Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements
Abstract
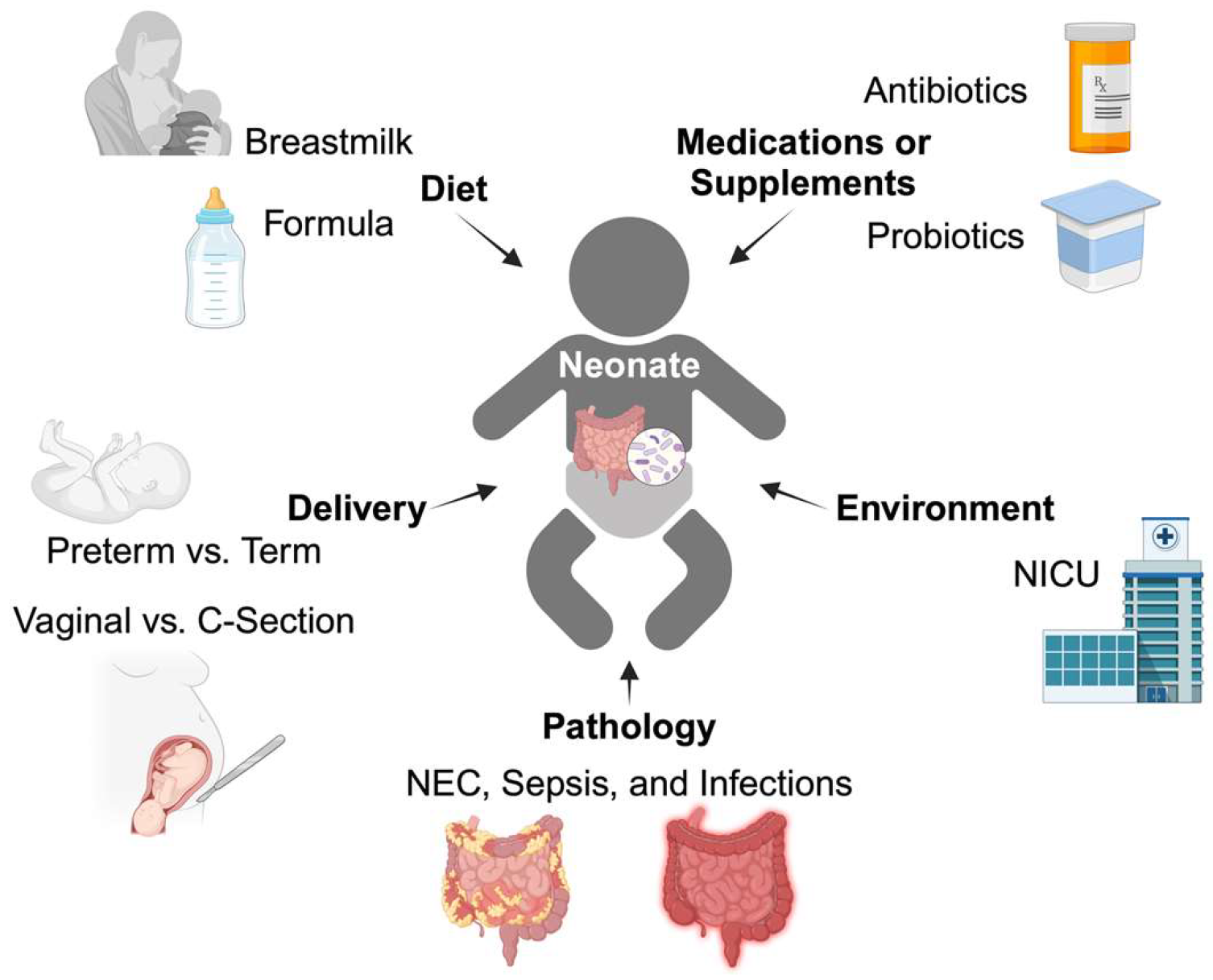
1. Introduction
2. Gut Microbiome Development
2.1. Colonization
2.2. Microbiome Development and Maturation
2.3. Nutritional Microbiome Modulators
| Preterm [33,46] | C-section [21,23] | Antibiotics [34,35] | Probiotics [47] | Formula [29] | NEC [48] | |
|---|---|---|---|---|---|---|
| Firmicutes | ↓ | ↑ | ↑ | ↑ | ↓ | |
| Lactobacilli | ↓ | ↓ | ↑ | ↓ | ||
| Staphylococcus Streptococcus Enterococcus | ↑ | ↑ | ↑ | |||
| Clostridium | ↓ | ↑ | ||||
| Bacteroidetes Bacteroides | ↓ | ↓ | ||||
| Proteobacteria | Depends on genus | Depends on genus | ↑ | ↑ | ↑ | |
| Actinobacteria | ↓ | ↓ | ↓ | ↓ | ||
| Bifidobacteria | ↓ | ↓ | ↑ | ↓ |
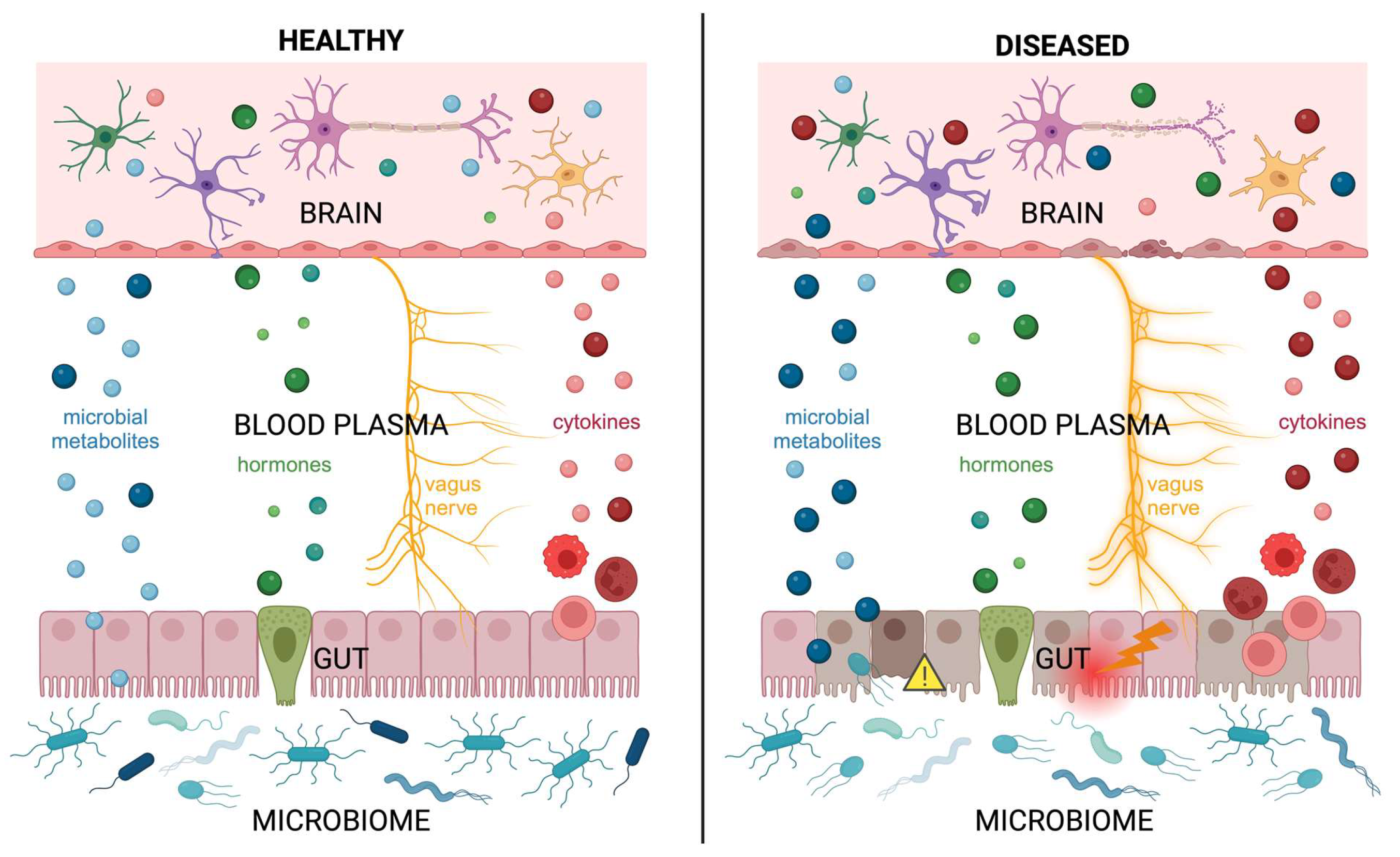
3. Gut–Microbiota–Brain Axis Signaling
3.1. Microbiome and Neurodevelopment
3.2. Nervous System: Autonomic
3.3. Endocrine System
3.4. Immune System: Inflammatory Mediators
4. Gut–Microbiota–Brain Axis Disruption in Neonatal and Pediatric Patients
4.1. Vulnerabilities of an Immature Gut
4.2. Abnormal Microbial Colonization of the Gut
4.3. Differential Nutritional Needs: Diet and the Microbiome
4.4. Disruption Impacts Neurodevelopment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.-A.M. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Vaher, K.; Bogaert, D.; Richardson, H.; Boardman, J.P. Microbiome-gut-brain axis in brain development, cognition and behavior during infancy and early childhood. Dev. Rev. 2022, 66, 101038. [Google Scholar] [CrossRef]
- Wakefield, A.J. The gut-brain axis in childhood developmental disorders. J. Pediatr. Gastroenterol. Nutr. 2002, 34 (Suppl. 1), S14–S17. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The microbiota-gut-brain axis and neurodevelopmental disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef]
- Jena, A.; Montoya, C.A.; Mullaney, J.A.; Dilger, R.N.; Young, W.; McNabb, W.C.; Roy, N.C. Gut-Brain Axis in the Early Postnatal Years of Life: A Developmental Perspective. Front. Integr. Neurosci. 2020, 14, 44. [Google Scholar] [CrossRef]
- Laue, H.E.; Coker, M.O.; Madan, J.C. The Developing Microbiome From Birth to 3 Years: The Gut-Brain Axis and Neurodevelopmental Outcomes. Front. Pediatr. 2022, 10, 815885. [Google Scholar] [CrossRef]
- Deng, W.; Yi, P.; Xiong, Y.; Ying, J.; Lin, Y.; Dong, Y.; Wei, G.; Wang, X.; Hua, F. Gut Metabolites Acting on the Gut-Brain Axis: Regulating the Functional State of Microglia. Aging Dis. 2024, 15, 480. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Bordeleau, M.; Carrier, M.; Luheshi, G.N.; Tremblay, M.E. Microglia along sex lines: From brain colonization, maturation and function, to implication in neurodevelopmental disorders. Semin. Cell Dev. Biol. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Sudo, N. Biogenic Amines: Signals Between Commensal Microbiota and Gut Physiology. Front. Endocrinol. 2019, 10, 504. [Google Scholar] [CrossRef]
