Fungal L-Methionine Biosynthesis Pathway Enzymes and Their Applications in Various Scientific and Commercial Fields
Abstract
:1. Introduction
2. L-Methionine Biosynthesis Pathway in Fungal Cells and Its Connections
3. Characterization of Fungal Enzymes Involved in the L-Methionine Biosynthesis Pathway
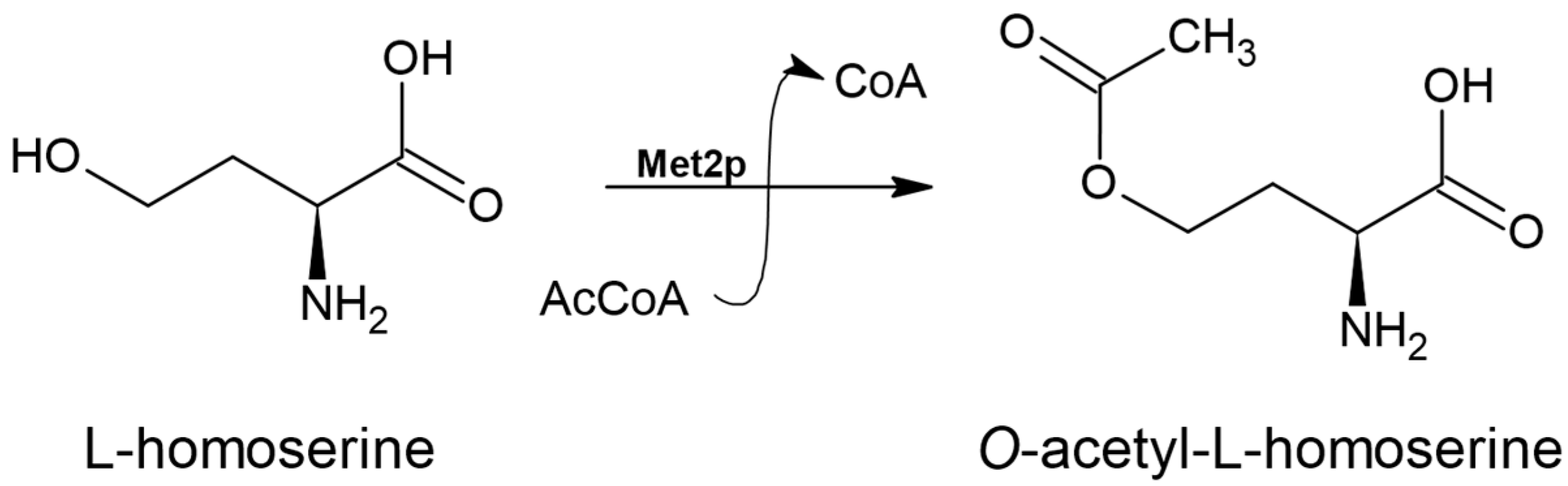
3.1. L-homoserine O-acetyltransferase (EC 2.3.1.31)
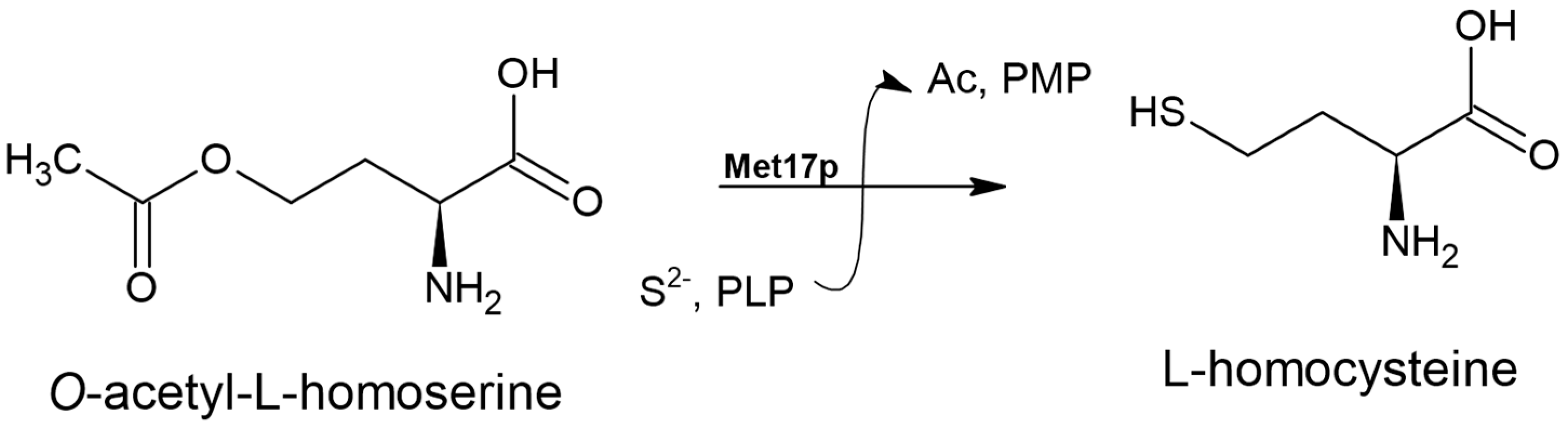
3.2. O-acetyl-L-homoserine Sulfhydrylase Enzyme (EC 2.5.1.49)
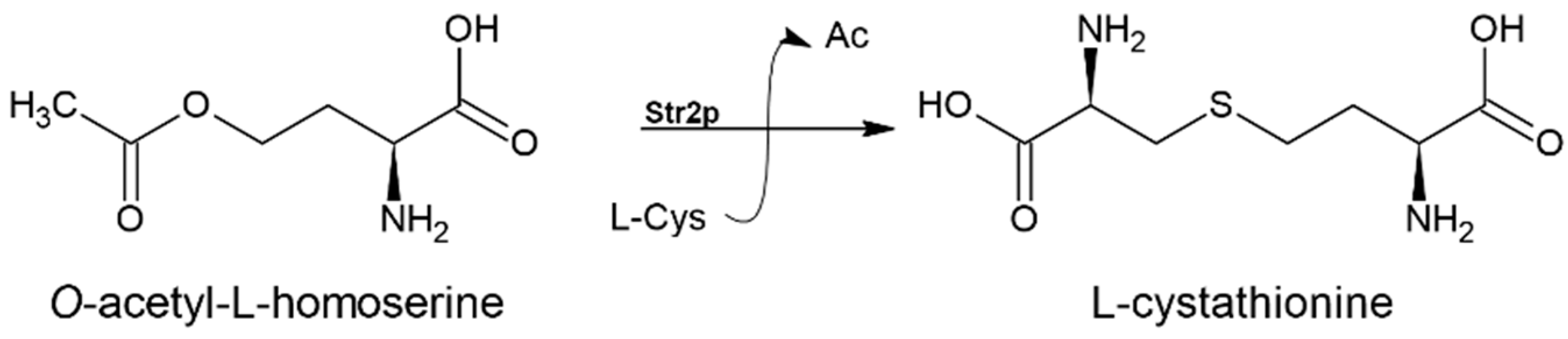
3.3. Cystathionine-γ-synthase (EC 2.5.1.48)
3.4. Cystathionine-β-lyase (EC 4.4.1.8)
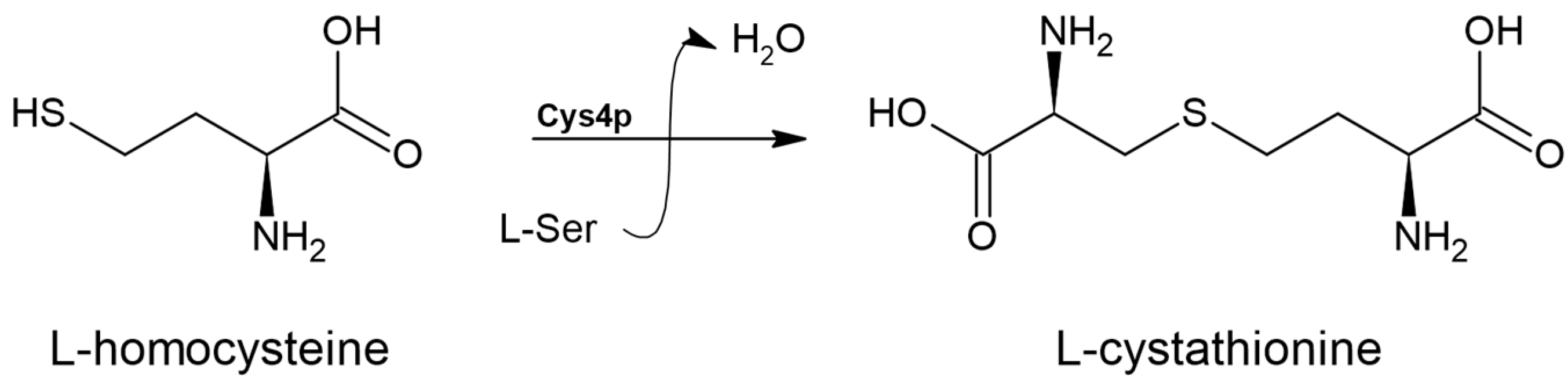
3.5. Cystathionine β-synthase (EC 4.2.1.22)
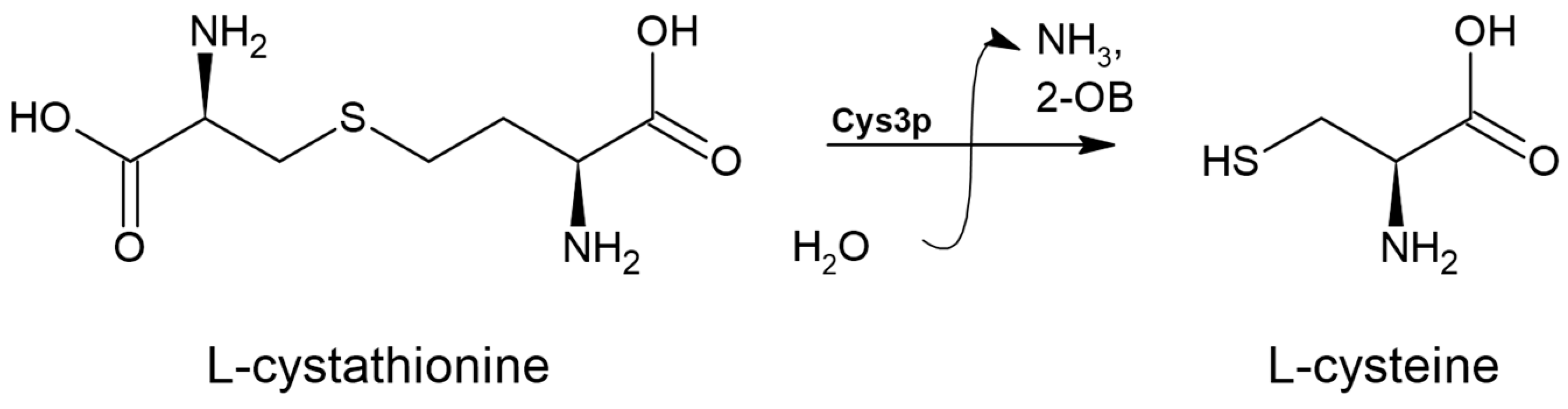
3.6. Cystathionine γ-lyase (EC 4.4.1.1)
3.7. Methionine Synthase (EC 2.1.1.13)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gophna, U.; Bapteste, E.; Doolittle, W.F.; Biran, D.; Ron, E.Z. Evolutionary Plasticity of Methionine Biosynthesis. Gene 2005, 355, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Martinez, A.; Gambin, Y.; Sierecki, E. Thinking Outside the Bug: Molecular Targets and Strategies to Overcome Antibiotic Resistance. Int. J. Mol. Sci. 2019, 20, 1255. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębowska, K.; Gabriel, I. Inhibitors of Amino Acids Biosynthesis as Antifungal Agents. Amino Acids 2015, 47, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, S.B.; Parikh, S.B.; Castilho Coelho, N.; Wacholder, A.; Belashov, I.; Zdancewicz, S.; Michaca, M.; Xu, J.; Kang, Y.P.; Ward, N.P.; et al. On the Illusion of Auxotrophy: Met15Δ Yeast Cells Can Grow on Inorganic Sulfur, Thanks to the Previously Uncharacterized Homocysteine Synthase Yll058w. J. Biol. Chem. 2022, 298, 102697. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Che, Y.; Yang, D.; Wang, Q.; Yang, H.; Boutet, J.; Huet, R.; Yin, S. Production of L -Methionine from 3-Methylthiopropionaldehyde and O-Acetylhomoserine by Catalysis of the Yeast O-Acetylhomoserine Sulfhydrylase. J. Agric. Food Chem. 2021, 69, 7932–7937. [Google Scholar] [CrossRef]
- Huang, C.W.; Walker, M.E.; Fedrizzi, B.; Roncoroni, M.; Gardner, R.C.; Jiranek, V. The Yeast TUM1 Affects Production of Hydrogen Sulfide from Cysteine Treatment during Fermentation. FEMS Yeast Res. 2016, 16, fow100. [Google Scholar] [CrossRef] [PubMed]
