Boosting Serotonin Synthesis Is Not Sufficient to Improve Motor Coordination of Mecp2 Heterozygous Mouse Model of Rett Syndrome
Abstract
1. Introduction
2. Materials and Methods
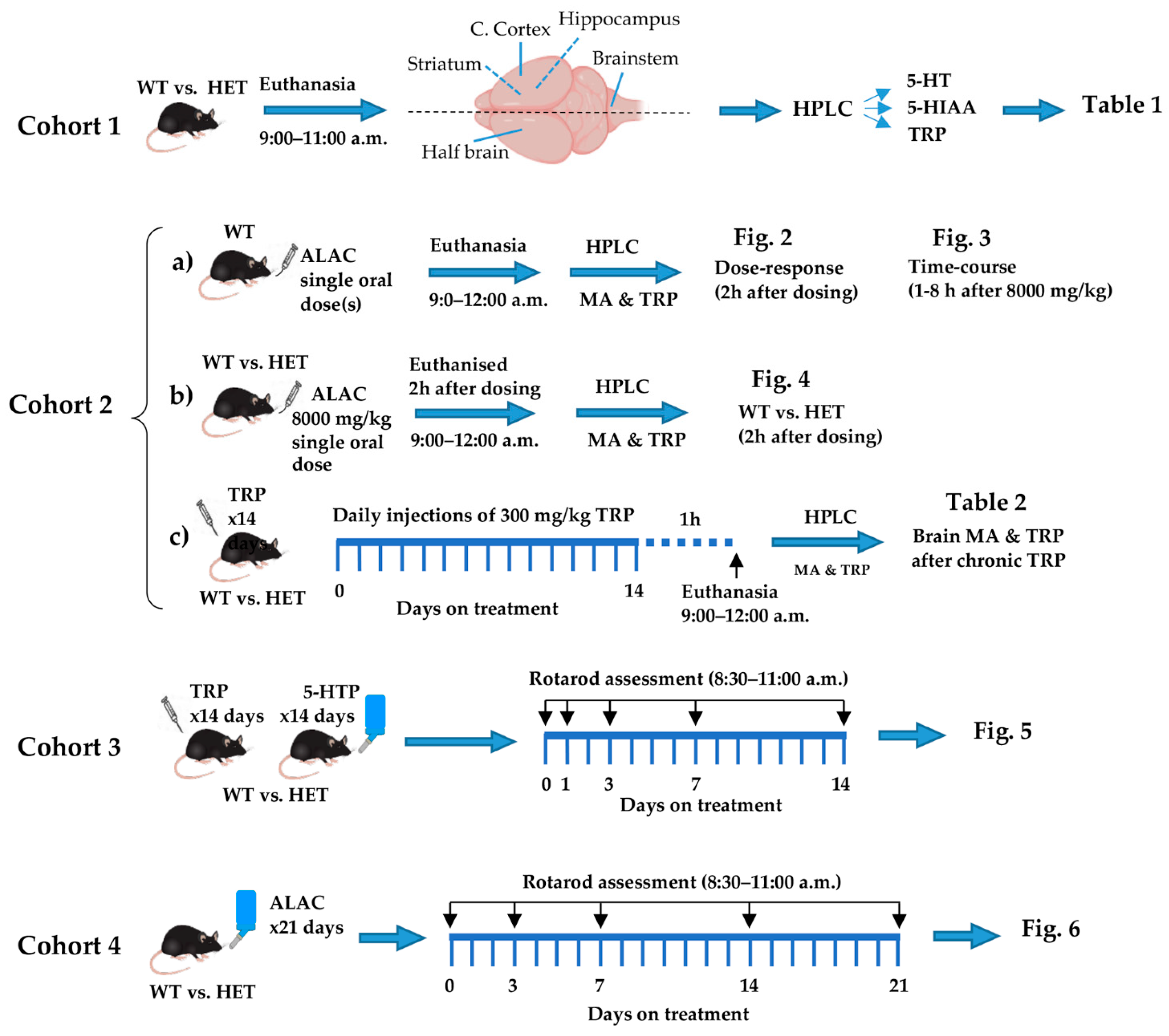
2.1. Experimental Design and Statistics
2.2. Drug Treatment
2.3. Monoamines and Tryptophan Assay
3. Results
3.1. Brain Levels of Monoamines and Tryptophan in Female WT and Mecp2 Het Mice
| 5-HT | 5-HIAA | TRP | ||
|---|---|---|---|---|
| ng/g | ng/g | μg/g | ||
| BRAIN | ||||
| WT (4) | 839 ± 26 | 456 ± 26 | 4.5 ± 0.2 | |
| HET (6) | 679 ± 21 | 391 ± 24 | 4.5 ± 0.3 | |
| t(df) | 4.763(8) | 1.803(8) | 0.0795(8) | |
| p | 0.0014 | 0.109 | 0.938 | |
| HIPP | ||||
| WT (4) | 792 ± 43 | 505 ± 21 | 5.2 ± 0.3 | |
| HET (6) | 617 ± 49 | 407 ± 18 | 4.9 ± 0.2 | |
| t(df) | 2.484(8) | 3.521(8) | 0.8481(8) | |
| p | 0.0379 | 0.0078 | 0.8481 | |
| CTX | ||||
| WT (4) | 709 ± 19 | 285 ± 8 | 3.3 ± 0.1 | |
| HET (6) | 580 ± 26 | 263 ± 13 | 3.6 ± 0.2 | |
| t(df) | 3.607(8) | 1.284(8) | 1.169(8) | |
| p | 0.0069 | 0.2351 | 0.2761 | |
| BST | ||||
| WT (4) | 1063 ± 37 | 556 ± 24 | 3.8 ± 0.1 | |
| HET (6) | 817 ± 34 | 493 ± 13 | 4.2 ± 0.2 | |
| t(df) | 4.778(8) | 2.549(8) | 1.290(8) | |
| p | 0.014 | 0.0342 | 0.2332 | |
| STR | ||||
| WT (4) | 674 ± 104 | 403 ± 55 | 5.0 ± 0.1 | |
| HET (5) | 601 ± 68 | 364 ± 24 | 5.0 ± 0.4 | |
| t(df) | 0.7250(7) | 0.6720(7) | 0.0668(7) | |
| p | 0.492 | 0.5231 | 0.9486 |
3.2. Dose-Dependent Effect of Single Oral Doses of ALAC on Plasma and Brain Levels of TRP and Brain Levels of 5-HT and 5-HIAA in WT Mice

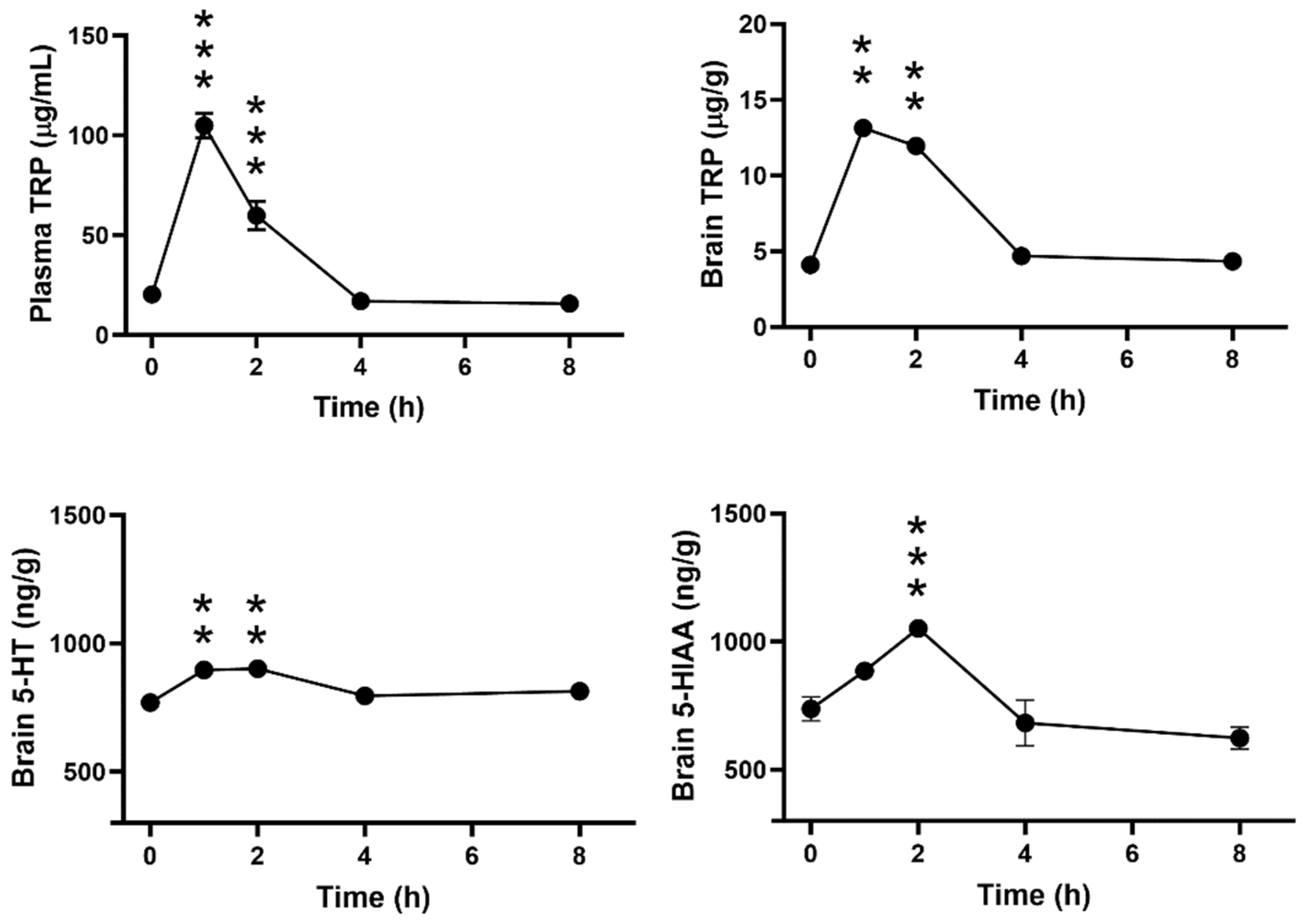
3.3. Time-Dependent Effect of ALAC on Plasma and Brain Levels of TRP and Brain Levels of 5-HT and 5-HIAA in WT Mice
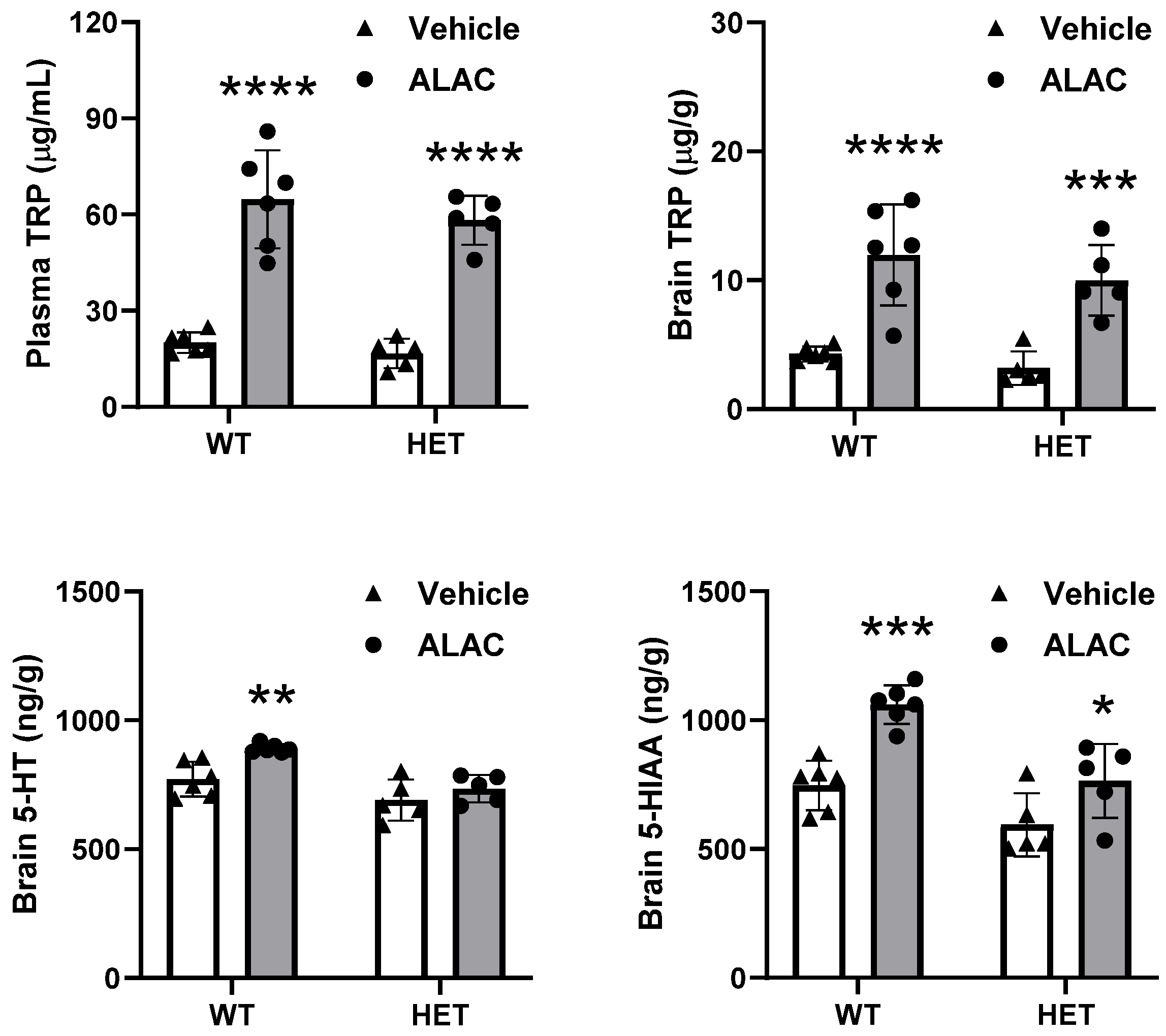
3.4. Effect of 8000 mg/kg ALAC on Plasma and Brain Levels of TRP and Brain Levels of 5-HT and 5-HIAA in WT and Mecp2 Het Mice
3.5. Effect of Repeated Treatment with Tryptophan on Brain Levels of TRP, 5-HT and 5-HIAA in WT and Mecp2 Het Mice
| TRP | 5-HT | 5-HIAA | |
|---|---|---|---|
| μg/g | ng/g tissue | ng/g tissue | |
| WT-Veh (5) | 3.4 ± 0.4 | 601 ± 31 | 668 ± 74 |
| WT-TRP (7) | 97.2 ± 6.3 *** | 773 ± 29 ** | 1602 ± 114 *** |
| HET-Veh (6) | 3.5 ± 0.2 | 631 ± 18 | 613 ± 31 |
