Metabolic and Proteomic Profiling of Coronary Microvascular Dysfunction: Insights from Rat Models
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Rats CMD Model Establishment
2.3. Transthoracic Echocardiography
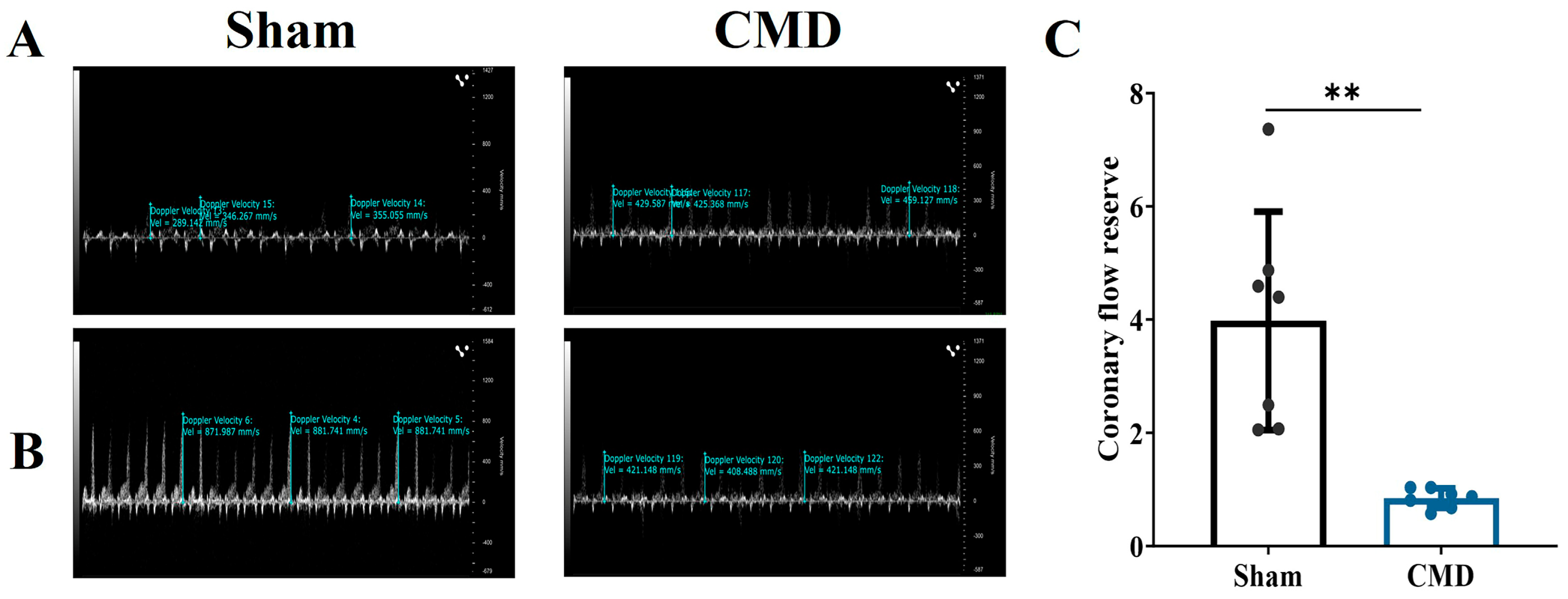
2.4. Coronary Flow Reserve Testing
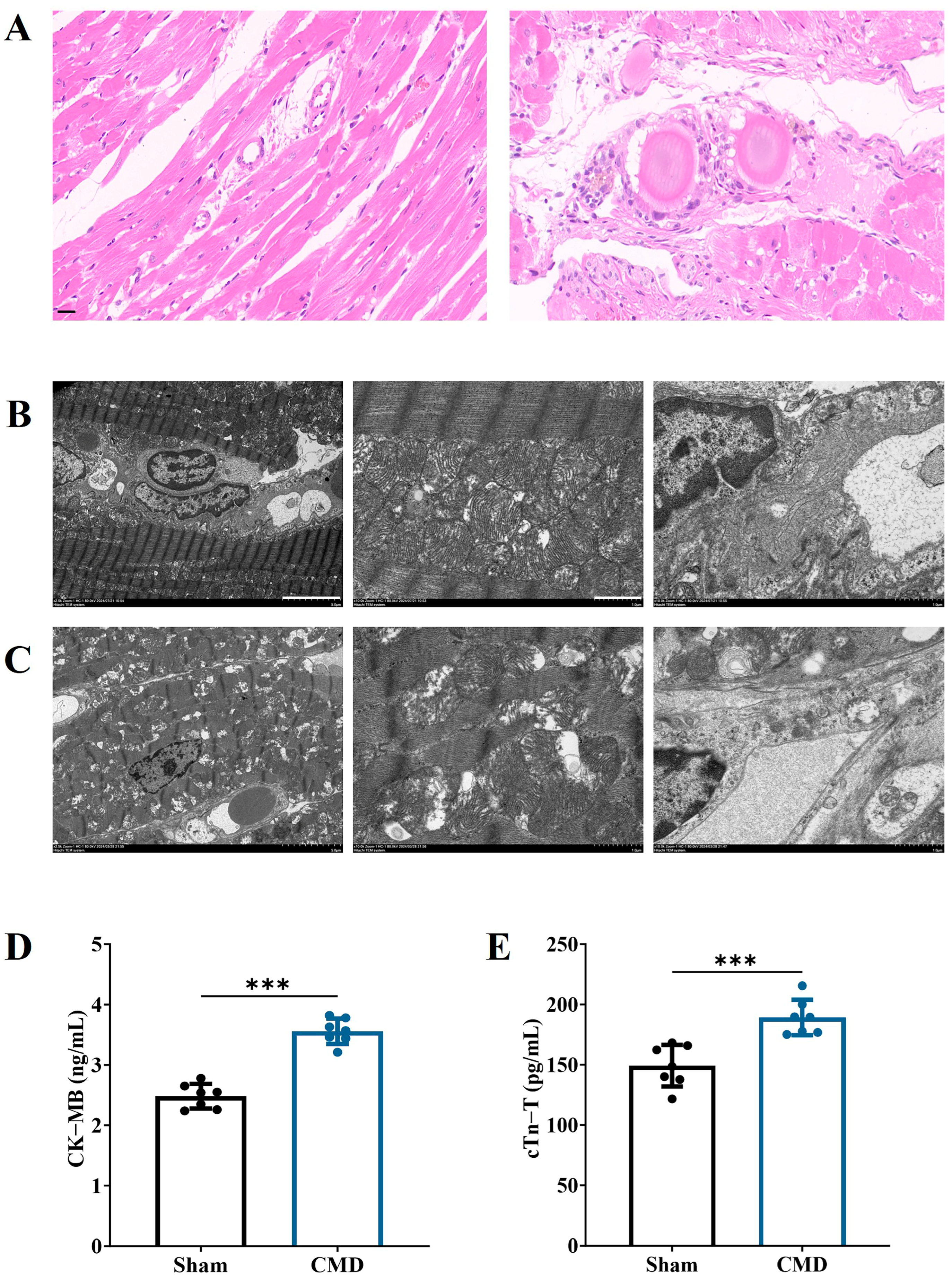
2.5. Heart Collection and Histological Analysis
2.6. Assessment of Myocardial Ultrastructure by Transmission Electron Microscopy
2.7. Serological Tests
2.8. Sample Preparation Analysis for Proteomics
2.9. Protein Identification and Analysis
2.10. Protein Co-Expression Network Analysis
2.11. Machine-Learning Prediction
2.12. Non-Targeted Metabolome Analysis
2.13. Joint Analysis of Metabolomics and Proteomics
2.14. Statistics
3. Results
3.1. Coronary Flow Reserve Evaluation Modeling Success
3.2. Echocardiographic Evaluation of Cardiac Function
3.3. Changes in Myocardial Injury in CMD Rats
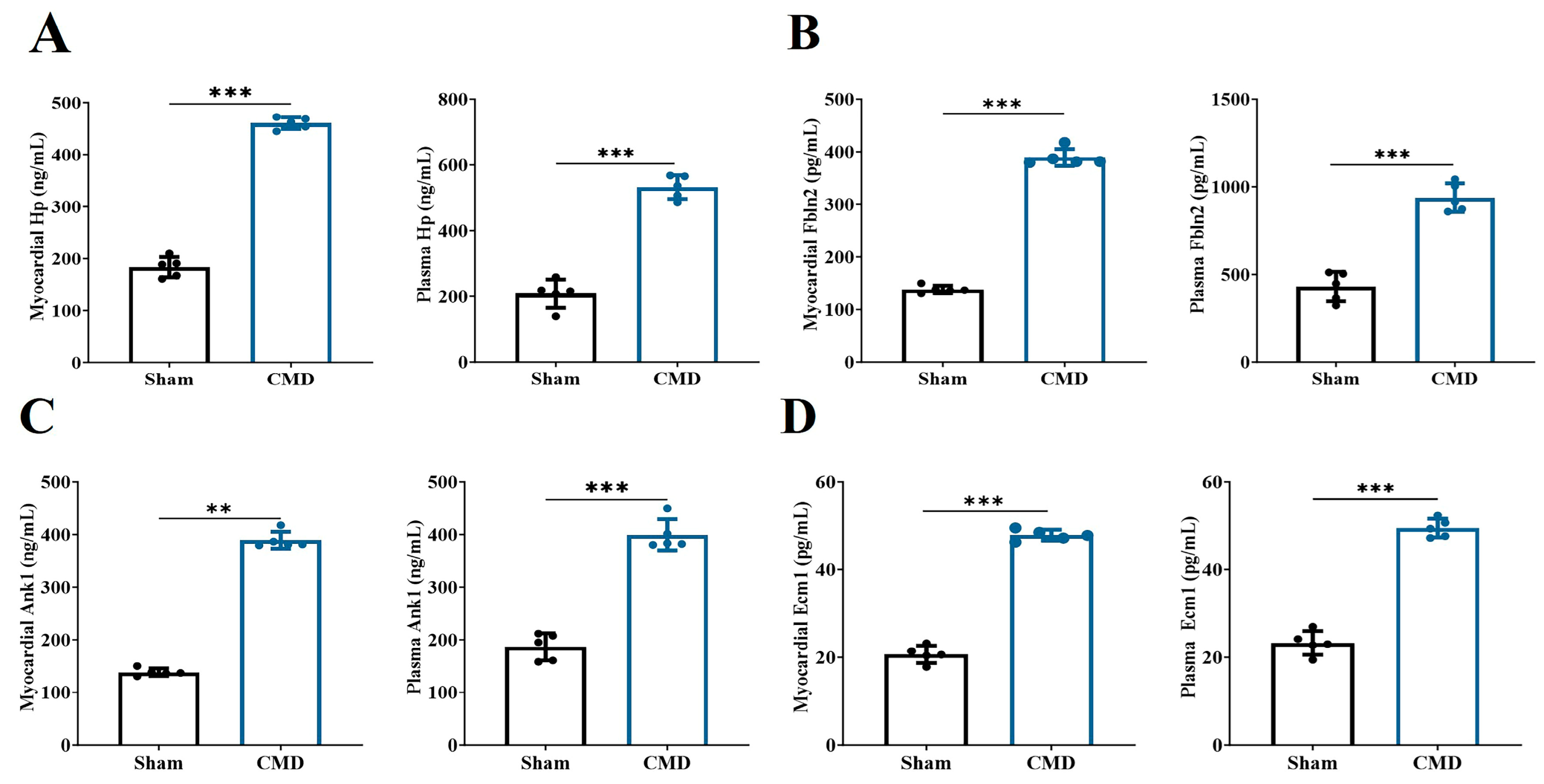
3.4. Screening for DEPs and Their Underlying Biological Mechanisms
3.5. Construction of Co-Expression Network and Identification of Key Modules in CMD
3.6. Selection of Potential Biomarkers Using Supervised Machine-Learning Algorithms
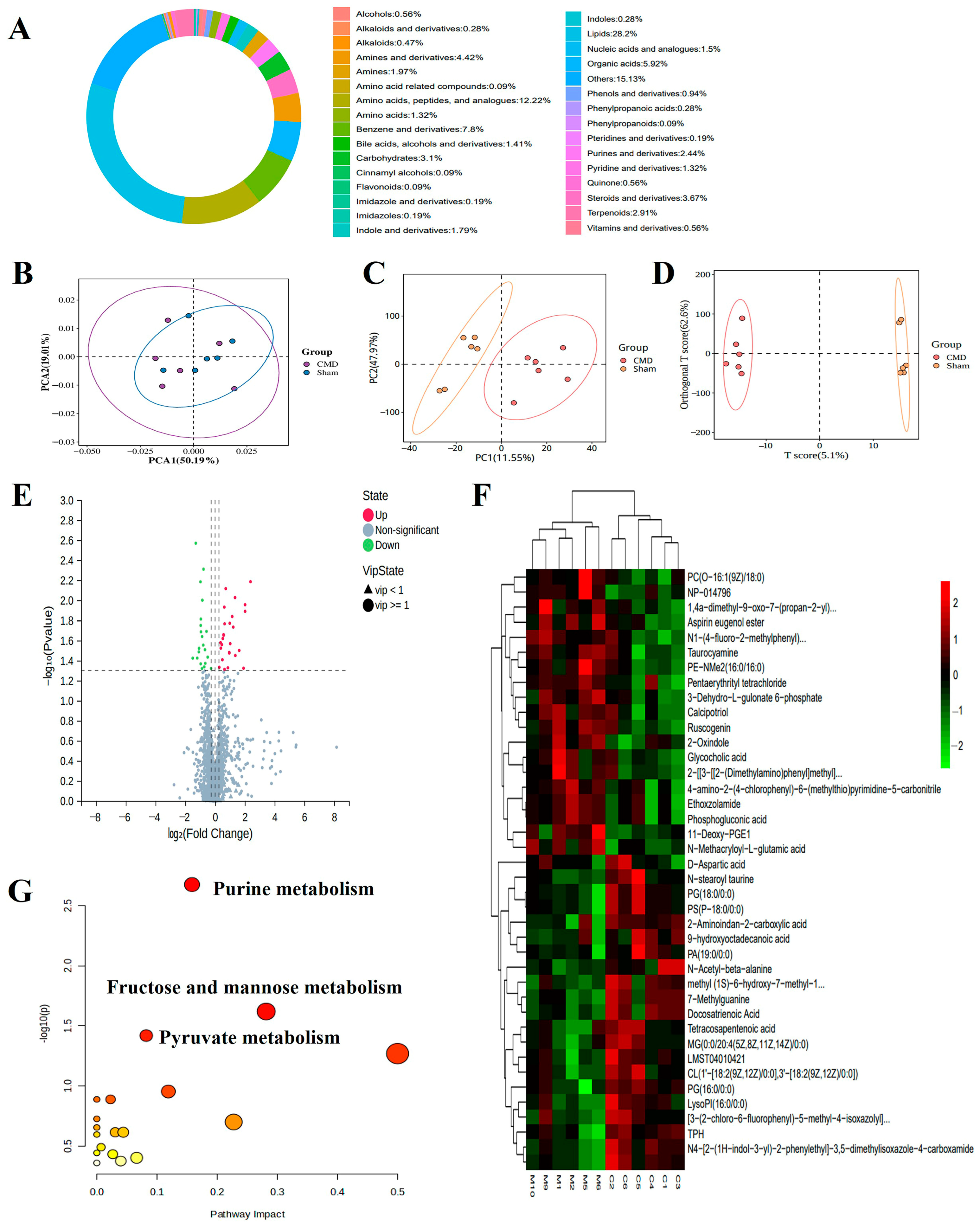
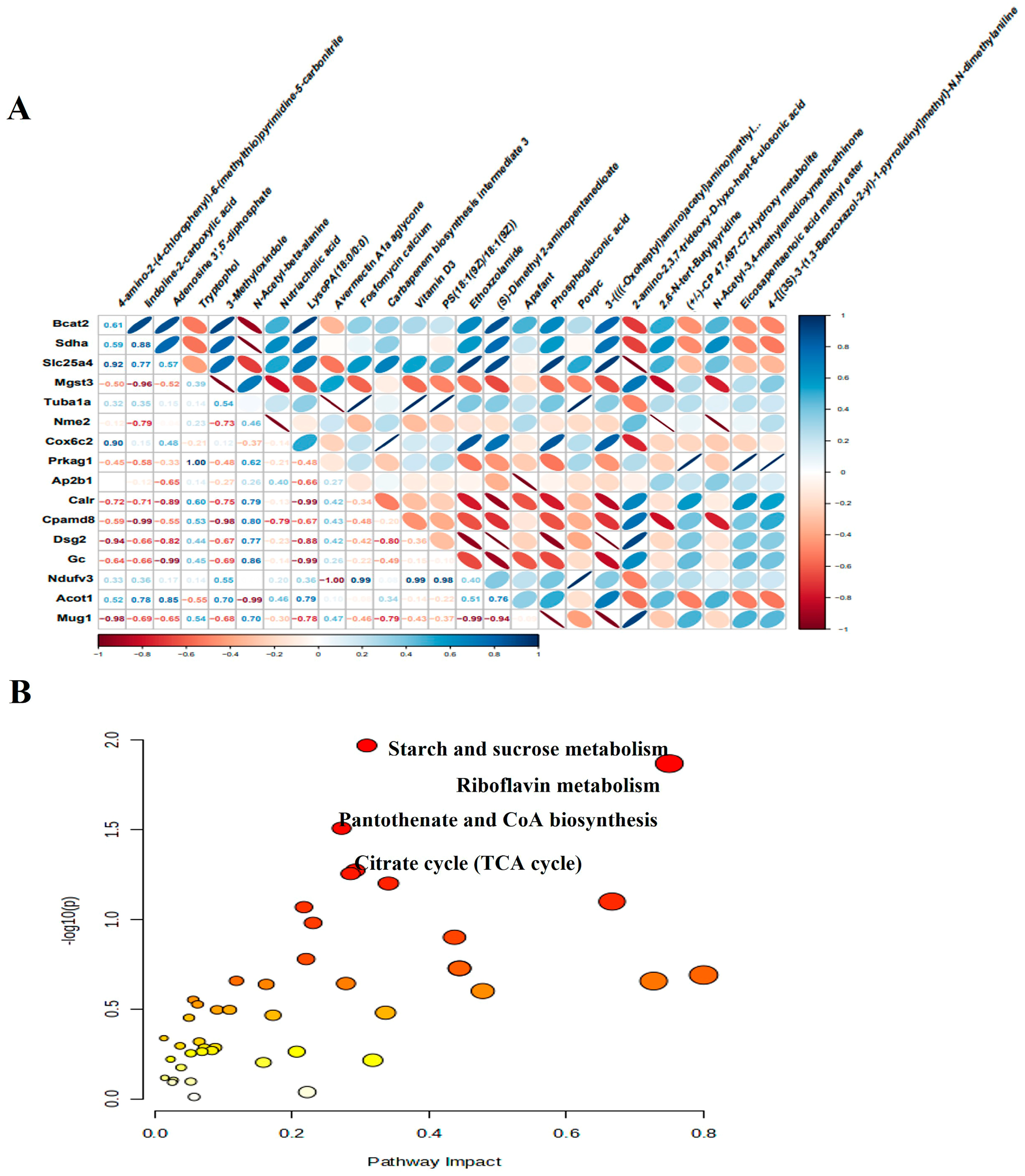
3.7. Untargeted Metabolomic Profiling
3.8. Integrated Analysis of Proteomics and Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMD | Coronary microvascular dysfunction. |
