Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight
Abstract
:1. Introduction
2. Plasticity in Adipocyte Size
3. Plasticity in Adipocyte Number
4. Plasticity of Adipocyte Species
4.1. Browning
4.1.1. Transcriptional and Epigenetic Regulation
4.1.2. Endocrine Hormone Regulation
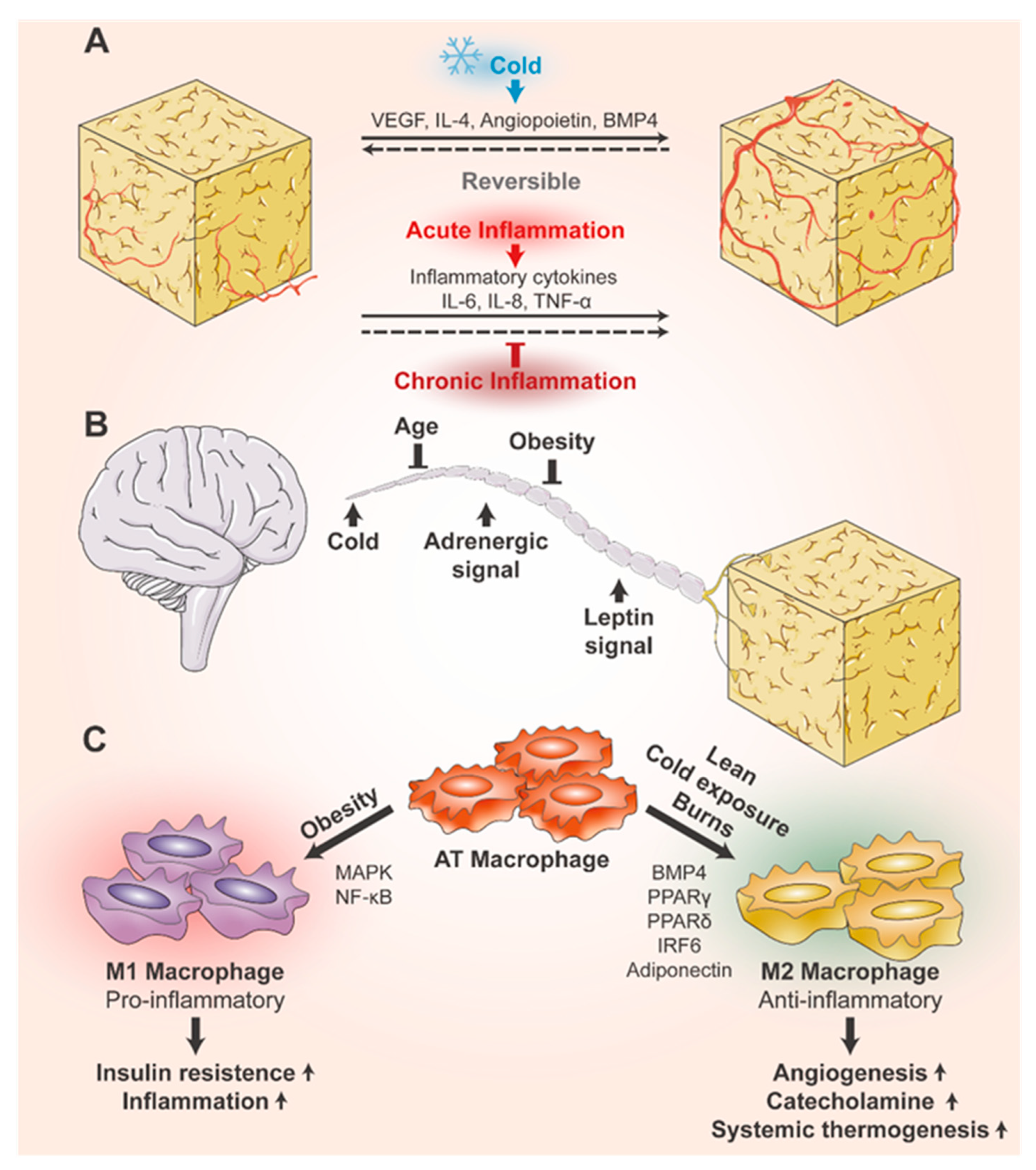
4.1.3. Environmental and Lifestyle Effects
4.2. Whitening
5. Plasticity of the Internal Structure of Adipocytes
6. Plasticity of the External Matrix of Adipocytes
6.1. Plasticity of Blood Vessels in Adipose Tissue
6.2. Plasticity of Nerves in Adipose Tissue
6.3. Plasticity of Immune Cells in Adipose Tissue
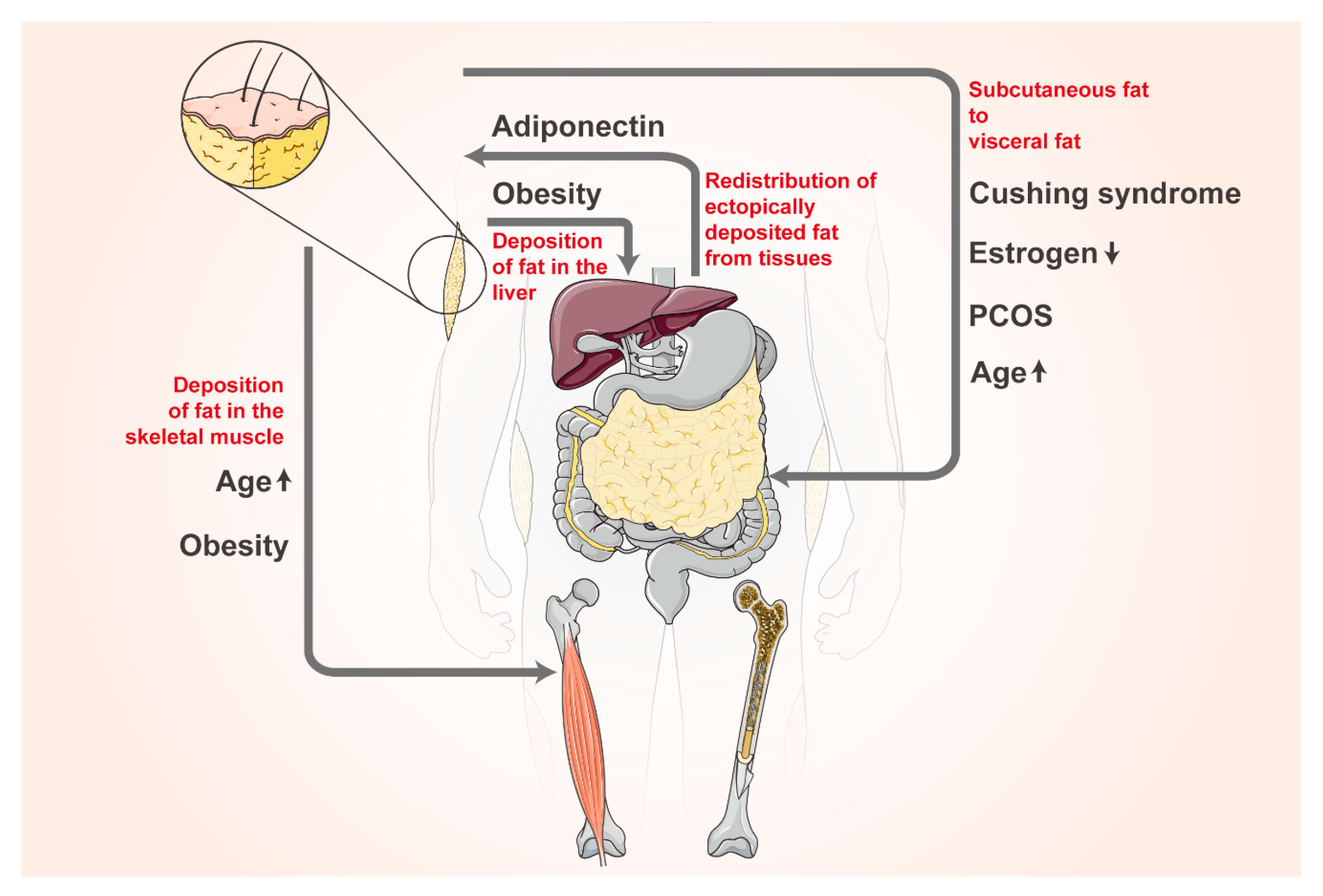
7. Plasticity of Adipose Tissue Distribution
8. Plasticity of Adipocyte Differentiation and Dedifferentiation Capacity
8.1. Plasticity of Adipocyte Differentiation Capacity
8.2. Plasticity of Adipocyte Dedifferentiation Capacity
9. Plasticity of Adipocytes Secreting Adipokines or Extracellular Vesicles
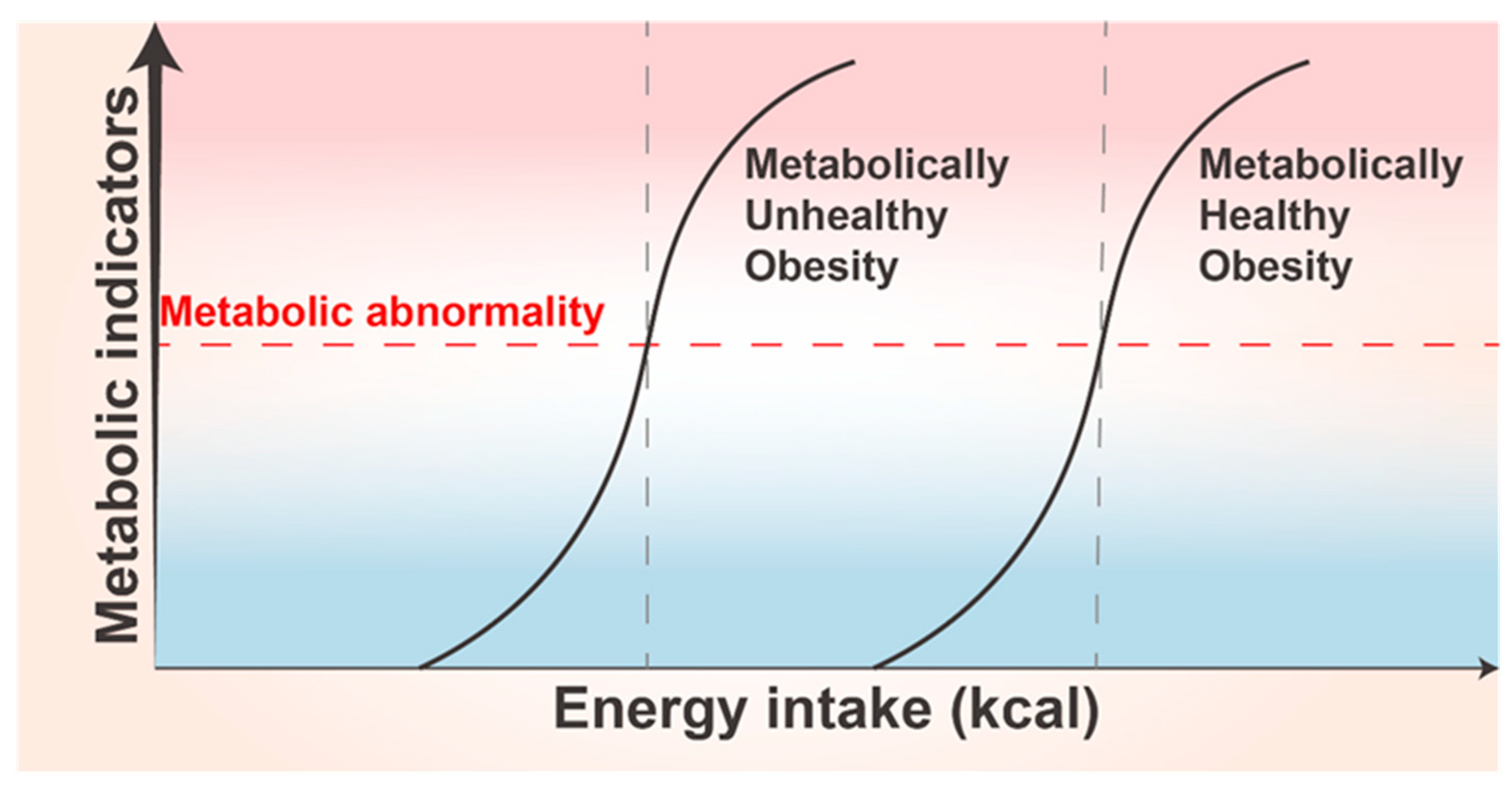
10. Thresholds of Adipose Tissue Plasticity and Their Individualized Differences
11. Relationship between Adipose Tissue Plasticity and Disease
11.1. Obesity
11.2. Lipodystrophy
11.3. Insulin Resistance
11.4. Polycystic Ovary Syndrome
11.5. HAIR-AN Syndrome
11.6. Atherosclerosis
11.7. Metabolic-Associated Fatty Liver Disease
11.8. Tumor
12. Mechanisms and Drugs Targeting Adipose Tissue Plasticity in Obesity Treatment
12.1. Regulation of Adipocyte Production and Adipose Tissue Expansion
12.2. Increase in BAT Mass and Activity
12.3. Regulation of Adipocyte Apoptosis
12.4. Improvement of Adipose Tissue Angiogenesis
12.5. Improvement of Inflammation in Adipose Tissue
13. Discussion
14. Prospect
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Fano, M.; Bartolini, D.; Tortoioli, C.; Vermigli, C.; Malara, M.; Galli, F.; Murdolo, G. Adipose Tissue Plasticity in Response to Pathophysiological Cues: A Connecting Link between Obesity and Its Associated Comorbidities. Int. J. Mol. Sci. 2022, 23, 5511. [Google Scholar] [CrossRef] [PubMed]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jørgensen, A.M.; Dashti, H.; Stefek, A.; et al. A single-cell atlas of human and mouse white adipose tissue. Nature 2022, 603, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.F.; Roesler, A.; Kazak, L. Regulation of adipocyte thermogenesis: Mechanisms controlling obesity. FEBS J. 2020, 287, 3370–3385. [Google Scholar] [CrossRef] [PubMed]
- Loyd, C.; Obici, S. Brown fat fuel use and regulation of energy homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 368–372. [Google Scholar] [CrossRef]
- Schreiber, R.; Diwoky, C.; Schoiswohl, G.; Feiler, U.; Wongsiriroj, N.; Abdellatif, M.; Kolb, D.; Hoeks, J.; Kershaw, E.E.; Sedej, S.; et al. Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab. 2017, 26, 753–763.e7. [Google Scholar] [CrossRef]
- Carrière, A.; Lagarde, D.; Jeanson, Y.; Portais, J.-C.; Galinier, A.; Ader, I.; Casteilla, L. The emerging roles of lactate as a redox substrate and signaling molecule in adipose tissues. J. Physiol. Biochem. 2020, 76, 241–250. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.; Rainer, P.Y.; Rumpler, M.; Russeil, J.; Zachara, M.; Pezoldt, J.; van Mierlo, G.; Gardeux, V.; Saelens, W.; Alpern, D.; et al. A human omentum-specific mesothelial-like stromal population inhibits adipogenesis through IGFBP2 secretion. Cell Metab. 2024, 36, 1566–1585.e9. [Google Scholar] [CrossRef]
- Sun, W.; Modica, S.; Dong, H.; Wolfrum, C. Plasticity and heterogeneity of thermogenic adipose tissue. Nat. Metab. 2021, 3, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sharma, A.K.; Wolfrum, C. Novel insights into adipose tissue heterogeneity. Rev. Endocr. Metab. Disord. 2022, 23, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Kuang, S. Adipocyte dedifferentiation in health and diseases. Clin. Sci. 2019, 133, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Stenkula, K.G.; Erlanson-Albertsson, C. Adipose cell size: Importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef]
- Weyer, C.; Foley, J.E.; Bogardus, C.; Tataranni, P.A.; Pratley, R.E. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts Type II diabetes independent of insulin resistance. Diabetologia 2000, 43, 1498–1506. [Google Scholar] [CrossRef]
