STING Promotes the Progression of ADPKD by Regulating Mitochondrial Function, Inflammation, Fibrosis, and Apoptosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture and Reagents
2.2. Western Blot Analysis
2.3. Histology and Immunohistochemistry (IHC)
2.4. Immunofluorescence Staining
2.5. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
2.6. Quantitative Reverse-Transcription PCR (qRT-PCR)
2.7. RNA Interference
2.8. Isolation and Analysis of Cytosolic DNA and mtDNA
2.9. Mouse Strain and Treatment
2.10. Measurement of Cyst Area
2.11. Quantitative BUN Determination
2.12. Ultrasonic Testing in Mice
2.13. Statistics
2.14. Study Approval
3. Result
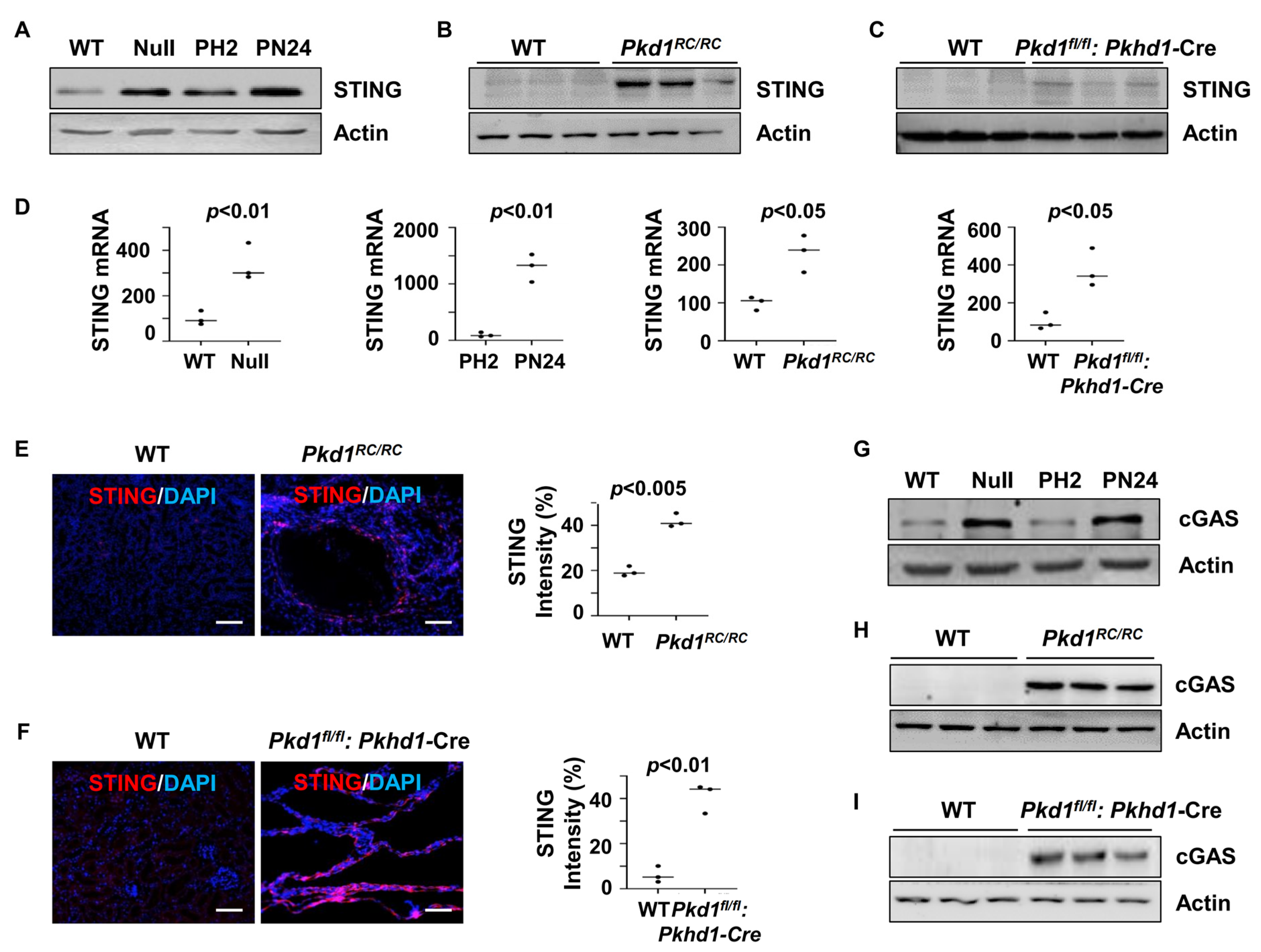
3.1. STING Expression Was Upregulated in Pkd1 Mutant Mouse Kidneys
3.2. Treatment with an Inhibitor of STING, C-176, Delays Cyst Growth in Pkd1 Mutant Mouse Kidneys
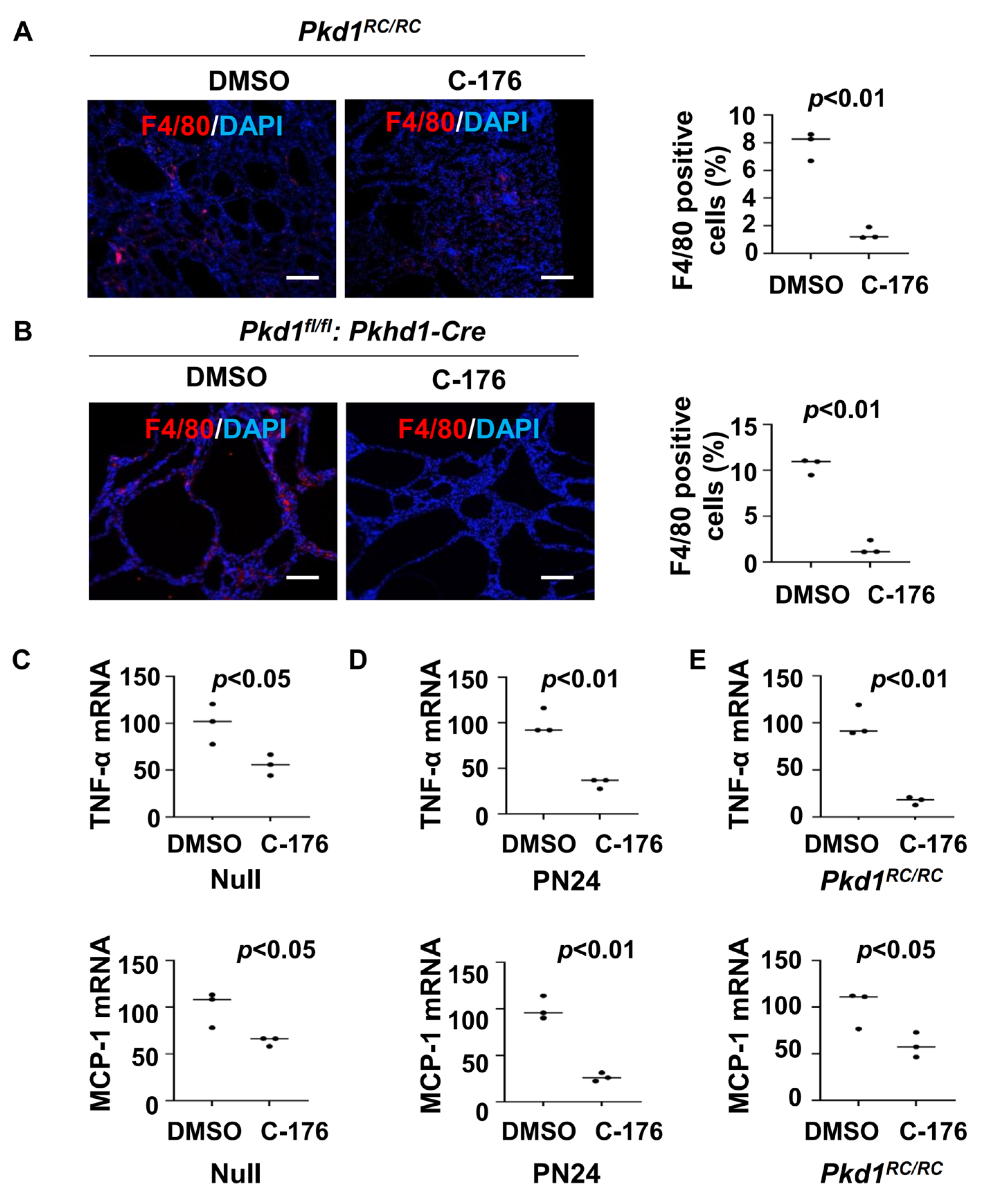
3.3. Treatment with the STING Inhibitor C-176 Reduces Cytokine Expression and Decreases Macrophage Recruitment in Pkd1 Mutant Kidneys
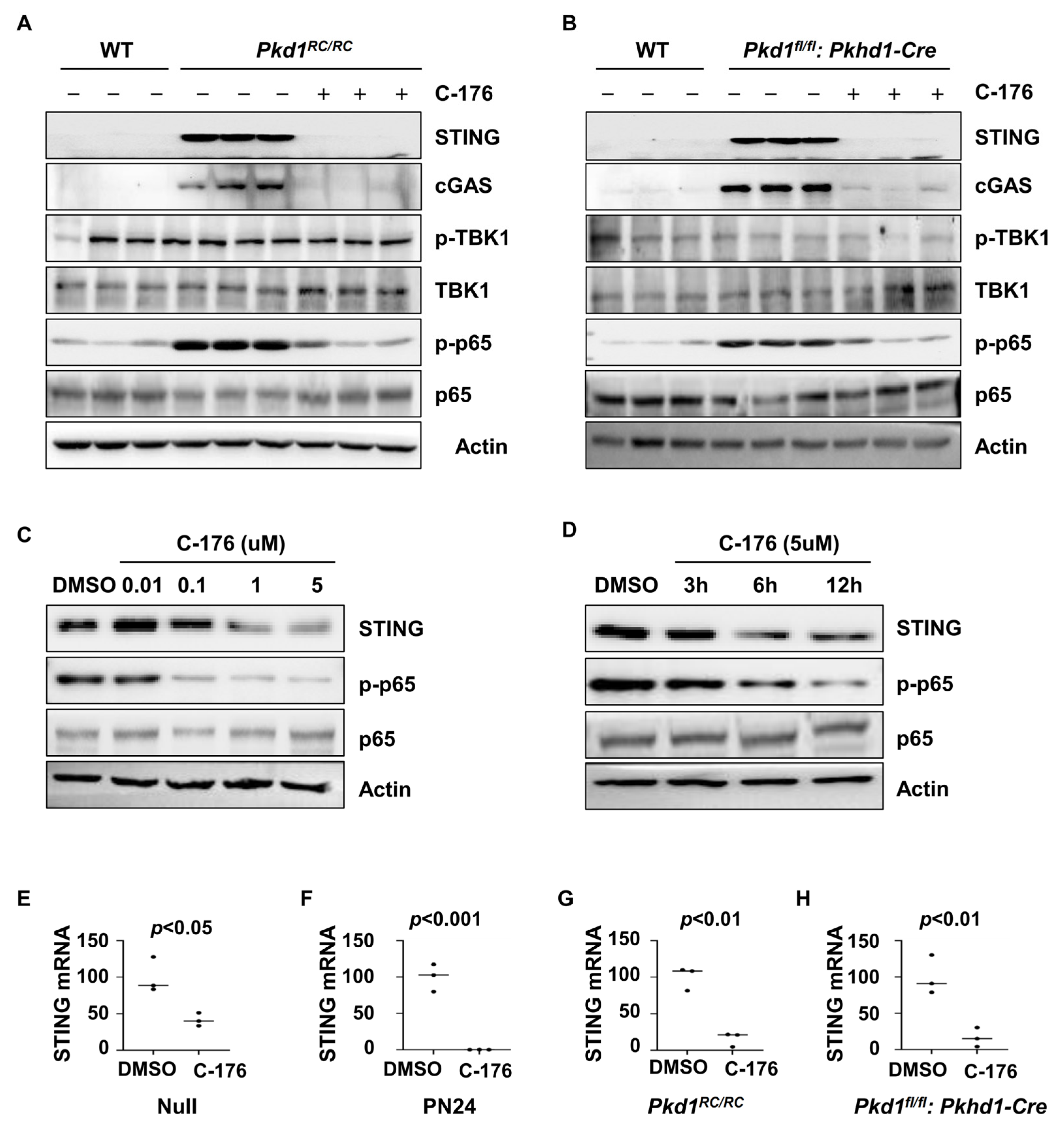
3.4. Treatment with STING Inhibitor C-176 Alleviates the Inflammatory Response Possibly through NF-κB Pathway
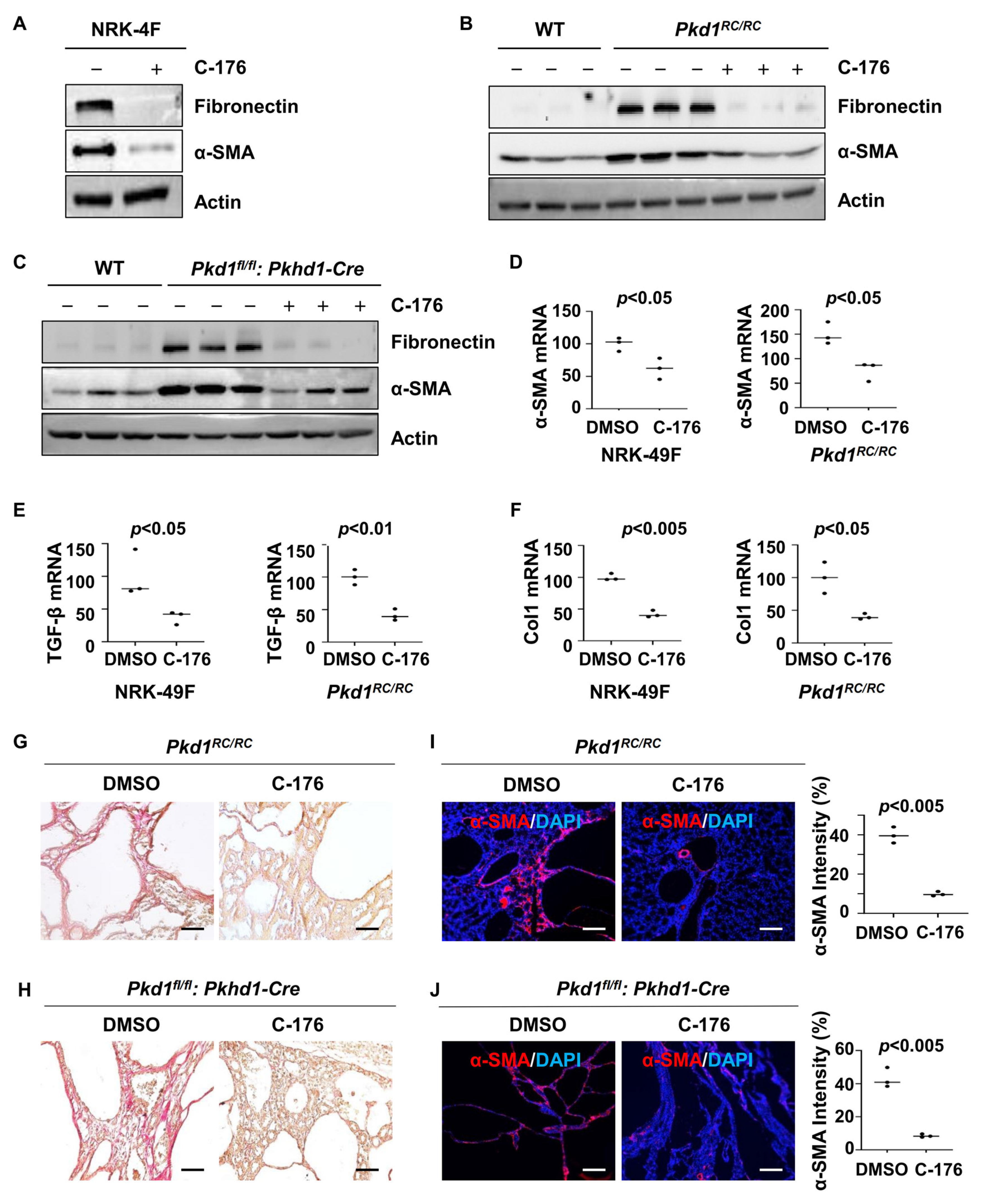
3.5. Treatment with STING Inhibitor C-176 Alleviates Renal Fibrosis in Pkd1 Mutant Mouse Kidneys
3.6. Inhibition of STING Induces p53-Dependent Cystic Renal Epithelial Cell Apoptosis
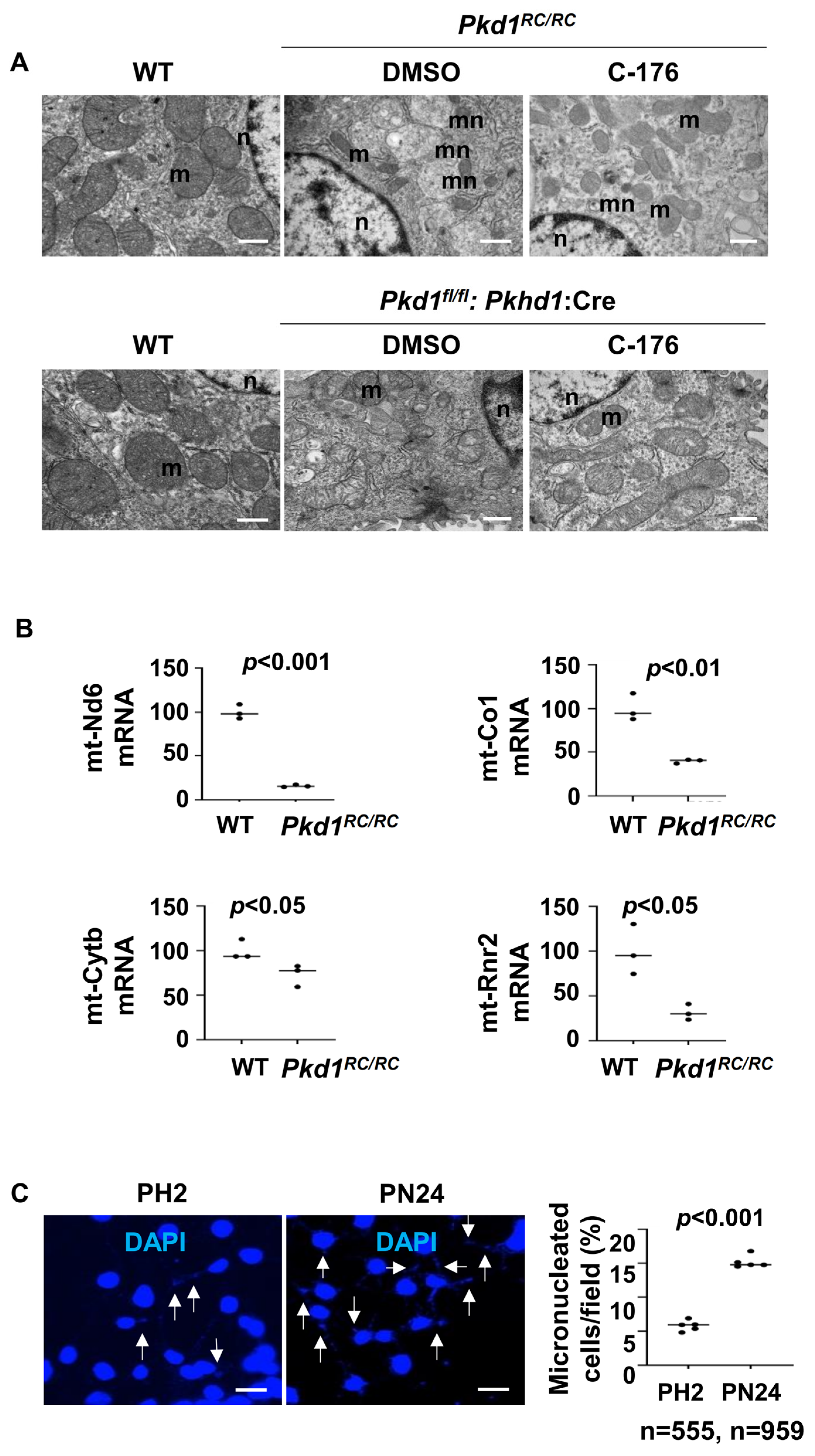
3.7. Mitochondria Impairment and Cytosolic DNA Trigger the Activation of STING in Pkd1 Mutant Renal Epithelial Cells and Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornec-Le, G.E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Germino, G.G.; Menezes, L.F. Mechanisms of Cyst Development in Polycystic Kidney Disease. Adv. Kidney Dis. Health 2023, 30, 209–219. [Google Scholar] [CrossRef]