| HET-TRP (6) | 86.3 ± 8.2 *** | 876 ± 41 *** | 1343 ± 89 *** |
| F treatment (df), p | 248.4 (1,20), p < 0.0001 | 44.33 (1,20), p < 0.0001 | 89.41 (1,20), p < 0.0001 |
| F genotype (df), p | 0.9199 (1,20), p = 0.3490 | 4.543 (1,20), p = 0.0457 | 3.176 (1,20), p = 0.0899 |
| F interaction (df), p | 0.9581 (1,20), p = 0.3394 | 1.406 (1,20), p = 0.2497 | 1.330 (1,20), p = 0.2623 |
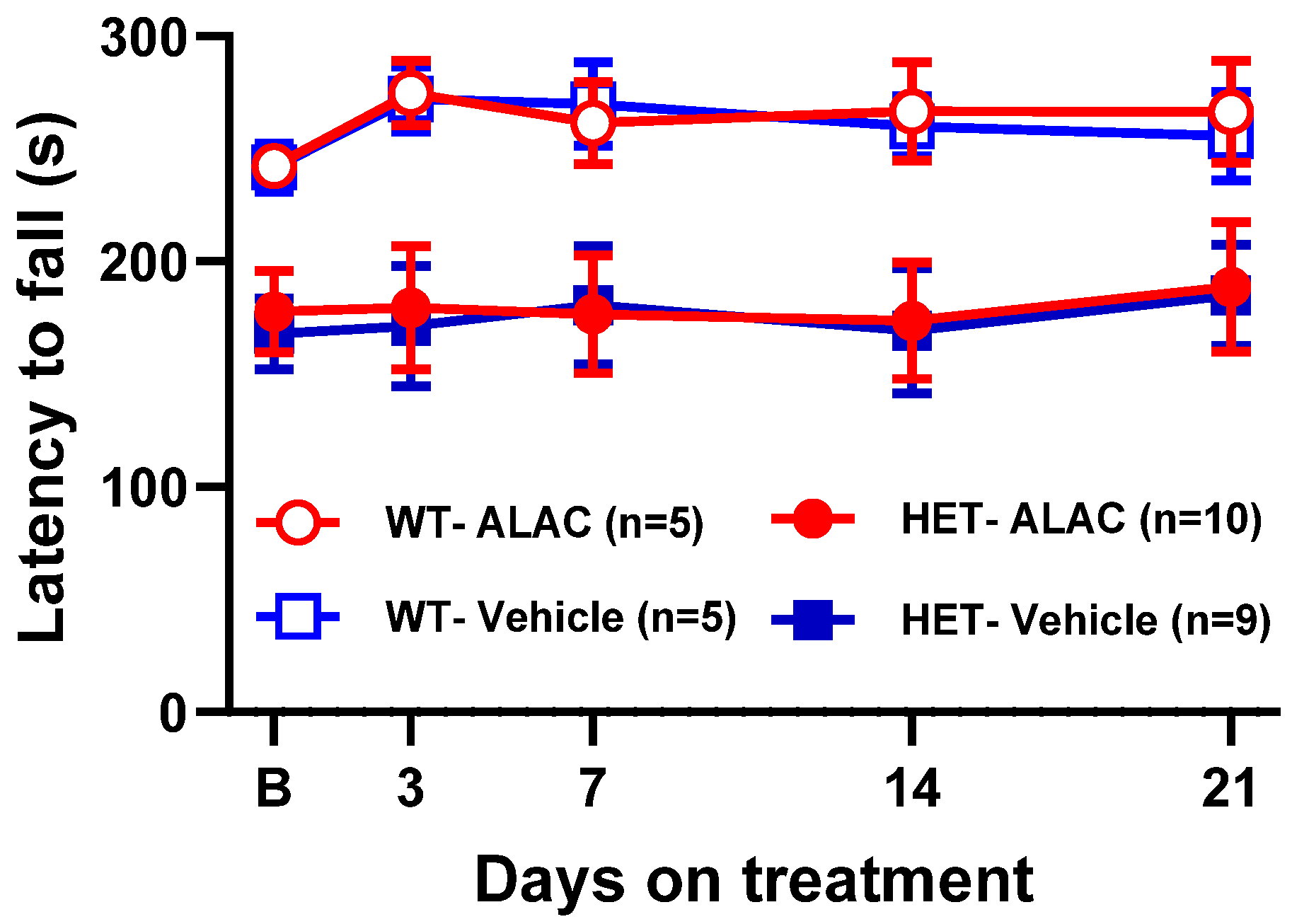
3.6. Effect of TRP, 5-HTP and ALAC on Rotarod Performance in Mecp2 Het Mice

4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl-CpG-Binding Protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.M.; Baker, S.A.; Zoghbi, H. MECP2 Disorders: From the Clinic to Mice and Back. J. Clin. Investig. 2015, 125, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Samaco, R.C.; Mandel-Brehm, C.; Chao, H.-T.; Ward, C.S.; Fyffe-Maricich, S.L.; Ren, J.; Hyland, K.; Thaller, C.; Maricich, S.M.; Humphreys, P.; et al. Loss of MeCP2 in Aminergic Neurons Causes Cell-Autonomous Defects in Neurotransmitter Synthesis and Specific Behavioral Abnormalities. Proc. Natl. Acad. Sci. USA 2009, 106, 21966–21971. [Google Scholar] [CrossRef] [PubMed]
- Villani, C.; Sacchetti, G.; Carli, M.; Invernizzi, R.W. Fluoxetine Rescues Rotarod Motor Deficits in Mecp2 Heterozygous Mouse Model of Rett Syndrome via Brain Serotonin. Neuropharmacology 2020, 176, 108221. [Google Scholar] [CrossRef] [PubMed]
- Philippe, T.J.; Vahid-Ansari, F.; Donaldson, Z.R.; Le François, B.; Zahrai, A.; Turcotte-Cardin, V.; Daigle, M.; James, J.; Hen, R.; Merali, Z.; et al. Loss of MeCP2 in Adult 5-HT Neurons Induces 5-HT1A Autoreceptors, with Opposite Sex-Dependent Anxiety and Depression Phenotypes. Sci. Rep. 2018, 8, 5788. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Summavielle, T.; Teixeira-Castro, A.; Silva-Fernandes, A.; Duarte-Silva, S.; Marques, F.; Martins, L.; Dierssen, M.; Oliveira, P.; Sousa, N.; et al. Monoamine Deficits in the Brain of Methyl-CpG Binding Protein 2 Null Mice Suggest the Involvement of the Cerebral Cortex in Early Stages of Rett Syndrome. Neuroscience 2010, 170, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Panayotis, N.; Ghata, A.; Villard, L.; Roux, J.C. Biogenic Amines and Their Metabolites Are Differentially Affected in the Mecp2-Deficient Mouse Brain. BMC Neurosci. 