The Formation of Stable Lung Tumor Spheroids during Random Positioning Involves Increased Estrogen Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Random Positioning
2.3. Static Spheroid Formation
2.4. Estrogen Treatment (Calu-3)
2.5. mRNA Isolation and Quantitative Real-Time PCR
2.6. Luminex® Multiplex Assay
2.7. Phase Contrast Microscopy
2.8. Immunofluorescence Microscopy and Analysis
2.9. Statistical Analysis
2.10. Interaction Networks of Chemicals and Proteins (STITCH Analysis)
3. Results
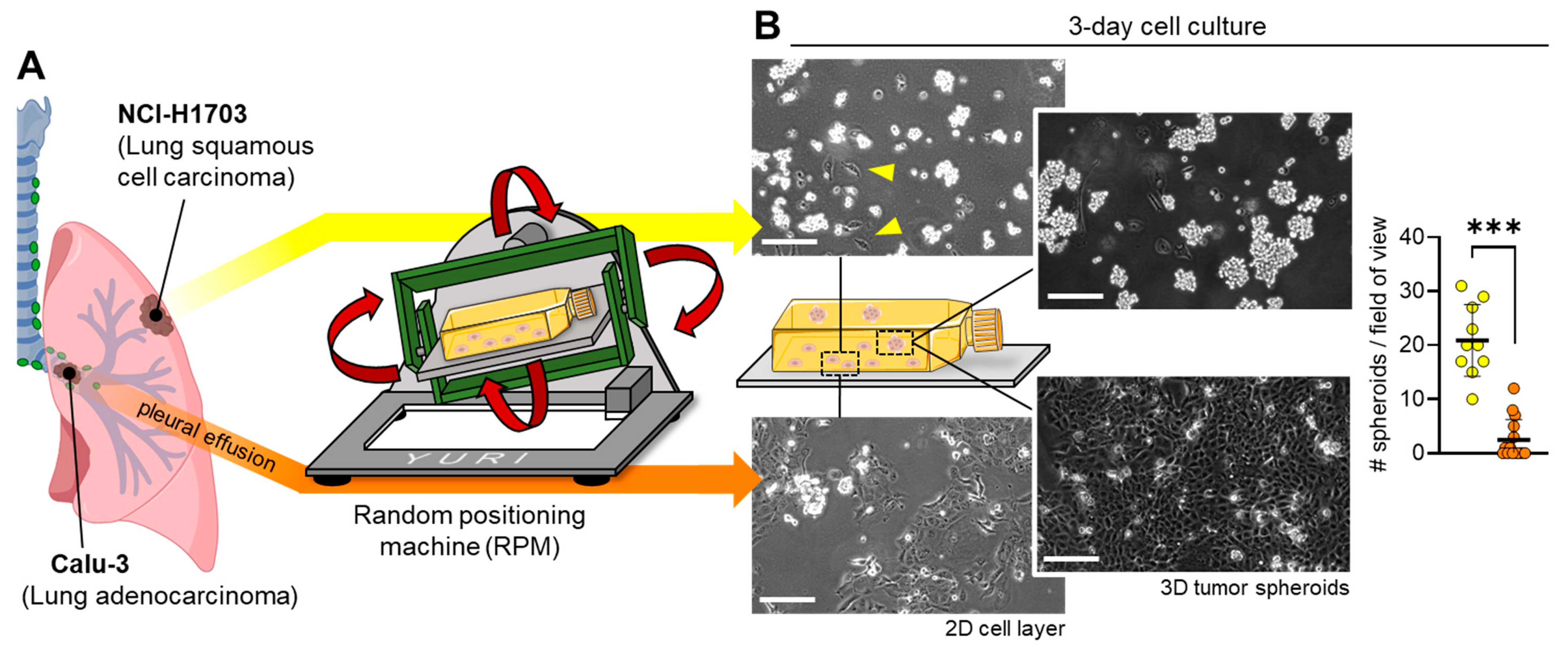
3.1. Different Lung Cancer Types Show Different Spheroid-Formation Ability When Exposed to the Random Positioning Machine
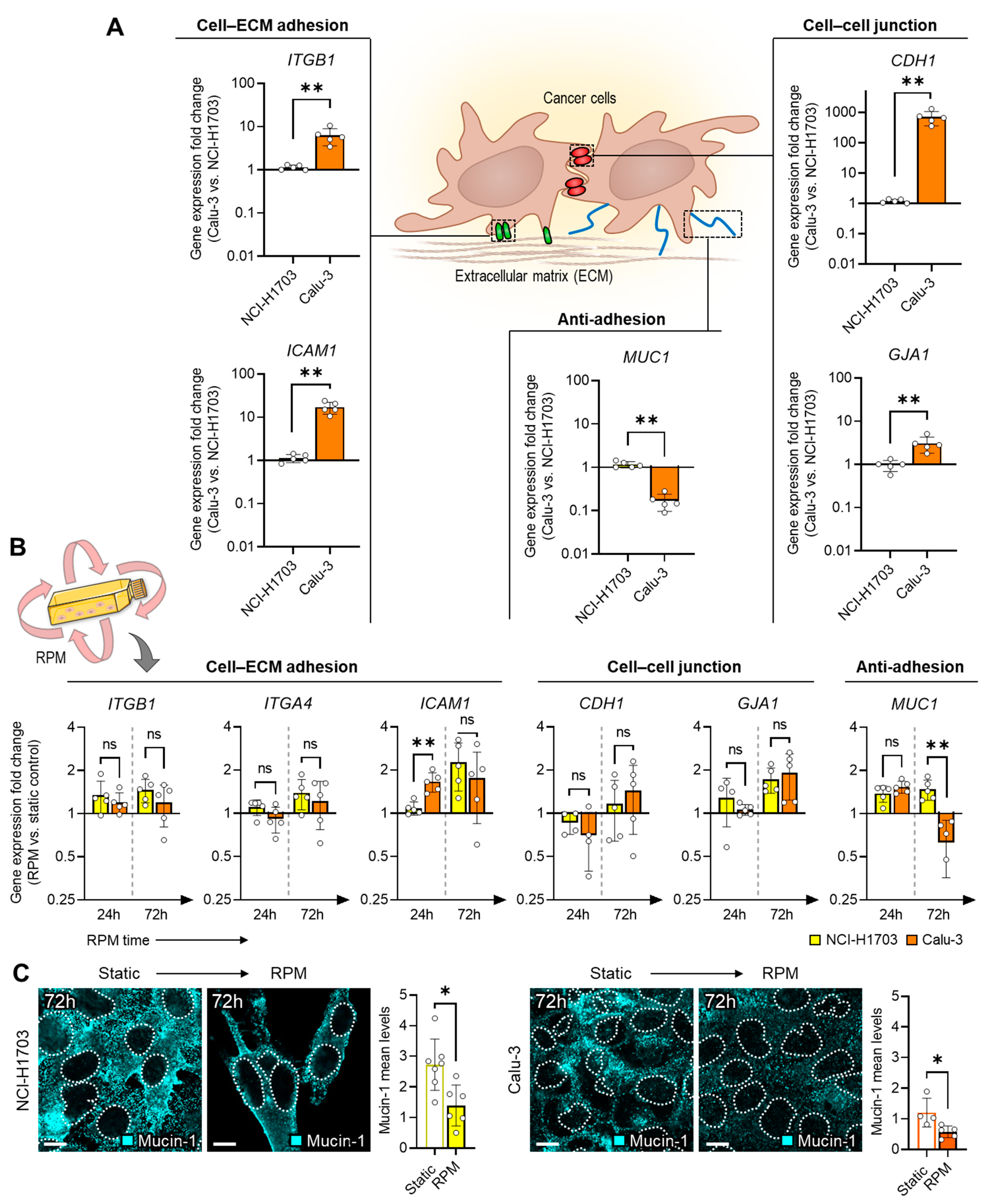
3.2. Spheroid Stability Due to the Presence of Adhesion and Anti-Adhesion Proteins
3.3. RPM-Induced Secretion of Multifunctional Signaling Molecules
3.4. Calu-3 Cells Upregulate ESR1 Expression during Random Positioning
4. Discussion
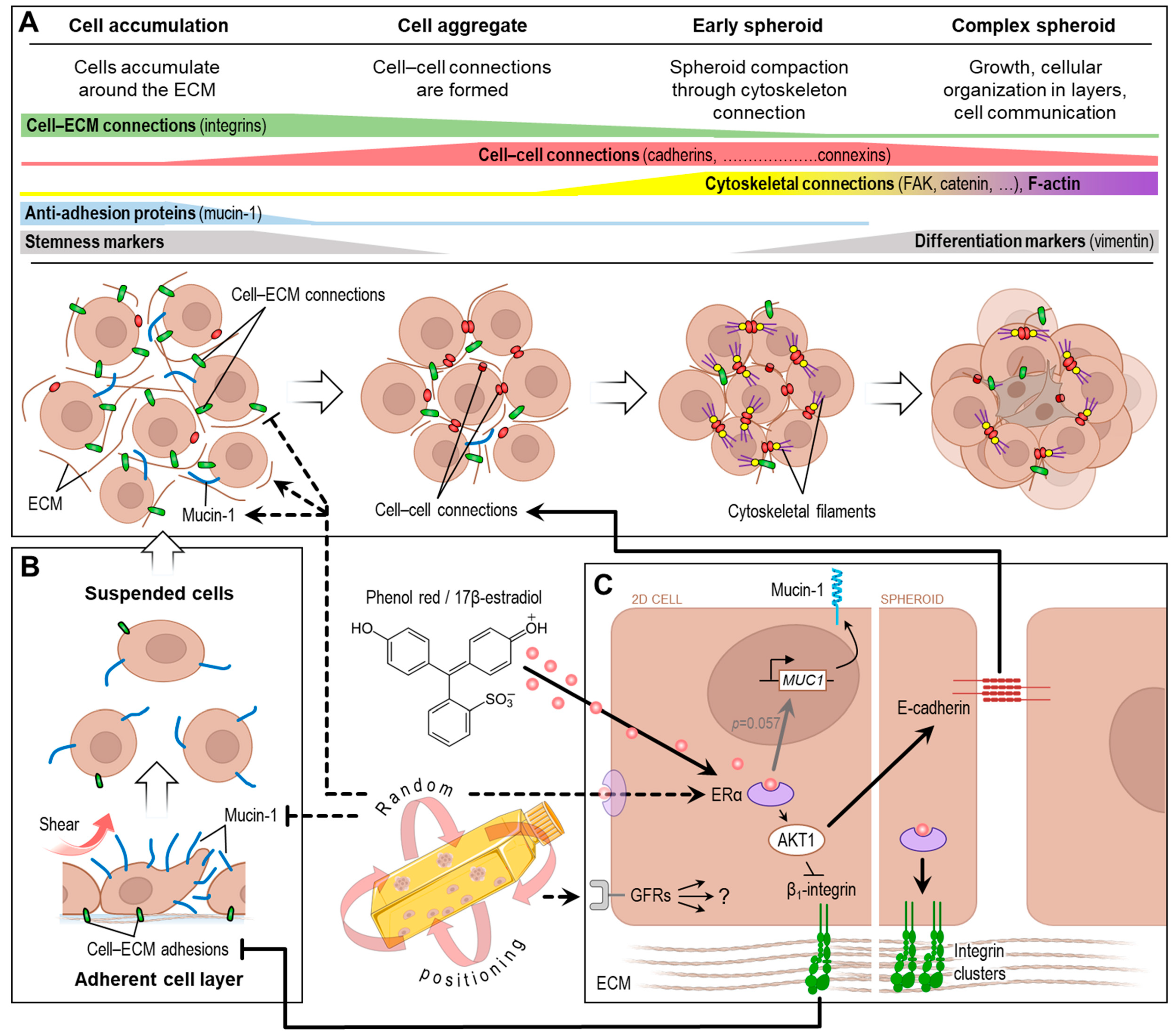
4.1. Spheroid Formation
4.2. Random Positioning and Estrogen Receptor
4.3. Random Positioning and Growth Factor Receptors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topal, U.; Zamur, C. Microgravity, stem cells, and cancer: A new hope for cancer treatment. Stem Cells Int. 2021, 2021, 5566872. [Google Scholar] [CrossRef]
- Sabbatini, M.; Bonetto, V.; Magnelli, V.; Lorusso, C.; Dondero, F.; Masini, M.A. Microgravity as an anti-metastatic agent in an in vitro glioma model. Biophysica 2023, 3, 636–650. [Google Scholar] [CrossRef]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Krüger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K.; et al. Simulated microgravity influences VEGF, MAPK, and PAM signaling in prostate cancer cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef]
- Masiello, M.G.; Cucina, A.; Proietti, S.; Palombo, A.; Coluccia, P.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Morini, V.; Bizzarri, M. Phenotypic switch induced by simulated microgravity on mda-mb-231 breast cancer cells. BioMed Res. Int. 2014, 2014, 652434. [Google Scholar] [CrossRef]
