The Important Role of Aquaglyceroporin 7 in Health and Disease
Abstract
:1. Introduction
2. Structure and Tissue Distribution
3. Role of AQP7 in Health
3.1. Transport of Glycerol and Other Substances
3.2. Regulation of Insulin Release
3.3. Involvement in Immune Responses
3.4. Facilitation of Cell Differentiation and Proliferation
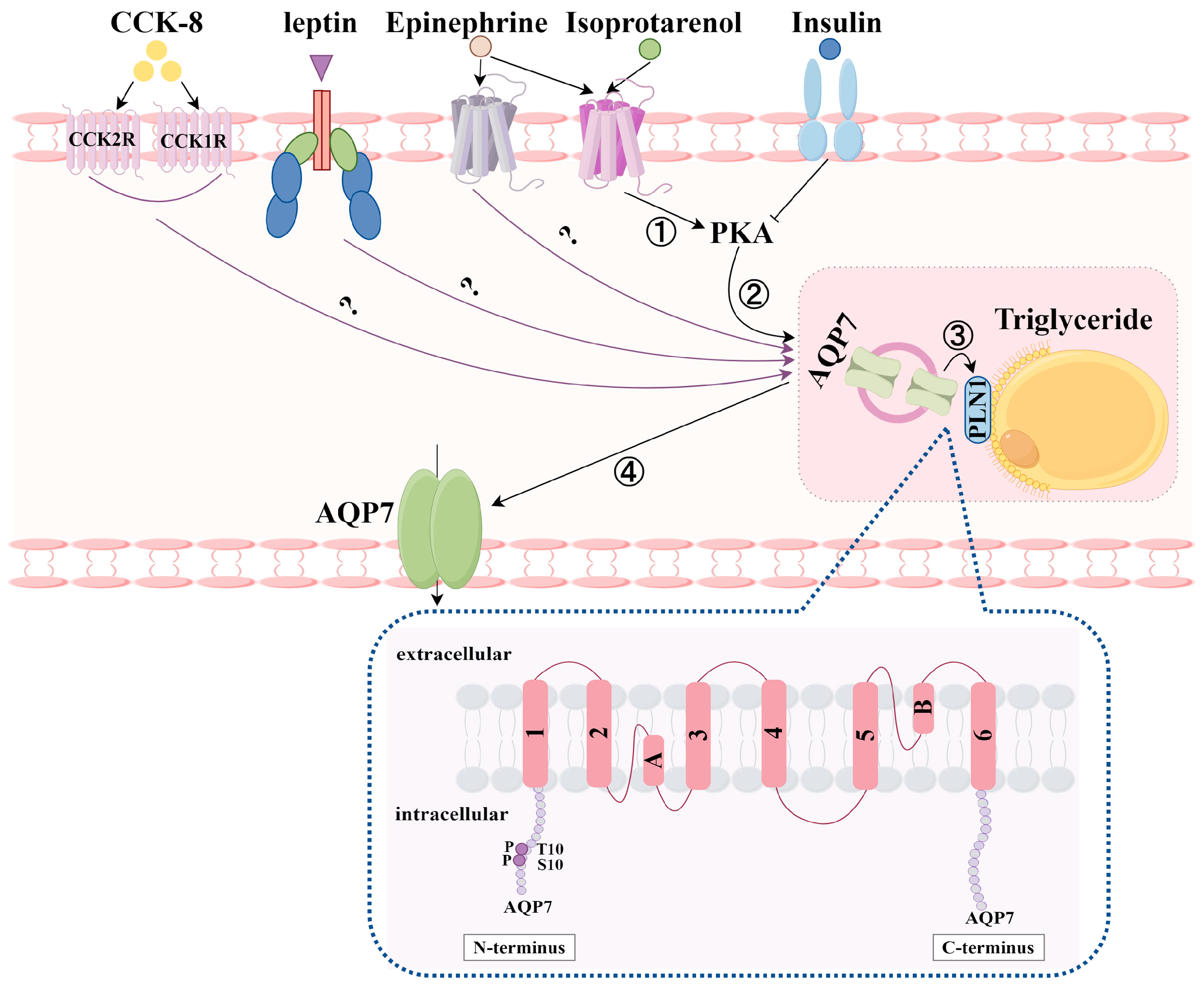
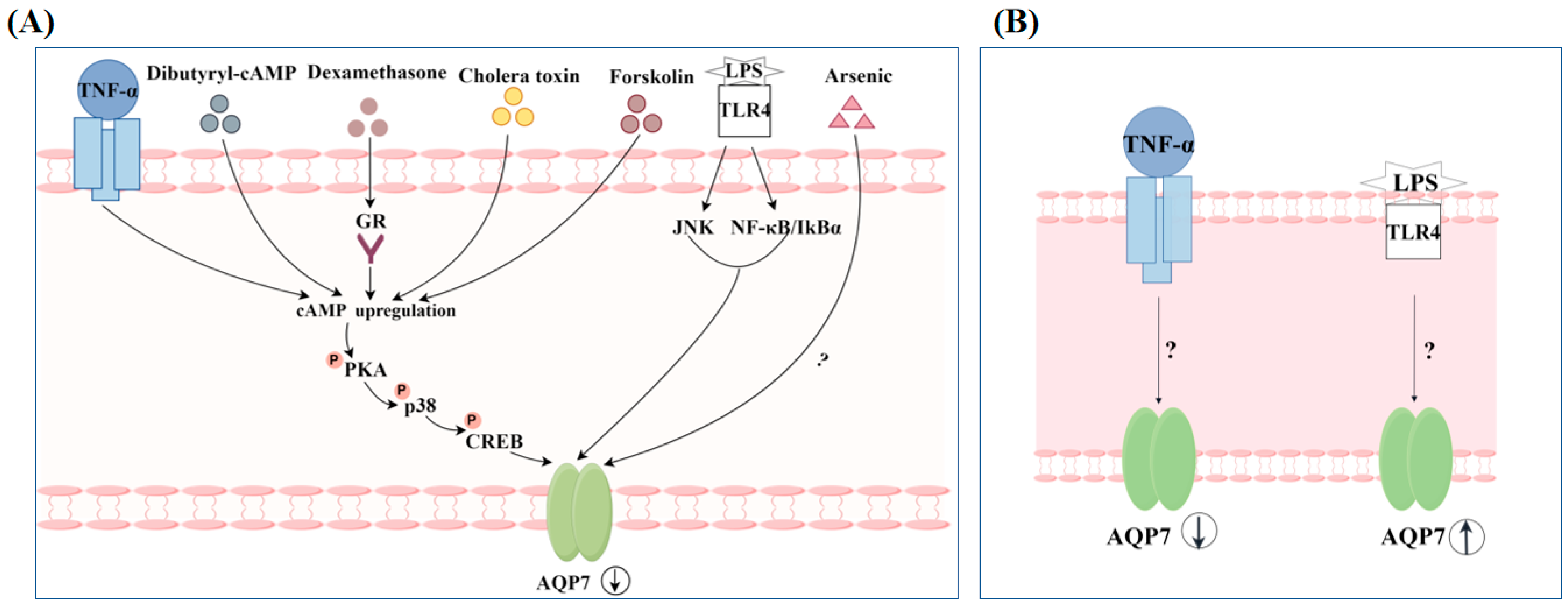
4. Regulation of AQP7 by Hormones and Non-Hormonal Substances
4.1. Regulation of AQP7 by Hormones
4.1.1. AQP7 Expression Levels Regulated by Hormones
- Insulin
- Leptin
- Cortisone
- Cholecystokinin
- Ghrelin
- Uroguanylin and guanylin
- Sex hormones
- Follicle-stimulating hormone
- Isoproterenol
- Glucagon-like peptide-1
4.1.2. Translocation of AQP7 by Hormones
4.2. Regulation of AQP7 by Non-Hormonal Substances
5. Involvement of AQP7 in Multiple Diseases
5.1. Obesity
5.2. T2DM and Its Complications
5.3. Cardiac Diseases
5.4. Cancer
5.5. Inflammatory Bowel Disease
6. AQP7 as Therapeutic Target
6.1. Treatment of Obesity
6.2. Treatment of T2DM and Its Complications
6.3. Treatment of Cardiac Diseases
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nejsum, L.N. The renal plumbing system: Aquaporin water channels. Cell. Mol. Life Sci. 2005, 62, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Knepper, M.A.; Kwon, T.-H.; Nielsen, S. Molecular Physiology of Water Balance Reply. N. Engl. J. Med. 2015, 373, 196. [Google Scholar] [PubMed]
- Verkman, A.S. Aquaporins at a glance. J. Cell Sci. 2011, 124 Pt 13, 2107–2112. [Google Scholar] [CrossRef]
- Bill, R.M.; Hedfalk, K. Aquaporins—Expression, purification and characterization. Biochim. Biophys. Acta. Biomembr. 2021, 1863, 183650. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Walz, T. The structure of aquaporins. Q. Rev. Biophys. 2006, 39, 361–396. [Google Scholar] [CrossRef]
- Jung, H.J.; Jang, H.J.; Kwon, T.H. Aquaporins implicated in the cell proliferation and the signaling pathways of cell stemness. Biochimie 2021, 188, 52–60. [Google Scholar] [CrossRef]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell: An update. Biochim. Biophys. Acta. Biomembr. 2021, 1863, 183617. [Google Scholar] [CrossRef]
- Brisson, D.; Vohl, M.C.; St-Pierre, J.; Hudson, T.J.; Gaudet, D. Glycerol: A neglected variable in metabolic processes? BioEssays News Rev. Mol. Cell. Dev. Biol. 2001, 23, 534–542. [Google Scholar] [CrossRef]
- Calamita, G.; Delporte, C. Involvement of aquaglyceroporins in energy metabolism in health and disease. Biochimie 2021, 188, 20–34. [Google Scholar] [CrossRef]
- da Silva, I.V.; Soveral, G. Aquaporins in Obesity. Adv. Exp. Med. Biol. 2023, 1398, 289–302. [Google Scholar]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Ren. Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef] [PubMed]
- Kreida, S.; Törnroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, D.; Xia, Y.; Zhou, F.; Zhang, Q.; Wang, Q.; Qin, A.; Zhao, J.; Li, D.; Li, Y.; et al. The structural basis for glycerol permeation by human AQP7. Sci. Bull. 2021, 66, 1550–1558. [Google Scholar] [CrossRef]
- de Maré, S.W.; Venskutonytė, R.; Eltschkner, S.; de Groot, B.L.; Lindkvist-Petersson, K. Structural Basis for Glycerol Efflux and Selectivity of Human Aquaporin 7. Structure 2020, 28, 215–222.e3. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Kageyama, Y.; Tohsaka, A.; Suzuki, F.; Marumo, F.; Sasaki, S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J. Biol. Chem. 1997, 272, 20782–20786. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Kawamoto, S.; Ishida, N.; Ohno, I.; Mita, S.; Matsuzawa, Y.; Matsubara, K.; Okubo, K. Molecular cloning and expression of a novel human aquaporin from adipose tissue with glycerol permeability. Biochem. Biophys. Res. Commun. 1997, 241, 53–58. [Google Scholar] [CrossRef]
- Madeira, A.; Camps, M.; Zorzano, A.; Moura, T.F.; Soveral, G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS ONE 2013, 8, e83442. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Yamamoto, H.; Abe, Y.; Yoshida, G.J.; Rojek, A.; Sohara, E.; Uchida, S.; Nielsen, S.; Yasui, M. Dynamic subcellular localization of aquaporin-7 in white adipocytes. FEBS Lett. 2015, 589, 608–614. [Google Scholar] [CrossRef]