- Celi, F.S. Human Brown Adipose Tissue Plasticity: Hormonal and Environmental Manipulation. In Hormones, Metabolism and the Benefits of Exercise; Spiegelman, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Chen, Y.-R.; Xiao, F.; Tang, H.-N.; Wang, T.; Zhou, Y.-H.; Iqbal, J.; Yang, S.-B.; Li, L.; Zhou, H. Plasticity of adipose tissues in response to fasting and refeeding declines with aging in mice. Aging 2023, 15, 4319–4333. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Tchernof, A.; Desmeules, A.; Richard, C.; Laberge, P.; Daris, M.; Mailloux, J.; Rhéaume, C.; Dupont, P. Ovarian Hormone Status and Abdominal Visceral Adipose Tissue Metabolism. J. Clin. Endocrinol. Metab. 2004, 89, 3425–3430. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-Inducible Factor 1α Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, L.; Gunasekar, S.K.; Tong, D.; Mishra, A.; Gibson, W.J.; Wang, C.; Fidler, T.; Marthaler, B.; Klingelhutz, A.; et al. SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat. Cell Biol. 2017, 19, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Bahceci, M.; Gokalp, D.; Bahceci, S.; Tuzcu, A.; Atmaca, S.; Arikan, S. The correlation between adiposity and adiponectin, tumor necrosis factor α, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J. Endocrinol. Investig. 2007, 30, 210–214. [Google Scholar] [CrossRef]
- Jernås, M.; Palming, J.; Sjöholm, K.; Jennische, E.; Svensson, P.-A.; Gabrielsson, B.G.; Levin, M.; Sjögren, A.; Rudemo, M.; Lystig, T.C.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef]
- Keller, P.; Petrie, J.T.; De Rose, P.; Gerin, I.; Wright, W.S.; Chiang, S.-H.; Nielsen, A.R.; Fischer, C.P.; Pedersen, B.K.; MacDougald, O.A. Fat-specific Protein 27 Regulates Storage of Triacylglycerol. J. Biol. Chem. 2008, 283, 14355–14365. [Google Scholar] [CrossRef]
- He, Y.; Taub, A.B.; Yu, L.; Yao, Y.; Zhang, R.; Zahr, T.; Aaron, N.; LeSauter, J.; Fan, L.; Liu, L.; et al. PPARγ Acetylation Orchestrates Adipose Plasticity and Metabolic Rhythms. Adv. Sci. 2023, 10, e2204190. [Google Scholar] [CrossRef]
- White, U.; Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019, 62, 17–23. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From Stem Cell to Adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef]
- Lu, Q.; Li, M.; Zou, Y.; Cao, T. Induction of adipocyte hyperplasia in subcutaneous fat depot alleviated type 2 diabetes symptoms in obese mice. Obesity 2014, 22, 1623–1631. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Arner, P.; Andersson, D.P.; Wirén, M.; Hoffstedt, J.; Näslund, E.; Thorell, A.; Rydén, M. Variations in the Size of the Major Omentum Are Primarily Determined by Fat Cell Number. J. Clin. Endocrinol. Metab. 2013, 98, E897–E901. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysio-logical cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect. Biol. 2016, 8, a006072. [Google Scholar] [CrossRef]
- Niemann, B.; Niemann, B.; Haufs-Brusberg, S.; Haufs-Brusberg, S.; Puetz, L.; Puetz, L.; Feickert, M.; Feickert, M.; Jaeckstein, M.Y.; Jaeckstein, M.Y.; et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature 2022, 609, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, X.; Yu, L.; Xu, Y.; Zhou, Y.; Qian, S.; Cao, X.; Ye, X.; Yang, J.; Jia, W.; et al. NF-κB regulates brown adipocyte function through suppression of ANT2. Acta Pharm. Sin. B 2022, 12, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.S.; Castro, R.E.; Machado, M.V.; Evangelista, T.; Silvestre, A.; Costa, A.; Coutinho, J.; Carepa, F.; Cortez-Pinto, H.; Rodrigues, C.M.P. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia 2011, 54, 1788–1798. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte Turnover: Relevance to Human Adipose Tissue Morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Li, M.; Liu, Y.; Shi, Y.; Zhang, J. Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis. Biomolecules 2024, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Wang, W.; Kissig, M.; Rajakumari, S.; Huang, L.; Lim, H.-W.; Won, K.-J.; Seale, P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl. Acad. Sci. USA 2014, 111, 14466–14471. [Google Scholar] [CrossRef]
- Qian, S.-W.; Tang, Y.; Li, X.; Liu, Y.; Zhang, Y.-Y.; Huang, H.-Y.; Xue, R.-D.; Yu, H.-Y.; Guo, L.; Gao, H.-D.; et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E798–E807. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Cai, J.; Quan, Y.; Zhu, S.; Lin, J.; Zhang, Q.; Liu, J.; Liang, Z.; Liao, Y.; Jiang, W.; He, Y.; et al. The browning and mobilization of subcutaneous white adipose tissue supports efficient skin repair. Cell Metab. 2024, 36, 1287–1301.e7. [Google Scholar] [CrossRef]
- Shi, L.; Tao, Z.; Zheng, L.; Yang, J.; Hu, X.; Scott, K.; de Kloet, A.; Krause, E.; Collins, J.F.; Cheng, Z. FoxO1 regulates adipose transdifferentiation and iron influx by mediating Tgfβ1 signaling pathway. Redox Biol. 2023, 63, 102727. [Google Scholar] [CrossRef]