- Agborbesong, E.; Li, L.X.; Li, L.; Li, X. Molecular Mechanisms of Epigenetic Regulation, Inflammation, and Cell Death in ADPKD. Front. Mol. Biosci. 2022, 9, 922428. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Magenheimer, B.S.; Xia, S.; Johnson, T.; Wallace, D.P.; Calvet, J.P.; Li, R. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 2008, 14, 863–868. [Google Scholar] [CrossRef]
- Cassini, M.F.; Kakade, V.R.; Kurtz, E.; Sulkowski, P.; Glazer, P.; Torres, R.; Somlo, S.; Cantley, L.G. Mcp1 Promotes Macrophage-Dependent Cyst Expansion in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 2471–2481. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Fan, L.X.; Yao, Y.; Swenson-Fields, K.I.; Gadjeva, M.; Wallace, D.P.; Peters, D.J.; Yu, A.; Grantham, J.J.; et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J. Clin. Investig. 2015, 125, 2399–2412. [Google Scholar] [CrossRef]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-L.; Chen, C.-F.; Lin, H.Y.-H.; Riley, D.J.; Chen, Y. The Link between Autosomal Dominant Polycystic Kidney Disease and Chromosomal Instability: Exploring the Relationship. Int. J. Mol. Sci. 2024, 25, 2936. [Google Scholar] [CrossRef]
- Ma, J.; Setton, J.; Lee, N.Y.; Riaz, N.; Powell, S.N. The therapeutic significance of mutational signatures from DNA repair deficiency in cancer. Nat. Commun. 2018, 9, 3292. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Chung, J.H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp. Mol. Med. 2023, 55, 510–519. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhang, H.; Zhou, M.; Huang, C.; Xia, W.; Li, J.; You, H. The effects of cGAS-STING inhibition in liver disease, kidney disease, and cellular senescence. Front. Immunol. 2024, 15, 1346446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, Q.; Li, X.; Zhao, Z.; Hong, C.; Sun, Z.; Deng, B.; Li, C.; Zhang, J.; Wang, S. The cGAS-STING pathway in viral infections: A promising link between inflammation, oxidative stress and autophagy. Front. Immunol. 2024, 15, 1352479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhuang, Z.; Li, J.; Feng, Z. Significance of the cGAS-STING Pathway in Health and Disease. Int. J. Mol. Sci. 2023, 24, 13316. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, O.P.G.; Unterholzner, L. DNA sensing in cancer: Pro-tumour and anti-tumour functions of cGAS-STING signalling. Essays Biochem. 2023, 67, 905–918. [Google Scholar]
- Smith, J.A. STING, the Endoplasmic Reticulum, and Mitochondria: Is Three a Crowd or a Conversation? Front. Immunol. 2020, 11, 611347. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chelvanambi, M.; Filderman, J.N.; Storkus, W.J.; Luke, J.J. STING Agonists as Cancer Therapeutics. Cancers 2021, 13, 2695. [Google Scholar] [CrossRef]
- Couillin, I.; Riteau, N. STING Signaling and Sterile Inflammation. Front. Immunol. 2021, 12, 753789. [Google Scholar] [CrossRef]
- Ou, L.; Zhang, A.; Cheng, Y.; Chen, Y. The cGAS-STING Pathway: A Promising Immunotherapy Target. Front. Immunol. 2021, 12, 795048. [Google Scholar] [CrossRef]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; van der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef]
