Investigating the Suitability of Mare’s Milk-Derived Exosomes as Potential Drug Carriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Mare’s Milk Purchase
2.2. Exosomes Isolation Methods
2.2.1. Total Exosome Isolation
2.2.2. Isoelectric Precipitation
2.2.3. Size Exclusion Chromatography
2.3. Characterization of Exosomes
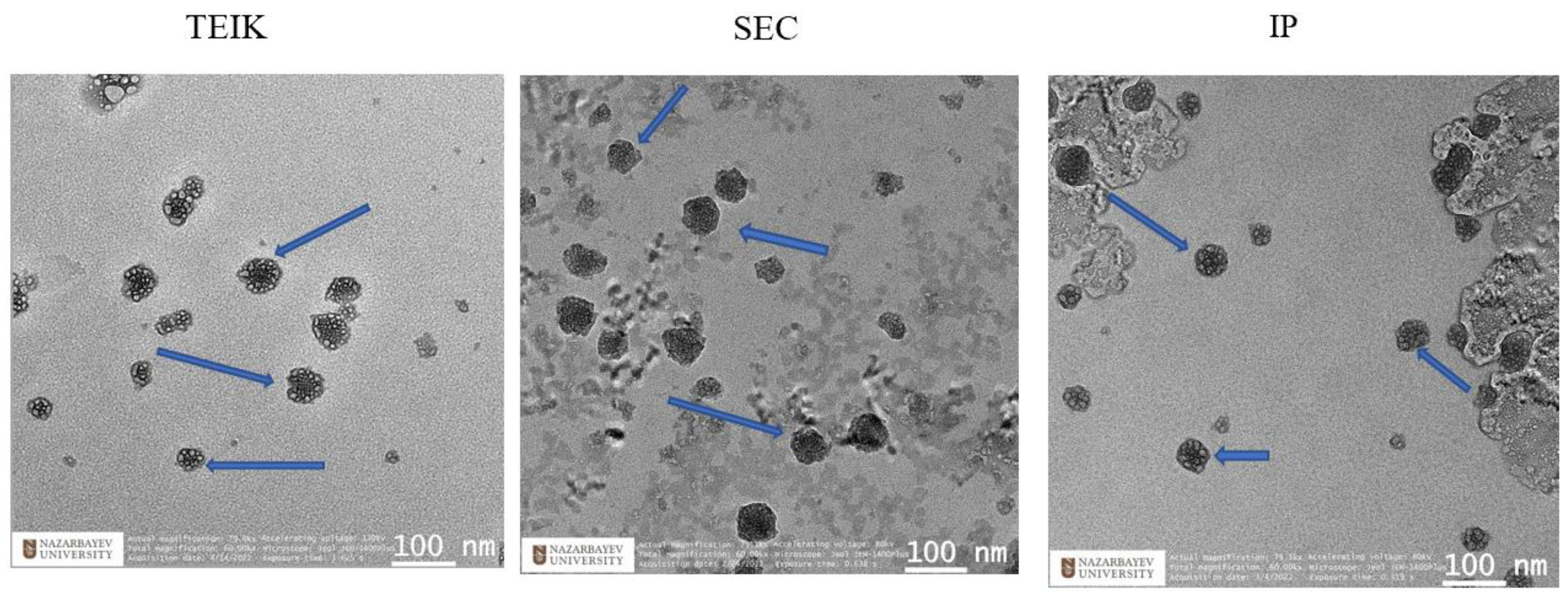
2.3.1. Transmission Electron Microscopy
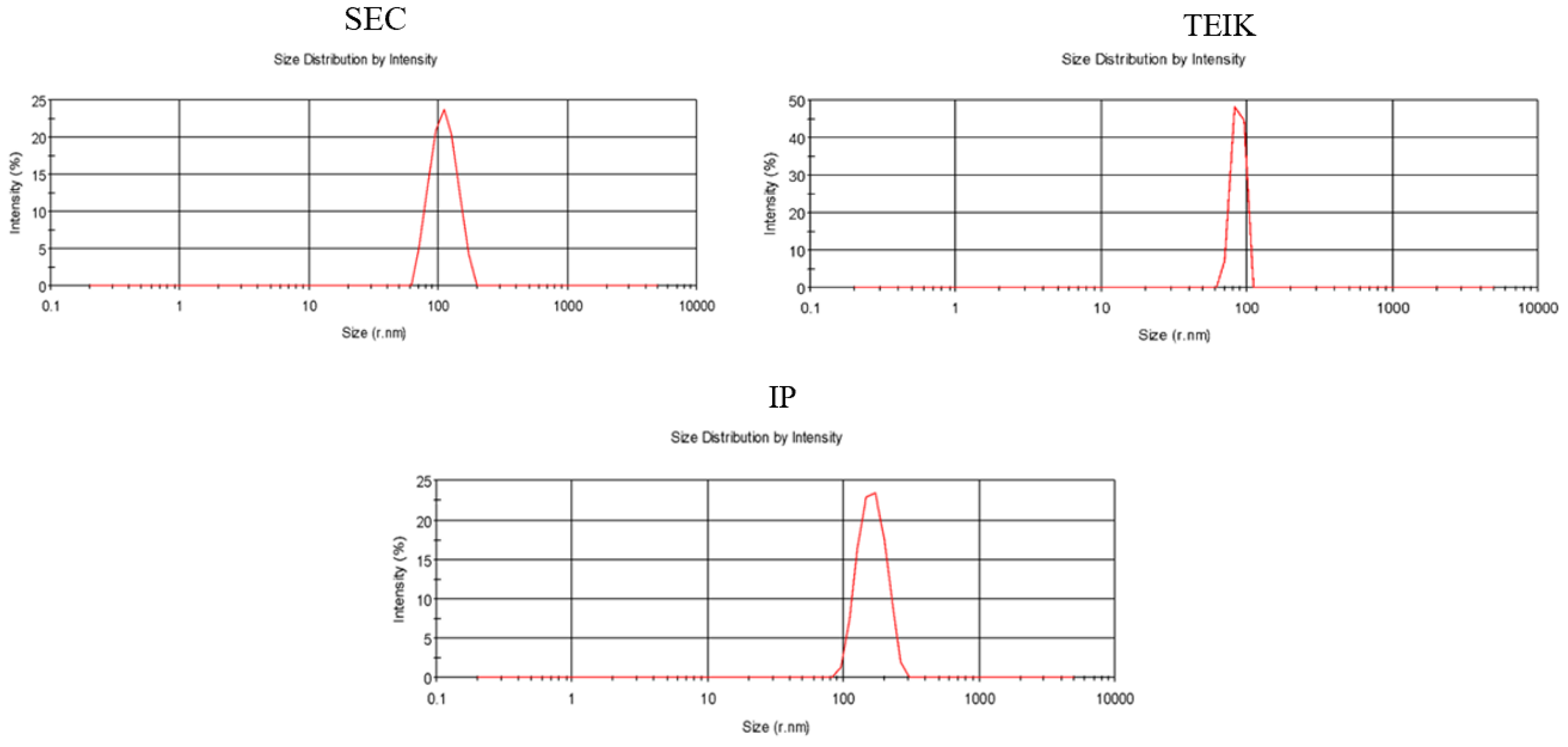
2.3.2. Nanoparticle Determination
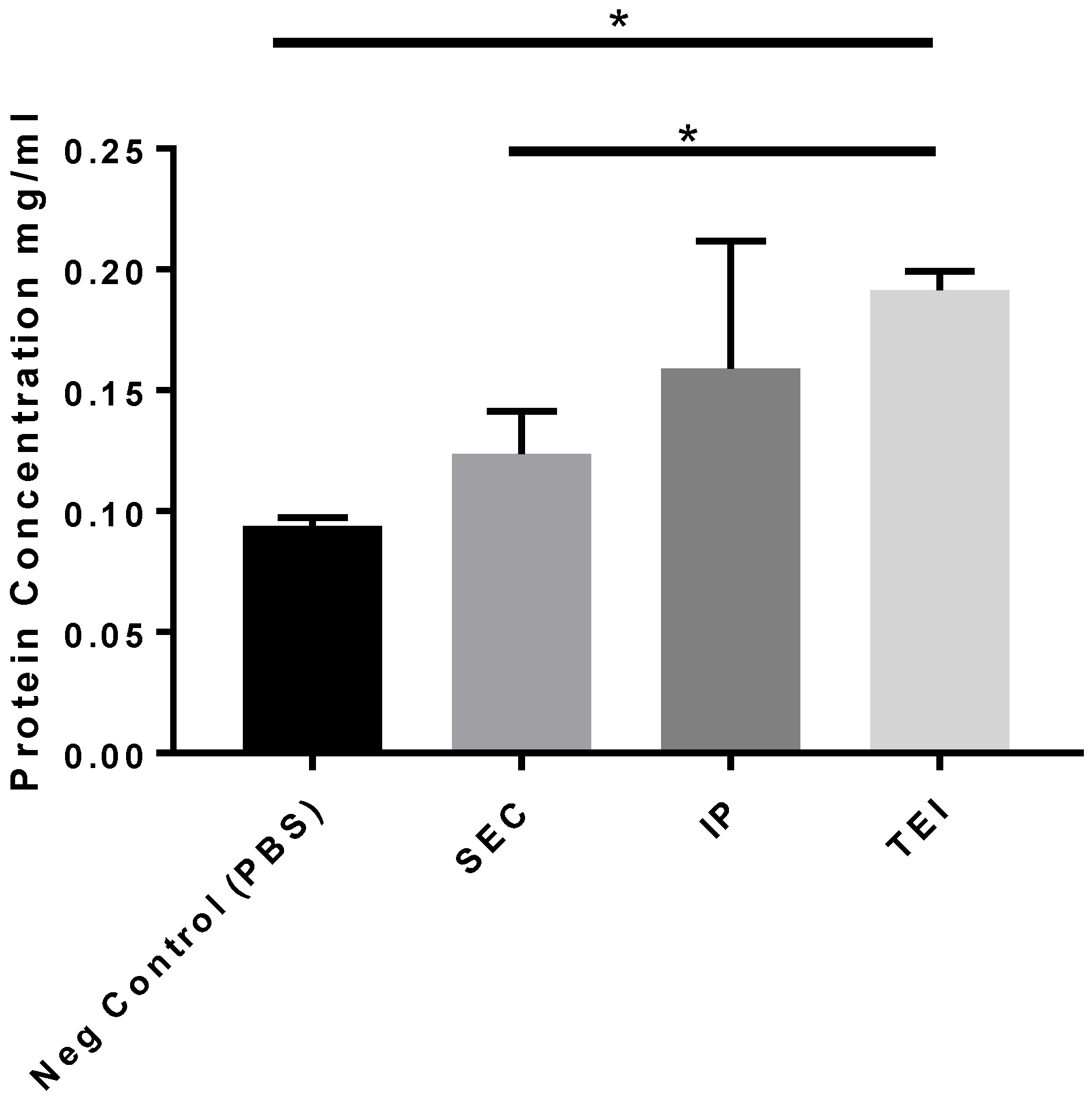
2.3.3. Protein Determination
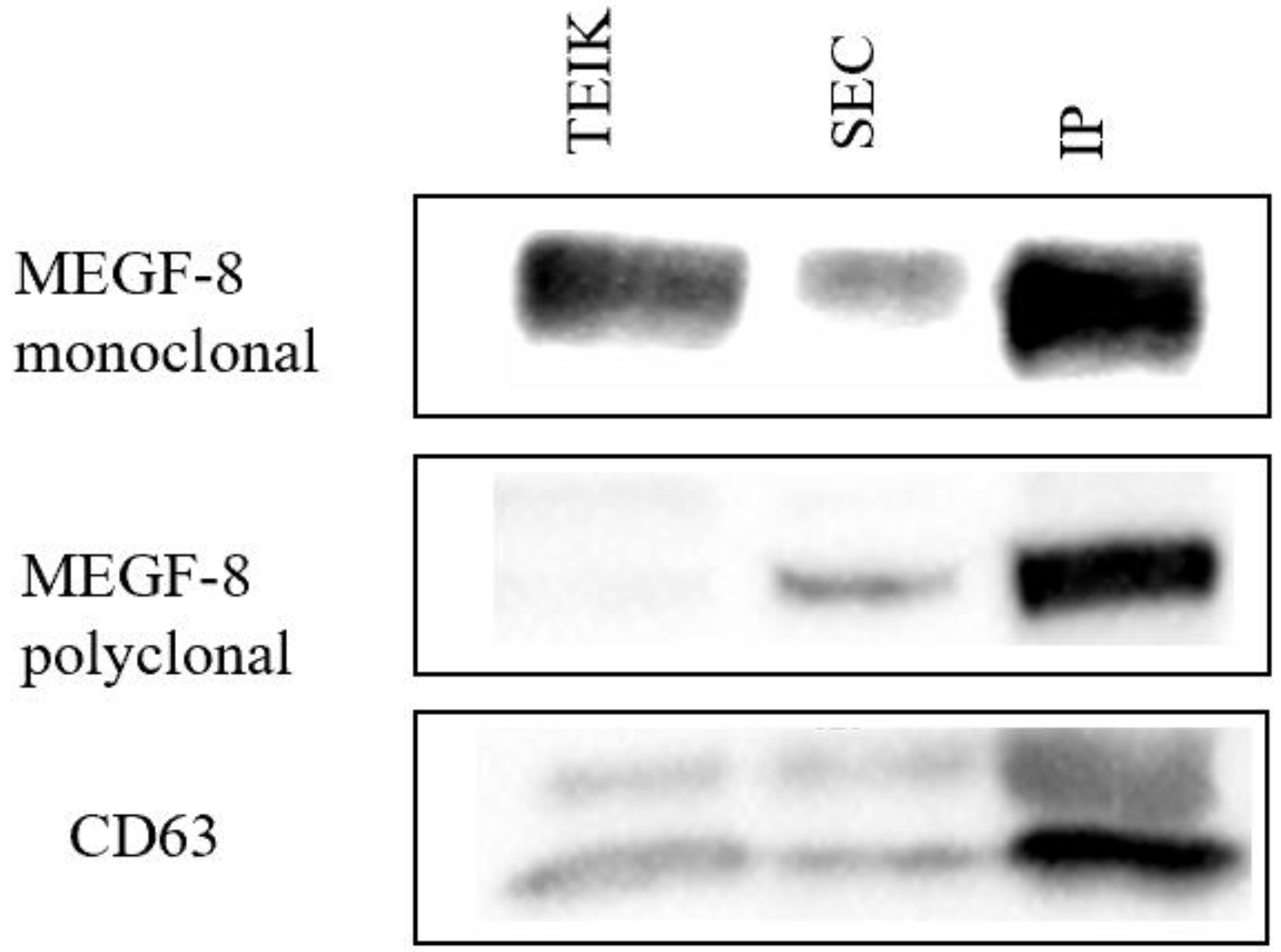
2.3.4. Western Blot Analysis
2.3.5. Exosome Loading with Quercetin
2.4. In Vitro Assays
2.4.1. Cell Cultures
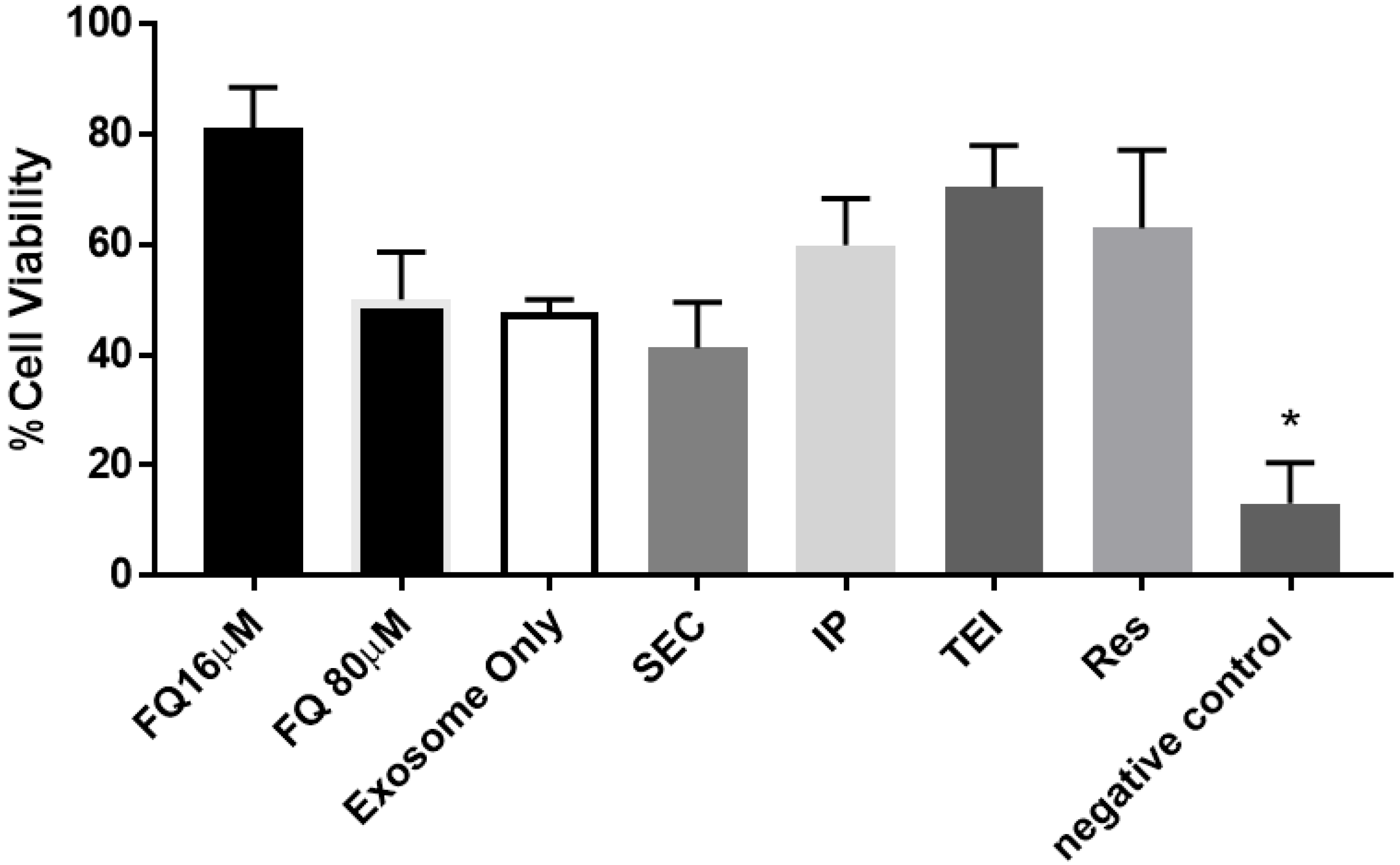
2.4.2. Cell Viability
2.4.3. β-Galactosidase Assay
2.5. Animal Experimentations
2.5.1. Efficacy of Quercetin-Loaded Exosomes from Mare’s Milk Compared to Free Quercetin in an Aging Male Rat Model
2.5.2. Experimental Design
2.5.3. Histopathological Examination
2.5.4. Morphometric Study
2.6. Statistical Analysis
3. Results
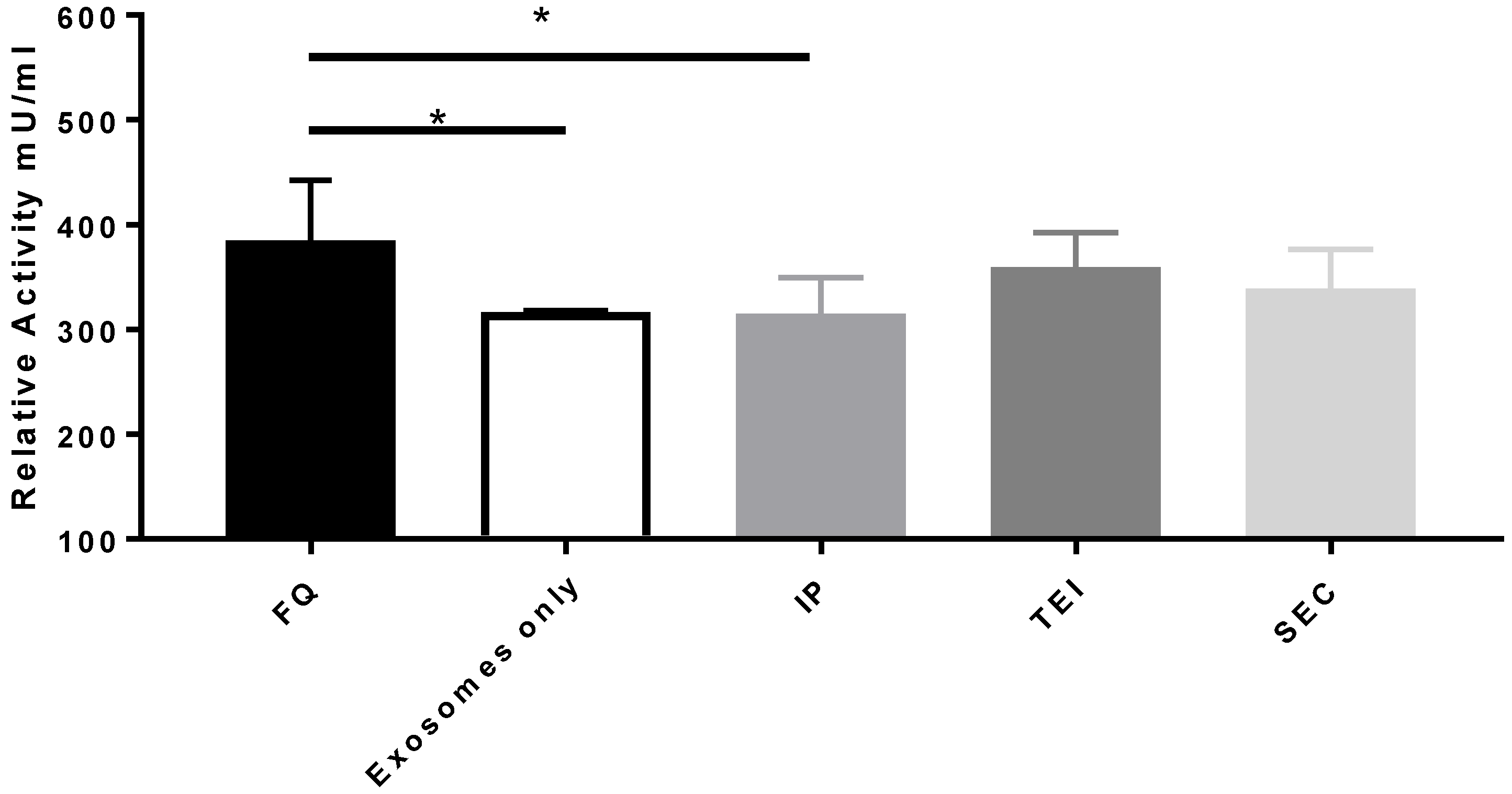
3.1. Comparison of Exosome Isolation Methods
3.2. Therapeutic Cargo Properties of Isolated Exosomes
3.3. Organ Histological and Histochemical Analysis
3.3.1. Heart
3.3.2. Kidneys
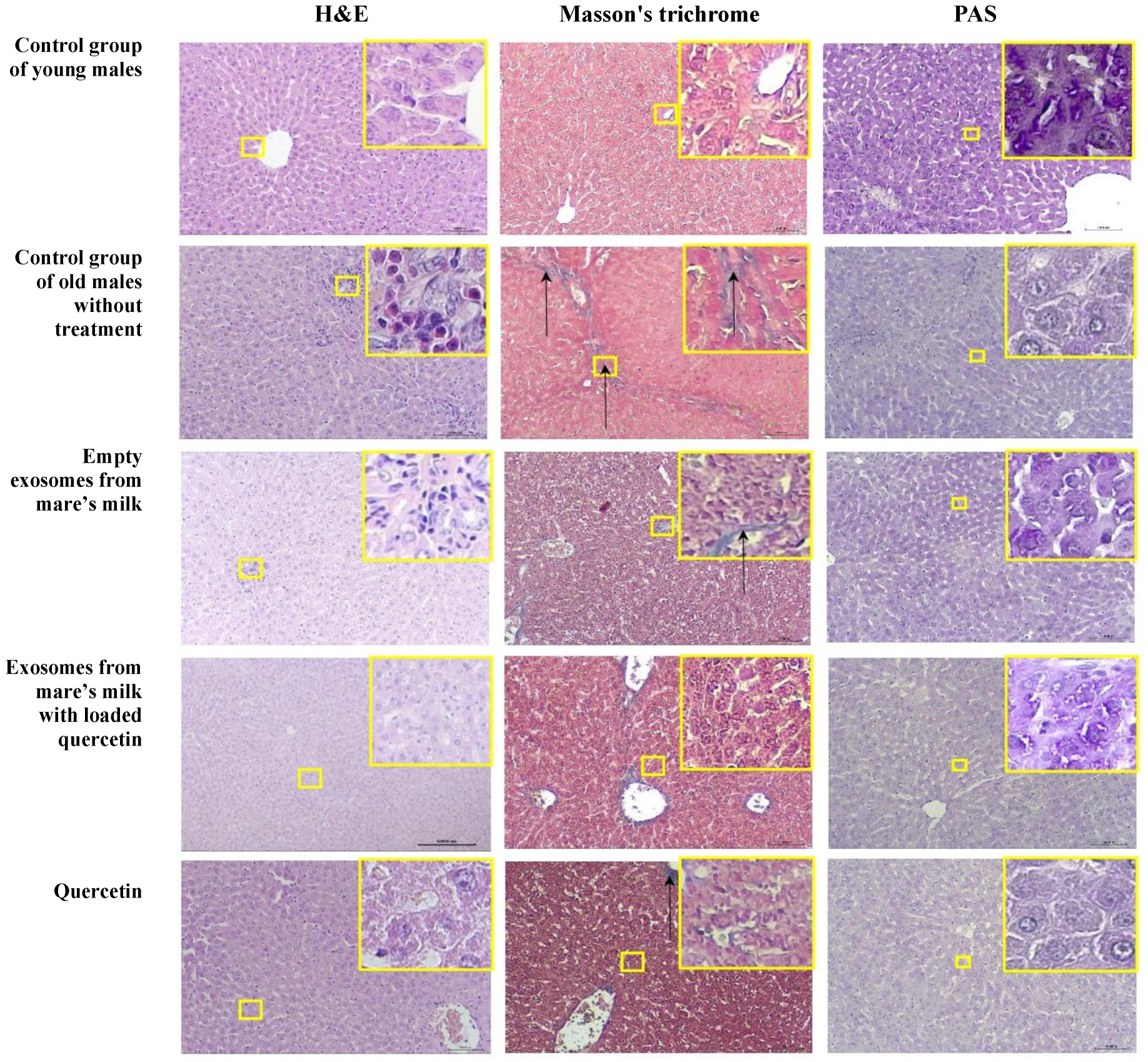
3.3.3. Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 15, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Liang, W. ASCs -derived exosomes loaded with vitamin A and quercetin inhibit rapid senescence-like response after acute liver injury. Biochem. Biophys. Res. Commun. 2021, 572, 125–130. [Google Scholar] [CrossRef]