- Sahana, J.; Corydon, T.J.; Wehland, M.; Krüger, M.; Kopp, S.; Melnik, D.; Kahlert, S.; Relja, B.; Infanger, M.; Grimm, D. Alterations of growth and focal adhesion molecules in human breast cancer cells exposed to the random positioning machine. Front. Cell Dev. Biol. 2021, 9, 672098. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Jiang, N.; Guo, S.; Li, B.B.; Yang, J.Q.; Chai, S.B.; Yan, H.F.; Sun, P.M.; Zhang, T.; Sun, H.W.; et al. Effect of simulated microgravity on metabolism of hgc-27 gastric cancer cells. Oncol. Lett. 2020, 19, 3439–3450. [Google Scholar] [CrossRef]
- Masini, M.A.; Bonetto, V.; Manfredi, M.; Pastò, A.; Barberis, E.; Timo, S.; Vanella, V.V.; Robotti, E.; Masetto, F.; Andreoli, F.; et al. Prolonged exposure to simulated microgravity promotes stemness impairing morphological, metabolic and migratory profile of pancreatic cancer cells: A comprehensive proteomic, lipidomic and transcriptomic analysis. Cell. Mol. Life Sci. 2022, 79, 226. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeong, A.J.; Kim, M.; Lee, C.; Ye, S.-K.; Kim, S. Time-averaged simulated microgravity (tasmg) inhibits proliferation of lymphoma cells, l-540 and hdlm-2, using a 3d clinostat. Biomed. Eng. Online 2017, 16, 48. [Google Scholar] [CrossRef]
- Calvaruso, M.; Militello, C.; Minafra, L.; La Regina, V.; Torrisi, F.; Pucci, G.; Cammarata, F.P.; Bravatà, V.; Forte, G.I.; Russo, G. Biological and mechanical characterization of the random positioning machine (rpm) for microgravity simulations. Life 2021, 11, 1190. [Google Scholar] [CrossRef]
- Kopp, S.; Warnke, E.; Wehland, M.; Aleshcheva, G.; Magnusson, N.E.; Hemmersbach, R.; Corydon, T.J.; Bauer, J.; Infanger, M.; Grimm, D. Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci. Rep. 2015, 5, 16691. [Google Scholar] [CrossRef]
- Kopp, S.; Slumstrup, L.; Corydon, T.J.; Sahana, J.; Aleshcheva, G.; Islam, T.; Magnusson, N.E.; Wehland, M.; Bauer, J.; Infanger, M.; et al. Identifications of novel mechanisms in breast cancer cells involving duct-like multicellular spheroid formation after exposure to the random positioning machine. Sci. Rep. 2016, 6, 26887. [Google Scholar] [CrossRef]
- Cortés-Sánchez, J.L.; Melnik, D.; Sandt, V.; Kahlert, S.; Marchal, S.; Johnson, I.R.D.; Calvaruso, M.; Liemersdorf, C.; Wuest, S.L.; Grimm, D.; et al. Fluid and bubble flow detach adherent cancer cells to form spheroids on a random positioning machine. Cells 2023, 12, 2665. [Google Scholar] [CrossRef]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The fight against cancer by microgravity: The multicellular spheroid as a metastasis model. Int. J. Mol. Sci. 2022, 23, 3073. [Google Scholar] [CrossRef]
