Increased Cardiometabolic Risk in Men with Hypoprolactinemia: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
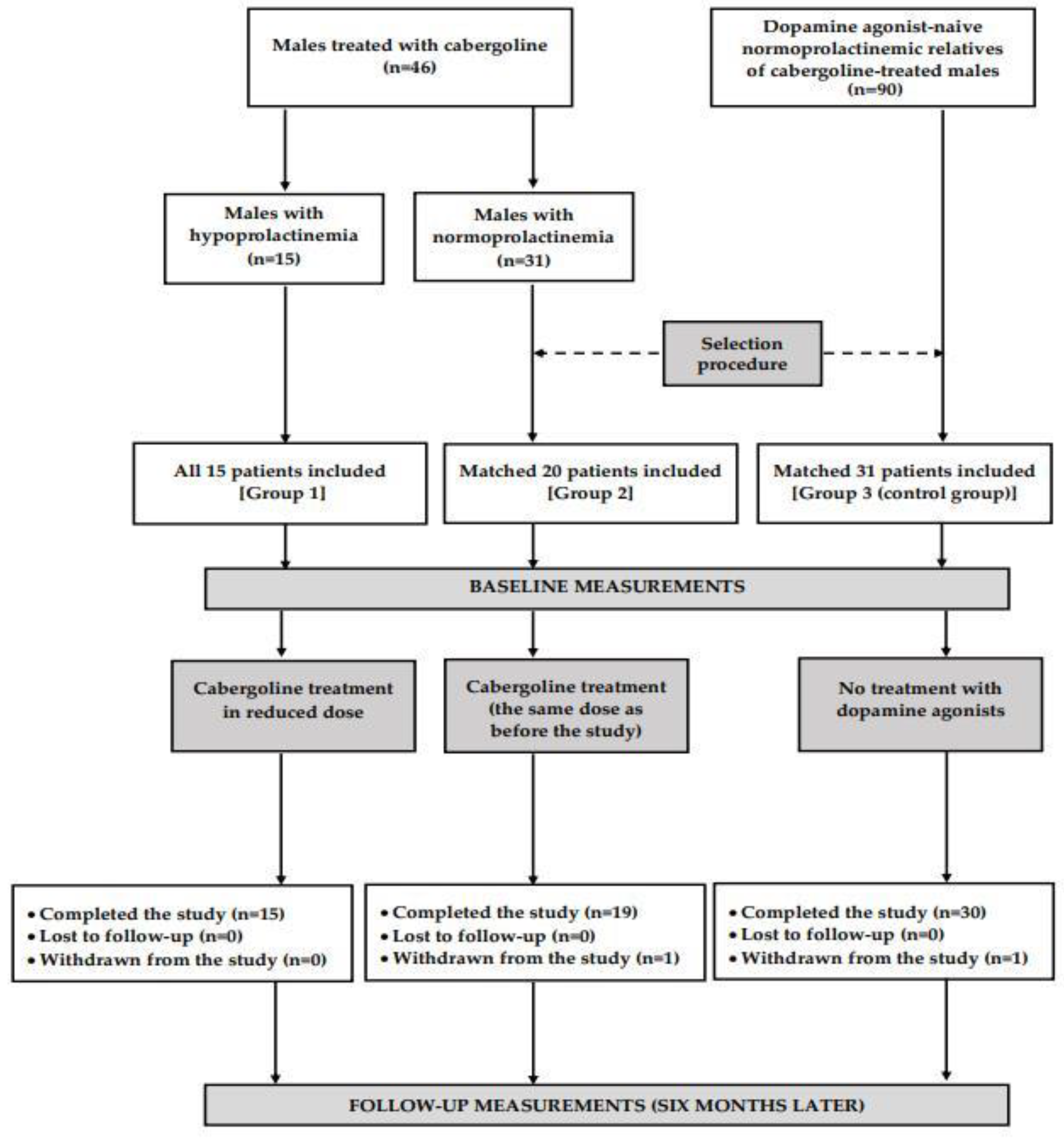
2.1. Study Population
2.2. Study Design
2.3. Measurements
2.4. Laboratory Assays
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| HbA1C | glycated hemoglobin |
| HDL | high-density lipoprotein |
| HOMA1-IR | homeostasis model assessment 1 of insulin resistance ratio |
| LDL | low-density lipoprotein |
| UACR | urinary albumin-to-creatinine ratio |
References
- Bernard, V.; Young, J.; Binart, N. Prolactin—A pleiotropic factor in health and disease. Nat. Rev. Endocrinol. 2019, 15, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Di Sarno, A.; Guerra, E.; Pivonello, R.; Cappabianca, P.; Caranci, F.; Elefante, A.; Cavallo, L.M.; Briganti, F.; Cirillo, S.; et al. Predictors of remission of hyperprolactinaemia after long-term withdrawal of cabergoline therapy. Clin. Endocrinol. 2007, 67, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Mannucci, E.; Jannini, E.A.; Lotti, F.; Ricca, V.; Monami, M.; Boddi, V.; Bandini, E.; Balercia, G.; Forti, G. Hypoprolactinemia: A new clinical syndrome in patients with sexual dysfunction. J. Sex. Med. 2009, 6, 1457–1466. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Sexual function and depressive symptoms in men with hypoprolactinaemia secondary to overtreatment of prolactin excess: A pilot study. Endocrinol. Diabetes Nutr. 2022, 69, 279–288. [Google Scholar] [CrossRef]
- Uzun, I.; Karaca, Z.; Hacioğlu, A.; Unluhizarci, K.; Kelestimur, F. The diagnosis and prevalence of hypoprolactinemia in patients with panhypopituitarism and the effects on depression and sexual functions. Pituitary 2024, 27, 277–286. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Sexual function and depressive symptoms in young women with hypoprolactinaemia. Clin. Endocrinol. 2020, 93, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Maseroli, E.; Verde, N.; Cipriani, S.; Rastrelli, G.; Alfaroli, C.; Ravelli, S.A.; Costeniero, D.; Scairati, R.; Minnetti, M.; Petraglia, F.; et al. Low prolactin level identifies hypoactive sexual desire disorder women with a reduced inhibition profile. J. Endocrinol. Invest. 2023, 46, 2481–2492. [Google Scholar] [CrossRef]
- Han, T.S.; Antonio, L.; Bartfai, G.; O’Neill, T.W.; Punab, M.; Rastrelli, G.; Maggi, M.; Słowikowska-Hilczer, J.; Tournoy, J.; Vanderschueren, D.; et al. Evidence-Based Definition of Hypoprolactinemia in European Men Aged 40–86 Years: The European Male Ageing Study. Rev. Endocr. Metab. Disord. 2024. [Google Scholar] [CrossRef]
- Ken-Dror, G.; Fluck, D.; Lean, M.E.; Casanueva, F.F.; Han, T.S. The relationship between low prolactin and type 2 diabetes. Rev. Endocr. Metab. Disord. 2024. [Google Scholar] [CrossRef]
