The Imbalance of Homocysteine, Vitamin B12 and Folic Acid in Parkinson Plus Syndromes: A Review beyond Parkinson Disease
Abstract
1. Introduction
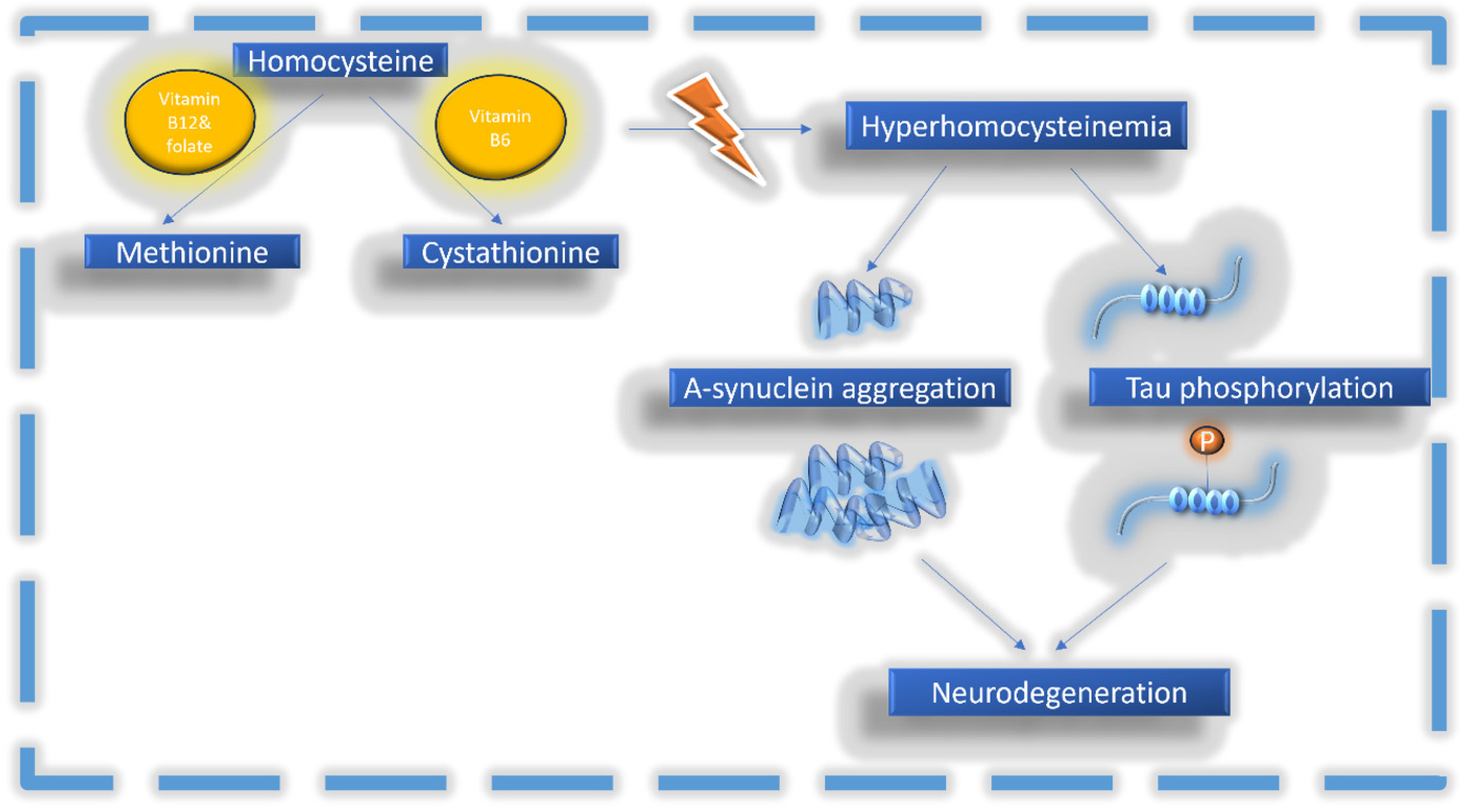
2. Homocysteine in Health and Disease
Homocysteine and Neurodegenerative Diseases
3. Parkinson Plus Syndromes
3.1. Synucleinopathies
3.1.1. Multiple System Atrophy
3.1.2. Dementia with Lewy Bodies
3.2. Tauopathies
3.2.1. Progressive Supranuclear Palsy
3.2.2. Corticobasal Degeneration
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rekik, A.; Santoro, C.; Poplawska-Domaszewicz, K.; Qamar, M.A.; Batzu, L.; Landolfo, S.; Rota, S.; Falup-Pecurariu, C.; Murasan, I.; Chaudhuri, K.R. Parkinson’s disease and vitamins: A focus on vitamin B12. J. Neural. Transm. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Schorr, H.; Eckert, R.; Herrmann, W. Vitamin B12 status in the elderly as judged by available biochemical markers. Clin. Chem. 2004, 50, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, X.; Sun, Y.; Zhou, F. Blood and CSF Homocysteine Levels in Alzheimer’s Disease: A Meta-Analysis and Meta-Regression of Case-Control Studies. Neuropsychiatr. Dis. Treat. 2022, 18, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Cervellati, C.; Brombo, G.; Trentini, A.; Roncon, L.; Zuliani, G. Elevated Blood Homocysteine and Risk of Alzheimer’s Dementia: An Updated Systematic Review and Meta-Analysis Based on Prospective Studies. J. Prev. Alzheimer’s Dis. 2021, 8, 329–334. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Chang, H.; Liu, X.; Zhu, R. Homocysteine and Folic Acid: Risk Factors for Alzheimer’s Disease-An Updated Meta-Analysis. Front. Aging Neurosci. 2021, 13, 665114. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, L.; Li, H.; Chen, G.; Qi, G.; Ma, X.; Jin, Y. Role of homocysteine in the development and progression of Parkinson’s disease. Ann. Clin. Transl. Neurol. 2020, 7, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Homocysteine and Parkinson’s disease. CNS Neurosci. Ther. 2024, 30, e14420. [Google Scholar] [CrossRef]
- Murray, L.K.; Jadavji, N.M. The role of one-carbon metabolism and homocysteine in Parkinson’s disease onset, pathology and mechanisms. Nutr. Res. Rev. 2019, 32, 218–230. [Google Scholar] [CrossRef]
- Periñán, M.T.; Macías-García, D.; Jesús, S.; Martín-Rodríguez, J.F.; Muñoz-Delgado, L.; Jimenez-Jaraba, M.V.; Buiza-Rueda, D.; Bonilla-Toribio, M.; Adarmes-Gómez, A.D.; Gómez-Garre, P.; et al. Homocysteine levels, genetic background, and cognitive impairment in Parkinson’s disease. J. Neurol. 2023, 270, 477–485. [Google Scholar] [CrossRef]
- Kumar, R.R.; Singh, L.; Thakur, A.; Singh, S.; Kumar, B. Role of Vitamins in Neurodegenerative Diseases: A Review. CNS Neurol. Disord. Drug Targets 2022, 21, 766–773. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, A.; Merola, A.; Artusi, C.A.; Rizzone, M.G.; Zibetti, M.; Lopiano, L. Levodopa-Induced Neuropathy: A Systematic Review. Mov. Disord. Clin. Pract. 2019, 6, 96–103. [Google Scholar] [CrossRef] [PubMed]
- O’Suilleabhain, P.E.; Bottiglieri, T.; Dewey, R.B., Jr.; Sharma, S.; Diaz-Arrastia, R. Modest increase in plasma homocysteine follows levodopa initiation in Parkinson’s disease. Mov. Disord. 2004, 19, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Isobe, C.; Abe, T.; Terayama, Y. L-Dopa therapy increases homocysteine concentration in cerebrospinal fluid from patients with Parkinson’s disease. J. Clin. Neurosci. 2010, 17, 717–721. [Google Scholar] [CrossRef]