| IHD | Ischemic heart disease. |
| CFR | Coronary flow reserve. |
| CK-MB | Creatine kinase MB isoenzyme. |
| cTnT | Cardiac troponin T. |
| ELISA | Enzyme linked immunosorbent assay. |
| FDR | False discovery rate. |
| PCA | Principal component analysis. |
| DEPs | Differentially expressed proteins. |
| FC | Fold change. |
| KEGG | Kyoto encyclopedia of genes and genomes. |
| WGCNA | Weighted gene co-expression network analysis. |
| SVM | Support vector machine. |
| ROC | Receiver operating characteristic. |
| AUC | Area under the curve. |
| UPLC | Ultra Performance Liquid Chromatography. |
| DEMs | Differential metabolites. |
| VIP | Variable importance. |
| OPLS-DA | Orthogonal partial least squares discriminant analysis. |
| LVESV | Left ventricular end-systolic volume. |
| LVIDs | Left ventricular end-systolic diameter. |
| LV Mass | Left ventricular mass. |
| LVPWs | Left ventricular posterior wall thickness. |
| EF | Ejection fraction. |
| FS | Fractional shortening. |
| GO | Gene ontology. |
| Emc1 | Endoplasmic reticulum membrane protein complex 1. |
| Ank1 | Ankyrin-1. |
| Fbln2 | Fibronectin 2. |
| Hp | Hemoglobin-binding protein. |
| TCA | cycle: Citrate cycle. |
| Enpp1 | Ectonucleotide pyrophosphatase phosphodiesterase 1. |
| Gbe1 | 1, 4-alpha-glucan branching enzyme 1. |
| Pygm | Myophosphorylase. |
| Sdha | Succinate dehydrogenase complex flavoprotein subunit A. |