- Ponce, A.J.; Galván-Salas, T.; Lerma-Alvarado, R.M.; Ruiz-Herrera, X.; Hernández-Cortés, T.; Valencia-Jiménez, R.; Cárdenas-Rodríguez, L.E.; Martínez de la Escalera, G.; Clapp, C.; Macotela, Y. Low prolactin levels are associated with visceral adipocyte hypertrophy and insulin resistance in humans. Endocrine 2020, 67, 331–343. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Cardiometabolic profile of young women with hypoprolactinemia. Endocrine 2022, 78, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Cabergoline-induced hypoprolactinemia may attenuate cardiometabolic effects of atorvastatin: A pilot study. Cardiology 2022, 147, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ge, Z.; Wang, H.; Feng, W.; Sun, X.; Chu, X.; Jiang, C.; Wang, Y.; Zhu, D.; Bi, Y. Prolactin improves hepatic steatosis via CD36 pathway. J. Hepatol. 2018, 68, 1247–1255. [Google Scholar] [CrossRef]
- Ruiz-Herrera, X.; de Los Ríos, E.A.; Díaz, J.M.; Lerma-Alvarado, R.M.; Martínez de la Escalera, L.; López-Barrera, F.; Lemini, M.; Arnold, E.; Martínez de la Escalera, G.; Clapp, C.; et al. Prolactin promotes adipose tissue fitness and insulin sensitivity in obese males. Endocrinology 2017, 158, 56–68. [Google Scholar] [CrossRef]
- Higham, C.E.; Johannsson, G.; Shalet, S.M. Hypopituitarism. Lancet 2016, 388, 2403–2415. [Google Scholar] [CrossRef]
- Tasaki, M.; Yasui-Furukori, N.; Yokoyama, S.; Shinozaki, M.; Sugawara, N.; Shimoda, K. Hypoprolactinemia and hyperprolactinemia in male schizophrenia patients treated with aripiprazole and risperidone and their relationships with testosterone levels. Neuropsychopharmacol. Rep. 2021, 41, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Clinical Investigators, Sponsors, and IRBs Adverse Effect Reporting to IRBs—Improving Human Subject Protection; FDA: Silver Spring, MD, USA, 2009. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Posawetz, A.S.; Trummer, C.; Pandis, M.; Aberer, F.; Pieber, T.R.; Obermayer-Pietsch, B.; Pilz, S.; Theiler-Schwetz, V. Adverse body composition and lipid parameters in patients with prolactinoma: A case-control study. BMC Endocr. Disord. 2021, 21, 81. [Google Scholar] [CrossRef]
- Greenman, Y.; Tordjman, K.; Stern, N. Increased body weight associated with prolactin secreting pituitary adenomas: Weight loss with normalization of prolactin levels. Clin. Endocrinol. 1998, 48, 547–553. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Galdiero, M.; Vitale, P.; Granieri, L.; Lo Calzo, F.; Salzano, C.; Ferreri, L.; Pivonello, C.; Cariati, F.; Coppola, G.; et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology 2013, 98, 299–310. [Google Scholar] [CrossRef]