- Viaene, J.; Tiels, P.; Logghe, M.; Dewaele, S.; Martinet, W.; Contreras, R. MET15 as a Visual Selection Marker for Candida Albicans. Yeast 2000, 16, 1205–1215. [Google Scholar] [CrossRef]
- Suliman, H.S.; Appling, D.R.; Robertus, J.D. The Gene for Cobalamin-Independent Methionine Synthase Is Essential in Candida Albicans: A Potential Antifungal Target. Arch. Biochem. Biophys. 2007, 467, 218–226. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, A.K. Metabolic Pathways as Drug Targets: Targeting the Sulphur Assimilatory Pathways of Yeast and Fungi for Novel Drug Discovery. In Combating Fungal Infections: Problems and Remedy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 327–346. [Google Scholar] [CrossRef]
- Takagi, H.; Yoshioka, K.; Awano, N.; Nakamori, S.; Ono, B.I. Role of Saccharomyces Cerevisiae Serine O-Acetyltransferase in Cysteine Biosynthesis. FEMS Microbiol. Lett. 2003, 218, 291–297. [Google Scholar] [CrossRef]
- Hébert, A.; Casaregola, S.; Beckerich, J.M. Biodiversity in Sulfur Metabolism in Hemiascomycetous Yeasts. FEMS Yeast Res. 2011, 11, 366–378. [Google Scholar] [CrossRef]
- Brzywczy, J.; Paszewski, A. Sulfur Amino Acid Metabolism in Schizosaccharomyces Pombe: Occurrence of Two O-Acetylhomoserine Sulfhydrylases and the Lack of the Reverse Transfulfuration Pathway. FEMS Microbiol. Lett. 1994, 121, 171–174. [Google Scholar] [CrossRef]
- Nazi, I.; Wright, G.D. Catalytic Mechanism of Fungal Homoserine Transacetylase. Biochemistry 2005, 44, 13560–13566. [Google Scholar] [CrossRef] [PubMed]
- Sagong, H.Y.; Hong, J.; Kim, K.J. Crystal Structure and Biochemical Characterization of O-Acetylhomoserine Acetyltransferase from Mycobacterium Smegmatis ATCC 19420. Biochem. Biophys. Res. Commun. 2019, 517, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kuplińska, A.; Rząd, K.; Wojciechowski, M.; Milewski, S.; Gabriel, I. Antifungal Effect of Penicillamine Due to the Selective Targeting of L-Homoserine O-Acetyltransferase. Int. J. Mol. Sci. 2022, 23, 7763. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, J.M.; McCusker, J.H. Cytocidal Amino Acid Starvation of Saccharomyces Cerevisiae and Candida Albicans Acetolactate Synthase (Ilv2Δ) Mutants Is Influenced by the Carbon Source and Rapamycin. Microbiology 2010, 156, 929–939. [Google Scholar] [CrossRef]