| Mdh2 | Malate dehydrogenase 2. |
References
- Uthman, O.A. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef] [PubMed]
- Konijnenberg, L.S.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; Van Geuns, R.J.M.; Berry, C.; Riksen, N.P.; Escaned, J.; van Royen, N. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Thakker, R.A.; Rodriguez Lozano, J.; Rodriguez Lozano, P.; Motiwala, A.; Rangasetty, U.; Khalife, W.; Chatila, K. Coronary Microvascular Disease. Cardiol. Ther. 2022, 11, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; Maron, B.A.; Loscalzo, J. Multiomics Network Medicine Approaches to Precision Medicine and Therapeutics in Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 493–503. [Google Scholar] [CrossRef]
- Benincasa, G.; Suades, R.; Padró, T.; Badimon, L.; Napoli, C. Bioinformatic platforms for clinical stratification of natural history of atherosclerotic cardiovascular diseases. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 758–769. [Google Scholar] [CrossRef]
- Ahmad, A.; Corban, M.T.; Moriarty, J.P.; Kanaji, Y.; Rosedahl, J.K.; Gulati, R.; Rihal, C.S.; Prasad, A.; Sara, J.D.; Toya, T.; et al. Coronary Reactivity Assessment Is Associated with Lower Health Care-Associated Costs in Patients Presenting with Angina and Nonobstructive Coronary Artery Disease. Circ. Cardiovasc. Interv. 2023, 16, e012387. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc. Interv. 2020, 13, 33–45. [Google Scholar] [CrossRef]