- Melnik, D.; Cortés-Sánchez, J.L.; Sandt, V.; Kahlert, S.; Kopp, S.; Grimm, D.; Krüger, M. Dexamethasone selectively inhibits detachment of metastatic thyroid cancer cells during random positioning. Cancers 2023, 15, 1641. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; An update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef]
- Popper, H.H. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, J.-H.; Han, D.G.; Kang, H.-W.; Lee, S.-H.; Lee, J.-I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef]
- Benavides Damm, T.; Walther, I.; Wüest, S.L.; Sekler, J.; Egli, M. Cell cultivation under different gravitational loads using a novel random positioning incubator. Biotechnol. Bioeng. 2014, 111, 1180–1190. [Google Scholar] [CrossRef]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. Stitch: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2008, 36, D684–D688. [Google Scholar] [CrossRef]
- Brungs, S.; Hauslage, J.; Hemmersbach, R. Validation of random positioning versus clinorotation using a macrophage model system. Microgravity Sci. Technol. 2019, 31, 223–230. [Google Scholar] [CrossRef]
- Hauslage, J.; Cevik, V.; Hemmersbach, R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). NPJ Microgravity 2017, 3, 12. [Google Scholar] [CrossRef]
- Wuest, S.L.; Stern, P.; Casartelli, E.; Egli, M. Fluid dynamics appearing during simulated microgravity using random positioning machines. PLoS ONE 2017, 12, e0170826. [Google Scholar] [CrossRef]
- Berthois, Y.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Phenol red in tissue culture media is a weak estrogen: Implications concerning the study of estrogen-responsive cells in culture. Proc. Natl. Acad. Sci. USA 1986, 83, 2496–2500. [Google Scholar] [CrossRef]
- Lan, Y.; Ni, W.; Tai, G. Expression of muc1 in different tumours and its clinical significance (review). Mol. Clin. Oncol. 2022, 17, 161. [Google Scholar] [CrossRef]
- Horm, T.M.; Schroeder, J.A. Muc1 and metastatic cancer: Expression, function and therapeutic targeting. Cell Adh. Migr. 2013, 7, 187–198. [Google Scholar] [CrossRef]