- Auriemma, R.S.; De Alcubierre, D.; Pirchio, R.; Pivonello, R.; Colao, A. The effects of hyperprolactinemia and its control on metabolic diseases. Expert Rev. Endocrinol. Metab. 2018, 13, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Berinder, K.; Nyström, T.; Höybye, C.; Hall, K.; Hulting, A.L. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary 2011, 14, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ciresi, A.; Amato, M.C.; Guarnotta, V.; Lo Castro, F.; Giordano, C. Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clin. Endocrinol. 2013, 79, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, D.; Deyneli, O.; Akpinar, I.; Yildiz, E.; Gözü, H.; Sezgin, O.; Haklar, G.; Akalin, S. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic premenopausal women. Eur. J. Endocrinol. 2003, 149, 187–193. [Google Scholar] [CrossRef][Green Version]
- Stumpe, K.O.; Kolloch, R.; Higuchi, M.; Kruck, F.; Vetter, H. Hyperprolactinaemia and antihypertensive effect of bromocriptine in essential hypertension. Identification of abnormal central dopamine control. Lancet 1977, 2, 211–214. [Google Scholar] [CrossRef]
- Inancli, S.S.; Usluogullari, A.; Ustu, Y.; Caner, S.; Tam, A.A.; Ersoy, R.; Cakir, B. Effect of cabergoline on insulin sensitivity, inflammation, and carotid intima media thickness in patients with prolactinoma. Endocrine 2013, 44, 193–199. [Google Scholar] [CrossRef]
- Krysiak, R.; Okopień, B. Different effects of cabergoline and bromocriptine on metabolic and cardiovascular risk factors in patients with elevated prolactin levels. Basic Clin. Pharmacol. Toxicol. 2015, 116, 251–256. [Google Scholar] [CrossRef]
- Doğan, B.A.; Arduç, A.; Tuna, M.M.; Nasıroğlu, N.I.; Işık, S.; Berker, D.; Güler, S. Evaluation of atherosclerosis after cessation of cabergoline therapy in patients with prolactinoma. Anatol. J. Cardiol. 2016, 16, 440–447. [Google Scholar]
- Auriemma, R.S.; Pivonello, R.; Ferreri, L.; Priscitelli, P.; Colao, A. Cabergoline use for pituitary tumors and valvular disorders. Endocrinol. Metab. Clin. N. Am. 2015, 44, 89–97. [Google Scholar] [CrossRef]
- Kars, M.; Pereira, A.M.; Bax, J.J.; Romijn, J.A. Cabergoline and cardiac valve disease in prolactinoma patients: Additional studies during long-term treatment are required. Eur. J. Endocrinol. 2008, 159, 363–367. [Google Scholar] [CrossRef]
- Bogazzi, F.; Manetti, L.; Raffaelli, V.; Lombardi, M.; Rossi, G.; Martino, E. Cabergoline therapy and the risk of cardiac valve regurgitation in patients with hyperprolactinemia: A meta-analysis from clinical studies. J. Endocrinol. Investig. 2008, 31, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Bancos, I.; Nippoldt, T.B.; Erickson, D. Hypersexuality in men with prolactinomas treated with dopamine agonists. Endocrine 2017, 56, 456–457. [Google Scholar] [CrossRef]