- Cong, X.; Henderson, W.A.; Graf, J.; McGrath, J.M. Early life experience and gut microbiome: The brain–gut–microbiota signaling system. Adv. Neonatal Care 2015, 15, 314–323. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.e1394. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Demers-Mathieu, V. The immature intestinal epithelial cells in preterm infants play a role in the necrotizing enterocolitis pathogenesis: A review. Health Sci. Rev. 2022, 4, 100033. [Google Scholar] [CrossRef]
- Walker, R.W.; Clemente, J.C.; Peter, I.; Loos, R.J.F. The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediatr. Obes. 2017, 12 (Suppl. 1), 3–17. [Google Scholar] [CrossRef]
- Elgin, T.G.; Kern, S.L.; McElroy, S.J. Development of the Neonatal Intestinal Microbiome and Its Association With Necrotizing Enterocolitis. Clin. Ther. 2016, 38, 706–715. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Bae, J.; Kim, M.J.; Kwon, H.; Park, G.; Kim, S.J.; Choe, Y.H.; Kim, J.; Park, S.H.; Choe, B.H.; et al. Delayed Establishment of Gut Microbiota in Infants Delivered by Cesarean Section. Front. Microbiol. 2020, 11, 2099. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Lou, W.; Tun, H.M.; Konya, T.B.; Morales-Lizcano, N.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Mandal, R.; Wishart, D.S.; et al. From Birth to Overweight and Atopic Disease: Multiple and Common Pathways of the Infant Gut Microbiome. Gastroenterology 2021, 160, 128–144.e110. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Boer, C.G.; Beth, S.A.; van der Wal, P.; Kiefte-De Jong, J.C.; Jansen, M.A.E.; Konstantinov, S.R.; Peppelenbosch, M.P.; Hays, J.P.; Jaddoe, V.W.V.; et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020, 10, 1040. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Ho, T.T.B.; Groer, M.W.; Kane, B.; Yee, A.L.; Torres, B.A.; Gilbert, J.A.; Maheshwari, A. Dichotomous development of the gut microbiome in preterm infants. Microbiome 2018, 6, 157. [Google Scholar] [CrossRef]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strømmen, K.; Nakstad, B.; Rønnestad, A.E.; Brække, K.; Iversen, P.O.; Drevon, C.A.; de Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 2453. [Google Scholar] [CrossRef]
- Lee, C.C.; Feng, Y.; Yeh, Y.M.; Lien, R.; Chen, C.L.; Zhou, Y.L.; Chiu, C.H. Gut Dysbiosis, Bacterial Colonization and Translocation, and Neonatal Sepsis in Very-Low-Birth-Weight Preterm Infants. Front. Microbiol. 2021, 12, 746111. [Google Scholar] [CrossRef]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E.; Shaikh, N.; Linneman, L.A.; et al. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef]
- Tapiainen, T.; Koivusaari, P.; Brinkac, L.; Lorenzi, H.A.; Salo, J.; Renko, M.; Pruikkonen, H.; Pokka, T.; Li, W.; Nelson, K.; et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci. Rep. 2019, 9, 10635. [Google Scholar] [CrossRef]