- Chung, K.W.; Dhillon, P.; Huang, S.; Sheng, X.; Shrestha, R.; Qiu, C.; Kaufman, B.A.; Park, J.; Pei, L.; Baur, J.; et al. Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab. 2019, 30, 784–799.e5. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Tada, T.; Hashimoto, M.; Nishimura, T.; Kiriki, M.; Higashiura, A.; Iwasaki, A.; Honda, M.; Nagasawa, Y.; Yamakado, K. Utility of ultrasonography for predicting indications for tolvaptan in patients with autosomal dominant polycystic kidney disease. J. Med. Ultrason. 2023, 50, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Riyahi, S.; Dev, H.; Blumenfeld, J.D.; Rennert, H.; Yin, X.; Attari, H.; Barash, I.; Chicos, I.; Bobb, W.; Donahue, S.; et al. Hemorrhagic Cysts and Other MR Biomarkers for Predicting Renal Dysfunction Progression in Autosomal Dominant Polycystic Kidney Disease. J. Magn. Reson. Imaging 2021, 53, 564–576. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, S.; Sun, J.; Cheng, D.; Guo, H.; Mei, J.; Zhang, X.; Maimaiti, M.; Hao, H.; Cao, P.; et al. STING signaling in islet macrophages impairs insulin secretion in obesity. Sci. China Life Sci. 2024, 67, 345–359. [Google Scholar] [CrossRef]
- Fan, X.; Han, J.; Zhong, L.; Zheng, W.; Shao, R.; Zhang, Y.; Shi, S.; Lin, S.; Huang, Z.; Huang, W.; et al. Macrophage-Derived GSDMD Plays an Essential Role in Atherosclerosis and Cross Talk Between Macrophages via the Mitochondria-STING-IRF3/NF-kappaB Axis. Arter. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1365–1378. [Google Scholar] [CrossRef]
- Zhou, J.X.; Cheng, A.S.; Chen, L.; Li, L.X.; Agborbesong, E.; Torres, V.E.; Harris, P.C.; Li, X. CD74 Promotes Cyst Growth and Renal Fibrosis in Autosomal Dominant Polycystic Kidney Disease. Cells 2024, 13, 489. [Google Scholar] [CrossRef] [PubMed]