- Zhu, N.; Feng, X.; He, C.; Gao, H.; Yang, L.; Ma, Q.; Guo, L.; Qiao, Y.; Yang, H.; Ma, T. Defective macrophage function in aquaporin-3 deficiency. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 4233–4239. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Lebeck, J.; Rojek, A.; Praetorius, J.; Füchtbauer, E.M.; Frøkiaer, J.; Nielsen, S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: Implications in glycerol metabolism. Am. J. Physiol. Ren. Physiol. 2007, 292, F956–F965. [Google Scholar] [CrossRef]
- Wakayama, Y.; Inoue, M.; Kojima, H.; Jimi, T.; Shibuya, S.; Hara, H.; Oniki, H. Expression and localization of aquaporin 7 in normal skeletal myofiber. Cell Tissue Res. 2004, 316, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Nakatsuji, H.; Tochino, Y.; Fujita, K.; Kihara, S.; Funahashi, T.; Shimomura, I. The heart requires glycerol as an energy substrate through aquaporin 7, a glycerol facilitator. Cardiovasc. Res. 2009, 83, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Sugiyama, Y.; Kabashima, K.; Sohara, E.; Uchida, S.; Sasaki, S.; Inoue, S.; Miyachi, Y. Involvement of aquaporin-7 in the cutaneous primary immune through modulation of antigen uptake and migration in dendritic cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 211–218. [Google Scholar] [CrossRef]
- Hardin, J.A.; Wallace, L.E.; Wong, J.F.; O’Loughlin, E.V.; Urbanski, S.J.; Gall, D.G.; MacNaughton, W.K.; Beck, P.L. Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn’s disease and infectious colitis. Cell Tissue Res. 2004, 318, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Chang, B.H.; Fujimiya, M.; Chen, W.; Kulkarni, R.N.; Eguchi, Y.; Kimura, H.; Kojima, H.; Chan, L. Aquaporin 7 is a beta-cell protein and regulator of intraislet glycerol content and glycerol kinase activity, beta-cell mass, and insulin production and secretion. Mol. Cell. Biol. 2007, 27, 6026–6037. [Google Scholar] [CrossRef]
- Best, L.; Brown, P.D.; Yates, A.P.; Perret, J.; Virreira, M.; Beauwens, R.; Malaisse, W.J.; Sener, A.; Delporte, C. Contrasting effects of glycerol and urea transport on rat pancreatic beta-cell function. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2009, 23, 255–264. [Google Scholar] [CrossRef]
- Nejsum, L.N.; Elkjaer, M.; Hager, H.; Frokiaer, J.; Kwon, T.H.; Nielsen, S. Localization of aquaporin-7 in rat and mouse kidney using RT-PCR, immunoblotting, and immunocytochemistry. Biochem. Biophys. Res. Commun. 2000, 277, 164–170. [Google Scholar] [CrossRef]
- Tritto, S.; Gastaldi, G.; Zelenin, S.; Grazioli, M.; Orsenigo, M.N.; Ventura, U.; Laforenza, U.; Zelenina, M. Osmotic water permeability of rat intestinal brush border membrane vesicles: Involvement of aquaporin-7 and aquaporin-8 and effect of metal ions. Biochem. Cell Biol. Biochim. Biol. Cell. 2007, 85, 675–684. [Google Scholar] [CrossRef]
- Laforenza, U.; Gastaldi, G.; Grazioli, M.; Cova, E.; Tritto, S.; Faelli, A.; Calamita, G.; Ventura, U. Expression and immunolocalization of aquaporin-7 in rat gastrointestinal tract. Biol. Cell 2005, 97, 605–613. [Google Scholar] [CrossRef]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef]
- Iena, F.M.; Kalucka, J.; Nielsen, L.; Søndergaard, E.; Nielsen, S.; Lebeck, J. Localization of aquaglyceroporins in human and murine white adipose tissue. Histochem. Cell Biol. 2022, 157, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Venskutonytė, R.; Prasad, R.B.; Ardalani, H.; de Maré, S.W.; Fan, X.; Li, P.; Spégel, P.; Yan, N.; Gourdon, P.; et al. Cryo-EM structure supports a role of AQP7 as a junction protein. Nat. Commun. 2023, 14, 600. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Asp. Med. 2012, 33, 642–650. [Google Scholar] [CrossRef]
- Ricanek, P.; Lunde, L.K.; Frye, S.A.; Støen, M.; Nygård, S.; Morth, J.P.; Rydning, A.; Vatn, M.H.; Amiry-Moghaddam, M.; Tønjum, T. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin. Exp. Gastroenterol. 2015, 8, 49–67. [Google Scholar] [CrossRef]