- Yadav, H.; Quijano, C.; Kamaraju, A.K.; Gavrilova, O.; Malek, R.; Chen, W.; Zerfas, P.; Zhigang, D.; Wright, E.C.; Stuelten, C.; et al. Protection from Obesity and Diabetes by Blockade of TGF-β/Smad3 Signaling. Cell Metab. 2011, 14, 67–79. [Google Scholar] [CrossRef]
- Bi, P.; Shan, T.; Liu, W.; Yue, F.; Yang, X.; Liang, X.-R.; Wang, J.; Li, J.; Carlesso, N.; Liu, X.; et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat. Med. 2014, 20, 911–918. [Google Scholar] [CrossRef]
- Nanduri, R. Epigenetic Regulators of White Adipocyte Browning. Epigenomes 2021, 5, 3. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; González-Muniesa, P.; Milagro, F.I.; Martínez, J.A. MicroRNAs and other non-coding RNAs in adipose tissue and obesity: Emerging roles as biomarkers and therapeutic targets. Clin. Sci. 2019, 133, 23–40. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, M.; Townsend, K.L.; Huang, T.L.; An, D.; Yan, X.; Xue, R.; Schulz, T.J.; Winnay, J.; Mori, M.; et al. Micro RNA-455 regulates brown adipogenesis via a novel HIF 1an-AMPK—PGC 1α signaling network. Embo Rep. 2015, 16, 1378–1393. [Google Scholar] [CrossRef]
- He, L.; Tang, M.; Xiao, T.; Liu, H.; Liu, W.; Li, G.; Zhang, F.; Xiao, Y.; Zhou, Z.; Liu, F.; et al. Obesity-Associated miR-199a/214 Cluster Inhibits Adipose Browning via PRDM16–PGC-1α Transcriptional Network. Diabetes 2018, 67, 2585–2600. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Gushchina, L.V.; Aubrecht, T.G.; Maurya, S.K.; Periasamy, M.; Nelson, R.J.; Popovich, P.G. miR-155 Deletion in Female Mice Prevents Diet-Induced Obesity. Sci. Rep. 2016, 6, 22862. [Google Scholar] [CrossRef]
- Fischer, C.; Seki, T.; Lim, S.; Nakamura, M.; Andersson, P.; Yang, Y.; Honek, J.; Wang, Y.; Gao, Y.; Chen, F.; et al. A miR-327–FGF10–FGFR2-mediated autocrine signaling mechanism controls white fat browning. Nat. Commun. 2017, 8, 2079. [Google Scholar] [CrossRef]
- Lv, Y.-F.; Yu, J.; Sheng, Y.-L.; Huang, M.; Kong, X.-C.; Di, W.-J.; Liu, J.; Zhou, H.; Liang, H.; Ding, G.-X. Glucocorticoids Suppress the Browning of Adipose Tissue via miR-19b in Male Mice. Endocrinology 2018, 159, 310–322. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.W.; Lee, M.G.; Nam, K.H.; Park, J.H.; Oh, H.; Lee, J.; Han, J.; Yi, S.A.; Han, J. Reversine promotes browning of white adipocytes by suppressing miR-133a. J. Cell. Physiol. 2019, 234, 3800–3813. [Google Scholar] [CrossRef]
- Fu, T.; Seok, S.; Choi, S.; Huang, Z.; Suino-Powell, K.; Xu, H.E.; Kemper, B.; Kemper, J.K. MicroRNA 34a Inhibits Beige and Brown Fat Formation in Obesity in Part by Suppressing Adipocyte Fibroblast Growth Factor 21 Signaling and SIRT1 Function. Mol. Cell. Biol. 2014, 34, 4130–4142. [Google Scholar] [CrossRef]
- Shi, F.; Collins, S. Second messenger signaling mechanisms of the brown adipocyte thermogenic program: An integrative perspective. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170062. [Google Scholar] [CrossRef]
- Collins, S. β-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol. 2011, 2, 102. [Google Scholar] [CrossRef]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Leiva, M.; Matesanz, N.; Pulgarín-Alfaro, M.; Nikolic, I.; Sabio, G. Uncovering the Role of p38 Family Members in Adipose Tissue Physiology. Front. Endocrinol. 2020, 11, 572089. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253. [Google Scholar] [CrossRef]
- Chen, Y.; Ikeda, K.; Yoneshiro, T.; Scaramozza, A.; Tajima, K.; Wang, Q.; Kim, K.; Shinoda, K.; Sponton, C.H.; Brown, Z.; et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 2019, 565, 180–185. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N.; Moreno-Navarrete, J.M.; Contreras, C.; Rial-Pensado, E.; Fernø, J.; Nogueiras, R.; Diéguez, C.; Fernández-Real, J.-M.; López, M. Thyroid hormones induce browning of white fat. J. Endocrinol. 2017, 232, 351–362. [Google Scholar] [CrossRef]
- Lin, J.Z.; Martagón, A.J.; Cimini, S.L.; Gonzalez, D.D.; Tinkey, D.W.; Biter, A.; Baxter, J.D.; Webb, P.; Gustafsson, J.; Hartig, S.M.; et al. Pharmacological Activation of Thyroid Hormone Receptors Elicits a Functional Conversion of White to Brown Fat. Cell Rep. 2015, 13, 1528–1537. [Google Scholar] [CrossRef]
- Finan, B.; Clemmensen, C.; Zhu, Z.; Stemmer, K.; Gauthier, K.; Müller, L.; Moreth, K.; Neff, F.; Perez-Tilve, D.; Fischer, K.; et al. Chemical Hybridization of Glucagon and Thyroid Hormone Optimizes Therapeutic Impact for Metabolic Disease. Cell 2016, 167, 843–857.e14. [Google Scholar] [CrossRef]
- Mahajan, R.D.; Patra, S.K. Irisin, a Novel Myokine Responsible for Exercise Induced Browning of White Adipose Tissue. Indian J. Clin. Biochem. 2013, 28, 102–103. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Castillo-Quan, J.I. From white to brown fat through the PGC-1α-dependent myokine irisin: Implications for diabetes and obesity. Dis. Model. Mech. 2012, 5, 293–295. [Google Scholar] [CrossRef]