- Dvorkin, S.; Cambier, S.; Volkman, H.E.; Stetson, D.B. New frontiers in the cGAS-STING intracellular DNA-sensing pathway. Immunity 2024, 57, 718–730. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Padovano, V.; Podrini, C.; Boletta, A.; Caplan, M.J. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat. Rev. Nephrol. 2018, 14, 678–687. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.X.; Ding, H.; Torres, V.E.; Yu, C.; Li, X. Ferroptosis Promotes Cyst Growth in Autosomal Dominant Polycystic Kidney Disease Mouse Models. J. Am. Soc. Nephrol. 2021, 32, 2759–2776. [Google Scholar] [CrossRef]
- Hu, M.-M.; Shu, H.-B. Mitochondrial DNA-triggered innate immune response: Mechanisms and diseases. Cell Mol. Immunol. 2023, 20, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Fei, C.J.; Hu, Y.; Wu, X.Y.; Nie, L.; Chen, J. Current understanding of the cGAS-STING signaling pathway: Structure, regulatory mechanisms, and related diseases. Zool. Res. 2023, 44, 183–218. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Mukai, K.; Takaya, E.; Shindo, R. STING Operation at the ER/Golgi Interface. Front. Immunol. 2021, 12, 646304. [Google Scholar] [CrossRef]
- Bai, J.; Liu, F. Nuclear cGAS: Sequestration and beyond. Protein Cell 2022, 13, 90–101. [Google Scholar] [CrossRef]
- Di Bona, M.; Bakhoum, S.F. Micronuclei and Cancer. Cancer Discov. 2024, 14, 214–226. [Google Scholar] [CrossRef]
- Yoo, M.; Haydak, J.C.; Azeloglu, E.U.; Lee, K.; Gusella, G.L. cGAS Activation Accelerates the Progression of Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2024, 35, 466–4682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.J.; Saravanabavan, S.; Chandra, A.N.; Munt, A.; Wong, A.T.Y.; Harris, P.C.; Harris, D.C.; McKenzie, P.; Wang, Y.; Rangan, G.K. Up-Regulation of DNA Damage Response Signaling in Autosomal Dominant Polycystic Kidney Disease. Am. J. Pathol. 2021, 191, 902–9020. [Google Scholar] [CrossRef]