- Gionet-Gonzales, M.A.; Leach, J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018, 13, 034109. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Kouroupis, D.; Correa, D. Increased mesenchymal stem cell functionalization in three-dimensional manufacturing settings for enhanced therapeutic applications. Front. Bioeng. Biotechnol. 2021, 9, 621748. [Google Scholar] [CrossRef]
- Baghoum, H.; Alahmed, H.; Hachim, M.; Senok, A.; Jalaleddine, N.; Al Heialy, S. Simulated microgravity influences immunity-related biomarkers in lung cancer. Int. J. Mol. Sci. 2022, 24, 155. [Google Scholar] [CrossRef]
- Kopp, S.; Sahana, J.; Islam, T.; Petersen, A.G.; Bauer, J.; Corydon, T.J.; Schulz, H.; Saar, K.; Huebner, N.; Slumstrup, L.; et al. The role of nfκb in spheroid formation of human breast cancer cells cultured on the random positioning machine. Sci. Rep. 2018, 8, 921. [Google Scholar] [CrossRef]
- Tuck, A.B.; Chambers, A.F.; Allan, A.L. Osteopontin overexpression in breast cancer: Knowledge gained and possible implications for clinical management. J. Cell Biochem. 2007, 102, 859–868. [Google Scholar] [CrossRef]
- Graf, J.; Schulz, H.; Wehland, M.; Corydon, T.J.; Sahana, J.; Abdelfattah, F.; Wuest, S.L.; Egli, M.; Krüger, M.; Kraus, A.; et al. Omics studies of tumor cells under microgravity conditions. Int. J. Mol. Sci. 2024, 25, 926. [Google Scholar] [CrossRef]
- Lung, D.K.; Reese, R.M.; Alarid, E.T. Intrinsic and extrinsic factors governing the transcriptional regulation of esr1. Horm. Cancer 2020, 11, 129–147. [Google Scholar] [CrossRef]
- Sherbet, G.V. Hormonal influences on cancer progression and prognosis. Vitam. Horm. 2005, 71, 147–200. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ganti, A.K.; Marr, A.; Batra, S.K. Lung cancer in women: Role of estrogens. Expert. Rev. Respir. Med. 2010, 4, 509–518. [Google Scholar] [CrossRef]
- Morabito, C.; Guarnieri, S.; Catizone, A.; Schiraldi, C.; Ricci, G.; Mariggiò, M.A. Transient increases in intracellular calcium and reactive oxygen species levels in tcam-2 cells exposed to microgravity. Sci. Rep. 2017, 7, 15648. [Google Scholar] [CrossRef]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via fak/rhoa-regulated mtorc1 and ampk pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef]