- Luthra, N.S.; Marcus, A.H.; Hills, N.K.; Christine, C.W. Vitamin B12 measurements across neurodegenerative disorders. J. Clin. Mov. Disord. 2020, 7, 3. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Schalinske, K.L.; Smazal, A.L. Homocysteine imbalance: A pathological metabolic marker. Adv. Nutr. 2012, 3, 755–762. [Google Scholar] [CrossRef]
- Dardiotis, E.; Arseniou, S.; Sokratous, M.; Tsouris, Z.; Siokas, V.; Mentis, A.A.; Michalopoulou, A.; Andravizou, A.; Dastamani, M.; Paterakis, K.; et al. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2017, 17, 190–197. [Google Scholar] [CrossRef]
- Oberley, M.J.; Yang, D.T. Laboratory testing for cobalamin deficiency in megaloblastic anemia. Am. J. Hematol. 2013, 88, 522–526. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elewa, Y.H.A.; Zahran, M.H.; Alexiou, A.; Papadakis, M.; Batiha, G.E. Parkinson’s Disease Risk and Hyperhomocysteinemia: The Possible Link. Cell. Mol. Neurobiol. 2023, 43, 2743–2759. [Google Scholar] [CrossRef]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693s–696s. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.N.; Siokas, V.; Aloizou, A.-M.; Tsouris, Z.; Dastamani, M.; Aslanidou, P.; Brotis, A.; Dardiotis, E. Pyridoxine, folate and cobalamin for migraine: A systematic review. Acta Neurol. Scand. 2020, 142, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Cherubini, V.; Falsetti, L.; Viticchi, G.; Silvestrini, M.; Toraldo, A. Homocysteine, Cognitive Functions, and Degenerative Dementias: State of the Art. Biomedicines 2022, 10, 2741. [Google Scholar] [CrossRef]
- Kang, S.S.; Wong, P.W.; Malinow, M.R. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef]
- Ivanova, M.A.; Kokorina, A.D.; Timofeeva, P.D.; Karelina, T.V.; Abushik, P.A.; Stepanenko, J.D.; Sibarov, D.A.; Antonov, S.M. Calcium Export from Neurons and Multi-Kinase Signaling Cascades Contribute to Ouabain Neuroprotection in Hyperhomocysteinemia. Biomolecules 2020, 10, 1104. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Boikov, S.I.; Karelina, T.V.; Antonov, S.M. GluN2 Subunit-Dependent Redox Modulation of NMDA Receptor Activation by Homocysteine. Biomolecules 2020, 10, 1441. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.; Feng, X.; Chen, Y.; Shi, J.; Weng, H.; Wang, D. Homocysteine aggravates DNA damage by impairing the FA/Brca1 Pathway in NE4C murine neural stem cells. Int. J. Med. Sci. 2020, 17, 2477–2486. [Google Scholar] [CrossRef]
- Lee, E.S.; Chen, H.; Soliman, K.F.; Charlton, C.G. Effects of homocysteine on the dopaminergic system and behavior in rodents. Neurotoxicology 2005, 26, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, F.; Brombo, G.; Zuliani, G. The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegener. Dis. Manag. 2016, 6, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Gangopadhaya, P.K.; Das, S.K. Parkinsonism plus syndrome—A review. Neurol. India 2003, 51, 183–188. [Google Scholar]
- Mark, M.H. Lumping and splitting the parkinson plus syndromes: Dementia with lewy bodies, multiple system atrophy, progressive supranuclear palsy, and cortical-basal ganglionic degeneration. Neurol. Clin. 2001, 19, 607–627. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Mead, S.; Ellis, M.; Wadsworth, J.D.; Nicoll, A.J.; Kenny, J.; Launchbury, F.; Linehan, J.; Richard-Loendt, A.; Walker, A.S.; et al. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 2015, 525, 247–250. [Google Scholar] [CrossRef]