- Montoya, E.J.O.; Melin, C.; Blanc, N.; Lanoue, A.; Foureau, E.; Boudesocque, L.; Gildas, P.; Simkin, A.J.; Creche, J.; Atehortua, L.; et al. Disrupting the Methionine Biosynthetic Pathway in Candida guilliermondii: Characterization of the MET2 Gene as Counter-Selectable Marker. Yeast 2014, 31, 234–251. [Google Scholar] [CrossRef]
- Singh, A.; Sherman, F. Characteristics and Relationships of Mercury Resistant Mutants and Methionine Auxotrophs of Yeast. J. Bacteriol. 1974, 118, 911–918. [Google Scholar] [CrossRef]
- Nazi, I.; Scott, A.; Sham, A.; Rossi, L.; Williamson, P.R.; Kronstad, J.W.; Wright, G.D. Role of Homoserine Transacetylase as a New Target for Antifungal Agents. Antimicrob. Agents Chemother. 2007, 51, 1731–1736. [Google Scholar] [CrossRef]
- Berney, M.; Berney-Meyer, L.; Wong, K.W.; Chen, B.; Chen, M.; Kim, J.; Wang, J.; Harris, D.; Parkhill, J.; Chan, J.; et al. Essential Roles of Methionine and S-Adenosylmethionine in the Autarkic Lifestyle of Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. USA 2015, 112, 10008–10013. [Google Scholar] [CrossRef]
- Li, H.; Mo, P.; Zhang, J.; Xie, Z.; Liu, X.; Chen, H.; Yang, L.; Liu, M.; Zhang, H.; Wang, P.; et al. Methionine Biosynthesis Enzyme MoMet2 Is Required for Rice Blast Fungus Pathogenicity by Promoting Virulence Gene Expression via Reducing 5mC Modification. PLoS Genet. 2023, 19, e1010927. [Google Scholar] [CrossRef]
- Yamagata, S. Roles of O-Acetyl-l-Homoserine Sulfhydrylases in Microorganisms. Biochimie 1989, 71, 1125–1143. [Google Scholar] [CrossRef]
- Thomas, D.; Surdin-Kerjan, Y. Metabolism of Sulfur Amino Acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1997, 61, 503–532. [Google Scholar] [CrossRef] [PubMed]
- Brzywczy, J.; Paszewski, A. Role of O-acetylhomoserine Sulfhydrylase in Sulfur Amino Acid Synthesis in Various Yeasts. Yeast 1993, 9, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Mohr, M.K.F.; Saleem-Batcha, R.; Cornelissen, N.V.; Andexer, J.N. Enzymatic Synthesis of L-Methionine Analogues and Application in a Methyltransferase Catalysed Alkylation Cascade. Chem.-A Eur. J. 2023, 29, e202301503. [Google Scholar] [CrossRef]