- Taga, M.; Yamauchi, K.; Odle, J.; Furian, L.; Sundaresan, A.; Ramesh, G.T.; Pellis, N.R.; Andrassy, R.J.; Kulkarni, A.D. Melanoma growth and tumorigenicity in models of microgravity. Aviat. Space Environ. Med. 2006, 77, 1113–1116. [Google Scholar]
- Ma, X.; Pietsch, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Bauer, J.; Braun, M.; Schwarzwälder, A.; Segerer, J.; et al. Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J. 2014, 28, 813–835. [Google Scholar] [CrossRef]
- Chang, D.; Xu, H.; Guo, Y.; Jiang, X.; Liu, Y.; Li, K.; Pan, C.; Yuan, M.; Wang, J.; Li, T.; et al. Simulated microgravity alters the metastatic potential of a human lung adenocarcinoma cell line. Vitr. Cell. Dev. Biol.-Anim. 2013, 49, 170–177. [Google Scholar] [CrossRef]
- Chung, J.H.; Ahn, C.B.; Son, K.H.; Yi, E.; Son, H.S.; Kim, H.-S.; Lee, S.H. Simulated microgravity effects on nonsmall cell lung cancer cell proliferation and migration. Aerosp. Med. Hum. Perform. 2017, 88, 82–89. [Google Scholar] [CrossRef]
- Vora, P.M.; Prabhu, S. Exploring the influence of microgravity on chemotherapeutic drug response in cancer: Unveiling new perspectives. J. Cell Mol. Med. 2024, 28, e18347. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Baczyńska, D.; Dubińska-Magiera, M.; Choromańska, A.; Bieżuńska-Kusiak, K.; Gajewska-Naryniecka, A.; Novickij, V.; Saczko, J.; Przystupski, D.; Kulbacka, J. Rccs bioreactor-based modeled microgravity affects gastric cancer cells and improves the chemotherapeutic effect. Membranes 2022, 12, 448. [Google Scholar] [CrossRef]
- McKinley, S.; Taylor, A.; Peeples, C.; Jacob, M.; Khaparde, G.; Walter, Y.; Ekpenyong, A. Simulated microgravity-induced changes to drug response in cancer cells quantified using fluorescence morphometry. Life 2023, 13, 1683. [Google Scholar] [CrossRef]
- Prasanth, D.; Suresh, S.; Prathivadhi-Bhayankaram, S.; Mimlitz, M.; Zetocha, N.; Lee, B.; Ekpenyong, A. Microgravity modulates effects of chemotherapeutic drugs on cancer cell migration. Life 2020, 10, 162. [Google Scholar] [CrossRef]
- Przystupski, D.; Górska, A.; Szewczyk, A.; Drąg-Zalesińska, M.; Kulbacka, J. 3d clinorotation affects drug sensitivity of human ovarian cancer cells. Microgravity Sci. Technol. 2021, 33, 42. [Google Scholar] [CrossRef]
- Maynadier, M.; Chambon, M.; Basile, I.; Gleizes, M.; Nirde, P.; Gary-Bobo, M.; Garcia, M. Estrogens promote cell–cell adhesion of normal and malignant mammary cells through increased desmosome formation. Mol. Cell Endocrinol. 2012, 364, 126–133. [Google Scholar] [CrossRef]
