhong, J.; Xiao, Y.; Wang, B.; Li, S.; Deng, Y.; He, D.; Yuan, J. Therapeutic effects of an anti-IL-6 antibody in fungal keratitis: Macrophage inhibition and T cell subset regulation. Int. Immunopharmacol. 2020, 85, 106649. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, P.; Wang, P.; Wang, W.; Liu, J. IL-6/ERK signaling pathway participates in type I IFN-programmed, unconventional M2-like macrophage polarization. Sci. Rep. 2023, 13, 1827. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chang, P.-Y.; Chen, J.-Y.; Wu, B.-S.; Yang, A.-H.; Lee, O.K.-S. Adipose-derived mesenchymal stem cells attenuate dialysis-induced peritoneal fibrosis by modulating macrophage polarization via interleukin-6. Stem Cell Res. Ther. 2021, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, C.; Cheng, Y.; Wang, J.; Zhang, S.; Yan, S.; He, F.; Yin, T.; Yang, J. Trophoblast-derived IL-6 serves as an important factor for normal pregnancy by activating Stat3-mediated M2 macrophages polarization. Int. Immunopharmacol. 2021, 90, 106788. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Xu, W.; Liu, Y.; Peng, Y.; Xu, W.; Yin, Y.; Zhang, X. IL-6 Prevents Lung Macrophage Death and Lung Inflammation Injury by Inhibiting GSDME- and GSDMD-Mediated Pyroptosis during Pneumococcal Pneumosepsis. Microbiol. Spectr. 2022, 10, e0204921. [Google Scholar] [CrossRef] [PubMed]
- Valença-Pereira, F.; Fang, Q.; Marié, I.J.; Giddings, E.L.; Fortner, K.A.; Yang, R.; Villarino, A.V.; Huang, Y.H.; Frank, D.A.; Wen, H.; et al. IL-6 enhances CD4 cell motility by sustaining mitochondrial Ca2+ through the noncanonical STAT3 pathway. Proc. Natl. Acad. Sci. USA 2021, 118, e2103444118. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jones, L.L.; Geiger, T.L. IL-6 Promotes T Cell Proliferation and Expansion under Inflammatory Conditions in Association with Low-Level RORγt Expression. J. Immunol. 2018, 201, 2934–2946. [Google Scholar] [CrossRef] [PubMed]
- Hop, H.T.; Huy, T.X.N.; Reyes, A.W.B.; Arayan, L.T.; Vu, S.H.; Min, W.; Lee, H.J.; Kang, C.K.; Kim, D.H.; Tark, D.S.; et al. Interleukin 6 Promotes Brucella abortus Clearance by Controlling Bactericidal Activity of Macrophages and CD8+ T Cell Differentiation. Infect. Immun. 2019, 87, e00431-19. [Google Scholar] [CrossRef]
- Liu, K.; He, K.; Xue, T.; Liu, P.; Xu, L.X. The cryo-thermal therapy-induced IL-6-rich acute pro-inflammatory response promoted DCs phenotypic maturation as the prerequisite to CD4+ T cell differentiation. Int. J. Hyperth. 2018, 34, 261–272. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Wang, Y.; Cui, Y.; Yu, Y.; Li, W.; Liu, F.; Liu, T. Tumor-derived LIF promotes chemoresistance via activating tumor-associated macrophages in gastric cancers. Exp. Cell Res. 2021, 406, 112734. [Google Scholar] [CrossRef]
- Jung, M.; Ma, Y.; Iyer, R.P.; DeLeon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic. Res. Cardiol. 2017, 112, 33. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Yang, K.; Kim, M.; Kim, H.-S.; Kang, J.L. Apoptosis inhibitor of macrophage (AIM) contributes to IL-10-induced anti-inflammatory response through inhibition of inflammasome activation. Cell Death Dis. 2021, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, K.; Zhu, J.; Wang, Y.; Sun, P.; Yang, Q.; Zhang, T.; Han, W.; Hu, W.; Yang, W.; et al. Sarsasapogenin Suppresses RANKL-Induced Osteoclastogenesis in vitro and Prevents Lipopolysaccharide-Induced Bone Loss in vivo. Drug Des. Dev. Ther. 2020, 14, 3435–3447. [Google Scholar] [CrossRef]