2011, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Vogelgesang, S.; Niebert, S.; Renner, U.; Möbius, W.; Hülsmann, S.; Manzke, T.; Niebert, M. Analysis of the Serotonergic System in a Mouse Model of Rett Syndrome Reveals Unusual Upregulation of Serotonin Receptor 5b. Front. Mol. Neurosci. 2017, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Morimoto, M.; Matsui, F.; Hasegawa, T.; Tozawa, T.; Morioka, S.; Chiyonobu, T.; Nishimura, A.; Yoshimoto, K.; Hosoi, H. Postnatal Changes in Serotonergic Innervation to the Hippocampus of Methyl-CpG-Binding Protein 2-Null Mice. Neuroscience 2010, 165, 1254–1260. [Google Scholar] [CrossRef]
- De Filippis, B.; Musto, M.; Altabella, L.; Romano, E.; Canese, R.; Laviola, G. Deficient Purposeful Use of Forepaws in Female Mice Modelling Rett Syndrome. Neural Plast. 2015, 2015, 326184. [Google Scholar] [CrossRef]
- Abdala, A.P.; Bissonnette, J.M.; Newman-Tancredi, A. Pinpointing Brainstem Mechanisms Responsible for Autonomic Dysfunction in Rett Syndrome: Therapeutic Perspectives for 5-HT1A Agonists. Front. Physiol. 2014, 5, 205. [Google Scholar] [CrossRef] [PubMed]
- Flores Gutiérrez, J.; De Felice, C.; Natali, G.; Leoncini, S.; Signorini, C.; Hayek, J.; Tongiorgi, E. Protective Role of Mirtazapine in Adult Female Mecp2+/− Mice and Patients with Rett Syndrome. J. Neurodev. Disord. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Gokben, S.; Ardic, U.A.; Serdaroglu, G. Use of Buspirone and Fluoxetine for Breathing Problems in Rett Syndrome. Pediatr. Neurol. 2012, 46, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Saito, Y.; Ueda, R.; Togawa, M.; Ohmae, T.; Matsuda, E.; Fujiyama, M.; Maegaki, Y. Effect of Serotonin 1A Agonists and Selective Serotonin Reuptake Inhibitors on Behavioral and Nighttime Respiratory Symptoms in Rett Syndrome. Pediatr. Neurol. 2016, 60, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Temudo, T.; Rios, M.; Prior, C.; Carrilho, I.; Santos, M.; Maciel, P.; Sequeiros, J.; Fonseca, M.; Monteiro, J.; Cabral, P.; et al. Evaluation of CSF Neurotransmitters and Folate in 25 Patients with Rett Disorder and Effects of Treatment. Brain Dev. 2009, 31, 46–51. [Google Scholar] [CrossRef]