- Li, W.; Tapiainen, T.; Brinkac, L.; Lorenzi, H.A.; Moncera, K.; Tejesvi, M.V.; Salo, J.; Nelson, K.E. Vertical Transmission of Gut Microbiome and Antimicrobial Resistance Genes in Infants Exposed to Antibiotics at Birth. J. Infect. Dis. 2021, 224, 1236–1246. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 77, 1–14. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; Brooker, S.L.; Peterson, H.K.; Steinkamp, K.M.; York, M.A.; Shafii, B.; Price, W.J.; et al. Strong Multivariate Relations Exist Among Milk, Oral, and Fecal Microbiomes in Mother-Infant Dyads during the First Six Months Postpartum. J. Nutr. 2019, 149, 902–914. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e324. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.M.; Sitarik, A.R.; Havstad, S.L.; Fujimura, K.E.; Wegienka, G.; Cassidy-Bushrow, A.E.; Kim, H.; Zoratti, E.M.; Lukacs, N.W.; Boushey, H.A.; et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci. Rep. 2016, 6, 31775. [Google Scholar] [CrossRef] [PubMed]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal microbiota of 6-week-old infants across Europe: Geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Xiang, Q.; Yan, X.; Shi, W.; Li, H.; Zhou, K. Early gut microbiota intervention in premature infants: Application perspectives. J. Adv. Res. 2023, 51, 59–72. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef]
- Zarei, I.; Koistinen, V.M.; Kokla, M.; Klåvus, A.; Babu, A.F.; Lehtonen, M.; Auriola, S.; Hanhineva, K. Tissue-wide metabolomics reveals wide impact of gut microbiota on mice metabolite composition. Sci. Rep. 2022, 12, 15018. [Google Scholar] [CrossRef]
- Scott, G.A.; Terstege, D.J.; Vu, A.P.; Law, S.; Evans, A.; Epp, J.R. Disrupted Neurogenesis in Germ-Free Mice: Effects of Age and Sex. Front. Cell. Dev. Biol. 2020, 8, 407. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, L.; Yu, Y.; Cluette-Brown, J.; Martin, C.R.; Claud, E.C. Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice. Sci. Rep. 2018, 8, 5443. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Munakomi, S. Embryology, Neural Tube. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Butler, S.J.; Bronner, M.E. From classical to current: Analyzing peripheral nervous system and spinal cord lineage and fate. Dev. Biol. 2015, 398, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Scott-Solomon, E.; Boehm, E.; Kuruvilla, R. The sympathetic nervous system in development and disease. Nat. Rev. Neurosci. 2021, 22, 685–702. [Google Scholar] [CrossRef]