- Anand, N.; Sehgal, R.; Kanwar, R.; Dubey, M.; Vasishta, R.; Kanwar, J. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite Toxoplasma gondii. Int. J. Nanomed. 2015, 10, 6355–6369. [Google Scholar]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, 346. [Google Scholar] [CrossRef]
- Sharma, P.; Ludwig, S.; Muller, L.; Hong, C.; Kirkwood, J.; Ferrone, S.; Whiteside, T.L. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J. Extracell. Vesicles 2018, 7, 1435138. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- Reif, S.; Elbaum Shiff, Y.; Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J. Transl. Med. 2019, 7, 325. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Norman Salem, J.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. BBA Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes From Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef]
- Vaswani, K.; Koh, Y.Q.; Almughlliq, F.B.; Peiris, H.N.; Mitchell, M.D. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 2017, 17, 341–348. [Google Scholar] [CrossRef]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Woźniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Alavian, S.M.; Nabavi, S.M. Hepatoprotective effect of quercetin: From chemistry to medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Bientinesi, E.; Lulli, M.; Becatti, M.; Ristori, S.; Margheri, F.; Monti, D. Doxorubicin-induced senescence in normal fibroblasts promotes in vitro tumour cell growth and invasiveness: The role of Quercetin in modulating these processes. Mech. Ageing Dev. 2022, 206, 111689. [Google Scholar] [CrossRef]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated β-Galactosidase Detection in Pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef]