- Sanders, D.W.; Kaufman, S.K.; DeVos, S.L.; Sharma, A.M.; Mirbaha, H.; Li, A.; Barker, S.J.; Foley, A.C.; Thorpe, J.R.; Serpell, L.C.; et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 2014, 82, 1271–1288. [Google Scholar] [CrossRef]
- Woerman, A.L.; Stöhr, J.; Aoyagi, A.; Rampersaud, R.; Krejciova, Z.; Watts, J.C.; Ohyama, T.; Patel, S.; Widjaja, K.; Oehler, A.; et al. Propagation of prions causing synucleinopathies in cultured cells. Proc. Natl. Acad. Sci. USA 2015, 112, E4949–E4958. [Google Scholar] [CrossRef]
- Angot, E.; Steiner, J.A.; Hansen, C.; Li, J.-Y.; Brundin, P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010, 9, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- McCann, H.; Stevens, C.H.; Cartwright, H.; Halliday, G.M. α-Synucleinopathy phenotypes. Park. Relat. Disord. 2014, 20 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef]
- Milanese, C.; Cerri, S.; Ulusoy, A.; Gornati, S.V.; Plat, A.; Gabriels, S.; Blandini, F.; Di Monte, D.A.; Hoeijmakers, J.H.; Mastroberardino, P.G. Activation of the DNA damage response in vivo in synucleinopathy models of Parkinson’s disease. Cell Death Dis. 2018, 9, 818. [Google Scholar] [CrossRef]
- Huang, Y.; Song, Y.J.; Murphy, K.; Holton, J.L.; Lashley, T.; Revesz, T.; Gai, W.P.; Halliday, G.M. LRRK2 and parkin immunoreactivity in multiple system atrophy inclusions. Neuropathology 2008, 116, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gong, C.X. Tau exon 10 alternative splicing and tauopathies. Mol. Neurodegener. 2008, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J. Tauopathies as clinicopathological entities. Park. Relat. Disord. 2016, 22 (Suppl. S1), S29–S33. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, T.; Meng, L.; Zhang, X.; Tian, Y.; Dai, L.; Niu, X.; Li, Y.; Liu, C.; Chen, G.; et al. N-homocysteinylation of α-synuclein promotes its aggregation and neurotoxicity. Aging Cell 2023, 22, e13745. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z. α-Synuclein pathology from the body to the brain: So many seeds so close to the central soil. Neural Regen. Res. 2024, 19, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, Y.; Wei, W.; Zhao, W.; Lu, F.; Liu, F. Vitamin B12 inhibits α-synuclein fibrillogenesis and protects against amyloid-induced cytotoxicity. Food Funct. 2019, 10, 2861–2870. [Google Scholar] [CrossRef]
- Schaffner, A.; Li, X.; Gomez-Llorente, Y.; Leandrou, E.; Memou, A.; Clemente, N.; Yao, C.; Afsari, F.; Zhi, L.; Pan, N.; et al. Vitamin B(12) modulates Parkinson’s disease LRRK2 kinase activity through allosteric regulation and confers neuroprotection. Cell Res. 2019, 29, 313–329. [Google Scholar] [CrossRef]
- Heckman, M.G.; Schottlaender, L.; Soto-Ortolaza, A.I.; Diehl, N.N.; Rayaprolu, S.; Ogaki, K.; Fujioka, S.; Murray, M.E.; Cheshire, W.P.; Uitti, R.J.; et al. LRRK2 exonic variants and risk of multiple system atrophy. Neurology 2014, 83, 2256–2261. [Google Scholar] [CrossRef]
- Lee, K.; Nguyen, K.D.; Sun, C.; Liu, M.; Zafar, F.; Saetern, J.; Flierl, A.; Tetrud, J.W.; Langston, J.W.; Dickson, D.; et al. LRRK2 p.Ile1371Val Mutation in a Case with Neuropathologically Confirmed Multi-System Atrophy. J. Park. Dis. 2018, 8, 93–100. [Google Scholar] [CrossRef]