- Agborbesong, E.; Zhou, J.X.; Zhang, H.; Li, L.X.; Harris, P.C.; Calvet, J.P.; Li, X. Overexpression of SMYD3 Promotes Autosomal Dominant Polycystic Kidney Disease by Mediating Cell Proliferation and Genome Instability. Biomedicines 2024, 12, 603. [Google Scholar] [CrossRef]
- Krupina, K.; Goginashvili, A.; Cleveland, D.W. Causes and consequences of micronuclei. Curr. Opin. Cell Biol. 2021, 70, 91–99. [Google Scholar] [CrossRef]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jønsson, K.L.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-κB Signaling after Nuclear DNA Damage. Mol. Cell 2018, 71, 745–760.e5. [Google Scholar] [CrossRef]
- Balka, K.R.; Louis, C.; Saunders, T.L.; Smith, A.M.; Calleja, D.J.; D’Silva, D.B.; Moghaddas, F.; Tailler, M.; Lawlor, K.E.; Zhan, Y.; et al. TBK1 and IKKepsilon Act Redundantly to Mediate STING-Induced NF-kappaB Responses in Myeloid Cells. Cell Rep. 2020, 31, 107492. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, A.K.; Cottle, D.L.; Runting, J.; Rodrigues, G.; Tham, M.S.; Jones, L.K.; Cumming, H.E.; Short, K.M.; Zaph, C.; Smyth, I.M. Modulating inflammation with interleukin 37 treatment ameliorates murine Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2024, 105, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qu, X.; Wang, X.; Xu, D.; Sheng, M.; Lin, Y.; Ke, M.; Song, C.; Xia, Q.; Jiang, L.; et al. The macrophage STING-YAP axis controls hepatic steatosis by promoting the autophagic degradation of lipid droplets. Hepatology 2023, 10, 1097. [Google Scholar] [CrossRef]