- Giuffrè, M.; Moretti, R.; Campisciano, G.; da Silveira, A.B.M.; Monda, V.M.; Comar, M.; Di Bella, S.; Antonello, R.M.; Luzzati, R.; Crocè, L.S. You Talking to Me? Says the Enteric Nervous System (ENS) to the Microbe. How Intestinal Microbes Interact with the ENS. J. Clin. Med. 2020, 9, 3705. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.H.; Zhu, Y.; Li, Q.L.; Zhao, C.; Zhou, P.H. Enteric Nervous System: The Bridge Between the Gut Microbiota and Neurological Disorders. Front. Aging Neurosci. 2022, 14, 810483. [Google Scholar] [CrossRef]
- Nagy, N.; Goldstein, A.M. Enteric nervous system development: A crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 2017, 66, 94–106. [Google Scholar] [CrossRef]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Câmara, R.; Griessenauer, C.J. Anatomy of the vagus nerve. In Nerves and Nerve Injuries; Elsevier: Amsterdam, The Netherlands, 2015; pp. 385–397. [Google Scholar]
- Tobias, A.; Sadiq, N.M. Physiology, Gastrointestinal Nervous Control. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Porges, S.W.; Furman, S.A. The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behavior: A Polyvagal Perspective. Infant Child Dev. 2011, 20, 106–118. [Google Scholar] [CrossRef]
- Harry, G.J.; Toews, A.D. Myelination, dysmyelination, and demyelination. In Handbook of Developmental Neurotoxicology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 87–115. [Google Scholar]
- Bartzokis, G. Brain Myelination in Prevalent Neuropsychiatric Developmental Disorders: Primary and Comorbid Addiction. Adolesc. Psychiatry 2005, 29, 55–96. [Google Scholar]
- Field, T.; Diego, M. Vagal activity, early growth and emotional development. Infant Behav. Dev. 2008, 31, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12 (Suppl. 2), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- May, C.L.; Kaestner, K.H. Gut endocrine cell development. Mol. Cell Endocrinol. 2010, 323, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Bany Bakar, R.; Reimann, F.; Gribble, F.M. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 784–796. [Google Scholar] [CrossRef]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef]
- Rawdon, B.B.; Andrew, A. Origin and differentiation of gut endocrine cells. Histol. Histopathol. 1993, 8, 567–580. [Google Scholar]
- Koo, D.J.; Zhou, M.; Jackman, D.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H.; Wang, P. Is gut the major source of proinflammatory cytokine release during polymicrobial sepsis? Biochim. Biophys. Acta 1999, 1454, 289–295. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Coombes, J.L.; Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef]
- Batterman, K.V.; Cabrera, P.E.; Moore, T.L.; Rosene, D.L. T Cells Actively Infiltrate the White Matter of the Aging Monkey Brain in Relation to Increased Microglial Reactivity and Cognitive Decline. Front. Immunol. 2021, 12, 607691. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Chen, H.; Jin, C.; Jin, Z.; Lu, J.; Xu, L.; Lu, Y.; Zhang, J.; Shi, L. Aged microglia promote peripheral T cell infiltration by reprogramming the microenvironment of neurogenic niches. Immun. Ageing 2022, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.W.; Yong, V.W. B cells in central nervous system disease: Diversity, locations and pathophysiology. Nat. Rev. Immunol. 