- Piao, H.; Fu, L.; Wang, Y.; Liu, Y.; Wang, Y.; Meng, X.; Yang, D.; Xiao, X.; Zhang, J. A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces malignancy progression. J. Exp. Clin. Cancer Res. 2022, 41, 174. [Google Scholar] [CrossRef]
- Rousset, F.; Garcia, E.; Defrance, T.; Péronne, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal Centers. Annu. Rev. Immunol. 2022, 40, 413–442. [Google Scholar] [CrossRef]
- Choe, J.; Choi, Y.S. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur. J. Immunol. 1998, 28, 508–515. [Google Scholar] [CrossRef]
- Singh, K.; Chang, C.; Gershwin, M.E. IgA deficiency and autoimmunity. Autoimmun. Rev. 2014, 13, 163–177. [Google Scholar] [CrossRef]
- Burdin, N.; Van Kooten, C.; Galibert, L.; Abrams, J.S.; Wijdenes, J.; Banchereau, J.; Rousset, F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J. Immunol. 1995, 154, 2533–2544. [Google Scholar] [CrossRef]
- Brière, F.; Bridon, J.M.; Chevet, D.; Souillet, G.; Bienvenu, F.; Guret, C.; Martinez-Valdez, H.; Banchereau, J. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J. Clin. Investig. 1994, 94, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Ma, R.; Yang, S.; Fan, T.; Cao, J.; Wang, Y.; Ma, W.; Yang, W.; Wang, F.; et al. IL-10 enhances T cell survival and is associated with faster relapse in patients with inactive ulcerative colitis. Mol. Immunol. 2020, 121, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yogev, N.; Bedke, T.; Kobayashi, Y.; Brockmann, L.; Lukas, D.; Regen, T.; Croxford, A.L.; Nikolav, A.; Hövelmeyer, N.; von Stebut, E.; et al. CD4+ T-cell-derived IL-10 promotes CNS inflammation in mice by sustaining effector T cell survival. Cell Rep. 2022, 38, 110565. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Darrow, M.A.; Thorpe, S.W.; Gingrich, A.A.; O’Donnell, E.F.; Bellini, A.R.; Sturgill, I.R.; Vick, L.V.; Dunai, C.; Stoffel, K.M.; et al. Analysis of tumor-infiltrating NK and T cells highlights IL-15 stimulation and TIGIT blockade as a combination immunotherapy strategy for soft tissue sarcomas. J. Immunother. Cancer 2020, 8, e001355. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, H.; Zhou, J.; Xu, S.; Gao, Y. Treatment with Recombinant Interleukin-15 (IL-15) Increases the Number of T Cells and Natural Killer (NK) Cells and Levels of Interferon-γ (IFN-γ) in a Rat Model of Sepsis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4450–4456. [Google Scholar] [CrossRef]
- Watkinson, F.; Nayar, S.K.; Rani, A.; Sakellariou, C.A.; Elhage, O.; Papaevangelou, E.; Dasgupta, P.; Galustian, C. IL-15 Upregulates Telomerase Expression and Potently Increases Proliferative Capacity of NK, NKT-Like, and CD8 T Cells. Front. Immunol. 2020, 11, 594620. [Google Scholar] [CrossRef]
- Dubois, S.P.; Miljkovic, M.D.; Fleisher, T.A.; Pittaluga, S.; Hsu-Albert, J.; Bryant, B.R.; Petrus, M.N.; Perera, L.P.; Müller, J.R.; Shih, J.H.; et al. Short-course IL-15 given as a continuous infusion led to a massive expansion of effective NK cells: Implications for combination therapy with antitumor antibodies. J. Immunother. Cancer 2021, 9, e002193. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, H.; Kim, J.H.; Kim, S.-Y.; Koh, J.-Y.; Sa, M.; Park, S.-H.; Shin, E.-C. CD5 Suppresses IL-15-Induced Proliferation of Human Memory CD8 T Cells by Inhibiting mTOR Pathways. J. Immunol. 2022, 209, 1108–1117. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Chang, Y.; Chen, J.; Kang, P.; Yi, X.; Cui, T.; Guo, S.; Xiao, Q.; Jian, Z.; et al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8 T cells activation via JAK-STAT pathway in vitiligo. Free Radic. Biol. Med. 2019, 139, 80–91. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Becker-Hapak, M.; Cashen, A.F.; Jacobs, M.; Wong, P.; Foster, M.; McClain, E.; Desai, S.; Pence, P.; Cooley, S.; et al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood 2022, 139, 1177–1183. [Google Scholar] [CrossRef]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Inoue, S.; Yamashita, K.; Kakeji, Y.; Fukumoto, T.; Kotani, J. IL-15 Improves Aging-Induced Persistent T Cell Exhaustion in Mouse Models of Repeated Sepsis. Shock 2020, 53, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Takeda, K.; Ouhara, K.; Shirawachi, S.; Kajiya, M.; Matsuda, S.; Kono, S.; Shiba, H.; Kurihara, H.; Mizuno, N. Involvement of Rac1 in macrophage activation by brain-derived neurotrophic factor. Mol. Biol. Rep. 2021, 48, 5249–5257. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-C.; Dang, Y.-Y.; Gao, H.-Y.; Wang, Z.-T.; Gao, M.; Yang, Y.; Zhang, H.-T.; Xu, R.-X. Local Injection of Lenti-BDNF at the Lesion Site Promotes M2 Macrophage Polarization and Inhibits Inflammatory Response After Spinal Cord Injury in Mice. Cell. Mol. Neurobiol. 2015, 35, 881–890. [Google Scholar] [CrossRef]
- Bi, C.; Fu, Y.; Li, B. Brain-derived neurotrophic factor alleviates diabetes mellitus-accelerated atherosclerosis by promoting M2 polarization of macrophages through repressing the STAT3 pathway. Cell. Signal. 2020, 70, 109569. [Google Scholar] [CrossRef]
- Bi, C.; Fu, Y.; Zhang, Z.; Li, B. Prostaglandin E2 confers protection against diabetic coronary atherosclerosis by stimulating M2 macrophage polarization via the activation of the CREB/BDNF/TrkB signaling pathway. FASEB J. 2020, 34, 7360–7371. [Google Scholar] [CrossRef]
- Chen, L.; Fu, L.; Sun, J.; Huang, Z.; Fang, M.; Zinkle, A.; Liu, X.; Lu, J.; Pan, Z.; Wang, Y.; et al. Structural basis for FGF hormone signalling. Nature 2023, 618, 862–870. [Google Scholar] [CrossRef]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef]
- Im, J.H.; Buzzelli, J.N.; Jones, K.; Franchini, F.; Gordon-Weeks, A.; Markelc, B.; Chen, J.; Kim, J.; Cao, Y.; Muschel, R.J. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat. Commun. 2020, 11, 4064. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Z.; Huang, C.; Huang, P.; Li, D. Serine protease PRSS23 drives gastric cancer by enhancing tumor associated macrophage infiltration FGF2. Front. Immunol. 2022, 13, 955841. [Google Scholar] [CrossRef]
- Yu, Y.; He, J.; Li, S.; Song, L.; Guo, X.; Yao, W.; Zou, D.; Gao, X.; Liu, Y.; Bai, F.; et al. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. Int. Immunopharmacol. 2016, 38, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, F.; Zhu, L.; Lin, P.; Han, F.; Wang, X.; Tan, X.; Lin, L.; Xiong, Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflam. 2020, 17, 257. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, T.-T.; Li, S.-M.; Li, Y.-H.; Wang, Y.-J.; Li, D.-S.; Wang, W.-F. Fibroblast growth factor 21 ameliorates pancreatic fibrogenesis via regulating polarization of macrophages. Exp. Cell Res. 2019, 382, 111457. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Xia, A.; Meng, F.; Chunyu, J.; Sun, X.; Ren, G.; Yu, D.; Jiang, X.; Tang, L.; Xiao, W.; et al. FGF21 alleviates chronic inflammatory injury in the aging process through modulating polarization of macrophages. Int. Immunopharmacol. 2021, 96, 107634. [Google Scholar] [CrossRef]