- Maeda, N.; Funahashi, T.; Hibuse, T.; Nagasawa, A.; Kishida, K.; Kuriyama, H.; Nakamura, T.; Kihara, S.; Shimomura, I.; Matsuzawa, Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. USA 2004, 101, 17801–17806. [Google Scholar] [CrossRef]
- Guo, Z.; Jensen, M.D. Blood glycerol is an important precursor for intramuscular triacylglycerol synthesis. J. Biol. Chem. 1999, 274, 23702–23706. [Google Scholar] [CrossRef] [PubMed]
- Montell, E.; Lerín, C.; Newgard, C.B.; Gómez-Foix, A.M. Effects of modulation of glycerol kinase expression on lipid and carbohydrate metabolism in human muscle cells. J. Biol. Chem. 2002, 277, 2682–2686. [Google Scholar] [CrossRef]
- Sohara, E.; Rai, T.; Miyazaki, J.; Verkman, A.S.; Sasaki, S.; Uchida, S. Defective water and glycerol transport in the proximal tubules of AQP7 knockout mice. Am. J. Physiology. Ren. Physiol. 2005, 289, F1195–F1200. [Google Scholar] [CrossRef]
- Londos, C.; Brasaemle, D.L.; Schultz, C.J.; Adler-Wailes, D.C.; Levin, D.M.; Kimmel, A.R.; Rondinone, C.M. On the control of lipolysis in adipocytes. Ann. N. Y. Acad. Sci. 1999, 892, 155–168. [Google Scholar] [CrossRef]
- Tansey, J.T.; Sztalryd, C.; Hlavin, E.M.; Kimmel, A.R.; Londos, C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 2004, 56, 379–385. [Google Scholar] [CrossRef]
- Pagnon, J.; Matzaris, M.; Stark, R.; Meex, R.C.; Macaulay, S.L.; Brown, W.; O’Brien, P.E.; Tiganis, T.; Watt, M.J. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology 2012, 153, 4278–4289. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, B.M. The kidney and acid-base regulation. Adv. Physiol. Educ. 2009, 33, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Litman, T.; Søgaard, R.; Zeuthen, T. Ammonia and urea permeability of mammalian aquaporins. Handb. Exp. Pharmacol. 2009, 190, 327–358. [Google Scholar]
- Skelly, R.H.; Wicksteed, B.; Antinozzi, P.A.; Rhodes, C.J. Glycerol-stimulated proinsulin biosynthesis in isolated pancreatic rat islets via adenoviral-induced expression of glycerol kinase is mediated via mitochondrial metabolism. Diabetes 2001, 50, 1791–1798. [Google Scholar] [CrossRef]
- Kishida, K.; Kuriyama, H.; Funahashi, T.; Shimomura, I.; Kihara, S.; Ouchi, N.; Nishida, M.; Nishizawa, H.; Matsuda, M.; Takahashi, M.; et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000, 275, 20896–20902. [Google Scholar] [CrossRef]
- da Silva, I.V.; Díaz-Sáez, F.; Zorzano, A.; Gumà, A.; Camps, M.; Soveral, G. Aquaglyceroporins Are Differentially Expressed in Beige and White Adipocytes. Int. J. Mol. Sci. 2020, 21, 610. [Google Scholar] [CrossRef]
- da Silva, I.V.; Cardoso, C.; Méndez-Giménez, L.; Camoes, S.P.; Frühbeck, G.; Rodríguez, A.; Miranda, J.P.; Soveral, G. Aquaporin-7 and aquaporin-12 modulate the inflammatory phenotype of endocrine pancreatic beta-cells. Arch. Biochem. Biophys. 2020, 691, 108481. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Li, Y.; Hu, H.; Ye, Q.; Yang, C.; Yang, L.; Zhang, B.; Ma, T. Aquaporin-7 Facilitates Proliferation and Adipogenic Differentiation of Mouse Bone Marrow Mesenchymal Stem Cells by Regulating Hydrogen Peroxide Transport. Stem Cell Rev. Rep. 2023, 19, 2378–2390. [Google Scholar] [CrossRef]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Reviews. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metab. Clin. Exp. 2022, 129, 155142. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Q.Q.; Zhu, Y.H.; Bi, Y.; Sun, W.P.; Liang, H.; Cai, M.Y.; He, X.Y.; Weng, J.P. Insulin therapy stimulates lipid synthesis and improves endocrine functions of adipocytes in dietary obese C57BL/6 mice. Acta Pharmacol. Sin. 2010, 31, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Shimomura, I.; Kondo, H.; Kuriyama, H.; Makino, Y.; Nishizawa, H.; Maeda, N.; Matsuda, M.; Ouchi, N.; Kihara, S.; et al. Genomic structure and insulin-mediated repression of the aquaporin adipose (AQPap), adipose-specific glycerol channel. J. Biol. Chem. 2001, 276, 36251–36260. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Shimomura, I.; Kishida, K.; Kuriyama, H.; Makino, Y.; Nishizawa, H.; Matsuda, M.; Maeda, N.; Nagaretani, H.; Kihara, S.; et al. Human aquaporin adipose (AQPap) gene. Genomic structure, promoter analysis and functional mutation. Eur. J. Biochem. 2002, 269, 1814–1826. [Google Scholar] [CrossRef]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Rodríguez, A.; Moreno, N.R.; Balaguer, I.; Méndez-Giménez, L.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Portincasa, P.; Calamita, G.; Soveral, G.; et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci. Rep. 2015, 5, 12067. [Google Scholar] [CrossRef]