- Persico, A.M.; Ricciardello, A.; Cucinotta, F. The Psychopharmacology of Autism Spectrum Disorder and Rett Syndrome. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 165, pp. 391–414. ISBN 978-0-444-64012-3. [Google Scholar]
- Villani, C.; Carli, M.; Castaldo, A.M.; Sacchetti, G.; Invernizzi, R.W. Fluoxetine Increases Brain MeCP2 Immuno-Positive Cells in a Female Mecp2 Heterozygous Mouse Model of Rett Syndrome through Endogenous Serotonin. Sci. Rep. 2021, 11, 14690. [Google Scholar] [CrossRef]
- Bianchi, M.T. Non-Serotonin Anti-Depressant Actions: Direct Ion Channel Modulation by SSRIs and the Concept of Single Agent Poly-Pharmacy. Med. Hypotheses 2008, 70, 951–956. [Google Scholar] [CrossRef]
- Pinna, G.; Costa, E.; Guidotti, A. SSRIs Act as Selective Brain Steroidogenic Stimulants (SBSSs) at Low Doses That Are Inactive on 5-HT Reuptake. Curr. Opin. Pharmacol. 2009, 9, 24–30. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hortnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Gijsman, H.J.; van Gerven, J.M.A.; de Kam, M.L.; Schoemaker, R.C.; Pieters, M.S.M.; Weemaes, M.; de Rijk, R.; van der Post, J.; Cohen, A.F. Placebo-Controlled Comparison of Three Dose-Regimens of 5-Hydroxytryptophan Challenge Test in Healthy Volunteers. J. Clin. Psychopharmacol. 2002, 22, 183–189. [Google Scholar] [CrossRef]
- Green, A.R.; Aronson, J.K.; Curzon, G.; Woods, H.F. Metabolism of an Oral Tryptophan Load. I: Effects of Dose and Pretreatment with Tryptophan. Br. J. Clin. Pharmacol. 1980, 10, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Sarna, G.S.; Hutson, P.H.; O’Connell, M.T.; Curzon, G. Effect of Tryptophan on Extracellular Concentrations of Tryptophan and 5-Hydroxyindoleacetic Acid in the Striatum and Cerebellum. J. Neurochem. 1991, 56, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.P.; Rudder, M.L.; Roberts, W.; Royer, E.L.; Robinson, T.J.; Oh, A.; Spasojevic, I.; Sachs, B.D.; Caron, M.G. SSRI Augmentation by 5-Hydroxytryptophan Slow Release: Mouse Pharmacodynamic Proof of Concept. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Heine, W.; Radke, M.; Wutzke, K.D.; Peters, E.; Kundt, G. Alpha-Lactalbumin-Enriched Low-Protein Infant Formulas: A Comparison to Breast Milk Feeding. Acta Paediatr. 1996, 85, 1024–1028. [Google Scholar] [CrossRef]