- Lovati, C.; Galimberti, D.; Pomati, S.; Capiluppi, E.; Dolci, A.; Scapellato, L.; Rosa, S.; Mailland, E.; Suardelli, M.; Vanotti, A.; et al. Serum folate concentrations in patients with cortical and subcortical dementias. Neurosci. Lett. 2007, 420, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, C.; Mao, C.; Song, B.; Hou, H.; Wu, J.; Liu, X.; Luo, H.; Sun, S.; Xu, Y. Plasma Homocysteine, Vitamin B12 and Folate Levels in Multiple System Atrophy: A Case-Control Study. PLoS ONE 2015, 10, e0136468. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wei, X.; Zou, J.; Wang, R.; Liu, X.; Xu, X.; Lu, J.; Wang, Z.; Tang, B.; Wang, B.; et al. Contra-Directional Expression of Serum Homocysteine and Uric Acid as Important Biomarkers of Multiple System Atrophy Severity: A Cross-Sectional Study. Front. Cell. Neurosci. 2015, 9, 247. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, Y.; Zhu, C.; Ma, L.; Huang, Q.; Chen, X. Oxidative Stress and Environmental Exposures are Associated with Multiple System Atrophy in Chinese Patients. Can. J. Neurol. Sci. 2016, 43, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhuang, X.D.; Xian, W.B.; Wu, L.L.; Huang, Z.N.; Hu, X.; Zhang, X.S.; Chen, L.; Liao, X.X. Serum Klotho, vitamin D, and homocysteine in combination predict the outcomes of Chinese patients with multiple system atrophy. CNS Neurosci. Ther. 2017, 23, 657–666. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, S.; Chen, Z.; Shi, Z.; Hu, W.; Ma, L.; Wang, X.; Li, X.; Ji, Y. Association of Elevated Plasma Total Homocysteine With Dementia With Lewy Bodies: A Case-Control Study. Front. Aging Neurosci. 2021, 13, 724990. [Google Scholar] [CrossRef]
- Hoffmann, J.; Busse, S.; von Hoff, F.; Borucki, K.; Frodl, T.; Busse, M. Association Between Homocysteine and Vitamin Levels in Demented Patients. J. Alzheimer’s Dis. 2021, 81, 1781–1792. [Google Scholar] [CrossRef]
- Song, Y.; Quan, M.; Li, T.; Jia, J. Serum Homocysteine, Vitamin B12, Folate, and Their Association with Mild Cognitive Impairment and Subtypes of Dementia. J. Alzheimer’s Dis. 2022, 90, 681–691. [Google Scholar] [CrossRef]
- Chmiela, T.; Węgrzynek, J.; Kasprzyk, A.; Waksmundzki, D.; Wilczek, D.; Gorzkowska, A. If Not Insulin Resistance so What?—Comparison of Fasting Glycemia in Idiopathic Parkinson’s Disease and Atypical Parkinsonism. Diabetes Metab. Syndr. Obes. 2022, 15, 1451–1460. [Google Scholar] [CrossRef]
- Chen, D.; Wan, L.; Chen, Z.; Yuan, X.; Liu, M.; Tang, Z.; Fu, Y.; Zhu, S.; Zhang, X.; Qiu, R.; et al. Serum vitamin levels in multiple system atrophy: A case-control study. Front. Aging Neurosci. 2022, 14, 1105019. [Google Scholar] [CrossRef]
- Fanciulli, A.; Wenning, G.K. Multiple-system atrophy. N. Engl. J. Med. 2015, 372, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.A.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Xiang, C.; Wang, H.; Cong, S. Diagnostic utility of fluid biomarkers in multiple system atrophy: A systematic review and meta-analysis. J. Neurol. 2021, 268, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.J.; Coon, E.A.; Savica, R.; St Louis, E.K.; Bower, J.H.; Benarroch, E.E.; Sandroni, P.; Low, P.; Singer, W. Lower Vitamin B12 Level at Multiple System Atrophy Diagnosis Is Associated With Shorter Survival. Mov. Disord. 2020, 35, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, R. Gastrointestinal dysfunction in movement disorders. Neurol. Sci. 2021, 42, 1355–1365. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Ginter, E.; Blazicek, P.; Klvanova, J. Homocysteine and vitamin C. Bratisl. Lekárske Listy 2002, 103, 171–173. [Google Scholar]