- Riggio, M.; Perrone, M.C.; Polo, M.L.; Rodriguez, M.J.; May, M.; Abba, M.; Lanari, C.; Novaro, V. Akt1 and akt2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci. Rep. 2017, 7, 44244. [Google Scholar] [CrossRef]
- Changede, R.; Sheetz, M. Integrin and cadherin clusters: A robust way to organize adhesions for cell mechanics. BioEssays 2017, 39, e201600123. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.Z.; Barnea, I.; Aylon, Y.; Gorivodsky, M.; Wreschner, D.H.; Keydar, I. Muc1 gene overexpressed in breast cancer: Structure and transcriptional activity of the muc1 promoter and role of estrogen receptor alpha (erα) in regulation of the muc1 gene expression. Mol. Cancer 2006, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Seregni, E.; Botti, C.; Bajetta, E.; Ferrari, L.; Martinetti, A.; Nerini-Molteni, S.; Bombardieri, E. Hormonal regulation of muc1 expression. Int. J. Biol. Markers 1999, 14, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-X.; Tian, W.-M.; Yan, H.-J.; Jiang, H.-D.; Liu, S.-S.; Yue, L.; Han, F.; Wei, L.-J.; Chen, X.-B.; Li, Y. Expression of estrogen receptor α in human breast cancer cells regulates mitochondrial oxidative stress under simulated microgravity. Adv. Space Res. 2012, 49, 1432–1440. [Google Scholar] [CrossRef]
- Tou, J.C.; Grindeland, R.E.; Wade, C.E. Effects of diet and exposure to hindlimb suspension on estrous cycling in sprague-dawley rats. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E425–E433. [Google Scholar] [CrossRef]
- Mark, S.; Scott, G.B.I.; Donoviel, D.B.; Leveton, L.B.; Mahoney, E.; Charles, J.B.; Siegel, B. The impact of sex and gender on adaptation to space: Executive summary. J. Women Health 2014, 23, 941–947. [Google Scholar] [CrossRef]
- Mathyk, B.A.; Tabetah, M.; Karim, R.; Zaksas, V.; Kim, J.; Anu, R.I.; Muratani, M.; Tasoula, A.; Singh, R.S.; Chen, Y.-K.; et al. Spaceflight induces changes in gene expression profiles linked to insulin and estrogen. Commun. Biol. 2024, 7, 692. [Google Scholar] [CrossRef]
- Holets, L.M.; Gupta, V.; Roby, K.F.; Tash, J.S. Spaceflight inhibits ovarian follicle development, induces down regulation of estrogen receptor alpha, and alters metabolic pathways and gene expression in mouse uterus. Biol. Reprod. 2012, 87, 18. [Google Scholar] [CrossRef]