2022, 22, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.B.; Torres, V.O.; Latchney, S.E.; Whoolery, C.W.; Noorbhai, I.Z.; Poinsatte, K.; Selvaraj, U.M.; Benson, M.A.; Meeuwissen, A.J.M.; Plautz, E.J.; et al. B cells migrate into remote brain areas and support neurogenesis and functional recovery after focal stroke in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 4983–4993. [Google Scholar] [CrossRef] [PubMed]
- Aspden, J.W.; Murphy, M.A.; Kashlan, R.D.; Xiong, Y.; Poznansky, M.C.; Sîrbulescu, R.F. Intruders or protectors—The multifaceted role of B cells in CNS disorders. Front. Cell Neurosci. 2023, 17, 1329823. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123. [Google Scholar] [CrossRef]
- Duess, J.W.; Sampah, M.E.; Lopez, C.M.; Tsuboi, K.; Scheese, D.J.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes 2023, 15, 2221470. [Google Scholar] [CrossRef]
- Neu, J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am. J. Clin. Nutr. 2007, 85, 629S–634S. [Google Scholar] [CrossRef]
- Tirone, C.; Pezza, L.; Paladini, A.; Tana, M.; Aurilia, C.; Lio, A.; D’Ippolito, S.; Tersigni, C.; Posteraro, B.; Sanguinetti, M.; et al. Gut and Lung Microbiota in Preterm Infants: Immunological Modulation and Implication in Neonatal Outcomes. Front. Immunol. 2019, 10, 2910. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, L.; Butel, M.J.; Campeotto, F.; Vodovar, M.; Roze, J.C.; Aires, J. Clostridia in premature neonates’ gut: Incidence, antibiotic susceptibility, and perinatal determinants influencing colonization. PLoS ONE 2012, 7, e30594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hoenig, J.D.; Malin, K.J.; Qamar, S.; Petrof, E.O.; Sun, J.; Antonopoulos, D.A.; Chang, E.B.; Claud, E.C. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009, 3, 944–954. [Google Scholar] [CrossRef]
- Costello, E.K.; Carlisle, E.M.; Bik, E.M.; Morowitz, M.J.; Relman, D.A. Microbiome assembly across multiple body sites in low-birthweight infants. mBio 2013, 4, e00782-13. [Google Scholar] [CrossRef] [PubMed]
- Chernikova, D.A.; Madan, J.C.; Housman, M.L.; Zain-Ul-Abideen, M.; Lundgren, S.N.; Morrison, H.G.; Sogin, M.L.; Williams, S.M.; Moore, J.H.; Karagas, M.R.; et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr. Res. 2018, 84, 71–79. [Google Scholar] [CrossRef]
- Janvier, A.; Malo, J.; Barrington, K.J. Cohort study of probiotics in a North American neonatal intensive care unit. J. Pediatr. 2014, 164, 980–985. [Google Scholar] [CrossRef]
- AlFaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid. Based Child Health 2014, 9, 584–671. [Google Scholar] [CrossRef]