- Tran, T.H.; Krishnamoorthy, K.; Begley, T.P.; Ealick, S.E. A Novel Mechanism of Sulfur Transfer Catalyzed by O-Acetylhomoserine Sulfhydrylase in the Methionine-Biosynthetic Pathway of Wolinella Succinogenes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 831–838. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, H.; Li, F.; Jiang, X.; Wang, J.; Wei, S.; Sun, Y.; Tian, Y.; Chu, H.; Shi, Y.; et al. O-Acetyl-Homoserine Sulfhydrylase Deficient Streptococcus Suis Serotype 2 Strain SC19 Becomes an Avirulent Strain and Provides Immune Protection against Homotype Infection in Mice. Vet. Microbiol. 2024, 288, 109943. [Google Scholar] [CrossRef] [PubMed]
- Brzywczy, J.; Yamagata, S.; Paszewski, A. Comparative Studies on O-Acetylhomoserine Sulfhydrylase Physiological Role and Characterization of the A Nidulans Enzyme. Acta Biochim. Pol. 1993, 40, 421–428. [Google Scholar] [CrossRef]
- Sonal; Yuan, A.E.; Yang, X.; Shou, W. Collective Production of Hydrogen Sulfide Gas Enables Budding Yeast Lacking MET17 to Overcome Their Metabolic Defect. PLoS Biol. 2023, 21, e3002439. [Google Scholar] [CrossRef]
- Yu, J.S.L.; Heineike, B.M.; Hartl, J.; Aulakh, S.K.; Correia-Melo, C.; Lehmann, A.; Lemke, O.; Agostini, F.; Lee, C.T.; Demichev, V.; et al. Inorganic Sulfur Fixation via a New Homocysteine Synthase Allows Yeast Cells to Cooperatively Compensate for Methionine Auxotrophy. PLoS Biol. 2022, 20, e3001912. [Google Scholar] [CrossRef]
- Willke, T. Methionine Production—A Critical Review. Appl. Microbiol. Biotechnol. 2014, 98, 9893–9914. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Niu, K.; Liu, P.; Cai, X.; Liu, Z.Q.; Zheng, Y.G. Combining Fermentation to Produce O-Succinyl-L-Homoserine and Enzyme Catalysis for the Synthesis of L-Methionine in One Pot. J. Biosci. Bioeng. 2021, 132, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Cao, S.; Yang, M.; Xu, L. Enzymatic Synthesis of S-Adenosylmethionine Using Immobilized Methionine Adenosyltransferase Variants on the 50-MM Scale. Catalysts 2017, 7, 238. [Google Scholar] [CrossRef]
- Rudenko, A.Y.; Mariasina, S.S.; Bolikhova, A.K.; Nikulin, M.V.; Ozhiganov, R.M.; Vasil’ev, V.G.; Ikhalaynen, Y.A.; Khandazhinskaya, A.L.; Khomutov, M.A.; Sergiev, P.V.; et al. Organophosphorus S-Adenosyl-L-Methionine Mimetics: Synthesis, Stability, and Substrate Properties. Front. Chem. 2024, 12, 1448747. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zheng, T.; Chang, X. Biosynthesis of S-Adenosylmethionine by Magnetically Immobilized Escherichia Coli Cells Highly Expressing a Methionine Adenosyltransferase Variant. Molecules 2017, 22, 1365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sultan, S.A.; Rehka, T.; Chen, X. Biotechnological Applications of S-Adenosyl-Methionine-Dependent Methyltransferases for Natural Products Biosynthesis and Diversification. Bioresour. Bioprocess. 2021, 8, 72. [Google Scholar] [CrossRef]