- Denby, K.J.; Zmaili, M.; Datta, S.; Das, T.; Ellis, S.; Ziada, K.; Lerman, A.; Raphael, C.E. Developments and Controversies in Invasive Diagnosis of Coronary Microvascular Dysfunction in Angina with Nonobstructive Coronary Arteries. Mayo Clin. Proc. 2024, 99, 1469–1481. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Ling, H.; Li, Y.; Fu, S.; Xu, M.; Li, B.; Liu, X.; Wang, Q.; Li, A.; et al. Navigating the Landscape of Coronary Microvascular Research: Trends, Triumphs, and Challenges Ahead. Rev. Cardiovasc. Med. 2024, 25, 288. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.W.; Zhang, X.; Shen, C.C. The shared circulating diagnostic biomarkers and molecular mechanisms of systemic lupus erythematosus and inflammatory bowel disease. Front. Immunol. 2024, 15, 1354348. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, H.; Hu, P.; Pan, Y.; Wang, S.; Liu, L.; Liu, X. Identification and validation of immune and oxidative stress-related diagnostic markers for diabetic nephropathy by WGCNA and machine learning. Front. Immunol. 2023, 14, 1084531. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; Berry, C. Treatment of coronary microvascular dysfunction. Cardiovasc. Res. 2020, 116, 856–870. [Google Scholar] [CrossRef]
- Schindler, T.H.; Dilsizian, V. Coronary Microvascular Dysfunction: Clinical Considerations and Noninvasive Diagnosis. JACC Cardiovasc. Imaging 2020, 13, 140–155. [Google Scholar] [CrossRef]