- Patel, R.M.; Underwood, M.A. Probiotics and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef]
- Sajankila, N.; Wala, S.J.; Ragan, M.V.; Volpe, S.G.; Dumbauld, Z.; Purayil, N.; Mihi, B.; Besner, G.E. Current and future methods of probiotic therapy for necrotizing enterocolitis. Front. Pediatr. 2023, 11, 1120459. [Google Scholar] [CrossRef]
- Wala, S.J.; Ragan, M.V.; Sajankila, N.; Volpe, S.G.; Purayil, N.; Dumbauld, Z.; Besner, G.E. Probiotics and novel probiotic delivery systems. Semin. Pediatr. Surg. 2023, 32, 151307. [Google Scholar] [CrossRef]
- Bergonzelli, G.E.; Blum, S.; Brüssow, H.; Corthésy-Theulaz, I. Probiotics as a treatment strategy for gastrointestinal diseases? Digestion 2005, 72, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, Z.; Triantafyllou, A.R.; Tsaousi, M.; Pouliakis, A.; Petropoulou, C.; Sokou, R.; Volaki, P.; Boutsikou, T.; Iacovidou, N. Gut Microbiome and Neurodevelopmental Disorders: A Link Yet to Be Disclosed. Microorganisms 2023, 11, 487. [Google Scholar] [CrossRef]
- McGuire, W.; Henderson, G.; Fowlie, P.W. Feeding the preterm infant. BMJ 2004, 329, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Smink, C.J.; Robinson, R.C.; Tian, T.; Guerrero, A.; Parker, E.A.; Smilowitz, J.T.; Hettinga, K.A.; Underwood, M.A.; Lebrilla, C.B.; et al. Endogenous human milk peptide release is greater after preterm birth than term birth. J. Nutr. 2015, 145, 425–433. [Google Scholar] [CrossRef]
- Henderickx, J.G.E.; Zwittink, R.D.; van Lingen, R.A.; Knol, J.; Belzer, C. The Preterm Gut Microbiota: An Inconspicuous Challenge in Nutritional Neonatal Care. Front. Cell Infect. Microbiol. 2019, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W., Jr. Aggressive Nutrition of the Preterm Infant. Curr. Pediatr. Rep. 2013, 1, 229–239. [Google Scholar] [CrossRef]
- Fanaro, S. Feeding intolerance in the preterm infant. Early Hum. Dev. 2013, 89 (Suppl. 2), S13–S20. [Google Scholar] [CrossRef]
- Ghoneim, N.; Bauchart-Thevret, C.; Oosterloo, B.; Stoll, B.; Kulkarni, M.; de Pipaon, M.S.; Zamora, I.J.; Olutoye, O.O.; Berg, B.; Wittke, A.; et al. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS ONE 2014, 9, e106888. [Google Scholar] [CrossRef]
- Patel, A.L.; Taylor, S.N. Dilemmas in initiation of very preterm infant enteral feeds-when, what, how? J. Perinatol. 2023, 43, 108–113. [Google Scholar] [CrossRef]
- Dahlin, M.; Prast-Nielsen, S. The gut microbiome and epilepsy. eBiomedicine 2019, 44, 741–746. [Google Scholar] [CrossRef]
- Yang, R.; Liu, J.; Diao, L.; Wei, L.; Luo, H.; Cai, L. A meta-analysis of the changes in the Gut microbiota in patients with intractable epilepsy compared to healthy controls. J. Clin. Neurosci. 2024, 120, 213–220. [Google Scholar] [CrossRef]
- Riva, A.; Sahin, E.; Volpedo, G.; Petretto, A.; Lavarello, C.; Di Sapia, R.; Barbarossa, D.; Zaniani, N.R.; Craparotta, I.; Barbera, M.C.; et al. Identification of an epilepsy-linked gut microbiota signature in a pediatric rat model of acquired epilepsy. Neurobiol. Dis. 2024, 194, 106469. [Google Scholar] [CrossRef]