- Snyder, J.M.; Ward, J.M.; Treuting, P.M. Cause-of-Death Analysis in Rodent Aging Studies. Vet. Pathol. 2016, 53, 233–243. [Google Scholar] [CrossRef]
- Keenan, C.M.; Baker, J.; Bradley, A.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; Mahler, B.; Meseck, E.; et al. International Harmonization of Nomenclature and Diagnostic Criteria (INHAND): Progress to Date and Future Plans. Toxicol. Pathol. 2015, 43, 730–732. [Google Scholar] [CrossRef]
- Snyder, J.M.; Snider, T.A.; Ciol, M.A.; Wilkinson, J.E.; Imai, D.M.; Casey, K.M.; Vilches-Moure, J.G.; Pettan-Brewer, C.; Pillai, S.P.S.; Carrasco, S.E.; et al. Validation of a geropathology grading system for aging mouse studies. Geroscience 2019, 41, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Sergazy, S.; Shulgau, Z.; Fedotovskikh, G.; Chulenbayeva, L.; Nurgozhina, A.; Nurgaziyev, M.; Krivyh, E.; Kamyshanskiy, Y.; Kushugulova, A.; Gulyayev, A.; et al. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci. Rep. 2020, 10, 14720. [Google Scholar] [CrossRef] [PubMed]
- Sergazy, S.; Shulgau, Z.; Kamyshanskiy, Y.; Zhumadilov, Z.; Krivyh, E.; Gulyayev, A.; Aljofan, M. Blueberry and cranberry extracts mitigate CCL4-induced liver damage, suppressing liver fibrosis, inflammation and oxidative stress. Heliyon 2023, 9, 15370. [Google Scholar] [CrossRef] [PubMed]