- Winter, G.; Henschke, P.A.; Higgins, V.J.; Ugliano, M.; Curtin, C.D. Effects of Rehydration Nutrients on H2S Metabolism and Formation of Volatile Sulfur Compounds by the Wine Yeast VL3. AMB Express 2011, 1, 36. [Google Scholar] [CrossRef]
- Steegborn, C.; Laber, B.; Messerschmidt, A.; Huber, R.; Clausen, T. Crystal Structures of Cystathionine γ-Synthase Inhibitor Complexes Rationalize the Increased Affinity of a Novel Inhibitor. J. Mol. Biol. 2001, 311, 789–801. [Google Scholar] [CrossRef]
- Kerr, D.S.; Flavin, M. The Regulation of Methionine Synthesis and the Nature of Cystathionine Gamma-Synthase in Neurospora. J. Biol. Chem. 1970, 245, 1842–1855. [Google Scholar] [CrossRef]
- Shao, W.; Yang, Y.; Zhang, Y.; Lv, C.; Ren, W.; Chen, C. Involvement of BcStr2 in Methionine Biosynthesis, Vegetative Differentiation, Multiple Stress Tolerance and Virulence in Botrytis Cinerea. Mol. Plant Pathol. 2016, 17, 438–447. [Google Scholar] [CrossRef]
- Sieńko, M.; Paszewski, A. The MetG Gene of Aspergillus Nidulans Encoding Cystathionine β-Lyase: Cloning and Analysis. Curr. Genet. 1999, 35, 638–646. [Google Scholar] [CrossRef]
- Seong, K.; Hou, Z.; Tracy, M.; Kistler, H.C.; Xu, J.R. Random Insertional Mutagenesis Identifies Genes Associated with Virulence in the Wheat Scab Fungus Fusarium Graminearum. Phytopathology 2005, 95, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.H.; Shringi, S.; Desai, A.R.; Heo, E.J.; Park, J.H.; Chae, J.S. Effect of MetC Mutation on Salmonella Gallinarum Virulence and Invasiveness in 1-Day-Old White Leghorn Chickens. Vet. Microbiol. 2007, 119, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kuplińska, A.; Rząd, K. Molecular Targets for Antifungals in Amino Acid and Protein Biosynthetic Pathways. Amino Acids 2021, 53, 961–991. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhang, M.; Jin, X.; Zhang, H.; Zheng, H.; Zheng, S.; Qiao, Y.; Yu, H.; Sun, B.; Hou, X.; et al. Inhibition of Fungal Pathogenicity by Targeting the H2Ssynthesizing Enzyme Cystathionine β-Synthase. Sci. Adv. 2022, 8, eadd5366. [Google Scholar] [CrossRef] [PubMed]