- Petersen, H.; Nomayo, A.; Zelenka, R.; Foster, J.; Tvrdík, J.; Jochum, F. Adequacy and Safety of α-Lactalbumin-Enriched Low-Protein Infant Formula: A Randomized Controlled Trial. Nutrition 2020, 74, 110728. [Google Scholar] [CrossRef]
- Dupont, C.; Rivero, M.; Grillon, C.; Belaroussi, N.; Kalindjian, A.; Marin, V. Alpha-Lactalbumin-Enriched and Probiotic-Supplemented Infant Formula in Infants with Colic: Growth and Gastrointestinal Tolerance. Eur. J. Clin. Nutr. 2010, 64, 765–767. [Google Scholar] [CrossRef]
- Choi, S.; DiSilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Meal Ingestion, Amino Acids and Brain Neurotransmitters: Effects of Dietary Protein Source on Serotonin and Catecholamine Synthesis Rates. Physiol. Behav. 2009, 98, 156–162. [Google Scholar] [CrossRef]
- Orosco, M.; Rouch, C.; Beslot, F.; Feurte, S.; Regnault, A.; Dauge, V. Alpha-Lactalbumin-Enriched Diets Enhance Serotonin Release and Induce Anxiolytic and Rewarding Effects in the Rat. Behav. Brain Res. 2004, 148, 1–10. [Google Scholar] [CrossRef]
- De Caro, C.; Leo, A.; Nesci, V.; Ghelardini, C.; di Cesare Mannelli, L.; Striano, P.; Avagliano, C.; Calignano, A.; Mainardi, P.; Constanti, A.; et al. Intestinal Inflammation Increases Convulsant Activity and Reduces Antiepileptic Drug Efficacy in a Mouse Model of Epilepsy. Sci. Rep. 2019, 9, 13983. [Google Scholar] [CrossRef]
- Citraro, R.; Scicchitano, F.; De Fazio, S.; Raggio, R.; Mainardi, P.; Perucca, E.; De Sarro, G.; Russo, E. Preclinical Activity Profile of α-Lactoalbumin, a Whey Protein Rich in Tryptophan, in Rodent Models of Seizures and Epilepsy. Epilepsy Res. 2011, 95, 60–69. [Google Scholar] [CrossRef]
- Leo, A.; De Caro, C.; Mainardi, P.; Tallarico, M.; Nesci, V.; Marascio, N.; Striano, P.; Russo, E.; Constanti, A.; De Sarro, G.; et al. Increased Efficacy of Combining Prebiotic and Postbiotic in Mouse Models Relevant to Autism and Depression. Neuropharmacology 2021, 198, 108782. [Google Scholar] [CrossRef] [PubMed]
- Cervo, L.; Canetta, A.; Calcagno, E.; Burbassi, S.; Sacchetti, G.; Caccia, S.; Fracasso, C.; Albani, D.; Forloni, G.; Invernizzi, R.W. Genotype-Dependent Activity of Tryptophan Hydroxylase-2 Determines the Response to Citalopram in a Mouse Model of Depression. J. Neurosci. 2005, 25, 8165–8172. [Google Scholar] [CrossRef] [PubMed]