- Sedykh, S.; Kuleshova, A.; Nevinsky, G. Milk Exosomes: Perspective Agents for Anticancer Drug Delivery. Int. J. Mol. Sci. 2020, 21, 6646. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Rao, L.; Chen, Y.; Xiao, S.; Xia, H.; Yang, J.; Lv, X.; Qin, D.; Zhu, C. Controlled SPION-Exosomes Loaded with Quercetin Preserves Pancreatic Beta Cell Survival and Function in Type 2 Diabetes Mellitus. Int. J. Nanomed. 2023, 18, 5733–5748. [Google Scholar] [CrossRef]
- de Albuquerque, P.B.S.; de Souza, M.P.; Bourbon, A.I.; Cerqueira, M.A.; Pastrana, L.; Jauregi, P.; Teixeira, J.A.; das Graças Carneiro-da-Cunha, M. Production and Properties of Quercetin-Loaded Liposomes and Their Influence on the Properties of Galactomannan-Based Films. Appl. Nano 2023, 4, 159–177. [Google Scholar] [CrossRef]
- Abd El-Emam, M.M.; Mostafa, M.; Farag, A.A.; Youssef, H.S.; El-Demerdash, A.S.; Bayoumi, H.; Gebba, M.A.; El-Halawani, S.M.; Saleh, A.M.; Badr, A.M.; et al. The Potential Effects of Quercetin-Loaded Nanoliposomes on Amoxicillin/Clavulanate-Induced Hepatic Damage: Targeting the SIRT1/Nrf2/NF-κB Signaling Pathway and Microbiota Modulation. Antioxidants 2023, 12, 1487. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, Y.; Dong, L.; Ma, W.; Lv, L.; Zhang, J.; Wang, X. Glioblastoma Stem Cell-Derived Exosomes Enhance Stemness and Tumorigenicity of Glioma Cells by Transferring Notch1 Protein. Cell Mol. Neurobiol. 2020, 40, 767–784. [Google Scholar] [CrossRef]
- Aqil, F.; Kausar, H.; Agrawal, A.K.; Jeyabalan, J.; Kyakulaga, A.H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Ma, X.; Wang, W.; Zhao, B.; Chen, Y.; Chen, C.; Bihl, J.C. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J. Cell Mol. Med. 2018, 22, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Poe, A.J.; Knowlton, A.A. Exosomes as agents of change in the cardiovascular system. J. Mol. Cell Cardiol. 2017, 111, 40–50. [Google Scholar] [CrossRef]