- Cao, L.; Choi, E.Y.; Liu, X.; Martin, A.; Wang, C.; Xu, X.; During, M.J. White to Brown Fat Phenotypic Switch Induced by Genetic and Environmental Activation of a Hypothalamic-Adipocyte Axis. Cell Metab. 2011, 14, 324–338. [Google Scholar] [CrossRef]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef]
- Foltz, I.N.; Hu, S.; King, C.; Wu, X.; Yang, C.; Wang, W.; Weiszmann, J.; Stevens, J.; Chen, J.S.; Nuanmanee, N.; et al. Treating Diabetes and Obesity with an FGF21-Mimetic Antibody Activating the βKlotho/FGFR1c Receptor Complex. Sci. Transl. Med. 2012, 4, 162ra153. [Google Scholar] [CrossRef]
- Lee, P.; Brychta, R.J.; Linderman, J.; Smith, S.; Chen, K.Y.; Celi, F.S. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: Relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J. Clin. Endocrinol. Metab. 2013, 98, 98–102. [Google Scholar] [CrossRef]
- Morón-Ros, S.; Uriarte, I.; Berasain, C.; Avila, M.A.; Sabater-Masdeu, M.; Moreno-Navarrete, J.M.; Fernández-Real, J.M.; Giralt, M.; Villarroya, F.; Gavaldà-Navarro, A. FGF15/19 is required for adipose tissue plasticity in response to thermogenic adaptations. Mol. Metab. 2021, 43, 101113. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Abdullahi, A.; Knuth, C.M.; Auger, C.; Sivayoganathan, T.; Parousis, A.; Jeschke, M.G. Adipose browning response to burn trauma is impaired with aging. J. Clin. Investig. 2021, 6, 143451. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 2016, 40, 955–963. [Google Scholar] [CrossRef]
- Petersen, C.; Nielsen, M.D.; Andersen, E.S.; Basse, A.L.; Isidor, M.S.; Markussen, L.K.; Viuff, B.M.; Lambert, I.H.; Hansen, J.B.; Pedersen, S.F. MCT1 and MCT4 Expression and Lactate Flux Activity Increase During White and Brown Adipogenesis and Impact Adipocyte Metabolism. Sci. Rep. 2017, 7, 13101. [Google Scholar] [CrossRef]
- Rabiee, A. Beige Fat Maintenance; Toward a Sustained Metabolic Health. Front. Endocrinol. 2020, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.C.; Tsai, L.T.; Shao, M.; Tenen, D.; Shen, Y.; Kumari, M.; Lyubetskaya, A.; Jacobs, C.; Dawes, B.; Gupta, R.K.; et al. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab. 2018, 27, 1121–1137.e5. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. Mechanisms in endocrinology: White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Bordicchia, M.; Liu, D.; Amri, E.-Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef]
- Liang, X.; Pan, J.; Cao, C.; Zhang, L.; Zhao, Y.; Fan, Y.; Li, K.; Tao, C.; Wang, Y.; Liang, X.; et al. Transcriptional Response of Subcutaneous White Adipose Tissue to Acute Cold Exposure in Mice. Int. J. Mol. Sci. 2019, 20, 3968. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.; Sato, M.; Nowell, C.; Pakzad, M.; Fahey, R.; Gao, J.; Dehvari, N.; Summers, R.J.; Bengtsson, T.; Evans, B.A.; et al. The PPARγ agonist rosiglitazone promotes the induction of brite adipocytes, increasing β-adrenoceptor-mediated mitochondrial function and glucose uptake. Cell. Signal. 2018, 42, 54–66. [Google Scholar] [CrossRef]
- Krause, K. Novel Aspects of White Adipose Tissue Browning by Thyroid Hormones. Exp. Clin. Endocrinol. Diabetes 2020, 128, 446–449. [Google Scholar] [CrossRef]
- Cairó, M.; Villarroya, J. The role of autophagy in brown and beige adipose tissue plasticity. J. Physiol. Biochem. 2020, 76, 213–226. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Corvera, S.; Gealekman, O. Adipose tissue angiogenesis: Impact on obesity and type-2 diabetes. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.B.; Massihnia, D.; Grisafi, F.; Castiglia, M.; Toia, F.; Montesano, L.; Russo, A.; Moschella, F.; Cordova, A. Adipose tissue, angiogenesis and angio-MIR under physiological and pathological conditions. Eur. J. Cell Biol. 2019, 98, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef] [PubMed]
- Briot, A.; Decaunes, P.; Volat, F.; Belles, C.; Coupaye, M.; Ledoux, S.; Bouloumié, A. Senescence Alters PPARγ (Peroxisome Proliferator–Activated Receptor Gamma)-Dependent Fatty Acid Handling in Human Adipose Tissue Microvascular Endothelial Cells and Favors Inflammation. Arter. Thromb. Vasc. Biol. 2018, 38, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.W.; Heo, S.C.; Jeong, G.O.; Yoon, J.W.; Mo, W.M.; Lee, M.J.; Jang, I.-H.; Kwon, S.M.; Lee, J.S.; Kim, J.H. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.J.; Donners, M.M.P.C. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Three-Dimensional Volume Fluorescence-Imaging of Vascular Plasticity in Adipose Tissues—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29914852/ (accessed on 29 October 2022).