- Lasley, S.M.; Michaelson, I.A.; Greenland, R.D.; McGinnis, P.M. Simultaneous Measurement of Tyrosine, Tryptophan and Related Monoamines for Determination of Neurotransmitter Turnover in Discrete Rat Brain Regions by Liquid Chromatography with Electrochemical Detection. J. Chromatogr. 1984, 305, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Isingrini, E.; Perret, L.; Rainer, Q.; Sagueby, S.; Moquin, L.; Gratton, A.; Giros, B. Selective Genetic Disruption of Dopaminergic, Serotonergic and Noradrenergic Neurotransmission: Insights into Motor, Emotional and Addictive Behaviour. J. Psychiatry Neurosci. JPN 2016, 41, 169–181. [Google Scholar] [CrossRef]
- Carlsson, A.; Davis, J.N.; Kehr, W.; Lindqvist, M.; Atack, C.V. Simultaneous Measurement of Tyrosine and Tryptophan Hydroxylase Activities in Brain In Vivo Using an Inhibitor of the Aromatic Amino Acid Decarboxylase. Naunyn Schmiedeberg’s Arch. Pharmacol. 1972, 275, 153–168. [Google Scholar] [CrossRef]
- Carli, M.; Kostoula, C.; Sacchetti, G.; Mainolfi, P.; Anastasia, A.; Villani, C.; Invernizzi, R.W. Tph2 Gene Deletion Enhances Amphetamine-Induced Hypermotility: Effect of 5-HT Restoration and Role of Striatal Noradrenaline Release. J. Neurochem. 2015, 135, 674–685. [Google Scholar] [CrossRef]
- Baumann, M.H.; Williams, Z.; Zolkowska, D.; Rothman, R.B. Serotonin (5-HT) Precursor Loading with 5-Hydroxy-L-Tryptophan (5-HTP) Reduces Locomotor Activation Produced by (+)-Amphetamine in the Rat. Drug Alcohol. Depend. 2011, 114, 147–152. [Google Scholar] [CrossRef][Green Version]
- Westerink, B.H.; De Vries, J.B. Effect of Precursor Loading on the Synthesis Rate and Release of Dopamine and Serotonin in the Striatum: A Microdialysis Study in Conscious Rats. J. Neurochem. 1991, 56, 228–233. [Google Scholar] [CrossRef]
- Carneiro, I.B.C.; Toscano, A.E.; Lacerda, D.C.; da Cunha, M.d.S.B.; de Castro, R.M.; Deiró, T.C.B.d.J.; Medeiros, J.M.B. L-Tryptophan Administration and Increase in Cerebral Serotonin Levels: Systematic Review. Eur. J. Pharmacol. 2018, 836, 129–135. [Google Scholar] [CrossRef]