- Frühbeck, G.; Méndez-Giménez, L.; Becerril, S.; Ramírez, B.; Hernández-Pardos, A.W.; Cienfuegos, J.A.; Valentí, V.; Moncada, R.; Catalán, V.; Gómez-Ambrosi, J.; et al. Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery. Int. J. Mol. Sci. 2023, 24, 3412. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M. 11 beta-Hydroxysteroid dehydrogenases: Implications for clinical medicine. Clin. Endocrinol. 1996, 44, 493–499. [Google Scholar] [CrossRef]
- Staab, C.A.; Maser, E. 11beta-Hydroxysteroid dehydrogenase type 1 is an important regulator at the interface of obesity and inflammation. J. Steroid Biochem. Mol. Biol. 2010, 119, 56–72. [Google Scholar] [CrossRef]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Fasshauer, M.; Klein, J.; Lossner, U.; Klier, M.; Kralisch, S.; Paschke, R. Suppression of aquaporin adipose gene expression by isoproterenol, TNFalpha, and dexamethasone. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 2003, 35, 222–227. [Google Scholar]
- Quesada-López, T.; González-Dávalos, L.; Piña, E.; Mora, O. HSD1 and AQP7 short-term gene regulation by cortisone in 3T3-L1 adipocytes. Adipocyte 2016, 5, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Merino, B.; Cano, V.; Domínguez, G.; Pérez-Castells, J.; Fernández-Alfonso, M.S.; Sengenès, C.; Chowen, J.A.; Ruiz-Gayo, M. Cholecystokinin is involved in triglyceride fatty acid uptake by rat adipose tissue. J. Endocrinol. 2018, 236, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Merino, B.; Del Olmo, N.; Ruiz-Gayo, M. The cholecystokinin receptor agonist, CCK-8, induces adiponectin production in rat white adipose tissue. Br. J. Pharmacol. 2019, 176, 2678–2690. [Google Scholar] [CrossRef]
- Plaza, A.; Merino, B.; Ruiz-Gayo, M. Cholecystokinin promotes functional expression of the aquaglycerol channel aquaporin 7 in adipocytes. Br. J. Pharmacol. 2022, 179, 4092–4106. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.; Ghatei, M.A.; et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Gil, M.J.; Becerril, S.; Sáinz, N.; Silva, C.; Salvador, J.; Colina, I.; Frühbeck, G. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int. J. Obes. 2009, 33, 541–552. [Google Scholar] [CrossRef]
- Méndez-Giménez, L.; Becerril, S.; Camões, S.P.; da Silva, I.V.; Rodrigues, C.; Moncada, R.; Valentí, V.; Catalán, V.; Gómez-Ambrosi, J.; Miranda, J.P.; et al. Role of aquaporin-7 in ghrelin- and GLP-1-induced improvement of pancreatic β-cell function after sleeve gastrectomy in obese rats. Int. J. Obes. 2017, 41, 1394–1402. [Google Scholar] [CrossRef]
- Seeley, R.J.; Tschöp, M.H. Uroguanylin: How the gut got another satiety hormone. J. Clin. Investig. 2011, 121, 3384–3386. [Google Scholar] [CrossRef] [PubMed]
- Folgueira, C.; Sanchez-Rebordelo, E.; Barja-Fernandez, S.; Leis, R.; Tovar, S.; Casanueva, F.F.; Dieguez, C.; Nogueiras, R.; Seoane, L.M. Uroguanylin levels in intestine and plasma are regulated by nutritional status in a leptin-dependent manner. Eur. J. Nutr. 2016, 55, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Ibáñez, P.; Vila, N.; Margall, M.A.; Moncada, R.; et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int. J. Obes. 2016, 40, 1405–1415. [Google Scholar] [CrossRef]
- Jin, B.; Chen, X.; Xing, L.; Xu, W.; Fu, X.; Zhu, J.; Mou, X.; Wang, Z.; Shu, J. Tissue-specific effects of estrogen on glycerol channel aquaporin 7 expression in an ovariectomized mouse model of menopause. Climacteric J. Int. Menopause Soc. 2017, 20, 385–390. [Google Scholar] [CrossRef]