- Meng, X.; Qian, X.; Ding, X.; Wang, W.; Yin, X.; Zhuang, G.; Zeng, W. Eosinophils regulate intra-adipose axonal plasticity. Proc. Natl. Acad. Sci. USA 2022, 119, e2112281119. [Google Scholar] [CrossRef]
- Blaszkiewicz, M.; Wood, E.; Koizar, S.; Willows, J.; Anderson, R.; Tseng, Y.-H.; Godwin, J.; Townsend, K.L. The involvement of neuroimmune cells in adipose innervation. Mol. Med. 2020, 26, 126. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, H.; Zeng, W. Whole-tissue 3D imaging reveals intra-adipose sympathetic plasticity regulated by NGF-TrkA signal in cold-induced beiging. Protein Cell 2018, 9, 527–539. [Google Scholar] [CrossRef]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Townsend, K.L. The metabolic and functional roles of sensory nerves in adipose tissues. Nat. Metab. 2023, 5, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Blaszkiewicz, M.; Willows, J.W.; Dubois, A.L.; Waible, S.; DiBello, K.; Lyons, L.L.; Johnson, C.P.; Paradie, E.; Banks, N.; Motyl, K.; et al. Neuropathy and neural plasticity in the subcutaneous white adipose depot. PLoS ONE 2019, 14, e0221766. [Google Scholar] [CrossRef] [PubMed]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.e14. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, A.K.; Van Hauwaert, E.L.; Markussen, L.K.; Gammelmark, E.; Marcher, A.-B.; Ebbesen, M.F.; Nielsen, R.; Brewer, J.R.; Madsen, J.G.S.; Mandrup, S. Plasticity of Epididymal Adipose Tissue in Response to Diet-Induced Obesity at Single-Nucleus Resolution. Cell Metab. 2021, 33, 437–453.e5. [Google Scholar] [CrossRef]
- Liang, W.; Qi, Y.; Yi, H.; Mao, C.; Meng, Q.; Wang, H.; Zheng, C. The Roles of Adipose Tissue Macrophages in Human Disease. Front. Immunol. 2022, 13, 908749. [Google Scholar] [CrossRef]
- Blüher, M.; Bashan, N.; Shai, I.; Harman-Boehm, I.; Tarnovscki, T.; Avinaoch, E.; Stumvoll, M.; Dietrich, A.; Klöting, N.; Rudich, A. Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link mac-rophage infiltration to whole-body Insulin sensitivity. J. Clin. Endocrinol. Metab. 2009, 94, 2507–2515. [Google Scholar] [CrossRef]
- Park, K.S. Aucubin, a naturally occurring iridoid glycoside inhibits TNF-α-induced inflammatory responses through suppression of NF-κB activation in 3T3-L1 adipocytes. Cytokine 2013, 62, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Tang, Y.; Tang, Q.-Q. Adipose tissue plasticity and the pleiotropic roles of BMP signaling. J. Biol. Chem. 2021, 296, 100678. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and Lipid Signaling in the Etiology of Insulin Resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Eagle, A.R.; Vats, D.; Morel, C.R.; Goforth, M.H.; Subramanian, V.; Mukundan, L.; Ferrante, A.W.; Chawla, A. Alternative M2 Activation of Kupffer Cells by PPARδ Ameliorates Obesity-Induced Insulin Resistance. Cell Metab. 2008, 7, 496–507. [Google Scholar] [CrossRef]
- Li, C.; Ying, W.; Huang, Z.; Brehm, T.; Morin, A.; Vella, A.T.; Zhou, B. IRF6 Regulates Alternative Activation by Suppressing PPARγ in Male Murine Macrophages. Endocrinology 2017, 158, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin Promotes Macrophage Polarization toward an Anti-inflammatory Phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Heeren, J.; Scheja, L. Regulation of immunometabolism in adipose tissue. Semin. Immunopathol. 2018, 40, 189–202. [Google Scholar] [CrossRef]
- Abdullahi, A.P.; Auger, C.; Stanojcic, M.P.; Patsouris, D.; Parousis, A.M.; Epelman, S.; Jeschke, M.G. Alternatively Activated Macrophages Drive Browning of White Adipose Tissue in Burns. Ann. Surg. 2019, 269, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.S.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Caslin, H.L.; Bhanot, M.; Bolus, W.R.; Hasty, A.H. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunol. Rev. 2020, 295, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.; Michelet, X.; Zhang, S.; Brennan, P.J.; Moseman, A.; Lester, C.; Besra, G.; Vomhof-Dekrey, E.E.; Tighe, M.; Koay, H.-F.; et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat. Immunol. 2015, 16, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Pramyothin, P.; Karastergiou, K.; Fried, S.K. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta (BBA) 2014, 1842, 473–481. [Google Scholar] [CrossRef]
- Mancuso, P.; Mancuso, P.; Bouchard, B.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. 2019, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Akopians, A.L.; Madrigal, V.K.; Ramirez, E.; Margolis, D.J.; Sarma, M.K.; Thomas, A.M.; Grogan, T.R.; Haykal, R.; Schooler, T.A.; et al. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J. Clin. Endocrinol. Metab. 2016, 101, 4178–4188. [Google Scholar] [CrossRef]
- Wang, G.; Song, A.; Bae, M.; Wang, Q.A. Adipose Tissue Plasticity in Aging. Compr. Physiol. 2022, 12, 4119–4132. [Google Scholar] [CrossRef]
- Kim, J.-Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ryu, R.; Choi, J.-Y.; Choi, M.-S. Platycodon grandiflorus Root Ethanol Extract Induces Lipid Excretion, Lipolysis, and Thermogenesis in Diet-Induced Obese Mice. J. Med. Food 2019, 22, 1100–1109. [Google Scholar] [CrossRef]
- Zamboni, M.; Rossi, A.P.; Fantin, F.; Zamboni, G.; Chirumbolo, S.; Zoico, E.; Mazzali, G. Adipose tissue, diet and aging. Mech. Ageing Dev. 2014, 136–137, 129–137. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Latre, A.R.; Kolonin, M.G. Adipose Stromal Cell Expansion and Exhaustion: Mechanisms and Consequences. Cells 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Ge, G.; Yang, P.; Wang, L.; Qiao, Y.; Pan, G.; Yang, H.; Bai, J.; Cui, W.; Geng, D. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv. Sci. 2023, 10, e2207334. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, N.; Zhang, P.; Jiang, B. Local transplantation of osteogenic pre-differentiated autologous adipose-derived mesenchymal stem cells may accelerate non-union fracture healing with limited pro-metastatic potency. Int. J. Clin. Exp. Med. 2015, 8, 1406–1410. [Google Scholar]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The Role of C/EBP Genes in Adipocyte Differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef]
- Shan, B.; Barker, C.S.; Shao, M.; Zhang, Q.; Gupta, R.K.; Wu, Y. Multilayered omics reveal sex- and depot-dependent adipose progenitor cell heterogeneity. Cell Metab. 2022, 34, 783–799.e7. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S. Role of kruppel-like transcription factors in adipogenesis. Dev. Biol. 2013, 373, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Chen, Z.; Friedman, J. Transcriptional Regulation of Adipogenesis by KLF4. Cell Metab. 2008, 7, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Choy, L.; Derynck, R. Transforming Growth Factor-β Inhibits Adipocyte Differentiation by Smad3 Interacting with CCAAT/Enhancer-binding Protein (C/EBP) and Repressing C/EBP Transactivation Function. J. Biol. Chem. 2003, 278, 9609–9619. [Google Scholar] [CrossRef]
- BMP Signaling Pathway Is Required for Commitment of C3H10T1/2 Pluripotent Stem Cells to the Adipocyte Lineage—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19620713/ (accessed on 17 October 2022).