- Hong, X.; Ratri, A.; Choi, S.Y.; Tash, J.S.; Ronca, A.E.; Alwood, J.S.; Christenson, L.K. Effects of spaceflight aboard the international space station on mouse estrous cycle and ovarian gene expression. NPJ Microgravity 2021, 7, 11. [Google Scholar] [CrossRef]
- Sahana, J.; Cortés-Sánchez, J.L.; Sandt, V.; Melnik, D.; Corydon, T.J.; Schulz, H.; Cai, Z.; Evert, K.; Grimm, D.; Wehland, M. Long-term simulation of microgravity induces changes in gene expression in breast cancer cells. Int. J. Mol. Sci. 2023, 24, 1181. [Google Scholar] [CrossRef]
- Melnik, D.; Sahana, J.; Corydon, T.J.; Kopp, S.; Nassef, M.Z.; Wehland, M.; Infanger, M.; Grimm, D.; Krüger, M. Dexamethasone inhibits spheroid formation of thyroid cancer cells exposed to simulated microgravity. Cells 2020, 9, 367. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkia, B.; Sandt, V.; Melnik, D.; Cortés-Sánchez, J.L.; Marchal, S.; Baselet, B.; Baatout, S.; Sahana, J.; Grimm, D.; Wehland, M.; et al. The Formation of Stable Lung Tumor Spheroids during Random Positioning Involves Increased Estrogen Sensitivity. Biomolecules 2024, 14, 1292. https://doi.org/10.3390/biom14101292
Barkia B, Sandt V, Melnik D, Cortés-Sánchez JL, Marchal S, Baselet B, Baatout S, Sahana J, Grimm D, Wehland M, et al. The Formation of Stable Lung Tumor Spheroids during Random Positioning Involves Increased Estrogen Sensitivity. Biomolecules. 2024; 14(10):1292. https://doi.org/10.3390/biom14101292
Chicago/Turabian StyleBarkia, Balkis, Viviann Sandt, Daniela Melnik, José Luis Cortés-Sánchez, Shannon Marchal, Bjorn Baselet, Sarah Baatout, Jayashree Sahana, Daniela Grimm, Markus Wehland, and et al. 2024. "The Formation of Stable Lung Tumor Spheroids during Random Positioning Involves Increased Estrogen Sensitivity" Biomolecules 14, no. 10: 1292. https://doi.org/10.3390/biom14101292
APA StyleBarkia, B., Sandt, V., Melnik, D., Cortés-Sánchez, J. L., Marchal, S., Baselet, B., Baatout, S., Sahana, J., Grimm, D., Wehland, M., Schulz, H., Infanger, M., Kraus, A., & Krüger, M. (2024). The Formation of Stable Lung Tumor Spheroids during Random Positioning Involves Increased Estrogen Sensitivity. Biomolecules, 14(10), 1292. https://doi.org/10.3390/biom14101292