- Xing, L.; Jin, B.; Fu, X.; Zhu, J.; Guo, X.; Xu, W.; Mou, X.; Wang, Z.; Jiang, F.; Zhou, Y.; et al. Identification of functional estrogen response elements in glycerol channel Aquaporin-7 gene. Climacteric J. Int. Menopause Soc. 2019, 22, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhao, G.; Liu, R.; Zheng, M.; Chen, J.; Wen, J. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. J. Lipid Res. 2012, 53, 909–917. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Liu, X.; Li, J.; Gao, Q.; Shi, S.; Wang, T.; Ye, X.; Lu, Y.; Zhang, D.; et al. AQP7 mediates post-menopausal lipogenesis in adipocytes through FSH-induced transcriptional crosstalk with AP-1 sites. Reprod. Biomed. Online 2020, 41, 1122–1132. [Google Scholar] [CrossRef]
- Nesverova, V.; Tornroth-Horsefield, S. Phosphorylation-dependent regulation of mammalian aquaporins. Cells 2019, 8, 82. [Google Scholar] [CrossRef]
- Huang, P.; Hansen, J.S.; Saba, K.H.; Bergman, A.; Negoita, F.; Gourdon, P.; Hagström-Andersson, A.; Lindkvist-Petersson, K. Aquaglyceroporins and orthodox aquaporins in human adipocytes. Biochim. Biophys. Acta. Biomembr. 2022, 1864, 183795. [Google Scholar] [CrossRef]
- Hansen, J.S.; Krintel, C.; Hernebring, M.; Haataja, T.J.; de Marè, S.; Wasserstrom, S.; Kosinska-Eriksson, U.; Palmgren, M.; Holm, C.; Stenkula, K.G.; et al. Perilipin 1 binds to aquaporin 7 in human adipocytes and controls its mobility via protein kinase A mediated phosphorylation. Metab. Clin. Exp. 2016, 65, 1731–1742. [Google Scholar] [CrossRef]
- Zhang, J.; Hupfeld, C.J.; Taylor, S.S.; Olefsky, J.M.; Tsien, R.Y. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature 2005, 437, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.G.; Holness, M.J.; Gibbons, G.F.; Sugden, M.C. Fasting-induced increases in aquaporin 7 and adipose triglyceride lipase mRNA expression in adipose tissue are attenuated by peroxisome proliferator-activated receptor alpha deficiency. Int. J. Obes. 2007, 31, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Shimomura, I.; Nishizawa, H.; Maeda, N.; Kuriyama, H.; Kondo, H.; Matsuda, M.; Nagaretani, H.; Ouchi, N.; Hotta, K.; et al. Enhancement of the aquaporin adipose gene expression by a peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2001, 276, 48572–48579. [Google Scholar] [CrossRef]
- Calderón-DuPont, D.; Romero-Córdoba, S.L.; Tello, J.K.; Espinosa, A.; Guerrero, B.; Contreras, A.V.; Morán-Ramos, S.; Díaz-Villaseñor, A. Impaired white adipose tissue fatty acid metabolism in mice fed a high-fat diet worsened by arsenic exposure, primarily affecting retroperitoneal adipose tissue. Toxicol. Appl. Pharmacol. 2023, 468, 116428. [Google Scholar] [CrossRef] [PubMed]
- Chiadak, J.D.; Arsenijevic, T.; Gregoire, F.; Bolaky, N.; Delforge, V.; Perret, J.; Delporte, C. Involvement of JNK/NFκB Signaling Pathways in the Lipopolysaccharide-Induced Modulation of Aquaglyceroporin Expression in 3T3-L1 Cells Differentiated into Adipocytes. Int. J. Mol. Sci. 2016, 17, 1742. [Google Scholar] [CrossRef]
- Hibuse, T.; Maeda, N.; Funahashi, T.; Yamamoto, K.; Nagasawa, A.; Mizunoya, W.; Kishida, K.; Inoue, K.; Kuriyama, H.; Nakamura, T.; et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 10993–10998. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Sohara, E.; Rai, T.; Ikawa, M.; Okabe, M.; Sasaki, S.; Uchida, S.; Verkman, A.S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005, 280, 15493–15496. [Google Scholar] [CrossRef]
- Oikonomou, E.; Kostopoulou, E.; Rojas-Gil, A.P.; Georgiou, G.; Spiliotis, B.E. The metabolic implications of aquaporin 7 (AQP7) promoter variants in lean children and children with obesity. Hormones 2020, 19, 187–195. [Google Scholar] [CrossRef]
- Mourelatou, R.; Kostopoulou, E.; Rojas-Gil, A.P.; Kehagias, I.; Linos, D.; Kalfarentzos, F.E.; Spiliotis, B.E. Decreased adipocyte glucose transporter 4 (GLUT4) and aquaglyceroporin-7 (AQP7) in adults with morbid obesity: Possible early markers of metabolic dysfunction. Hormones 2019, 18, 297–306. [Google Scholar] [CrossRef]