- Lijnen, H.; Maquoi, E.; Hansen, L.; Van Hoef, B.; Frederix, L.; Collen, D. Matrix Metalloproteinase Inhibition Impairs Adipose Tissue Development in Mice. Arter. Thromb. Vasc. Biol. 2002, 22, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Guerrero-Juarez, C.F.; Ito, M.; Li, Y.R.; Dedhia, P.H.; Zheng, Y.; Shao, M.; Gay, D.L.; Ramos, R.; Hsi, T.-C.; et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 2017, 355, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Bochet, L.; Lehuédé, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef]
- Bi, P.; Yue, F.; Karki, A.; Castro, B.; Wirbisky, S.E.; Wang, C.; Durkes, A.; Elzey, B.D.; Andrisani, O.M.; Bidwell, C.A.; et al. Notch activation drives adipocyte dedifferentiation and tumorigenic transformation in mice. J. Exp. Med. 2016, 213, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Darra, E.; Rizzatti, V.; Budui, S.; Franceschetti, G.; Mazzali, G.; Rossi, A.P.; Fantin, F.; Menegazzi, M.; Cinti, S.; et al. Adipocytes WNT5a mediated dedifferentiation: A possible target in pancreatic cancer microenvironment. Oncotarget 2016, 7, 20223–20235. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.D.; Wei, J.; Wood, T.A.; Graham, L.V.; Whitfield, M.L.; Scherer, P.E.; Tourtellotte, W.G.; Varga, J. Myofibroblasts in Murine Cutaneous Fibrosis Originate from Adiponectin-Positive Intradermal Progenitors. Arthritis Rheumatol. 2015, 67, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Ohgo, S.; Hasegawa, S.; Hasebe, Y.; Mizutani, H.; Nakata, S.; Akamatsu, H. Bleomycin inhibits adipogenesis and accelerates fibrosis in the subcutaneous adipose layer through TGF-β1. Exp. Dermatol. 2013, 22, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-Reducing Effects of the Plasma Protein Encoded by the obese Gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; O’Rahilly, S. Mutations in ligands and receptors of the leptin–melanocortin pathway that lead to obesity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Pirzgalska, R.M.; Pereira, M.M.; Kubasova, N.; Barateiro, A.; Seixas, E.; Lu, Y.-H.; Kozlova, A.; Voss, H.; Martins, G.G.; et al. Sympathetic Neuro-adipose Connections Mediate Leptin-Driven Lipolysis. Cell 2015, 163, 84–94. [Google Scholar] [CrossRef]
- Li, R.-M.; Chen, S.-Q.; Zeng, N.-X.; Zheng, S.-H.; Guan, L.; Liu, H.-M.; Zhou, L.-Q.; Xu, J.-W. Browning of Abdominal Aorta Perivascular Adipose Tissue Inhibits Adipose Tissue Inflammation. Metab. Syndr. Relat. Disord. 2017, 15, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Gesmundo, I.; Pardini, B.; Gargantini, E.; Gamba, G.; Birolo, G.; Fanciulli, A.; Banfi, D.; Congiusta, N.; Favaro, E.; Deregibus, M.C.; et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic β cells. J. Clin. Investig. 2021, 6, 141962. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Lazic, M.; Armando, A.M.; Phillips, S.A.; Katebian, R.; Maraka, S.; Quehenberger, O.; Sears, D.D.; Feldstein, A.E. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J. Mol. Med. 2016, 94, 1241–1253. [Google Scholar] [CrossRef]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Huang-Doran, I.; Zhang, C.-Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Storti, G.; Scioli, M.G.; Kim, B.-S.; Terriaca, S.; Fiorelli, E.; Orlandi, A.; Cervelli, V. Mesenchymal Stem Cells in Adipose Tissue and Extracellular Vesicles in Ovarian Cancer Patients: A Bridge toward Metastatic Diffusion or a New Therapeutic Opportunity? Cells 2021, 10, 2117. [Google Scholar] [CrossRef]
- Röszer, T. Metabolic impact of adipose tissue macrophages in the early postnatal life. J. Leukoc. Biol. 2022, 112, 1515–1524. [Google Scholar] [CrossRef]
- McMorrow, A.M.; Connaughton, R.M.; Lithander, F.E.; Roche, H.M. Adipose tissue dysregulation and metabolic consequences in childhood and adolescent obesity: Potential impact of dietary fat quality. Proc. Nutr. Soc. 2015, 74, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef]
- Carobbio, S.; Pellegrinelli, V.; Vidal-Puig, A. Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 161–196. [Google Scholar] [CrossRef]
- Rydén, M.; Andersson, D.P.; Bergström, I.B.; Arner, P. Adipose tissue and metabolic alterations: Regional differences in fat cell size and number matter, but differently: A cross-sectional study. J. Clin. Endocrinol. Metab. 2014, 99, E1870–E1876. [Google Scholar] [CrossRef] [PubMed]
- Lacasa, D.; Taleb, S.; Keophiphath, M.; Miranville, A.; Clement, K. Macrophage-Secreted Factors Impair Human Adipogenesis: Involvement of Proinflammatory State in Preadipocytes. Endocrinology 2007, 148, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef]
- Yaghootkar, H.; Lotta, L.A.; Tyrrell, J.; Smit, R.A.; Jones, S.E.; Donnelly, L.; Beaumont, R.; Campbell, A.; Tuke, M.A.; Hayward, C.; et al. Genetic Evidence for a Link between Favorable Adiposity and Lower Risk of Type 2 Diabetes, Hypertension, and Heart Disease. Diabetes 2016, 65, 2448–2460. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.-Y.; Zhang, H.; Tan, P.-C.; Zhou, S.-B.; Li, Q.-F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, T.P.; Kogan, S.; Aouadi, M.; Hendricks, G.M.; Straubhaar, J.; Czech, M.P. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1425–H1437. [Google Scholar] [CrossRef]
- Sakamoto, T. Inflammation induced by RAW macrophages suppresses UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes. Am. J. Physiol. Cell. Physiol. 2013, 304, 729–738. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Heuvingh, J.; du Roure, O.; Rouault, C.; Devulder, A.; Klein, C.; Lacasa, M.; Clément, E.; Lacasa, D.; Clément, K. Human adipocyte function is impacted by mechanical cues. J. Pathol. 2014, 233, 183–195. [Google Scholar] [CrossRef]
- Abdennour, M.; Reggio, S.; Le Naour, G.; Liu, Y.; Poitou, C.; Aron-Wisnewsky, J.; Charlotte, F.; Bouillot, J.-L.; Torcivia, A.; Sasso, M.; et al. Association of Adipose Tissue and Liver Fibrosis with Tissue Stiffness in Morbid Obesity: Links with Diabetes and BMI Loss After Gastric Bypass. J. Clin. Endocrinol. Metab. 2014, 99, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Tchoukalova, Y.; Koutsari, C.; Jensen, M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia 2007, 50, 151–157. [Google Scholar] [CrossRef]
- Haque, W.A.; Oral, E.A.; Dietz, K.; Bowcock, A.M.; Agarwal, A.K.; Garg, A. Risk Factors for Diabetes in Familial Partial Lipodystrophy, Dunnigan Variety. Diabetes Care 2003, 26, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Wong, G.L.-H.; Yeung, D.K.-W.; Abrigo, J.M.; Kong, A.P.-S.; Chan, R.S.-M.; Chim, A.M.-L.; Shen, J.; Ho, C.-S.; Woo, J.; et al. Fatty Pancreas, Insulin Resistance, and β-Cell Function: A Population Study Using Fat-Water Magnetic Resonance Imaging. Am. J. Gastroenterol. 2014, 109, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Valencia, A.; Startzell, M.; Cochran, E.; Walter, P.J.; Garraffo, H.M.; Cai, H.; Gharib, A.M.; Ouwerkerk, R.; Courville, A.B.; et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J. Clin. Investig. 2018, 128, 3504–3516. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; Hebestreit, H.; Hofmann, A.K.; Strauss, G.; Möller, P.; Debatin, K.-M.; Wabitsch, M. Role of CD95-Mediated Adipocyte Loss in Autoimmune Lipodystrophy. J. Clin. Endocrinol. Metab. 2006, 91, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Torriani, M.; Srinivasa, S.; Fitch, K.V.; Thomou, T.; Wong, K.; Petrow, E.; Kahn, C.R.; Cypess, A.M.; Grinspoon, S.K. Dysfunctional Subcutaneous Fat with Reduced Dicer and Brown Adipose Tissue Gene Expression in HIV-Infected Patients. J. Clin. Endocrinol. Metab. 2016, 101, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, M.; Wang, S.; Chen, L.; Li, Z.; Li, C.; Cao, P.; Chen, Y. Lactate Is a Key Mediator That Links Obesity to Insulin Resistance via Modulating Cytokine Production from Adipose Tissue. Diabetes 2022, 71, 637–652. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rodriguez-Cuenca, S.; Rouault, C.; Figueroa-Juarez, E.; Schilbert, H.; Virtue, S.; Moreno-Navarrete, J.M.; Bidault, G.; Vázquez-Borrego, M.C.; Dias, A.R.; et al. Dysregulation of macrophage PEPD in obesity determines adipose tissue fibro-inflammation and insulin resistance. Nat. Metab. 2022, 4, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sun, W.; Shen, Y.; Baláz, M.; Balázová, L.; Ding, L.; Löffler, M.; Hamilton, B.; Klöting, N.; Blüher, M.; et al. Identification of a regulatory pathway inhibiting adipogenesis via RSPO2. Nat. Metab. 2022, 4, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Choi, W.; Oh, T.J.; Cha, H.-N.; Hwang, I.; Lee, Y.K.; Lee, S.Y.; Shin, H.; Lim, A.; Ryu, D.; et al. Inhibiting serotonin signaling through HTR2B in visceral adipose tissue improves obesity-related insulin resistance. J. Clin. Investig. 2021, 131, e145331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Gasek, N.S.; Zhou, Y.; Cohn, R.L.; Martin, D.E.; Zuo, W.; Flynn, W.F.; Guo, C.; Jellison, E.R.; et al. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 2022, 34, 75–89.e8. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Psilopanagioti, A.; Papadaki, H.; Kranioti, E.F.; Alexandrides, T.K.; Varakis, J.N. Expression of Adiponectin and Adiponectin Receptors in Human Pituitary Gland and Brain. Neuroendocrinology 2009, 89, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, K.; Moon, Y.S.; Sul, H.S. A Cysteine-rich Adipose Tissue-specific Secretory Factor Inhibits Adipocyte Differentiation. J. Biol. Chem. 2001, 276, 11252–11256. [Google Scholar] [CrossRef]