- Kosaka, K. Lewy body disease and dementia with Lewy bodies. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 301–306. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Jellinger, K.A. Dementia with Lewy bodies and Parkinson’s disease-dementia: Current concepts and controversies. J. Neural. Transm. 2018, 125, 615–650. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Attems, J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008, 115, 427–436. [Google Scholar] [CrossRef]
- Howlett, D.R.; Whitfield, D.; Johnson, M.; Attems, J.; O’Brien, J.T.; Aarsland, D.; Lai, M.K.; Lee, J.H.; Chen, C.; Ballard, C.; et al. Regional Multiple Pathology Scores Are Associated with Cognitive Decline in Lewy Body Dementias. Brain Pathol. 2015, 25, 401–408. [Google Scholar] [CrossRef] [PubMed]
- De Reuck, J.; Deramecourt, V.; Cordonnier, C.; Leys, D.; Pasquier, F.; Maurage, C.-A. Prevalence of cerebrovascular lesions in patients with Lewy body dementia: A neuropathological study. Clin. Neurol. Neurosurg. 2013, 115, 1094–1097. [Google Scholar] [CrossRef]
- Karanth, S.; Nelson, P.T.; Katsumata, Y.; Kryscio, R.J.; Schmitt, F.A.; Fardo, D.W.; Cykowski, M.D.; Jicha, G.A.; Van Eldik, L.J.; Abner, E.L. Prevalence and Clinical Phenotype of Quadruple Misfolded Proteins in Older Adults. JAMA Neurol. 2020, 77, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Gan, J.; Yang, Y.; Meng, Q.; Han, J.; Ji, Y. The vascular risk factors and vascular neuropathology in subjects with autopsy-confirmed dementia with Lewy bodies. Int. J. Geriatr. Psychiatry 2022, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Isojima, D.; Togo, T.; Kosaka, K.; Fujishiro, H.; Akatsu, H.; Katsuse, O.; Iritani, S.; Matsumoto, T.; Hirayasu, Y. Vascular complications in dementia with Lewy bodies: A postmortem study. Neuropathology 2006, 26, 293–297. [Google Scholar] [CrossRef]
- Lildballe, D.L.; Fedosov, S.; Sherliker, P.; Hin, H.; Clarke, R.; Nexo, E. Association of cognitive impairment with combinations of vitamin B₁₂-related parameters. Clin. Chem. 2011, 57, 1436–1443. [Google Scholar] [CrossRef]
- Hin, H.; Clarke, R.; Sherliker, P.; Atoyebi, W.; Emmens, K.; Birks, J.; Schneede, J.; Ueland, P.M.; Nexo, E.; Scott, J.; et al. Clinical relevance of low serum vitamin B12 concentrations in older people: The Banbury B12 study. Age Ageing 2006, 35, 416–422. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- de Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef]
- Raszewski, G.; Chwedorowicz, R.; Chwedorowicz, A.; Gustaw Rothenberg, K. Homocysteine, antioxidant vitamins and lipids as biomarkers of neurodegeneration in Alzheimer’s disease versus non-Alzheimer’s dementia. Ann. Agric. Environ. Med. 2016, 23, 193–196. [Google Scholar] [CrossRef][Green Version]
- Soysal, P.; Dokuzlar, O.; Erken, N.; Dost Günay, F.S.; Isik, A.T. The Relationship Between Dementia Subtypes and Nutritional Parameters in Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Shirafuji, N.; Hamano, T.; Yen, S.H.; Kanaan, N.M.; Yoshida, H.; Hayashi, K.; Ikawa, M.; Yamamura, O.; Kuriyama, M.; Nakamoto, Y. Homocysteine Increases Tau Phosphorylation, Truncation and Oligomerization. Int. J. Mol. Sci. 2018, 19, 891. [Google Scholar] [CrossRef] [PubMed]
- Di Meco, A.; Li, J.G.; Barrero, C.; Merali, S.; Praticò, D. Elevated levels of brain homocysteine directly modulate the pathological phenotype of a mouse model of tauopathy. Mol. Psychiatry 2019, 24, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- van Hummel, A.; Taleski, G.; Sontag, J.M.; Feiten, A.F.; Ke, Y.D.; Ittner, L.M.; Sontag, E. Methyl donor supplementation reduces phospho-Tau, Fyn and demethylated protein phosphatase 2A levels and mitigates learning and motor deficits in a mouse model of tauopathy. Neuropathol. Appl. Neurobiol. 2023, 49, e12931. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Bötzel, K.; Giese, A.; Vogeser, M.; Lorenzl, S. Elevated levels of methylmalonate and homocysteine in Parkinson’s disease, progressive supranuclear palsy and amyotrophic lateral sclerosis. Dement. Geriatr. Cogn. Disord. 2010, 29, 553–559. [Google Scholar] [CrossRef]