- Sueiro-Olivares, M.; Scott, J.; Gago, S.; Petrovic, D.; Kouroussis, E.; Zivanovic, J.; Yu, Y.; Strobel, M.; Cunha, C.; Thomson, D.; et al. Fungal and Host Protein Persulfidation Are Functionally Correlated and Modulate Both Virulence and Antifungal Response. PLoS Biol. 2021, 19, e3001247. [Google Scholar] [CrossRef]
- Goh, N.Y.; Mohamad, R.; Muhammad, F.; Yap, Y.H.Y.; Ng, C.L.; Fung, S.Y. In Silico Analysis and Characterization of Medicinal Mushroom Cystathionine Beta-Synthase as an Angiotensin Converting Enzyme (ACE) Inhibitory Protein. Comput. Biol. Chem. 2022, 96, 107620. [Google Scholar] [CrossRef]
- Shangguan, J.; Qiao, J.; Liu, H.; Zhu, L.; Han, X.; Shi, L.; Zhu, J.; Liu, R.; Ren, A.; Zhao, M. The CBS/H2S Signalling Pathway Regulated by the Carbon Repressor CreA Promotes Cellulose Utilization in Ganoderma Lucidum. Commun. Biol. 2024, 7, 466. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Abdel-Azeim, S.; Ibrahim, H.M.; Yassin, M.A.; Abdel-Ghany, S.E.; Esener, S.; Ali, G.S. Biochemical Stability and Molecular Dynamic Characterization of Aspergillus Fumigatus Cystathionine γ-Lyase in Response to Various Reaction Effectors. Enzyme Microb. Technol. 2015, 81, 31–46. [Google Scholar] [CrossRef]
- Messerchmidt, A.; Worbs, M.; Steegborn, C.; Wahl, M.C.; Huber, R.; Laber, B.; Clausen, T. Determinants of Enzymatic Specificity in the Cys-Met-Metabolism PLP-Dependent Enzymes Family: Crystal Structure of Cystathionine γ-Lyase from Yeast and Intrafamiliar Structure Comparison. Biol. Chem. 2003, 384, 373–386. [Google Scholar] [CrossRef]
- Gu, Z.; Sun, Y.; Wu, F. Mechanism of Growth Regulation of Yeast Involving Hydrogen Sulfide From S-Propargyl-Cysteine Catalyzed by Cystathionine-γ-Lyase. Front. Microbiol. 2021, 12, 679563. [Google Scholar] [CrossRef]
- Huang, C.W.; Walker, M.E.; Fedrizzi, B.; Gardner, R.C.; Jiranek, V. Hydrogen Sulfide and Its Roles in Saccharomyces Cerevisiae in a Winemaking Context. FEMS Yeast Res. 2017, 17, fox058. [Google Scholar] [CrossRef]
- Bregón-Villahoz, M.; Menéndez-Manjón, P.; Carrano, G.; Díez-Villalba, A.; Arrieta-Aguirre, I.; Fernandez-de-Larrinoa, I.; Moragues, M.D. Candida Albicans CDNA Library Screening Reveals Novel Potential Diagnostic Targets for Invasive Candidiasis. Diagn. Microbiol. Infect. Dis. 2024, 109, 116311. [Google Scholar] [CrossRef]