- Perry, K.W.; Fuller, R.W. Extracellular 5-Hydroxytryptamine Concentration in Rat Hypothalamus after Administration of Fluoxetine plus L-5-Hydroxytryptophan. J. Pharm. Pharmacol. 1993, 45, 759–761. [Google Scholar] [CrossRef]
- Choi, S.; DiSilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Effect of Chronic Protein Ingestion on Tyrosine and Tryptophan Levels and Catecholamine and Serotonin Synthesis in Rat Brain. Nutr. Neurosci. 2011, 14, 260–267. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-Lactalbumin in Human Nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Gaspar, P.; Cases, O.; Maroteaux, L. The Developmental Role of Serotonin: News from Mouse Molecular Genetics. Nat. Rev. Neurosci. 2003, 4, 1002–1012. [Google Scholar] [CrossRef]
- Jing, J.-Q.; Jia, S.-J.; Yang, C.-J. Physical Activity Promotes Brain Development through Serotonin during Early Childhood. Neuroscience 2024, 554, 34–42. [Google Scholar] [CrossRef]
- Meijer, A.; Königs, M.; Vermeulen, G.T.; Visscher, C.; Bosker, R.J.; Hartman, E.; Oosterlaan, J. The Effects of Physical Activity on Brain Structure and Neurophysiological Functioning in Children: A Systematic Review and Meta-Analysis. Dev. Cogn. Neurosci. 2020, 45, 100828. [Google Scholar] [CrossRef] [PubMed]
- Vitrac, C.; Benoit-Marand, M. Monoaminergic Modulation of Motor Cortex Function. Front. Neural Circuits 2017, 11, 72. [Google Scholar] [CrossRef]
- FitzGerald, P.M.; Jankovic, J.; Glaze, D.G.; Schultz, R.; Percy, A.K. Extrapyramidal Involvement in Rett’s Syndrome. Neurology 1990, 40, 293–295. [Google Scholar] [CrossRef]
- Hirano, K.; Kimura, R.; Sugimoto, Y.; Yamada, J.; Uchida, S.; Kato, Y.; Hashimoto, H.; Yamada, S. Relationship between Brain Serotonin Transporter Binding, Plasma Concentration and Behavioural Effect of Selective Serotonin Reuptake Inhibitors. Br. J. Pharmacol. 2005, 144, 695–702. [Google Scholar] [CrossRef]
- Eccleston, D.; Ashcroft, G.W.; Crawford, T.B. 5-Hydroxyindole Metabolism in Rat Brain. A Study of Intermediate Metabolism Using the Technique of Tryptophan Loading. II. Applications and Drug Studies. J. Neurochem. 1965, 12, 493–503. [Google Scholar] [CrossRef]