- Nederlof, R.; Reidel, S.; Spychala, A.; Gödecke, S.; Heinen, A.; Lautwein, T.; Petzsch, P.; Köhrer, K.; Gödecke, A. Insulin-Like Growth Factor 1 Attenuates the Pro-Inflammatory Phenotype of Neutrophils in Myocardial Infarction. Front. Immunol. 2022, 13, 908023. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- O’Riordan, C.; Clifford, A.; Van De Ven, P.; Nelson, J. Chronic neck pain and exercise interventions: Frequency, intensity, time, and type principle. Arch. Phys. Med. Rehabil. 2014, 95, 770–783. [Google Scholar] [CrossRef]
- Rao, J.; Wang, H.; Ni, M.; Wang, Z.; Wang, Z.; Wei, S.; Liu, M.; Wang, P.; Qiu, J.; Zhang, L.; et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 2022, 71, 2539–2550. [Google Scholar] [CrossRef]
- Li, G.; Ren, H.; Wu, X.; Hu, Q.; Hong, Z.; Wang, G.; Gu, G.; Ren, J.; Li, J. Follistatin like protein-1 modulates macrophage polarization and aggravates dextran sodium sulfate-induced colitis. Int. Immunopharmacol. 2020, 83, 106456. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Luo, J.; Xie, T.; Mor, G.; Liao, A. Decorin promotes decidual M1-like macrophage polarization via mitochondrial dysfunction resulting in recurrent pregnancy loss. Theranostics 2022, 12, 7216–7236. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Vachliotis, I.D.; Mantzoros, C.S. Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metabolism 2023, 147, 155676. [Google Scholar] [CrossRef]
- Ragland, T.J.; Malin, S.K. Exercise increases TCA intermediate concentrations during low-calorie diet independent of insulin resistance among women with obesity. Physiol. Rep. 2024, 12, e15987. [Google Scholar] [CrossRef]


| Myokines | Model | Stimulation | Myokine Alteration | Physical Signs | Ref. |
|---|---|---|---|---|---|
| Irisin | Mice | Swimming training (30 min/day, for 3 days/week for 8 weeks) | Irisin ↑ in muscle | Reduced the oxidative stress index (OSI), degeneration in the heart muscle, inflammation and cardiopathy | [46] |
| Mice | Treadmill exercise with moderate intensity (5 days/week for 8 weeks) | Irisin ↑ in muscle | Increased bone mineral density of trabecular bone in mice | [47] | |
| Rat | Swimming training (60 min/day, for 7 days/week for 8 weeks) | Irisin ↑ in serum | Effectively improved bone health caused by obesity | [48] | |
| Mice | Aerobic exercise, Resistance exercise, Vibration exercise, The Electrical stimulation (60 min/day, for 5 days/week for 4 weeks) | Irisin/FNDC5 ↑ in muscle | Promoted mitochondrial autophagy, improving heart function and resisting exercise. | [49] | |
| Human | Cycling on stationary bikes (20–35 min/week for 10 weeks) | Irisin ↑ in plasma | Improved glucose homeostasis and caused a small weight loss | [50] | |
| Human | Progressive resistance training (1 h/day, for 2 days/week for 12 weeks) | Irisin ↑ in serum | Increased grip strengthand leg strength | [51] | |
| Human | Strength and endurance training intervention (60 min/day, for 2 days/week for 12 weeks) | Irisin ↑ in serum | Not described | [52] | |
| Human | Moderate-intensity treadmill walking (180 min, 21.9 °C and 41.1 °C) | Irisin ↑ in serum | Reduced oxidative stress and inflammation | [53] | |
| Human | Winter swimming | Irisin ↓ in serum | Not described | [54] | |
| IL-6 | Human | Marathon | IL-6 ↑ in plasma | Not described | [55] |
| Rat | Treadmill running and ladder climbing (75 min/day, for 3 days/week for 12 weeks) | IL-6 ↑ in muscle | Helped reduce inflammation | [56] | |
| LIF | Rat | Interval exercise training (60 min/day, for 5 days/week for 8 weeks) | LIF ↑ in muscle | Reduced apoptosis and promoted proliferation in gastrocnemius muscle | [57] |
| Human | Heavy resistance exercise of 6–8 repetitions | LIF ↑ in muscle | Not described | [58] | |