- De Sousa, S.M.; Chapman, I.M.; Falhammar, H.; Torpy, D.J. Dopa-testotoxicosis: Disruptive hypersexuality in hypogonadal men with prolactinomas treated with dopamine agonists. Endocrine 2017, 55, 618–624. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Przewłocki, T. Clinical significance of carotid intima-media complex and carotid plaque assessment by ultrasound for the prediction of adverse cardiovascular events in primary and secondary care patients. J. Clin. Med. 2021, 10, 4628. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; Linares Sánchez, M.; López Alvarez, F.; Suárez Nieto, C.; Mäkitie, A.A.; Olsen, K.D.; Ferlito, A. Evaluation of intima-media thickness and arterial stiffness as early ultrasound biomarkers of carotid artery atherosclerosis. Cardiol. Ther. 2022, 11, 231–247. [Google Scholar] [CrossRef]
- Miljić, D.; Popovic, V. Metabolic syndrome in hypopituitarism. Front. Horm. Res. 2018, 49, 1–19. [Google Scholar] [PubMed]
- Ngaosuwan, K.; Johnston, D.G.; Godsland, I.F.; Cox, J.; Majeed, A.; Quint, J.K.; Oliver, N.; Robinson, S. Cardiovascular disease in patients with primary and secondary adrenal insufficiency and the role of comorbidities. J. Clin. Endocrinol. Metab. 2021, 106, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Quinton, R. The metabolic syndrome in central hypogonadotrophic hypogonadism. Front. Horm. Res. 2018, 49, 156–169. [Google Scholar]
- Grzeskowiak, L.E.; Wlodek, M.E.; Geddes, D.T. What evidence do we have for pharmaceutical galactagogues in the treatment of lactation insufficiency?—A narrative review. Nutrients 2019, 11, 974. [Google Scholar] [CrossRef]
- Oseko, F.; Nakano, A.; Morikawa, K.; Endo, J.; Taniguchi, A.; Usui, T. Effects of chronic bromocriptine-induced hypoprolactinemia on plasma testosterone responses to human chorionic gonadotropin stimulation in normal men. Fertil. Steril. 1991, 55, 355–357. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Velasquez, G.; Garcia-Hjarles, M. Hypoprolactinemia as related to seminal quality and serum testosterone. Arch. Androl. 1989, 23, 259–365. [Google Scholar] [CrossRef] [PubMed]
- Gorvin, C.M. The prolactin receptor: Diverse and emerging roles in pathophysiology. J. Clin. Transl. Endocrinol. 2015, 2, 85–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, R.B.; Gupta, S.; Dherange, P.; De Meester, F.; Wilczynska, A.; Alam, S.E.; Pella, D.; Wilson, D.W. Metabolic syndrome: A brain disease. Can. J. Physiol. Pharmacol. 2012, 90, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Streiner, D.L. Regression toward the mean: Its etiology, diagnosis, and treatment. Can. J. Psychiatry 2001, 46, 72–76. [Google Scholar] [CrossRef]
| Variable | Group 1 | Group 2 | Group 3 | p-Value | ||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||
| Number of patients | 15 | 19 | 30 | - | - | - |
| Age (years) | 46 ± 13 | 47 ± 12 | 48 ± 14 | 0.8176 | 0.6462 | 0.7983 |