- Yao, S.; Wei, C.; Lin, H.; Zhang, P.; Liu, Y.; Deng, Y.; Huang, Q.; Xie, B. Cystathionine Gamma-Lyase Regulate Psilocybin Biosynthesis in Gymnopilus Dilepis Mushroom via Amino Acid Metabolism Pathways. J. Fungi 2022, 8, 870. [Google Scholar] [CrossRef]
- Sahu, U.; Rajendra, V.K.H.; Kapnoor, S.S.; Bhagavat, R.; Chandra, N.; Rangarajan, P.N. Methionine Synthase Is Localized to the Nucleus in Pichia Pastoris and Candida Albicans and to the Cytoplasm in Saccharomyces Cerevisiae. J. Biol. Chem. 2017, 292, 14730–14746. [Google Scholar] [CrossRef]
- Ubhi, D.; Kavanagh, K.L.; Monzingo, A.F.; Robertus, J.D. Structure of Candida Albicans Methionine Synthase Determined by Employing Surface Residue Mutagenesis. Arch. Biochem. Biophys. 2011, 513, 19–26. [Google Scholar] [CrossRef]
- Kanchanapiboon, J.; Maiuthed, A.; Rukthong, P.; Thunyaharn, S.; Tuntoaw, S.; Poonsatha, S.; Santimaleeworagun, W. Metabolomics Profiling of Culture Medium Reveals Association of Methionine and Vitamin B Metabolisms with Virulent Phenotypes of Clinical Bloodstream-Isolated Candida Albicans. Res. Microbiol. 2023, 174, 104009. [Google Scholar] [CrossRef]
- Pascon, R.C.; Ganous, T.M.; Kingsbury, J.M.; Cox, G.M.; McCusker, J.H. Cryptococcus Neoformans Methionine Synthase: Expression Analysis and Requirement for Virulence. Microbiology 2004, 150, 3013–3023. [Google Scholar] [CrossRef]
- Amich, J.; Dümig, M.; O’Keeffe, G.; Binder, J.; Doyle, S.; Beilhack, A.; Krappmann, S. Exploration of Sulfur Assimilation of Aspergillus Fumigatus Reveals Biosynthesis of Sulfur-Containing Amino Acids as a Virulence Determinant. Infect. Immun. 2016, 84, 917–929. [Google Scholar] [CrossRef]
- Scott, J.; Sueiro-Olivares, M.; Thornton, B.P.; Owens, R.A.; Muhamadali, H.; Fortune-Grant, R.; Thomson, D.; Thomas, R.; Hollywood, K.; Doyle, S.; et al. Targeting Methionine Synthase in a Fungal Pathogen Causes a Metabolic Imbalance That Impacts Cell Energetics, Growth, and Virulence. MBio 2020, 11, e01985-20. [Google Scholar] [CrossRef]
- Saint-Macary, M.E.; Barbisan, C.; Gagey, M.J.; Frelin, O.; Beffa, R.; Lebrun, M.H.; Droux, M. Methionine Biosynthesis Is Essential for Infection in the Rice Blast Fungus Magnaporthe Oryzae. PLoS ONE 2015, 10, e0111108. [Google Scholar] [CrossRef]