- Boxer, A.L.; Yu, J.T.; Golbe, L.I.; Litvan, I.; Lang, A.E.; Höglinger, G.U. Advances in progressive supranuclear palsy: New diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017, 16, 552–563. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Tepedino, M.F.; Avallone, A.R.; Abate, F.; Serio, M.; Caterino, M.; Erro, R.; Pellecchia, M.T.; Barone, P.; Picillo, M. Longitudinal change of energy expenditure, body composition and dietary habits in Progressive Supranuclear Palsy patients. Neurol. Sci. 2024, 45, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Koga, S.; Kouri, N.; Walton, R.L.; Ebbert, M.T.W.; Josephs, K.A.; Litvan, I.; Graff-Radford, N.; Ahlskog, J.E.; Uitti, R.J.; van Gerpen, J.A.; et al. Corticobasal degeneration with TDP-43 pathology presenting with progressive supranuclear palsy syndrome: A distinct clinicopathologic subtype. Acta Neuropathol. 2018, 136, 389–404. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Kwon, Y.; Lee, S.; Kim, S.; Jo, M.; Lee, S.; Kim, S.R.; Kim, K.; Kim, H.J. Vitamin B12 Reduces TDP-43 Toxicity by Alleviating Oxidative Stress and Mitochondrial Dysfunction. Antioxidants 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Flores-Torres, M.H.; Christine, C.W.; Bjornevik, K.; Molsberry, S.A.; Hung, A.Y.; Healy, B.C.; Blacker, D.; Schwarzschild, M.A.; Ascherio, A. Long-Term Intake of Folate, Vitamin B6, and Vitamin B12 and the Incidence of Parkinson’s Disease in a Sample of U.S. Women and Men. Mov. Disord. 2023, 38, 866–879. [Google Scholar] [CrossRef] [PubMed]
- de Lau, L.M.; Koudstaal, P.J.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease. Neurology 2006, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.M.; Schwarzschild, M.A.; Hernán, M.A.; Logroscino, G.; Willett, W.C.; Ascherio, A. Folate intake and risk of Parkinson’s disease. Am. J. Epidemiol. 2004, 160, 368–375. [Google Scholar] [CrossRef]
| First Author, Year, Country | Measurement Method | Diagnostic Criteria | Participants, Number | Sex (m/f) | Age (Mean ± SD) | Serum Homocysteine (Mean ± SD) | Serum Folate (Mean ± SD) | Serum Vitamin B12 (Mean ± SD) |
|---|---|---|---|---|---|---|---|---|
| Lovati, 2007 [51], Italy | Chemiluminescent immunoassay | HC, 76 | 18/58 | 67.6 ± 7.2 | Not Measured | 6.87 ± 3.50 μg/L | Not Measured | |
| The second International Workshop of the Consortium on DLB, 1999 | LBD, 9 | 3/6 | 79.0 ± 4.0 | 4.26 ± 2.94 μg/L | ||||
| Zhang, 2015 [52], China | Not Mentioned | Consensus criteria for the clinical diagnosis of MSA (2008) | MSA, 161 | 82/79 | 57.99 ± 8.34 | 16.23 ± 8.09 μmol/L | 6.46 ± 3.14 ng/mL | 600.85 ± 515.69 pg/mL |
| HC, 161 | 78/83 | 57.34 ± 10.37 | 14.04 ± 4.25 μmol/L | 6.40 ± 3.28 ng/mL | 547.1 ± 479.56 pg/mL | |||
| Chen, 2015 [53], China | Solid-phase competitive chemiluminescent enzyme immunoassay | Consensus criteria for the clinical diagnosis of MSA (1999) | MSA, 47 | 31/16 | 58.74 ± 10.18 | 13.28 ± 4.13 μmol/L | Not Measured | Not Measured |
| HC, 50 | 27/23 | 55.64 ± 10.82 | 10.34 ± 3.07 μmol/L | |||||
| Zhou, 2016 [54], China | Not Mentioned | Consensus criteria for the clinical diagnosis of MSA (2008) | MSA, 55 | 37/18 | 59.33 ± 10.47 | 13.52 ± 4.56 μmol/L | Not Measured | Not Measured |