- Jansen, F.; Nickenig, G.; Werner, N. Extracellular Vesicles in Cardiovascular Disease: Potential Applications in Diagnosis, Prognosis, and Epidemiology. Circ. Res. 2017, 120, 1649–1657. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Wang, M.; Yan, M.; Jiang, J.; Li, Z. Exosomes derived miR-126 attenuates oxidative stress and apoptosis from ischemia and reperfusion injury by targeting ERRFI1. Gene 2019, 690, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C.; et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; Nonnecke, B.J.; Sacco, R.E. Bovine milk exosome proteome. J. Proteom. 2012, 75, 1486–1492. [Google Scholar] [CrossRef]


| Parameters | Scores | Control Group of Young Male Rats (n = 3) | Control Group of Old Male Rats without Treatment (n = 3) | Empty Exosomes from Mare’s Milk (n = 3) | Exosomes from Mare’s Milk Loaded with Quercetin (n = 3) | Quercetin (n = 3) |
|---|---|---|---|---|---|---|
| Hematoxylin and eosin staining | ||||||
| Cardiomyocyte hypertrophy * | 0 | 100% (3/3) | - | - | 33% (1/3) | - |
| 1 | - | 100% (3/3) | 100% (3/3) | 66% (2/3) | 100% (3/3) | |
| Focal hyper-eosinophilia * | 0 | 100% (3/3) | - | 33% (1/3) | 66% (2/3) | 66% (2/3) |
| 1 | - | 100% (3/3) | 66% (2/3) | 33% (1/3) | 33% (1/3) | |
| Increased size of cardiomyocyte nuclei/appearance of multinucleated cardiomyocytes * | 0 | 100% (3/3) | - | 33% (1/3) | 66% (2/3) | 66% (2/3) |
| 1 | - | 100% (3/3) | 66% (2/3) | 33% (1/3) | 33% (1/3) | |