- Jin, S.C.; Homsy, J.; Zaidi, S.; Lu, Q.; Morton, S.; DePalma, S.R.; Zeng, X.; Qi, H.; Chang, W.; Sierant, M.C.; et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat. Genet. 2017, 49, 1593–1601. [Google Scholar] [CrossRef]
- Chung, H.L.; Rump, P.; Lu, D.; Glassford, M.R.; Mok, J.W.; Fatih, J.; Basal, A.; Marcogliese, P.C.; Kanca, O.; Rapp, M.; et al. De novo variants in EMC1 lead to neurodevelopmental delay and cerebellar degeneration and affect glial function in Drosophila. Hum. Mol. Genet. 2022, 31, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Derrick, C.J.; Noël, E.S. The ECM as a driver of heart development and repair. Development 2021, 148, dev191320. [Google Scholar] [CrossRef]
- Bennett, V.; Lorenzo, D.N. An Adaptable Spectrin/Ankyrin-Based Mechanism for Long-Range Organization of Plasma Membranes in Vertebrate Tissues. Curr. Top. Membr. 2016, 77, 143–184. [Google Scholar] [CrossRef]
- Leterrier, C. The Axon Initial Segment: An Updated Viewpoint. J. Neurosci. 2018, 38, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Yuan, S.; Hu, G.; Zhan, J.; Jin, K.; Tang, Y.; Fan, J.; Zhao, Y.; Wang, F.; Chen, C.; et al. Nuclear AGO2 promotes myocardial remodeling by activating ANKRD1 transcription in failing hearts. Mol. Ther. 2024, 32, 1578–1594. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Dong, H.; Joyce, J.; Sasaki, T.; Chu, M.L.; Tsuda, T. Fibulin-2 is essential for angiotensin II-induced myocardial fibrosis mediated by transforming growth factor (TGF)-β. Lab. Investig. 