- Peng, A.; Qiu, X.; Lai, W.; Li, W.; Zhang, L.; Zhu, X.; He, S.; Duan, J.; Chen, L. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 2018, 147, 102–107. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e1713. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, Y.; Wang, X.; Zhao, D.; Wang, Z.; Wang, G.; Li, C.; Yang, L.; Ji, H.; Liu, K.; et al. Ketogenic Diets Alter the Gut Microbiome, Resulting in Decreased Susceptibility to and Cognitive Impairment in Rats with Pilocarpine-Induced Status Epilepticus. Neurochem. Res. 2024, 49, 2726–2742. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.A.J.E.; de Kinderen, R.J.A.; Vles, J.S.H.; de Louw, A.J.; Aldenkamp, A.P.; Majoie, H.J.M. A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol. Scand. 2017, 135, 678. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Chau, V.; Brant, R.; Poskitt, K.J.; Tam, E.W.; Synnes, A.; Miller, S.P. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr. Res. 2012, 71, 274–279. [Google Scholar] [CrossRef]
- Niemarkt, H.J.; De Meij, T.G.; van Ganzewinkel, C.J.; de Boer, N.K.H.; Andriessen, P.; Hutten, M.C.; Kramer, B.W. Necrotizing Enterocolitis, Gut Microbiota, and Brain Development: Role of the Brain-Gut Axis. Neonatology 2019, 115, 423–431. [Google Scholar] [CrossRef]
- Hintz, S.R.; Kendrick, D.E.; Stoll, B.J.; Vohr, B.R.; Fanaroff, A.A.; Donovan, E.F.; Poole, W.K.; Blakely, M.L.; Wright, L.; Higgins, R.; et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005, 115, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.; Georgieff, M.; Ramel, S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Merhar, S.L.; Ramos, Y.; Meinzen-Derr, J.; Kline-Fath, B.M. Brain magnetic resonance imaging in infants with surgical necrotizing enterocolitis or spontaneous intestinal perforation versus medical necrotizing enterocolitis. J. Pediatr. 2014, 164, 410–412.e411. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, E.K.; Yoo, H.; Choi, Y.H.; Kim, S.; Lee, B.K.; Jung, Y.H.; Kim, H.Y.; Kim, H.S.; Choi, J.H. Surgical Necrotizing Enterocolitis versus Spontaneous Intestinal Perforation in White Matter Injury on Brain Magnetic Resonance Imaging. Neonatology 2016, 110, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Schelonka, R.L.; Dimmitt, R.A.; Carlo, W.A.; Munoz-Hernandez, B.; Das, A.; McDonald, S.A.; Thorsen, P.; Skogstrand, K.; Hougaard, D.M.; et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr. Res. 2014, 76, 100–108. [Google Scholar] [CrossRef]
- MohanKumar, K.; Namachivayam, K.; Ho, T.T.; Torres, B.A.; Ohls, R.K.; Maheshwari, A. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin. Perinatol. 2017, 41, 52–60. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Yang, C.; Feng, Z.; Deng, H.; Dai, L.; He, L.; Yin, L.; Zhao, J. CXCL1/CXCR2 is involved in white matter injury in neonatal rats via the gut-brain axis. BMC Neurosci. 2022, 23, 67. [Google Scholar] [CrossRef]