| Cellular infiltrate * | 0 | 100% (3/3) | 66% (2/3) | 66% (2/3) | 66% (2/3) | 100% (3/3) |
| 1 | - | 33% (1/3) | 33% (1/3) | 33% (1/3) | - | |
| Masson’s trichrome staining | ||||||
| Atherosclerosis * | 0 | 100% (3/3) | - | - | - | - |
| 1 | - | 100% (3/3) | 100% (3/3) | 100% (3/3) | 100% (3/3) | |
| Perivascular fibrosis ** | 0 | 100% (3/3) | - | - | - | - |
| 1 | - | 33% (1/3) | 33% (1/3) | 33% (1/3) | 33% (1/3) | |
| 2 | - | 66% (2/3) | 66% (2/3) | 66% (2/3) | 66% (2/3) | |
| 3 | - | - | - | - | - | |
| 4 | - | - | - | - | - | |
| Interstitial fibrosis ** | 0 | 100% (3/3) | - | - | - | - |
| 1 | - | - | - | - | - | |
| 2 | - | 100% (3/3) | 100% (3/3) | 100% (3/3) | 100% (3/3) | |
| Parameters | Scores | Control Group of Young Male Rats (n = 3) | Control Group of old Male Rats without Treatment (n = 3) | Empty Exosomes from Mare’s Milk (n = 3) | Exosomes from Mare’s Milk Loaded with Quercetin (n = 3) | Quercetin (n = 3) |
|---|---|---|---|---|---|---|
| Hematoxylin and eosin staining | ||||||
| Vacuolization of the epithelium | 0 | 100% (3/3) | 100% (3/3) | 100% (3/3) | 100% (3/3) | 100% (3/3) |
| 1 | - | - | - | - | - | |
| Hyperplasia of tubular epithelial cells | 0 | 100% (3/3) | - | - | 33% (1/3) | 33% (1/3) |
| 1 | - | 100% (3/3) | 100% (3/3) | 66% (2/3) | 66% (2/3) | |
| Formation of protein casts in tubules | 0 | 100% (3/3) | - | - | - | 33% (1/3) |
| 1 | - | 100% (3/3) | 100% (3/3) | 100% (3/3) | 66% (2/3) | |
| Cellular infiltrate in the interstitium | 0 | 100% (3/3) | - | - | 33% (1/3) | 66% (2/3) |
| 1 | - | 100% (3/3) | 100% (3/3) | 66% (2/3) | 33% (1/3) | |
| Masson’s trichrome staining | ||||||