- Ho, I.K.; Brase, D.A.; Loh, H.H.; Way, E.L. Influence of L-Tryptophan on Morphine Analgesia, Tolerance and Physical Dependence. J. Pharmacol. Exp. Ther. 1975, 193, 35–43. [Google Scholar] [PubMed]
- Fredricson Overø, K. Kinetics of Citalopram in Test Animals; Drug Exposure in Safety Studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 1982, 6, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-H.; Kao, F.-C.; Huang, Y.-B.; Liao, W. MeCP2 in the Rostral Striatum Maintains Local Dopamine Content Critical for Psychomotor Control. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 6209–6220. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, S.; Calcagno, E.; Canetta, A.; Sacchetti, G.; Fracasso, C.; Caccia, S.; Cervo, L.; Invernizzi, R.W. Strain Differences in Paroxetine-Induced Reduction of Immobility Time in the Forced Swimming Test in Mice: Role of Serotonin. Eur. J. Pharmacol. 2008, 594, 117–124. [Google Scholar] [CrossRef]
- Calcagno, E.; Canetta, A.; Guzzetti, S.; Cervo, L.; Invernizzi, R.W. Strain Differences in Basal and Post-Citalopram Extracellular 5-HT in the Mouse Medial Prefrontal Cortex and Dorsal Hippocampus: Relation with Tryptophan Hydroxylase-2 Activity. J. Neurochem. 2007, 103, 1111–1120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villani, C.; Sacchetti, G.; Invernizzi, R.W. Boosting Serotonin Synthesis Is Not Sufficient to Improve Motor Coordination of Mecp2 Heterozygous Mouse Model of Rett Syndrome. Biomolecules 2024, 14, 1230. https://doi.org/10.3390/biom14101230
Villani C, Sacchetti G, Invernizzi RW. Boosting Serotonin Synthesis Is Not Sufficient to Improve Motor Coordination of Mecp2 Heterozygous Mouse Model of Rett Syndrome. Biomolecules. 2024; 14(10):1230. https://doi.org/10.3390/biom14101230
Chicago/Turabian StyleVillani, Claudia, Giuseppina Sacchetti, and Roberto W. Invernizzi. 2024. "Boosting Serotonin Synthesis Is Not Sufficient to Improve Motor Coordination of Mecp2 Heterozygous Mouse Model of Rett Syndrome" Biomolecules 14, no. 10: 1230. https://doi.org/10.3390/biom14101230
APA StyleVillani, C., Sacchetti, G., & Invernizzi, R. W. (2024). Boosting Serotonin Synthesis Is Not Sufficient to Improve Motor Coordination of Mecp2 Heterozygous Mouse Model of Rett Syndrome. Biomolecules, 14(10), 1230. https://doi.org/10.3390/biom14101230