- Spicer, L.J.; Schreiber, N.B.; Lagaly, D.V.; Aad, P.Y.; Douthit, L.B.; Grado-Ahuir, J.A. Effect of resistin on granulosa and theca cell function in cattle. Anim. Reprod. Sci. 2011, 124, 19–27. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; González, F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil. Steril. 2011, 95, 1048–1058.e2. [Google Scholar] [CrossRef]
- González, F.; Considine, R.V.; Abdelhadi, O.A.; Acton, A.J. Inflammation Triggered by Saturated Fat Ingestion Is Linked to Insulin Resistance and Hyperandrogenism in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, E2152–E2167. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L.; Ryan, K.J. Hyperandrogenism, insulin resistance, and acanthosis nigricans syndrome: A common endocrinopathy with distinct pathophysiologic features. Am. J. Obstet. Gynecol. 1983, 147, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Mathur, R.; Purwar, N.; Mathur, S.K.; Mathur, D.K. Hyperandrogenism, Insulin Resistance, and Acanthosis Nigricans (HAIR-AN) Syndrome Reflects Adipose Tissue Dysfunction (“Adiposopathy” or “Sick Fat”) in Asian Indian Girls. Dermatology 2021, 237, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Gołacki, J.; Matuszek, M.; Matyjaszek-Matuszek, B. Link between Insulin Resistance and Obesity—From Diagnosis to Treatment. Diagnostics 2022, 12, 1681. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.P.; Memije, M.V.; Tamayo, M.T.; Carreón, A.R. Skin disorders in overweight and obese patients and their relationship with insulin. Actas Dermosifiliogr. 2014, 105, 178–185. [Google Scholar] [CrossRef]
- Kutbay, N. An Unusual Case of Acquired Partial Lipodystrophy Presenting with Acanthosis Nigricans. Acta Endocrinol. 2019, 15, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, F.; Akcan, N.; Dastamani, A.; Morgan, K.; Semple, R.K.; Shah, P. Unusual Glycemic Presentations in a Child with a Novel Heterozygous Intragenic INSR Deletion. Horm. Res. Paediatr. 2020, 93, 396–401. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, P.; Zhang, S.-Y.; Dong, Y.; Zeng, G.; Yan, Y.; Sun, L.; Wu, Q.; Liu, H.; et al. Adipocyte Hypoxia-Inducible Factor 2α Suppresses Atherosclerosis by Promoting Adipose Ceramide Catabolism. Cell Metab. 2019, 30, 937–951.e5. [Google Scholar] [CrossRef]
- Hu, H.; Garcia-Barrio, M.; Jiang, Z.-S.; Chen, Y.E.; Chang, L. Roles of Perivascular Adipose Tissue in Hypertension and Atherosclerosis. Antioxid. Redox Signal. 2021, 34, 736–749. [Google Scholar] [CrossRef]
- Mancio, J.; Oikonomou, E.K.; Antoniades, C. Perivascular adipose tissue and coronary atherosclerosis. Heart 2018, 104, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ueda, K.; Nomura, S.; Ito, K.; Katoh, M.; Katagiri, M.; Yamada, S.; Hashimoto, M.; Zhai, B.; Numata, G.; et al. Beiging of perivascular adipose tissue regulates its inflammation and vascular remodeling. Nat. Commun. 2022, 13, 5117. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Qian, S.; Song, Y.; Yang, L.; Song, S.; Yang, Q.; Liu, H.; Liu, Y.; Pan, D.; Tang, Y.; et al. BMP4-mediated browning of perivascular adipose tissue governs an anti-inflammatory program and prevents atherosclerosis. Redox Biol. 2021, 43, 101979. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Alharthi, J.; Latchoumanin, O.; George, J.; Eslam, M. Macrophages in metabolic associated fatty liver disease. World J. Gastroenterol. 2020, 26, 1861–1878. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Fukushima, J.; Kamada, Y.; Matsumoto, H.; Yoshida, Y.; Ezaki, H.; Takemura, T.; Saji, Y.; Igura, T.; Tsutsui, S.; Kihara, S.; et al. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol. Res. 2009, 39, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leclercq, I.; Brymora, J.M.; Xu, N.; Ramezani–Moghadam, M.; London, R.M.; Brigstock, D.; George, J. Kupffer Cells Mediate Leptin-Induced Liver Fibrosis. Gastroenterology 2009, 137, 713–723.e1. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.C.; Chen, S.-C.; Jackson, J.V.; Rojas-Triana, A.; Kinsley, D.; Cui, L.; Fine, J.S.; Greenfeder, S.; Bober, L.A.; Jenh, C.-H. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. 2011, 8, 8. [Google Scholar] [CrossRef]
- Luo, W.; Xu, Q.; Wang, Q.; Wu, H.; Hua, J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci. Rep. 2017, 7, srep44612. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Miserocchi, G.; De Vita, A.; Spadazzi, C.; Cocchi, C.; Vanni, S.; Gabellone, S.; Martinelli, G.; Ranallo, N.; Bongiovanni, A.; et al. Lipids and adipocytes involvement in tumor progression with a focus on obesity and diet. Obes. Rev. 2024, e13833, online ahead of print. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T.; et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017, 5, 1. [Google Scholar] [CrossRef]
- Zhang, M.; Di Martino, J.S.; Bowman, R.L.; Campbell, N.R.; Baksh, S.C.; Simon-Vermot, T.; Kim, I.S.; Haldeman, P.; Mondal, C.; Yong-Gonzales, V.; et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov. 2018, 8, 1006–1025. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer—Mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013, 18, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta 2014, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of Ppary. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARγ agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lai, X.-R.; Lu, J.-Q.; Tang, L.-Z.; Zhang, J.-R.; Liu, H.-W. Decellularized adipose tissue: A key factor in promoting fat regeneration by recruiting and inducing mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2021, 541, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Zhu, X.; Meng, M.; Li, L.; Shen, Y.; Chi, Q.; Wang, D.; Zhang, Z.; Li, C.; et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013, 23, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; He, X.; Sheng, Y.; Yang, C.; Li, H.; Xu, J.; Xu, W.; Huang, K. Caulis Spatholobi Ameliorates Obesity through Activating Brown Adipose Tissue and Modulating the Composition of Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5150. [Google Scholar] [CrossRef]
- Quan, L.-H.; Zhang, C.; Dong, M.; Jiang, J.; Xu, H.; Yan, C.; Liu, X.; Zhou, H.; Zhang, H.; Chen, L.; et al. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut 2020, 69, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Cheong, L.Y.; Xu, A. Intercellular and inter-organ crosstalk in browning of white adipose tissue: Molecular mechanism and therapeutic complications. J. Mol. Cell Biol. 2021, 13, 466–479. [Google Scholar] [CrossRef]
- Singh, A.K.; Aryal, B.; Chaube, B.; Rotllan, N.; Varela, L.; Horvath, T.L.; Suárez, Y.; Fernández-Hernando, C. Brown adipose tissue derived ANGPTL4 controls glucose and lipid metabolism and regulates thermogenesis. Mol. Metab. 2018, 11, 59–69. [Google Scholar] [CrossRef]
- Giordano, A.; Frontini, A.; Cinti, S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 2016, 15, 405–424. [Google Scholar] [CrossRef]
- Liao, W.; Yin, X.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Resveratrol-Induced White Adipose Tissue Browning in Obese Mice by Remodeling Fecal Microbiota. Molecules 2018, 23, 3356. [Google Scholar] [CrossRef] [PubMed]
- Mader, I.; Wabitsch, M.; Debatin, K.; Fischer-Posovszky, P.; Fulda, S. Identification of a novel proapoptotic function of resveratrol in fat cells: SIRT1-independent sensitization to TRAIL-induced apoptosis. FASEB J. 2010, 24, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- The Key Role of a Glucagon-Like Peptide-1 Receptor Agonist in Body Fat Redistribution—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30530905/ (accessed on 9 October 2022).