- Rodríguez-Morales, P.; Franklin, R.A. Macrophage phenotypes and functions: Resolving inflammation and restoring homeostasis. Trends Immunol. 2023, 44, 986–998. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Li, Y.; Shi, T.; Xu, N.; Liu, R.; Evans, S.E. Mitochondrial (mt)DNA-cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling promotes pyroptosis of macrophages via interferon regulatory factor (IRF)7/IRF3 activation to aggravate lung injury during severe acute pancreatitis. Cell Mol. Biol. Lett. 2024, 29, 61. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, R.; Tang, D. The STING1 network regulates autophagy and cell death. Signal Transduct. Target. Ther. 2021, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Y.-J.; Dobbs, N.; Sakai, T.; Liou, J.; Miner, J.J.; Yan, N. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 2019, 216, 867–883. [Google Scholar] [CrossRef]
- Riley, J.S.; Quarato, G.; Cloix, C.; Lopez, J.; O’Prey, J.; Pearson, M.; Chapman, J.; Sesaki, H.; Carlin, L.M.; Passos, J.F.; et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 2018, 37, 17. [Google Scholar] [CrossRef] [PubMed]
- Lemos, H.; Mohamed, E.; Huang, L.; Ou, R.; Pacholczyk, G.; Arbab, A.S.; Munn, D.; Mellor, A.L. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016, 76, 2076–2081. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Ghosh, M.; Saha, S.; Li, J.; Montrose, D.C.; Martinez, L.A. p53 engages the cGAS/STING cytosolic DNA sensing pathway for tumor suppression. Mol. Cell 2023, 83, 266–280.