| Thickening and fiberization of basement membranes | 0 | 100% (3/3) | - | - | 33% (1/3) | 33% (1/3) |
| 1 | - | 100% (3/3) | 100% (3/3) | 66% (2/3) | 66% (2/3) | |
| Hyaline glomerulopathy | 0 | 100% (3/3) | 33% (1/3) | 33% (1/3) | 33% (1/3) | 66% (2/3) |
| 1 | - | 66% (2/3) | 66% (2/3) | 66% (2/3) | 33% (1/3) | |
| Glomerulosclerosis | 0 | 100% (3/3) | - | - | - | - |
| 1 | - | 100% (3/3) | 100% (3/3) | 100% (3/3) | 100% (3/3) | |
| Parameters | Scores | Control Group of Young Male Rats (n = 3) | Control Group of Old Male Rats without Treatment (n = 3) | Empty Exosomes from Mare’s Milk (n = 3) | Exosomes from Mare’s Milk Loaded with Quercetin (n = 3) | Quercetin (n = 3) |
|---|---|---|---|---|---|---|
| Hematoxylin and eosin staining | ||||||
| Vacuolization of hepatocytes * | 0 | 100% (3/3) | - | - | - | 33% (1/3) |
| 1 | - | 100% (3/3) | 100% (3/3) | 100% (3/3) | 66% (2/3) | |
| Karyocytomegaly and/or multinucleated hepatocytes * | 0 | 100% (3/3) | 33% (1/3) | 33% (1/3) | 33% (1/3) | 33% (1/3) |
| 1 | - | 66% (2/3) | 66% (2/3) | 66% (2/3) | 66% (2/3) | |
| Central venous stasis * | 0 | - | - | - | - | |
| 1 | 100% (3/3) | 100% (3/3) | 100% (3/3) | 100% (3/3) | ||
| Cellular infiltrate * | 0 | 100% (3/3) | - | - | - | 33% (1/3) |
| 1 | - | 100% (3/3) | 100% (3/3) | 100% (3/3) | 66% (2/3) | |
| Masson’s trichrome staining | ||||||
| Bile duct hyperplasia/cysts * | 0 | 100% (3/3) | - | 33% (1/3) | - | 33% (1/3) |
| 1 | - | 100% (3/3) | 66% (2/3) | 100% (3/3) | 66% (2/3) | |
| Periportal fibrosis * | 0 | 100% (3/3) | - | - | - | - |
| 1 | - | 100% (3/3) | 100% (3/3) | 100% (3/3) | 100% (3/3) | |