- Marrades, M.P.; Milagro, F.I.; Martínez, J.A.; Moreno-Aliaga, M.J. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem. Biophys. Res. Commun. 2006, 339, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Pastor, C.; Rotellar, F.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Vendrell, J.; et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes. Surg. 2008, 18, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Escoté, X.; Ceperuelo-Mallafré, V.; Alcaide, M.J.; Simón, I.; Vilarrasa, N.; Wabitsch, M.; Vendrell, J. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: Differences and similarities between depots. J. Clin. Endocrinol. Metab. 2010, 95, 3470–3479. [Google Scholar] [CrossRef]
- Miranda, M.; Ceperuelo-Mallafré, V.; Lecube, A.; Hernandez, C.; Chacon, M.R.; Fort, J.M.; Gallart, L.; Baena-Fustegueras, J.A.; Simó, R.; Vendrell, J. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity: Relationship with glucose abnormalities. Metab. Clin. Exp. 2009, 58, 1762–1768. [Google Scholar] [CrossRef]
- Sjöholm, K.; Palming, J.; Olofsson, L.E.; Gummesson, A.; Svensson, P.A.; Lystig, T.C.; Jennische, E.; Brandberg, J.; Torgerson, J.S.; Carlsson, B.; et al. A microarray search for genes predominantly expressed in human omental adipocytes: Adipose tissue as a major production site of serum amyloid A. J. Clin. Endocrinol. Metab. 2005, 90, 2233–2239. [Google Scholar] [CrossRef]
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, Y.; Hirako, S.; Ogawa, T.; Jimi, T.; Shioda, S. Upregulated Expression of AQP 7 in the Skeletal Muscles of Obese ob/ob Mice. Acta Histochem. Cytochem. 2014, 47, 27–33. [Google Scholar] [CrossRef]
- Oikonomou, E.; Kostopoulou, E.; Rojas-Gil, A.P.; Georgiou, G.; Spiliotis, B.E. Adipocyte aquaporin 7 (AQP7) expression in lean children and children with obesity. Possible involvement in molecular mechanisms of childhood obesity. J. Pediatr. Endocrinol. Metab. JPEM 2018, 31, 1081–1089. [Google Scholar] [CrossRef]
- Iena, F.M.; Jul, J.B.; Vegger, J.B.; Lodberg, A.; Thomsen, J.S.; Brüel, A.; Lebeck, J. Sex-Specific Effect of High-Fat Diet on Glycerol Metabolism in Murine Adipose Tissue and Liver. Front. Endocrinol. 2020, 11, 577650. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafré, V.; Miranda, M.; Chacón, M.R.; Vilarrasa, N.; Megia, A.; Gutiérrez, C.; Fernández-Real, J.M.; Gómez, J.M.; Caubet, E.; Frühbeck, G.; et al. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 3640–3645. [Google Scholar] [CrossRef]
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; De Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, G.; Paternain, L.; Milagro, F.I.; Martínez, J.A.; Campion, J. Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J. Physiol. Biochem. 2013, 69, 601–611. [Google Scholar] [CrossRef]
- Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Bañuelos, O.; Soria, A.C.; Rodríguez-Sánchez, S.; Martínez, J.A.; Campión, J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.; Tu, Q.; Wu, J.; Qin, Y.; Zhu, Z.; Shen, Y.; Yan, L.; Han, A.; Xiang, Q.; et al. Associations between Aquaglyceroporin Gene Polymorphisms and Risk of Type 2 Diabetes Mellitus. BioMed Res. Int. 2018, 2018, 8167538. [Google Scholar] [CrossRef]
- He, S.; Yang, M.; Yang, Y.; Wang, F.; Wang, X.; Lu, T.; Jiao, M.; Li, Y. Association study of single nucleotide polymorphisms of AQP7 and AQP9 genes with type 2 diabetes mellitus among ethnic Han Chinese population. Zhonghua Yi Xue Yi Chuan Xue Za Zhi Zhonghua Yixue Yichuanxue Zazhi Chin. J. Med. Genet. 2022, 39, 234–239. [Google Scholar]
- Frühbeck, G.; López, M.; Diéguez, C. Role of caveolins in body weight and insulin resistance regulation. Trends Endocrinol. Metab. TEM 2007, 18, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, S.; Krief, S.; Farnier, C.; Lefrère, I.; Le Liepvre, X.; Bazin, R.; Ferré, P.; Dugail, I. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J. Biol. Chem. 2001, 276, 16904–16910. [Google Scholar] [CrossRef]