| Smokers (n (%)) Number of cigarettes a day (n) Duration of smoking (years) | 6 (40) 11 ± 7 24 ± 10 | 7 (37) 12 ± 8 26 ± 11 | 12 (40) 10 ± 9 26 ± 12 | 0.8495 0.8156 0.7398 | 1.0000 0.8154 0.7295 | 0.8248 0.6445 1.0000 |
| Physical activity: total/once a week/several times a week/once a month (%) | 94/27/40/27 | 89/26/42/21 | 94/27/47/20 | 0.8769 | 0.7885 | 0.8038 |
| Primary or vocational/secondary/university education (%) | 20/40/40 | 21/37/42 | 7/40/43 | 0.8674 | 0.6534 | 0.6781 |
| Occupational activity/blue-collar/white-collar/pink-collar workers (%) | 94/47/47/0 | 100/42/53/5 | 97/43/47/7 | 0.8224 | 0.8628 | 0.8815 |
| Stress exposure (n (%)) | 11 (73) | 15 (79) | 23 (76) | 0.7012 | 0.8062 | 0.8521 |
| Concomitant disorders (n (%)) | 3 (20) | 4 (21) | 7 (23) | 0.9325 | 0.7988 | 0.8516 |
| Comedications (n (%)) | 2 (13) | 3 (16) | 5 (17) | 0.8415 | 0.7706 | 0.9383 |
| Variable | Group 1 (n = 15) | Group 2 (n = 19) | p-Value (1 vs. 2) |
|---|---|---|---|
| Cabergoline dose before this study (mg weekly) | 1.28 ± 0.51 | 1.09 ± 0.40 | 0.2318 |
| Cumulative cabergoline dose before this study (mg) | 49.8 ± 11.3 | 43.4 ± 9.5 | 0.0822 |
| Duration of cabergoline treatment before this study (months) | 10 ± 2 | 9 ± 2 | 0.1818 |
| Duration of hypoprolactinemia/normoprolactinemia before this study (months) | 7 ± 2 | 6 ± 2 | 0.1785 |
| Cabergoline dose during this study (mg weekly) | 0.92 ± 0.32 | 1.09 ± 0.40 | 0.1060 |
| Cumulative cabergoline dose at the end of this study (mg) | 73.6 ± 16.4 | 71.6 ± 14.8 | 0.7115 |
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| BMI (kg/m2) | |||
| At baseline | 27.0 ± 4.8 a | 23.8 ± 4.2 | 23.4 ± 4.4 |
| After 6 months | 25.3 ± 4.0 | 24.2 ± 4.1 | 23.5 ± 3.8 |
| Fat-free mass index (kg/m2) | |||
| At baseline | 19.5 ± 3.4 | 19.0 ± 3.0 | 18.9 ± 2.8 |
| After 6 months | 19.4± 2.9 | 19.1 ± 3.2 | 18.7 ± 3.8 |
| Fat content (%) | |||
| At baseline | 25.1 ± 6.0 a | 20.3 ± 5.5 | 19.8 ± 4.9 |
| After 6 months | 23.8 ± 5.8 | 21.2 ± 5.0 | 20.8 ± 5.3 |
| Waist circumference (cm) | |||
| At baseline | 101 ± 12 a | 92 ± 10 | 91 ± 15 |
| After 6 months | 97 ± 15 | 93 ± 13 | 92 ± 16 |
| Systolic blood pressure (mmHg) | |||
| At baseline | 134 ± 18 a | 123 ± 12 | 118 ± 14 |
| After 6 months | 123 ± 16 b | 119 ± 20 | 117 ± 15 |
| Diastolic blood pressure (mmHg) | |||
| At baseline | 82 ± 10 | 80 ± 6 | 79 ± 6 |
| After 6 months | 80 ± 9 | 81 ± 7 | 79 ± 8 |
| Carotid intima–media thickness (mm) | |||
| At baseline | 0.81 ± 0.10 a | 0.71 ± 0.07 | 0.70 ± 0.06 |
| After 6 months | 0.75 ± 0.09 | 0.72 ± 0.07 | 0.71 ± 0.06 |
| QRISK3 score (%) | |||
| At baseline | 5.6 ± 1.9 a | 3.7 ± 1.3 | 3.4 ± 1.2 |
| After 6 months | 3.7 ± 1.2 b | 3.5 ± 1.4 | 3.4 ± 1.1 |
| Variable | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Prolactin (ng/mL) | |||