2016, 96, 773–783. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhang, R.; Xia, Y. Knockdown of FBLN2 suppresses TGF-β1-induced MRC-5 cell migration and fibrosis by downregulating VTN. Tissue Cell 2023, 81, 102005. [Google Scholar] [CrossRef]
- Tsuda, T.; Wu, J.; Gao, E.; Joyce, J.; Markova, D.; Dong, H.; Liu, Y.; Zhang, H.; Zou, Y.; Gao, F.; et al. Loss of fibulin-2 protects against progressive ventricular dysfunction after myocardial infarction. J. Mol. Cell. Cardiol. 2012, 52, 273–282. [Google Scholar] [CrossRef]
- Holme, I.; Aastveit, A.H.; Hammar, N.; Jungner, I.; Walldius, G. Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Ann. Med. 2009, 41, 522–532. [Google Scholar] [CrossRef]
- Figtree, G.A.; Vernon, S.T.; Harmer, J.A.; Gray, M.P.; Arnott, C.; Bachour, E.; Barsha, G.; Brieger, D.; Brown, A.; Celermajer, D.S.; et al. Clinical Pathway for Coronary Atherosclerosis in Patients Without Conventional Modifiable Risk Factors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 1343–1359. [Google Scholar] [CrossRef]
- Matuszek, M.A.; Aristoteli, L.P.; Bannon, P.G.; Hendel, P.N.; Hughes, C.F.; Jessup, W.; Dean, R.T.; Kritharides, L. Haptoglobin elutes from human atherosclerotic coronary arteries—A potential marker of arterial pathology. Atherosclerosis 2003, 168, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Q.; Zhou, Y.; Yu, S.; Hong, L.; Zhao, S.; Yang, J.; Wan, H.; Xu, G.; Zhang, Y.; et al. Integration of Proteomics and Metabolomics Revealed Metabolite-Protein Networks in ACTH-Secreting Pituitary Adenoma. Front. Endocrinol. 2018, 9, 678. [Google Scholar] [CrossRef]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef]
- Bulló, M.; Papandreou, C.; García-Gavilán, J.; Ruiz-Canela, M.; Li, J.; Guasch-Ferré, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Tricarboxylic acid cycle related-metabolites and risk of atrial fibrillation and heart failure. Metabolism 2021, 125, 154915. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.K.; Jackson, B.T.; Paras, K.I.; Brunner, J.S.; Hart, M.L.; Newsom, O.J.; Alibeckoff, S.P.; Endress, J.; Drill, E.; Sullivan, L.B.; et al. A non-canonical tricarboxylic acid cycle underlies cellular identity. Nature 2022, 603, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.G.; Li, B.; Zhang, H.; Li, Q.X.; Lam, S.M.; Yin, C.L.; Tian, H.; Shui, G. Precise Metabolomics Defines Systemic Metabolic Dysregulation Distinct to Acute Myocardial Infarction Associated with Diabetes. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Mei, Z.; Zhao, J.; Prodhan, M.A.I.; Obal, D.; Katragadda, K.; Doelling, B.; Hoetker, D.; Posa, D.K.; He, L.; et al. Integrated Multilayer Omics Reveals the Genomic, Proteomic, and Metabolic Influences of Histidyl Dipeptides on the Heart. J. Am. Heart Assoc. 2022, 11, e023868. [Google Scholar] [CrossRef]