| Glycogen accumulation ** | 0 | - | 100% (3/3) | 66% (2/3) | 66% (2/3) | 100% (3/3) |
| 1 | - | - | - | 33% (1/3) | - | |
| 2 | - | - | 33% (1/3) | - | - | |
| 3 | 100% (3/3) | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergazy, S.; Zhetkenev, S.; Shulgau, Z.; Chulenbayeva, L.; Kamyshanskiy, Y.; Nurgaziyev, M.; Nurgozhina, A.; Mukhanbetzhanova, Z.; Berikkhanova, K.; Gulyayev, A.; et al. Investigating the Suitability of Mare’s Milk-Derived Exosomes as Potential Drug Carriers. Biomolecules 2024, 14, 1247. https://doi.org/10.3390/biom14101247
Sergazy S, Zhetkenev S, Shulgau Z, Chulenbayeva L, Kamyshanskiy Y, Nurgaziyev M, Nurgozhina A, Mukhanbetzhanova Z, Berikkhanova K, Gulyayev A, et al. Investigating the Suitability of Mare’s Milk-Derived Exosomes as Potential Drug Carriers. Biomolecules. 2024; 14(10):1247. https://doi.org/10.3390/biom14101247
Chicago/Turabian StyleSergazy, Shynggys, Sanzhar Zhetkenev, Zarina Shulgau, Laura Chulenbayeva, Yevgeniy Kamyshanskiy, Madiyar Nurgaziyev, Ayaulym Nurgozhina, Zhanel Mukhanbetzhanova, Kulzhan Berikkhanova, Alexander Gulyayev, and et al. 2024. "Investigating the Suitability of Mare’s Milk-Derived Exosomes as Potential Drug Carriers" Biomolecules 14, no. 10: 1247. https://doi.org/10.3390/biom14101247
APA StyleSergazy, S., Zhetkenev, S., Shulgau, Z., Chulenbayeva, L., Kamyshanskiy, Y., Nurgaziyev, M., Nurgozhina, A., Mukhanbetzhanova, Z., Berikkhanova, K., Gulyayev, A., & Aljofan, M. (2024). Investigating the Suitability of Mare’s Milk-Derived Exosomes as Potential Drug Carriers. Biomolecules, 14(10), 1247. https://doi.org/10.3390/biom14101247