| IL-10 | Rat | HIIT (Running with 8 m/min, 10 min/day for 5 days) | IL-10 ↑ in serum | The expression of pro-inflammatory factors was inhibited | [59] |
| Human | Physical activity of moderate intensity (12 weeks) | IL-10 ↑ in serum | Improvement of metabolic risk factors | [60] | |
| Human | Running 5 km intermittently; running 5 km continuously at 70% of MAS (determined in the incremental test) on the treadmill | IL-10 ↑ in serum | It effectively inhibits the progression of inflammatory response | [61] | |
| IL-15 | Human | A bilateral leg resistance exercise | IL-15 ↑ in muscle serum | Improved myofibrillar fractional synthetic rate | [62] |
| Human | High-intensity circuit training (HICT) (3 days/week for 5 weeks) | IL-15 ↑ in serum | Improved an insulin sensitivity | [63] | |
| Human | Moderate intensity exercise (60 min/day, for 3 days/week for 12 weeks) | IL-15 ↑ in serum | Decreased body fat | [64] | |
| BDNF | Mice | Swimming training (30 min/day, for 7 days/week for 12 weeks) | BDNF ↑ in cerebral cortex | Provided neuroprotective effects | [65] |
| Mice | Voluntary wheel-running exercise (30 days) | BDNF ↑ in striatum | Enhanced striatal dopamine (DA) release | [66] | |
| Mice | Rat cages equipped with running Wheels (3 h/day for 2 weeks) | BDNF ↑ in hippocampal tissues | Improved cognition in Alzheimer’s disease (AD) | [67] | |
| Human | Aerobic exercise (35 min) | BDNF ↑ in serum | Improved cognition in Alzheimer’s disease (AD) | [68] | |
| Human | Walk on a treadmill at light to moderate intensity (30 min) | mBDNF ↑ in serum | Enhancement of neuroplasticity and facilitate the improvement of motor performance | [69] | |
| FGF2 | Rat | Continuous exercise training (15 min at 65% maximal speed for 1 week, 20 min for 2 weeks, 25 min for three weeks at 70% maximal speed, and 30 min for 4, 5, and 6 weeks at 70% maximal speed) | FGF2↑ in heart tissue | Delayed age-related myocardial fibrosis | [70] |
| Human | Aerobic exercise (300 min/week for 12 months) | FGF2 ↑ in serum | Reduced postmenopausal breast cancer risk | [71] | |
| FGF21 | Mice | Resistance training (10 repetitions/day, 3 days/week for 8 weeks | FGF21 ↑ in muscle | Improved muscle strength | [72] |
| Mice | Performed treadmill exercises at 30 m/min for 60 min | FGF21 ↑ in plasma and muscle | Not described | [73] | |
| Human | A treadmill exercise test (following the Bruce’s protocol) (5 days/week for 2 weeks) | FGF21 ↑ in serum | Increased glucose intake | [74] | |
| IGF-1 | Mice | Ladder climbing (85-degree incline, 1.5 cm spacing), utilizing progressive overload (twice a day, every third day for 16–18 weeks) | IGF-1 ↑ in muscle | Compensatory growth of muscle | [75] |
| Human | Resistance training (RT), aerobic training (AT), combination training (CT) (60 min/day, for 3 days/week for 8 weeks) | IGF-1 ↑ in serum | Increased muscle mass and reduced total fat mass and visceral fat area (VFA) | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Wang, Z.; Zhang, X.-A.; Ning, K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules 2024, 14, 1205. https://doi.org/10.3390/biom14101205
Lu Z, Wang Z, Zhang X-A, Ning K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules. 2024; 14(10):1205. https://doi.org/10.3390/biom14101205
Chicago/Turabian StyleLu, Zheng, Zhuo Wang, Xin-An Zhang, and Ke Ning. 2024. "Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review" Biomolecules 14, no. 10: 1205. https://doi.org/10.3390/biom14101205
APA StyleLu, Z., Wang, Z., Zhang, X.-A., & Ning, K. (2024). Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules, 14(10), 1205. https://doi.org/10.3390/biom14101205