- Consegal, M.; Núñez, N.; Barba, I.; Benito, B.; Ruiz-Meana, M.; Inserte, J.; Ferreira-González, I.; Rodríguez-Sinovas, A. Citric Acid Cycle Metabolites Predict Infarct Size in Pigs Submitted to Transient Coronary Artery Occlusion and Treated with Succinate Dehydrogenase Inhibitors or Remote Ischemic Perconditioning. Int. J. Mol. Sci. 2021, 22, 4151. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Wang, Y.; Xin, Q.; Yuan, R.; Chen, K.; Chu, J.; Cong, W. Metabolic and Proteomic Profiling of Coronary Microvascular Dysfunction: Insights from Rat Models. Biomolecules 2024, 14, 1305. https://doi.org/10.3390/biom14101305
Lu Y, Wang Y, Xin Q, Yuan R, Chen K, Chu J, Cong W. Metabolic and Proteomic Profiling of Coronary Microvascular Dysfunction: Insights from Rat Models. Biomolecules. 2024; 14(10):1305. https://doi.org/10.3390/biom14101305
Chicago/Turabian StyleLu, Yan, Yuying Wang, Qiqi Xin, Rong Yuan, Keji Chen, Jianfeng Chu, and Weihong Cong. 2024. "Metabolic and Proteomic Profiling of Coronary Microvascular Dysfunction: Insights from Rat Models" Biomolecules 14, no. 10: 1305. https://doi.org/10.3390/biom14101305
APA StyleLu, Y., Wang, Y., Xin, Q., Yuan, R., Chen, K., Chu, J., & Cong, W. (2024). Metabolic and Proteomic Profiling of Coronary Microvascular Dysfunction: Insights from Rat Models. Biomolecules, 14(10), 1305. https://doi.org/10.3390/biom14101305