| HC, 76 | 42/34 | 60.41 ± 11.50 | 10.28 ± 3.31 μmol/L | |||||
| Guo, 2017 [55], China | Routine laboratory tests | Consensus criteria for the clinical diagnosis of MSA (2008) | MSA, 53 | 30/23 | 59.7 ± 10.1 | 15.2 ± 5.95 μmol/L | Not Measured | Not Measured |
| HC, 62 | 34/28 | 53.4 ± 9.08 | 10.4 ± 3.09 μmol/L | |||||
| Luthra, 2020 [15], USA | Chemiluminescent Assay | Not mentioned | DLB, 36 | 19/17 | 73 ± 8.6 | Not Measured | Not Measured | 518.7 ± 180.4 pg/mL |
| Not mentioned | MSA, 9 | 6/3 | 65.1 ± 3.8 | 549.8 ± 145.6 pg/mL | ||||
| Zhang, 2021 [56], China | Electrochemiluminescence immunoassays (ECLIA) | 2017 DLB Diagnostic criteria | DLB, 132 | 64/68 | 72.7 ± 7.8 | 22.9 ± 16.3 μmol/L | 8.0 ± 5.9 nmol/L | 337.2 ± 210.4 pmol/L |
| HC, 295 | 118/146 | 72.2 ± 6.1 | 13.0 ± 4.4 μmol/L | 14.3 ± 8.0 nmol/L | 457.5 ± 205.6 pmol/L | |||
| Hoffmann, 2021 [57], Germany | Electrochemiluminescence immunoassays (ECLIA) | HC, 54 | 13/41 | 72.00 | 16.45 * μmol/L | 8.700 * ng/mL | 415.0 * ng/L | |
| Not mentioned | LBD, 9 | 5/1 | 76.5 | 23.60 * μmol/L | 5.950 * ng/mL | 223.5 * ng/L | ||
| Song, 2022 [58], China | Enzymatic cycling assay | HC,62 | 34/28 | 61.40 ± 8.79 | 13.30 ± 6.29 μmol/L | 10.99 ± 6.82 ng/mL | 560.90 ± 3910.72 pg/mL | |
| 2017 DLB Diagnostic criteria | LBD, 23 | 15/8 | 67.61 ± 7.74 | 16.60 ± 7.68 μmol/L | 9.04 ± 5.23 ng/mL | 342.00 ± 2640.94 pg/mL | ||
| Chmiela, 2022 [59], Poland | Not mentioned | Not mentioned | MSA, 14 | 8/6 | 65.2 ± 11.0 | 17.0 ± 8.9 μmol/L | Not Measured | Not Measured |
| Chen, 2023 [60], China | Electrochemistry method | Consensus criteria for the clinical diagnosis of MSA (2008) | MSA, 224 | 145/99 | 56.56 ± 7.55 | Not measured | 9.74 ± 5.71 μg/L | 507.98 ± 339.80 ng/L |
| HC, 244 | 145/99 | 56.06 ± 8.13 | 15.06 ± 5.25 μg/L | 504.41 ± 217.30 ng/L |
| First Author, Year, Country | Measurement Method | Diagnostic Criteria | Participants, Number | Sex (m/f) | Age (Mean ± SD) | Serum Homocysteine (Mean ± SD) | Serum Folate (Mean ± SD) | Serum Vitamin B12 (Mean ± SD) |
|---|---|---|---|---|---|---|---|---|
| Lovati, 2007 [51], Italy | Chemiluminescent immunoassay | HC, 76 | 18/58 | 67.6 ± 7.2 | Not Measured | 6.87 ± 3.50 μg/L | Not Measured | |
| Accuracy of the clinical diagnosis of corticobasal degeneration, 1997 | CBD, 5 | 3/2 | 69.6 ± 9.6 | 4.30 ± 2.80 μg/L | ||||
| Clinical research criteria for the diagnosis of PSP, 1996 | PSP, 6 | 3/3 | 71.8 ± 4.8 | 5.74 ± 4.05 μg/L | ||||
| Levin, 2010 [85], Germany | Automated ligand- binding assays | Clinical and PET diagnostics | PSP, 22 | 12/10 | 66.95 ± 5.71 | 15.75 ± 5.75 μmol/L | Not Mentioned | Not Mentioned |
| HC, 30 | 13/12 | 63.62 ± 11.32 | 11.19 ± 1.21 μmol/L | |||||
| Luthra, 2020 [15], USA | Chemiluminescent Assay | Not mentioned | PSP, 20 | 11/9 | 69 ± 6.1 | Not Measured | Not Measured | 539.1 ± 178.1 pg/mL |
| Chmiela, 2022 [59], Poland | Not mentioned | Not mentioned | PSP, 33 | 15/18 | 70.1 ± 7.1 | 13.8 ± 3.4 μmol/L | Not Measured | Not Measured |
| First Author, Year, Country | All Participants | PPS Participants | Level of Significance Hcy between Disease and HC | Level of Significance Folic between Disease and HC | Level of Significance B12 between Disease and HC | Level of Significance Hcy between Diseases | Level of Significance Folic between Diseases | Level of Significance B12 between Diseases |
|---|---|---|---|---|---|---|---|---|