- Nino, D.F.; Zhou, Q.; Yamaguchi, Y.; Martin, L.Y.; Wang, S.; Fulton, W.B.; Jia, H.; Lu, P.; Prindle, T., Jr.; Zhang, F.; et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 2018, 10, eaan0237. [Google Scholar] [CrossRef]
- Manohar, K.; Mesfin, F.M.; Liu, J.; Shelley, W.C.; Brokaw, J.P.; Markel, T.A. Gut-Brain cross talk: The pathogenesis of neurodevelopmental impairment in necrotizing enterocolitis. Front. Pediatr. 2023, 11, 1104682. [Google Scholar] [CrossRef]
- Nino, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.S.; Sanderson, I.R.; Claesson, M.J. Paediatric inflammatory bowel disease and its relationship with the microbiome. Microb. Ecol. 2021, 82, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.; Stokes, P.; Keogh, C.E.; Brust-Mascher, I.; Hennessey, C.; Knotts, T.A.; Sladek, J.A.; Rude, K.M.; Swedek, M.; Rabasa, G. A murine model of pediatric inflammatory bowel disease causes microbiota-gut-brain axis deficits in adulthood. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G361–G374. [Google Scholar] [CrossRef] [PubMed]
| Colonization [29] | Infants, 4 Months [29] | Infants, 12 Months [29] | Children, 9 Years [28] | Adults [28] |
|---|---|---|---|---|
| Bacteroidetes Bacteroides (30%) | Actinobacteria Bifidobacteria (42%) | Bacteroidetes Bacteroides (45%) | Firmicutes (57%) | Firmicutes (75%) |
| Proteobacteria Escherichia/Shigella (22%) | Bacteroidetes Bacteroides (24%) | Firmicutes Ruminococcus (13%) | Bacteroidetes (30%) | Bacteroidetes (13%) |
| Actinobacteria Bifidobacteria (22%) | Proteobacteria Escherichia/Shigella (5%) | Firmicutes Clostridium (5%) | Proteobacteria (6%) | Proteobacteria (5%) |
| Actinobacteria (4%) | Actinobacteria (4%) | |||
| Verrucomicrobia (2%) | Verrucomicrobia (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, C.; Jin, Z.; Ku, S.Y.; Kogosov, A.S.; Yu, S.; Bergese, S.D.; Hsieh, H. Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements. Biomolecules 2024, 14, 1254. https://doi.org/10.3390/biom14101254
Sha C, Jin Z, Ku SY, Kogosov AS, Yu S, Bergese SD, Hsieh H. Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements. Biomolecules. 2024; 14(10):1254. https://doi.org/10.3390/biom14101254
Chicago/Turabian StyleSha, Cuilee, Zhaosheng Jin, Stella Y. Ku, Ann S. Kogosov, Sun Yu, Sergio D. Bergese, and Helen Hsieh. 2024. "Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements" Biomolecules 14, no. 10: 1254. https://doi.org/10.3390/biom14101254
APA StyleSha, C., Jin, Z., Ku, S. Y., Kogosov, A. S., Yu, S., Bergese, S. D., & Hsieh, H. (2024). Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements. Biomolecules, 14(10), 1254. https://doi.org/10.3390/biom14101254