- Ferrer, R.; Pardina, E.; Rossell, J.; Baena-Fustegueras, J.A.; Lecube, A.; Balibrea, J.M.; Caubet, E.; González, O.; Vilallonga, R.; Fort, J.M.; et al. Decreased lipases and fatty acid and glycerol transporter could explain reduced fat in diabetic morbidly obese. Obesity 2014, 22, 2379–2387. [Google Scholar] [CrossRef]
- Duan, Y.; Yuan, Z.; Yao, J.; Zhang, T.; Chen, C.; Guo, X. Expression of aquaporin 7 in perirenal adipose tissue of Otsuka Long-Evans Tokushima Fatty diabetic rats. Beijing Da Xue Xue Bao. Yi Xue Ban J. Peking Univ. Health Sci. 2011, 43, 117–122. [Google Scholar]
- Wakayama, Y.; Hirako, S.; Ohtaki, H.; Arata, S.; Jimi, T.; Honda, K. Histopathological and aquaporin7 mRNA expression analyzes in the skeletal and cardiac muscles of obese db/db mice. J. Vet. Med. Sci. 2021, 83, 1155–1160. [Google Scholar] [CrossRef]
- Lebeck, J.; Søndergaard, E.; Nielsen, S. Increased AQP7 abundance in skeletal muscle from obese men with type 2 diabetes. American journal of physiology. Endocrinol. Metab. 2018, 315, E367–E373. [Google Scholar]
- Hirako, S.; Wakayama, Y.; Kim, H.; Iizuka, Y.; Wada, N.; Kaibara, N.; Okabe, M.; Arata, S.; Matsumoto, A. Association of Aquaporin 7 and 9 with Obesity and Fatty Liver in db/db Mice. Zool. Sci. 2023, 40, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Shimomura, I.; Kishida, K.; Kondo, H.; Furuyama, N.; Nishizawa, H.; Maeda, N.; Matsuda, M.; Nagaretani, H.; Kihara, S.; et al. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 2002, 51, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, 1.e1–1.e18. [Google Scholar] [CrossRef]
- Huang, P.W.; Jeng, S.F.; Liu, C.M.; Chen, C.C.; Chang, L.R.; Shih, H.S.; Chen, H.F.; Yang, C.H.; Chen, J.A.; Feng, G.M. Involvement of Aquaporins in the Intense Pulsed Light-Enhanced Wound Healing in Diabetic Rats. Lasers Surg. Med. 2021, 53, 549–556. [Google Scholar] [CrossRef]
- Ishihama, S.; Yoshida, S.; Yoshida, T.; Mori, Y.; Ouchi, N.; Eguchi, S.; Sakaguchi, T.; Tsuda, T.; Kato, K.; Shimizu, Y.; et al. LPL/AQP7/GPD2 promotes glycerol metabolism under hypoxia and prevents cardiac dysfunction during ischemia. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e22048. [Google Scholar] [CrossRef]
- Heinig, M.; Adriaens, M.E.; Schafer, S.; van Deutekom, H.W.M.; Lodder, E.M.; Ware, J.S.; Schneider, V.; Felkin, L.E.; Creemers, E.E.; Meder, B.; et al. Natural genetic variation of the cardiac transcriptome in non-diseased donors and patients with dilated cardiomyopathy. Genome Biol. 2017, 18, 170. [Google Scholar] [CrossRef]
- Aggeli, I.K.; Kapogiannatou, A.; Paraskevopoulou, F.; Gaitanaki, C. Differential response of cardiac aquaporins to hyperosmotic stress; salutary role of AQP1 against the induced apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 313–325. [Google Scholar] [PubMed]
- Malvia, S.; Bagadi, S.A.R.; Pradhan, D.; Chintamani, C.; Bhatnagar, A.; Arora, D.; Sarin, R.; Saxena, S. Study of Gene Expression Profiles of Breast Cancers in Indian Women. Sci. Rep. 2019, 9, 10018. [Google Scholar] [CrossRef]
- Marino, N.; German, R.; Rao, X.; Simpson, E.; Liu, S.; Wan, J.; Liu, Y.; Sandusky, G.; Jacobsen, M.; Stoval, M.; et al. Upregulation of lipid metabolism genes in the breast prior to cancer diagnosis. NPJ Breast Cancer 2020, 6, 50. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Ding, Z.; Xu, T.; Zhang, X.; Xu, K. Comprehensive exploration of the expression and prognostic value of AQPs in clear cell renal cell carcinoma. Medicine 2022, 101, e29344. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; Fernández-Quintela, A.; González, M.; Portillo, M.P. Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue. Int. J. Mol. Sci. 2018, 19, 2654. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, L.; Zeng, X.; Liu, M.; Zhou, P.; Lu, H.; Lin, H.; Dong, M. Aquaporin 7 involved in GINSENOSIDE-RB1-mediated anti-obesity via peroxisome proliferator-activated receptor gamma pathway. Nutr. Metab. 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, J.A.; Bak, S.S.; Byun, H.G.; Kim, S.K. Anti-obesity effect of carboxymethyl chitin by AMPK and aquaporin-7 pathways in 3T3-L1 adipocytes. J. Nutr. Biochem. 2011, 22, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Ren, B.; Jia, Y.; Dong, Y.; Wang, Y.; Shan, J.; Wang, Y. Feprazone Displays Antiadipogenesis and Antiobesity Capacities in in Vitro 3 T3-L1 Cells and in Vivo Mice. ACS Omega 2021, 6, 6674–6680. [Google Scholar] [CrossRef]