| Lovati, 2007 [51], Italy | HC, AD, FTD, LBD, CBD, PSP, PD-d | LBD | Not Measured | Not Significant | Not Measured | Not Measured | Cortical (AD, FTD) vs. subcortical (LBD, CBD, PSP, PD-d) dementias: p < 0.01 | Not Measured |
| CBD | Not Significant | |||||||
| PSP | Not Significant | |||||||
| Levin, 2010 [85], Germany | PD, PSP, ALS, HC | PSP | p < 0.001 | Not Significant | Not Significant | Levodopa- treated PD patients vs. patient groups with other neurodegenerative diseases (PSP, ALS): Not Significant | Not Reported | Not Reported |
| Zhang, 2015 [52], China | MSA, HC | MSA | p < 0.05 | Not Significant | Not Significant | — | — | — |
| Chen, 2015 [53], China | MSA, PD, HC | MSA | p = 0.005 | Not Measured | Not Measured | MSA vs. PD: p = 0.897 | Not Measured | Not Measured |
| Zhou, 2016 [54], China | MSA, HC | MSA | p < 0.001 | Not Measured | Not Measured | - | Not Measured | Not Measured |
| Guo, 2017 [55], China | MSA, PD, HC | MSA | p = 0.005 | Not Measured | Not Measured | MSA vs. PD: p = 0.865 | Not Measured | Not Measured |
| Luthra, 2020 [15], USA | AD, DLB, FTD, MCI, MSA, PD, PSP | DLB | Not Measured | Not Measured | Not Measured | Not Measured | Not Measured | PD vs. either AD or MSA: Not Significant All disease groups (AD, DLB, FTD, MCI, MSA, PD, PSP) p = 0.015 |
| MSA | ||||||||
| PSP | ||||||||
| Zhang, 2021 [56], China | DLB, AD, HC | DLB | p < 0.01 | p < 0.01 | p < 0.01 | DLB vs. AD: p < 0.05 | DLB vs. AD: Not Significant | DLB vs. AD: p < 0.05 |
| Hoffmann, 2021 [57], Germany | FTD, LBD, AD, VaD, HC | LBD | p = 0.1390 | p = 0.0773 | p = 0.0207 | All disease groups (FTD, LBD, AD, VaD): Not Significant | All disease groups (FTD, LBD, AD, VaD): Not Significant | All disease groups (FTD, LBD, AD, VaD): Not Significant |
| Song, 2022 [58], China | HC, MCI, AD, VaD, FTD, LBD | LBD | p < 0.01 | Not Significant | p < 0.01 | All disease groups (MCI, AD, VaD, FTD, LBD): p < 0.001 | All disease groups (MCI, AD, VaD, FTD, LBD): p = 0.330 | All disease groups (MCI, AD, VaD, FTD, LBD): p < 0.001 |
| Chmiela, 2022 [59], Poland | PD, MSA, PSP | MSA | Not Measured | Not Measured | Not Measured | All disease groups (PD, MSA, PSP) p = 0.402 | Not Measured | Not Measured |
| PSP | ||||||||
| Chen, 2023 [60], China | MSA, PD, HC | MSA | Not Measured | <0.001 | 0.083 | Not Measured | MSA vs. PD 0.502 | MSA vs. PD 0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulidou, V.; Liampas, I.; Arnaoutoglou, M.; Dardiotis, E.; Siokas, V. The Imbalance of Homocysteine, Vitamin B12 and Folic Acid in Parkinson Plus Syndromes: A Review beyond Parkinson Disease. Biomolecules 2024, 14, 1213. https://doi.org/10.3390/biom14101213
Poulidou V, Liampas I, Arnaoutoglou M, Dardiotis E, Siokas V. The Imbalance of Homocysteine, Vitamin B12 and Folic Acid in Parkinson Plus Syndromes: A Review beyond Parkinson Disease. Biomolecules. 2024; 14(10):1213. https://doi.org/10.3390/biom14101213
Chicago/Turabian StylePoulidou, Vasiliki, Ioannis Liampas, Marianthi Arnaoutoglou, Efthimios Dardiotis, and Vasileios Siokas. 2024. "The Imbalance of Homocysteine, Vitamin B12 and Folic Acid in Parkinson Plus Syndromes: A Review beyond Parkinson Disease" Biomolecules 14, no. 10: 1213. https://doi.org/10.3390/biom14101213
APA StylePoulidou, V., Liampas, I., Arnaoutoglou, M., Dardiotis, E., & Siokas, V. (2024). The Imbalance of Homocysteine, Vitamin B12 and Folic Acid in Parkinson Plus Syndromes: A Review beyond Parkinson Disease. Biomolecules, 14(10), 1213. https://doi.org/10.3390/biom14101213