- Díez, A.; Carrano, G.; Bregón-Villahoz, M.; Cuétara, M.S.; García-Ruiz, J.C.; Fernandez-de-Larrinoa, I.; Moragues, M.D. Biomarkers for the Diagnosis of Invasive Candidiasis in Immunocompetent and Immunocompromised Patients. Diagn. Microbiol. Infect. Dis. 2021, 101, 115509. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, F.; Ghasemi, Y.; Atapour, A.; Zomorodian, K.; Ranjbar, M.; Monabati, A.; Nezafat, N.; Savardashtaki, A. B-Cell Epitope Mapping from Eight Antigens of Candida Albicans to Design a Novel Diagnostic Kit: An Immunoinformatics Approach. Int. J. Pept. Res. Ther. 2022, 28, 110. [Google Scholar] [CrossRef]
- Adams, A.L.; Eberle, K.; Colón, J.R.; Courville, E.; Xin, H. Synthetic Conjugate Peptide Fba-Met6 (MP12) Induces Complement-Mediated Resistance against Disseminated Candida Albicans. Vaccine 2021, 39, 4099–4107. [Google Scholar] [CrossRef]
- Xin, H. Effects of Immune Suppression in Murine Models of Disseminated Candida Glabrata and Candida Tropicalis Infection and Utility of a Synthetic Peptide Vaccine. Med. Mycol. 2019, 57, 745–756. [Google Scholar] [CrossRef]





| Enzyme | Application | |
|---|---|---|
| Met2p | L-homoserine O acetyltransferase | |
| Met15/Met17p | bi-functional O-acetyl-L-homoserine/O-acetyl-L-serine sulfhydrylase |
|
| Str2p | cystathionine-γ-synthase |
|
| Cys4p | cystathionine β- synthase |
|
| Cys3p | cystathionine γ-lyase |
|
| Met6p | methionine synthase |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rząd, K.; Kuplińska, A.; Gabriel, I. Fungal L-Methionine Biosynthesis Pathway Enzymes and Their Applications in Various Scientific and Commercial Fields. Biomolecules 2024, 14, 1315. https://doi.org/10.3390/biom14101315
Rząd K, Kuplińska A, Gabriel I. Fungal L-Methionine Biosynthesis Pathway Enzymes and Their Applications in Various Scientific and Commercial Fields. Biomolecules. 2024; 14(10):1315. https://doi.org/10.3390/biom14101315
Chicago/Turabian StyleRząd, Kamila, Aleksandra Kuplińska, and Iwona Gabriel. 2024. "Fungal L-Methionine Biosynthesis Pathway Enzymes and Their Applications in Various Scientific and Commercial Fields" Biomolecules 14, no. 10: 1315. https://doi.org/10.3390/biom14101315
APA StyleRząd, K., Kuplińska, A., & Gabriel, I. (2024). Fungal L-Methionine Biosynthesis Pathway Enzymes and Their Applications in Various Scientific and Commercial Fields. Biomolecules, 14(10), 1315. https://doi.org/10.3390/biom14101315