- Mehanna, E.T.; Barakat, B.M.; ElSayed, M.H.; Tawfik, M.K. An optimized dose of raspberry ketones controls hyperlipidemia and insulin resistance in male obese rats: Effect on adipose tissue expression of adipocytokines and Aquaporin 7. Eur. J. Pharmacol. 2018, 832, 81–89. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Shi, Y.; Liu, Y.; Lu, T. Abdominal Massage Ameliorates Inguinal Fat Accumulation via Augmentation of PPARγ Signaling in High-Fat Diet-Induced Obese Mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 2409–2418. [Google Scholar] [CrossRef]
- He, X.; Gao, F.; Hou, J.; Li, T.; Tan, J.; Wang, C.; Liu, X.; Wang, M.; Liu, H.; Chen, Y.; et al. Metformin inhibits MAPK signaling and rescues pancreatic aquaporin 7 expression to induce insulin secretion in type 2 diabetes mellitus. J. Biol. Chem. 2021, 297, 101002. [Google Scholar] [CrossRef]
- Shalaby, A.; Mennander, A.; Rinne, T.; Oksala, N.; Aanismaa, R.; Narkilahti, S.; Paavonen, T.; Laurikka, J.; Tarkka, M. Aquaporin-7 expression during coronary artery bypass grafting with diazoxide. Scand. Cardiovasc. J. SCJ 2011, 45, 354–359. [Google Scholar] [CrossRef]
- Tan, C.; Zeng, J.; Wu, G.; Zheng, L.; Huang, M.; Huang, X. Xinshuitong Capsule extract attenuates doxorubicin-induced myocardial edema via regulation of cardiac aquaporins in the chronic heart failure rats. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 144, 112261. [Google Scholar] [CrossRef]
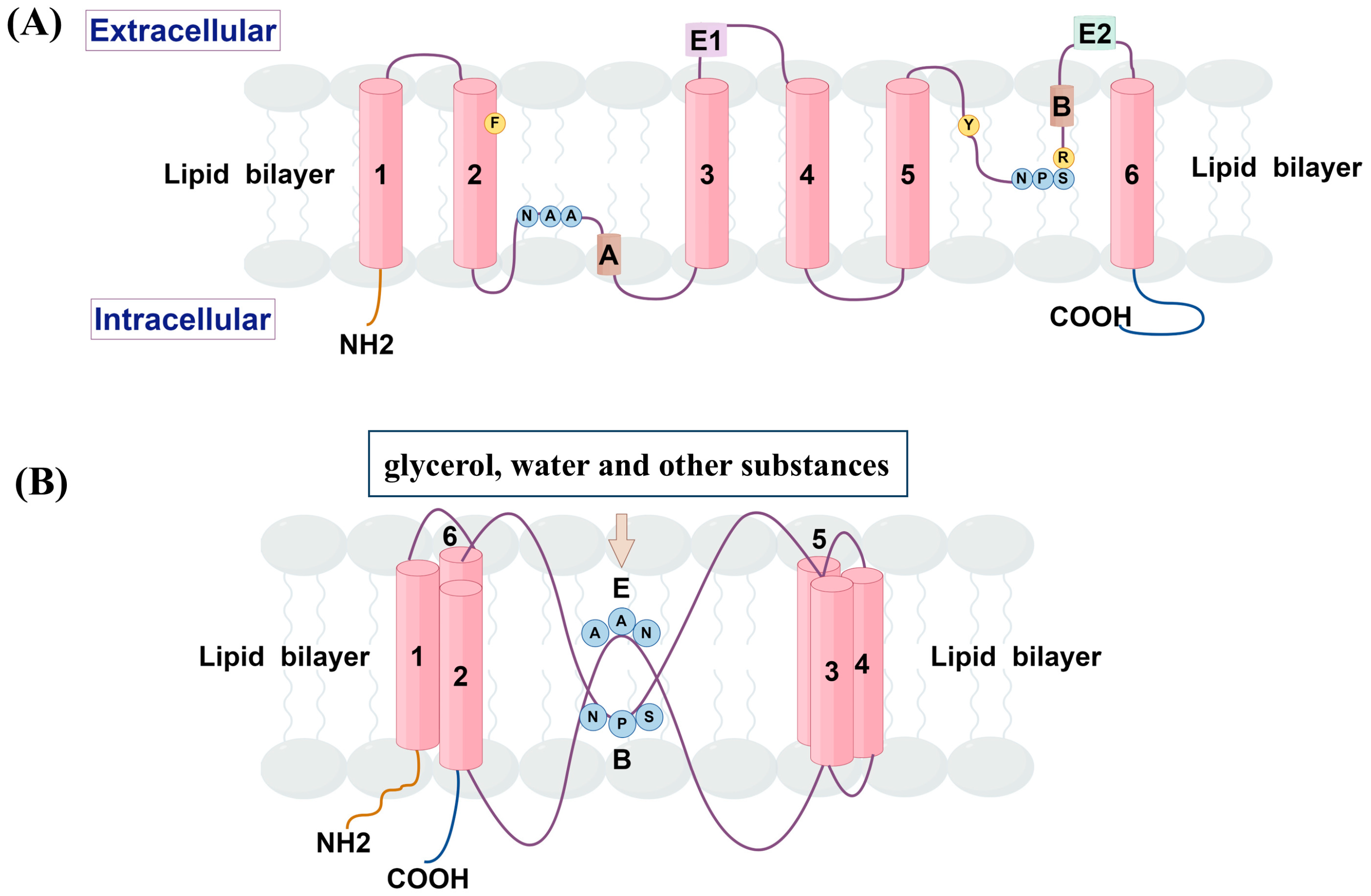
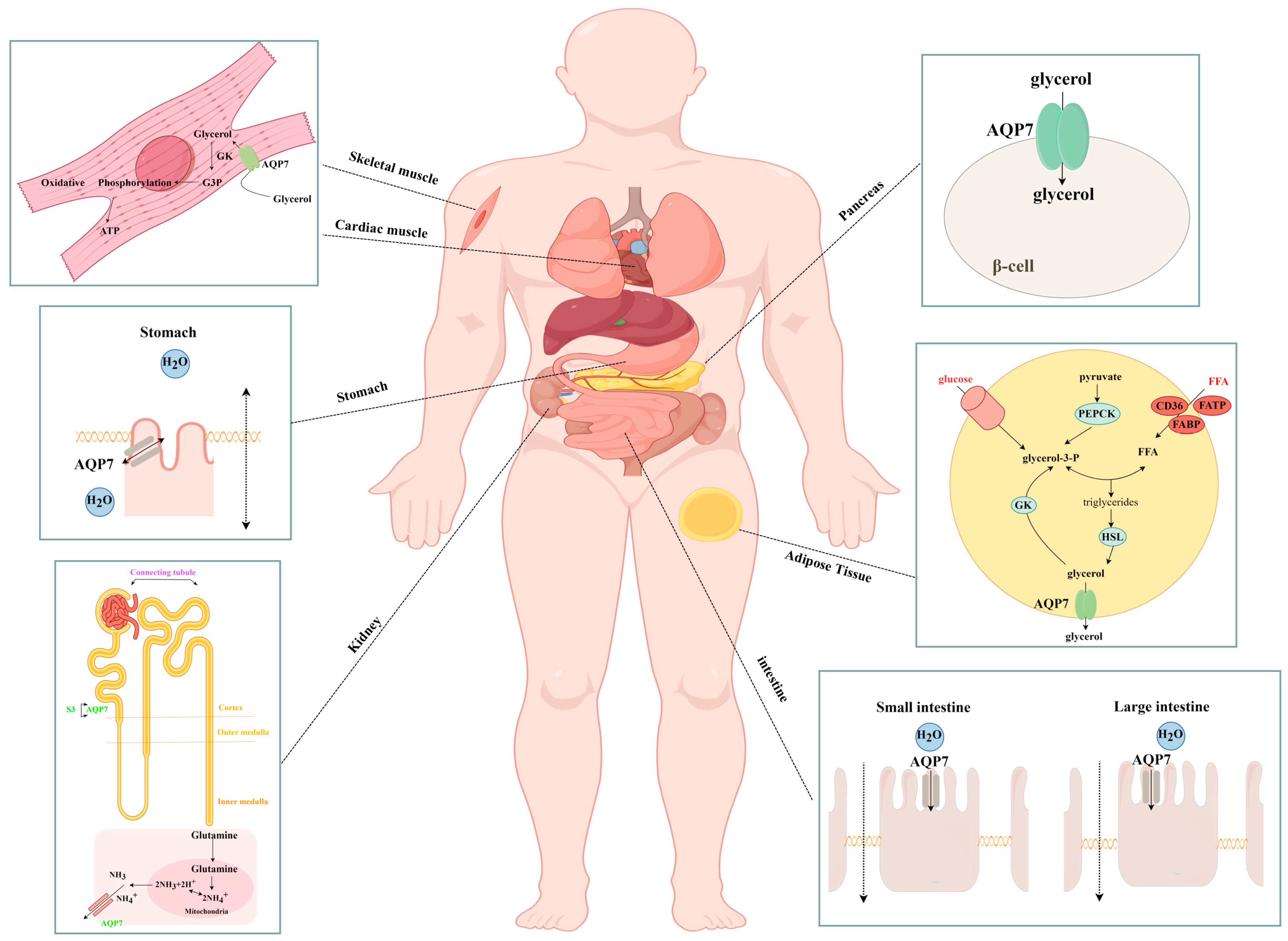
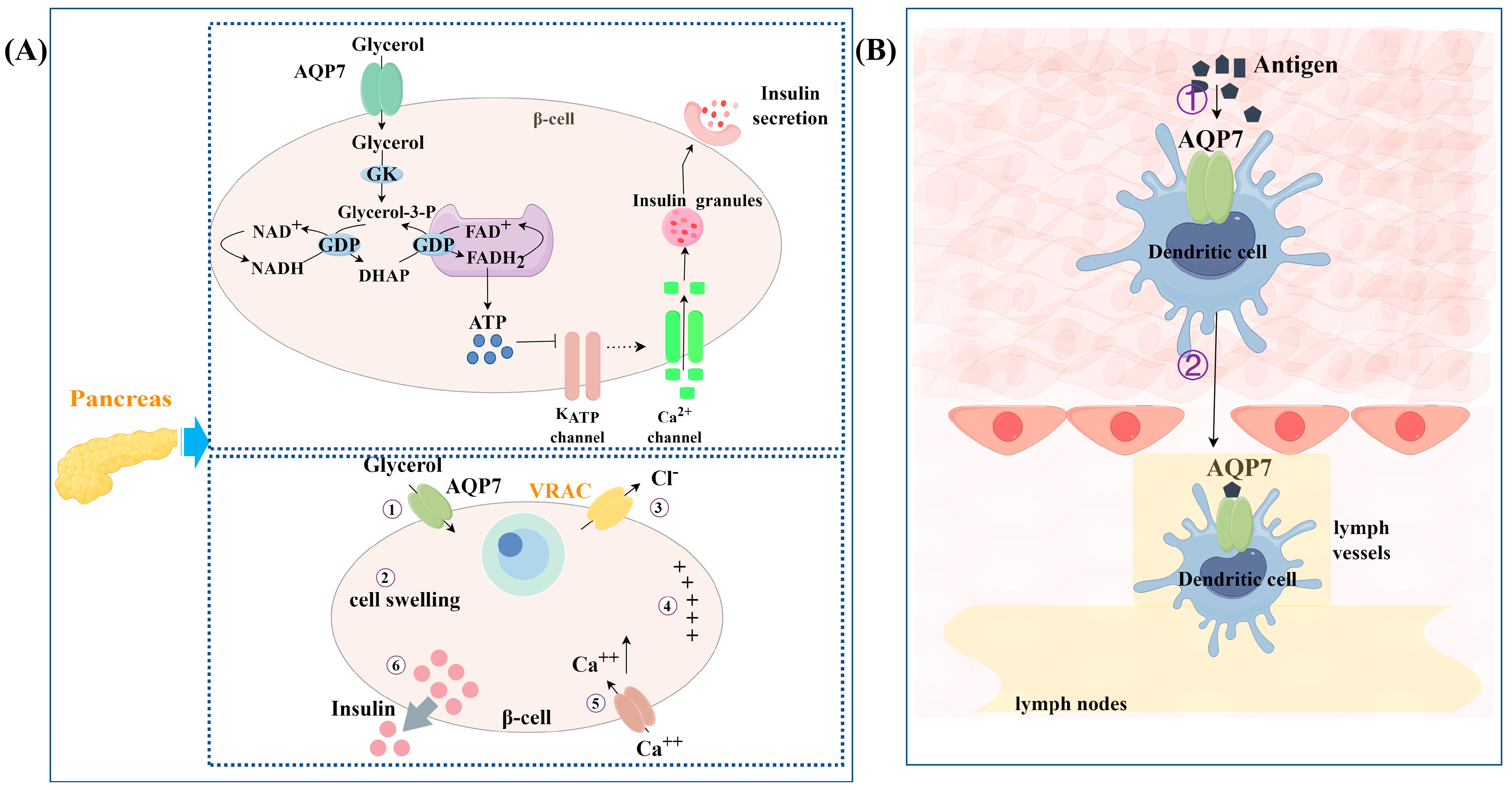
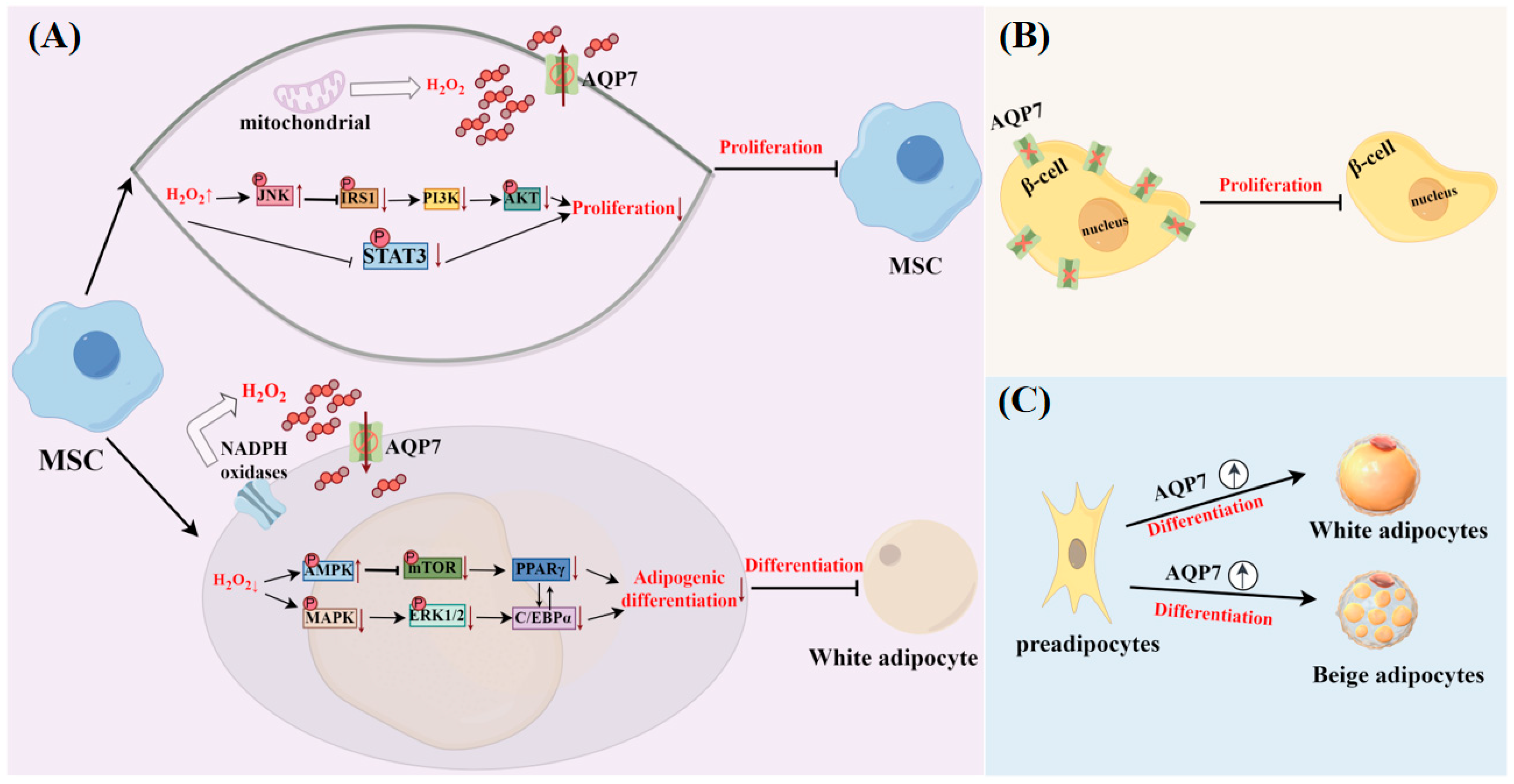

| Species | Tissue Localization | Subcellular Localization |
|---|---|---|
| Mice | visceral white adipose tissue [17,18], peritoneal cavity [19], interscapular brown adipose tissue [20] | adipocytes, stromal vascular fractions [17], endothelial cells [18], macrophage cells [19] |
| kidneys [20] | segment 3 of murine renal proximal tubules [20] | |
| muscle [20] | capillary network of skeletal muscle, capillaries of cardiac muscle [20], the myofiber surface of type 1 and type 2 fibers [21], cardiomyocytes [22] | |
| spermatocytes [20] | the surroundings of mouse spermatids in tails, testicular and epipidymal spermatozoa tails [20] | |
| skin [23] | epidermal and dermal dendritic cells [23] | |
| colon [24] | basolateral aspect of the colonic epithelium [24] | |
| pancreas [25] | Β cells [25] | |
| Rat | pancreas [26] | β cells [26] |
| kidneys [22,27] | segment 3 proximal tubules [22,27] | |
| small intestinal [28,29] | superficial epithelial cells [28], apical brush border membrane of intestinal epithelial cells [29] | |
| large intestine [28] | monolayer of epithelial cells [28] | |
| Human | subcutaneous adipose tissues [30,31] | adipocyte and capillary plasma membranes [30], macrophages/immune cells [31] |
| pancreas [32] | A cells, β cells [32] | |
| muscle [21] | the myofiber surface of type 1 and type 2 fibers [21] | |
| stomach body, pyloric antrum [33] | - | |
| ileum [34] | mucosal epithelium [34] | |
| colon [24] | the surface epithelium and upper half of the crypt epithelium [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xia, Z.; Peng, S.; Xia, J.; Xu, R.; Wang, X.; Li, F.; Zhu, W. The Important Role of Aquaglyceroporin 7 in Health and Disease. Biomolecules 2024, 14, 1228. https://doi.org/10.3390/biom14101228
Liu J, Xia Z, Peng S, Xia J, Xu R, Wang X, Li F, Zhu W. The Important Role of Aquaglyceroporin 7 in Health and Disease. Biomolecules. 2024; 14(10):1228. https://doi.org/10.3390/biom14101228
Chicago/Turabian StyleLiu, Jing, Ziwei Xia, Shuhong Peng, Juanjuan Xia, Ruixiang Xu, Xin Wang, Fei Li, and Weifeng Zhu. 2024. "The Important Role of Aquaglyceroporin 7 in Health and Disease" Biomolecules 14, no. 10: 1228. https://doi.org/10.3390/biom14101228