- Zhu, D.; Li, X.; Ma, J.; Zeng, J.; Gan, S.; Dong, X.; Yang, J.; Lin, X.; Cai, H.; Song, W.; et al. Dorzagliatin in drug-naïve patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 965–973. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Liñares-Pose, L.; Rial-Pensado, E.; Romero-Picó, A.; Moreno-Navarrete, J.M.; Martínez-Sánchez, N.; Garrido-Gil, P.; Iglesias-Rey, R.; Morgan, D.A.; Tomasini, N.; et al. Central nicotine induces browning through hypothalamic κ opioid receptor. Nat. Commun. 2019, 10, 4037. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Palou, A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, P.; Valente, A.; Carrillo, A.E.; Metsios, G.S.; Nadolnik, L.; Jamurtas, A.Z.; Koutedakis, Y.; Boguszewski, C.; Andrade, C.M.B.; Svensson, P.-A.; et al. Chronic l-menthol-induced browning of white adipose tissue hypothesis: A putative therapeutic regime for combating obesity and improving metabolic health. Med. Hypotheses 2016, 93, 21–26. [Google Scholar] [CrossRef]
- Lee, C.G.; Rhee, D.K.; Kim, B.O.; Um, S.H.; Pyo, S. Allicin induces beige-like adipocytes via KLF15 signal cascade. J. Nutr. Biochem. 2019, 64, 13–24. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.-Z.; Shen, J.-Z.; Chen, L.; He, T.; Jin, M.-W.; Liu, H. Pentamethylquercetin induces adipose browning and exerts beneficial effects in 3T3-L1 adipocytes and high-fat diet-fed mice. Sci. Rep. 2017, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P.; et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syn-drome in mice. Sci. Transl. Med. 2020, 12, eaaz8664. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Rennekampff, H.O.; Engeli, S. Apoptotic pathways in adipose tissue. Apoptosis 2013, 18, 911–916. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, C.T.; Kim, Y. Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expres-sion in adipose tissue of diet-induced obese mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Grigem, S.; Fischer-Posovszky, P.; Debatin, K.M.; Loizon, E.; Vidal, H.; Wabitsch, M. The effect of the HIV protease inhibitor ritonavir on proliferation, differentiation, lipogenesis, gene expression and apoptosis of human preadipocytes and adipocytes. Horm. Metab. Res. 2005, 37, 602–609. [Google Scholar] [CrossRef]
- Liao, Z.-Z.; Ran, L.; Qi, X.-Y.; Wang, Y.-D.; Wang, Y.-Y.; Yang, J.; Liu, J.-H.; Xiao, X.-H. Adipose endothelial cells mastering adipose tissues metabolic fate. Adipocyte 2022, 11, 108–119. [Google Scholar] [CrossRef]
- Xian, Y.; Chen, Z.; Deng, H.; Cai, M.; Liang, H.; Xu, W.; Weng, J.; Xu, F. Exenatide mitigates inflammation and hypoxia along with improved angiogenesis in obese fat tissue. J. Endocrinol. 2019, 242, 79–89. [Google Scholar] [CrossRef]
- Kito, T.; Shibata, R.; Kondo, M.; Yamamoto, T.; Suzuki, H.; Ishii, M.; Murohara, T. Nifedipine ameliorates ischemia-induced revascularization in diet-induced obese mice. Am. J. Hypertens. 2012, 25, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lin, F.; Wan, Q.; Wu, J.; Luo, M. Mechanisms of action of metformin and its regulatory effect on microRNAs related to angiogenesis. Pharmacol. Res. 2021, 164, 105390. [Google Scholar] [CrossRef] [PubMed]
- Duerre, D.J.; Galmozzi, A. Deconstructing Adipose Tissue Heterogeneity One Cell at a Time. Front. Endocrinol. 2022, 13, 847291. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Liang, S.; Guo, C.; Yu, X.; Zhao, J.; Zhang, H.; Shang, W. Inhibition of M1 macrophage activation in adipose tissue by berberine improves insulin resistance. Life Sci. 2016, 166, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.L.; Zanni, M.V.; Johnsen, S.; Rasheed, S.; Makimura, H.; Lee, H.; Khor, V.K.; Ahima, R.S.; Grinspoon, S.K. TNF-α Antagonism with Etanercept Decreases Glucose and Increases the Proportion of High Molecular Weight Adiponectin in Obese Subjects with Features of the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E146–E150. [Google Scholar] [CrossRef]
- Braun, K.; Oeckl, J.; Westermeier, J.; Li, Y.; Klingenspor, M. Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb165381. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef]
- Chan, M.; Lim, Y.C.; Yang, J.; Namwanje, M.; Liu, L.; Qiang, L. Identification of a natural beige adipose depot in mice. J. Biol. Chem. 2019, 294, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Pink Adipocytes. Trends Endocrinol. Metab. 2018, 29, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Prokesch, A.; Smorlesi, A.; Perugini, J.; Manieri, M.; Ciarmela, P.; Mondini, E.; Trajanoski, Z.; Kristiansen, K.; Giordano, A.; Bogner-Strauss, J.; et al. Molecular Aspects of Adipoepithelial Transdifferentiation in Mouse Mammary Gland. Stem Cells 2014, 32, 2756–2766. [Google Scholar] [CrossRef]
- Giordano, A.; Perugini, J.; Kristensen, D.M.; Sartini, L.; Frontini, A.; Kajimura, S.; Kristiansen, K.; Cinti, S. Mammary alveolar epithelial cells convert to brown adipocytes in post-lactating mice. J. Cell. Physiol. 2017, 232, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Ricquier, D. UCP1, the mitochondrial uncoupling protein of brown adipocyte: A personal contribution and a historical perspective. Biochimie 2017, 134, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sebo, Z.L.; Jeffery, E.; Holtrup, B.; Rodeheffer, M.S. A mesodermal fate map for adipose tissue. Development 2018, 145, dev.166801. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Sanchez-Gurmaches, J.; Guertin, D.A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 2014, 5, 4099. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Thaler, R.; Glasstetter, L.M.; Xing, L.; Zhu, X.-Y.; Osborne, A.C.; Mondesir, R.; Bhagwate, A.V.; Lerman, A.; van Wijnen, A.J.; et al. Obesity-driven mitochondrial dysfunction in human adipose tissue-derived mesenchymal stem/stromal cells involves epigenetic changes. Cell Death Dis. 2024, 15, 387. [Google Scholar] [CrossRef] [PubMed]
- Schwalie, P.C.; Dong, H.; Zachara, M.; Russeil, J.; Alpern, D.; Akchiche, N.; Caprara, C.; Sun, W.; Schlaudraff, K.-U.; Soldati, G.; et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018, 559, 103–108. [Google Scholar] [CrossRef]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.; Clément, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Bäckdahl, J.; Franzén, L.; Massier, L.; Li, Q.; Jalkanen, J.; Gao, H.; Andersson, A.; Bhalla, N.; Thorell, A.; Rydén, M.; et al. Spatial mapping reveals human adipocyte subpopulations with distinct sensitivities to insulin. Cell Metab. 2021, 33, 1869–1882.e6. [Google Scholar] [CrossRef] [PubMed]
- Rogal, J.; Roosz, J.; Teufel, C.; Cipriano, M.; Xu, R.; Eisler, W.; Weiss, M.; Schenke-Layland, K.; Loskill, P. Autologous Human Immunocompetent White Adipose Tissue-on-Chip. Adv. Sci. 2022, 9, e2104451. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Y.-Y.; Han, Y.-X.; Xu, S.-N.; Jiang, H.-L.; Wu, H.-X.; Cai, J.-M.; Li, L.; Bu, Y.-H.; Xiao, F.; Liang, H.-D.; et al. Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight. Biomolecules 2024, 14, 1223. https://doi.org/10.3390/biom14101223
Mo Y-Y, Han Y-X, Xu S-N, Jiang H-L, Wu H-X, Cai J-M, Li L, Bu Y-H, Xiao F, Liang H-D, et al. Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight. Biomolecules. 2024; 14(10):1223. https://doi.org/10.3390/biom14101223
Chicago/Turabian StyleMo, Yu-Yao, Yu-Xin Han, Shi-Na Xu, Hong-Li Jiang, Hui-Xuan Wu, Jun-Min Cai, Long Li, Yan-Hong Bu, Fen Xiao, Han-Dan Liang, and et al. 2024. "Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight" Biomolecules 14, no. 10: 1223. https://doi.org/10.3390/biom14101223
APA StyleMo, Y.-Y., Han, Y.-X., Xu, S.-N., Jiang, H.-L., Wu, H.-X., Cai, J.-M., Li, L., Bu, Y.-H., Xiao, F., Liang, H.-D., Wen, Y., Liu, Y.-Z., Yin, Y.-L., & Zhou, H.-D. (2024). Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight. Biomolecules, 14(10), 1223. https://doi.org/10.3390/biom14101223