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Li, L.X.; Zhang, H.; Agborbesong, E.; Harris, P.C.; Calvet, J.P.; Li, X. DNA methyltransferase 1 (DNMT1) promotes cyst growth and epigenetic age acceleration in autosomal dominant polycystic kidney disease. Kidney Int. 2024, 106, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-Y.; Liu, X.-S.; Lan, H.-Y. Therapeutic potential for renal fibrosis by targeting Smad3-dependent noncoding RNAs. Mol. Ther. 2024, 32, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Capili, A.; Choi, M.E. Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches. Kidney Res. Clin. Pract. 2020, 39, 244–258. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Cheng, S.; Lee, G.; Agborbesong, E.; Li, X.; Zhou, X.; Li, X. STING Promotes the Progression of ADPKD by Regulating Mitochondrial Function, Inflammation, Fibrosis, and Apoptosis. Biomolecules 2024, 14, 1215. https://doi.org/10.3390/biom14101215
Wu J, Cheng S, Lee G, Agborbesong E, Li X, Zhou X, Li X. STING Promotes the Progression of ADPKD by Regulating Mitochondrial Function, Inflammation, Fibrosis, and Apoptosis. Biomolecules. 2024; 14(10):1215. https://doi.org/10.3390/biom14101215
Chicago/Turabian StyleWu, Jiao, Shasha Cheng, Geoffray Lee, Ewud Agborbesong, Xiaoyan Li, Xia Zhou, and Xiaogang Li. 2024. "STING Promotes the Progression of ADPKD by Regulating Mitochondrial Function, Inflammation, Fibrosis, and Apoptosis" Biomolecules 14, no. 10: 1215. https://doi.org/10.3390/biom14101215
APA StyleWu, J., Cheng, S., Lee, G., Agborbesong, E., Li, X., Zhou, X., & Li, X. (2024). STING Promotes the Progression of ADPKD by Regulating Mitochondrial Function, Inflammation, Fibrosis, and Apoptosis. Biomolecules, 14(10), 1215. https://doi.org/10.3390/biom14101215