| At baseline | 1.8 ± 0.8 a | 12.5 ± 4.6 | 12.9 ± 4.0 |
| After 6 months | 12.4 ± 4.1 b | 12.8 ± 4.6 | 13.1 ± 4.2 |
| Fasting glucose (mg/dL) | |||
| At baseline | 97 ± 12 a | 87 ± 9 | 85 ± 10 |
| After 6 months | 88 ± 10 b | 86 ± 8 | 84 ± 7 |
| 2 h post-load glucose (mg/dL) | |||
| At baseline | 138 ± 18 a | 120 ± 15 | 116 ± 12 |
| After 6 months | 123 ± 20 b | 118 ± 17 | 118 ± 18 |
| HbA1C (%) | |||
| At baseline | 5.8 ± 0.5 a | 5.2 ± 0.4 | 5.4 ± 0.4 |
| After 6 months | 5.4 ± 0.5 b | 5.3 ± 0.5 | 5.3 ± 0.5 |
| HOMA1-IR | |||
| At baseline | 2.2 ± 0.8 a | 1.5 ± 0.4 | 1.4 ± 0.5 |
| After 6 months | 1.6 ± 0.6 b | 1.5 ± 0.6 | 1.5 ± 0.4 |
| Total cholesterol (mg/dL) | |||
| At baseline | 193 ± 37 | 190 ± 31 | 182 ± 28 |
| After 6 months | 192 ± 40 | 188 ± 29 | 185 ± 29 |
| HDL cholesterol (mg/dL) | |||
| At baseline | 39 ± 10 a | 52 ± 8 | 55 ± 9 |
| After 6 months | 51 ± 12 b | 55 ± 11 | 56 ± 10 |
| LDL cholesterol (mg/dL) | |||
| At baseline | 118 ± 32 | 108 ± 22 | 98 ± 18 |
| After 6 months | 110 ± 29 | 104 ± 19 | 100 ± 16 |
| Triglycerides (mg/dL) | |||
| At baseline | 168 ± 70 | 142 ± 63 | 132 ± 40 |
| After 6 months | 144 ± 58 | 130 ± 50 | 135 ± 48 |
| Uric acid (mg/dL) | |||
| At baseline | 5.5 ± 1.6 a | 4.0 ± 1.4 | 4.2 ± 1.2 |
| After 6 months | 4.1 ± 1.8 b | 4.3 ± 1.6 | 3.9 ± 1.4 |
| hsCRP (mg/L) | |||
| At baseline | 3.1 ± 1.2 a | 2.0 ± 0.8 | 1.9 ± 0.7 |
| After 6 months | 2.0 ± 0.9 b | 1.8 ± 0.7 | 1.7 ± 0.8 |
| Fibrinogen (mg/dL) | |||
| At baseline | 408 ± 120 a | 305 ± 80 | 324 ± 69 |
| After 6 months | 320 ± 93 b | 295 ± 81 | 310 ± 76 |
| Homocysteine (μmol/L) | |||
| At baseline | 34 ± 15 a | 17 ± 10 | 14 ± 8 |
| After 6 months | 20 ± 12 b | 18 ± 12 | 16 ± 9 |
| UACR (mg/g) | |||
| At baseline | 32.8 ± 14.6 a | 16.3 ± 12.6 | 12.6 ± 10.5 |
| After 6 months | 19.5 ± 12.5 b | 15.1 ± 12.3 | 14.9 ± 11.3 |
| Testosterone (ng/mL) | |||
| At baseline | 4.8 ± 1.3 a | 6.9 ± 2.5 | 7.1 ± 3.0 |
| After 6 months | 6.9 ± 2.1 b | 6.7 ± 2.8 | 7.3 ± 2.6 |
| Correlated Variables | Correlations at Baseline | Correlations Between Changes in Response to Cabergoline Dose Reduction | |||
|---|---|---|---|---|---|
| r Value | p-Value | r Value | p-Value | ||
| Prolactin | BMI | −0.328 | 0.0400 | 0.234 | 0.0688 |
| Prolactin | Fat content | −0.411 | 0.0026 | 0.242 | 0.0556 |
| Prolactin | Waist circumference | −0.352 | 0.0294 | 0.246 | 0.0510 |
| Prolactin | Systolic blood pressure | −0.428 | 0.0019 | 0.435 | 0.0010 |
| Prolactin | Fasting glucose | −0.302 | 0.0481 | 0.351 | 0.0254 |
| Prolactin | 2 h post-load glucose | −0.406 | 0.0016 | 0.395 | 0.0023 |
| Prolactin | HOMA1-IR | −0.423 | 0.0012 | 0.406 | 0.0018 |
| Prolactin | HbA1C | −0.345 | 0.0298 | 0.321 | 0.0418 |
| Prolactin | HDL cholesterol | −0.362 | 0.0208 | 0.310 | 0.0480 |
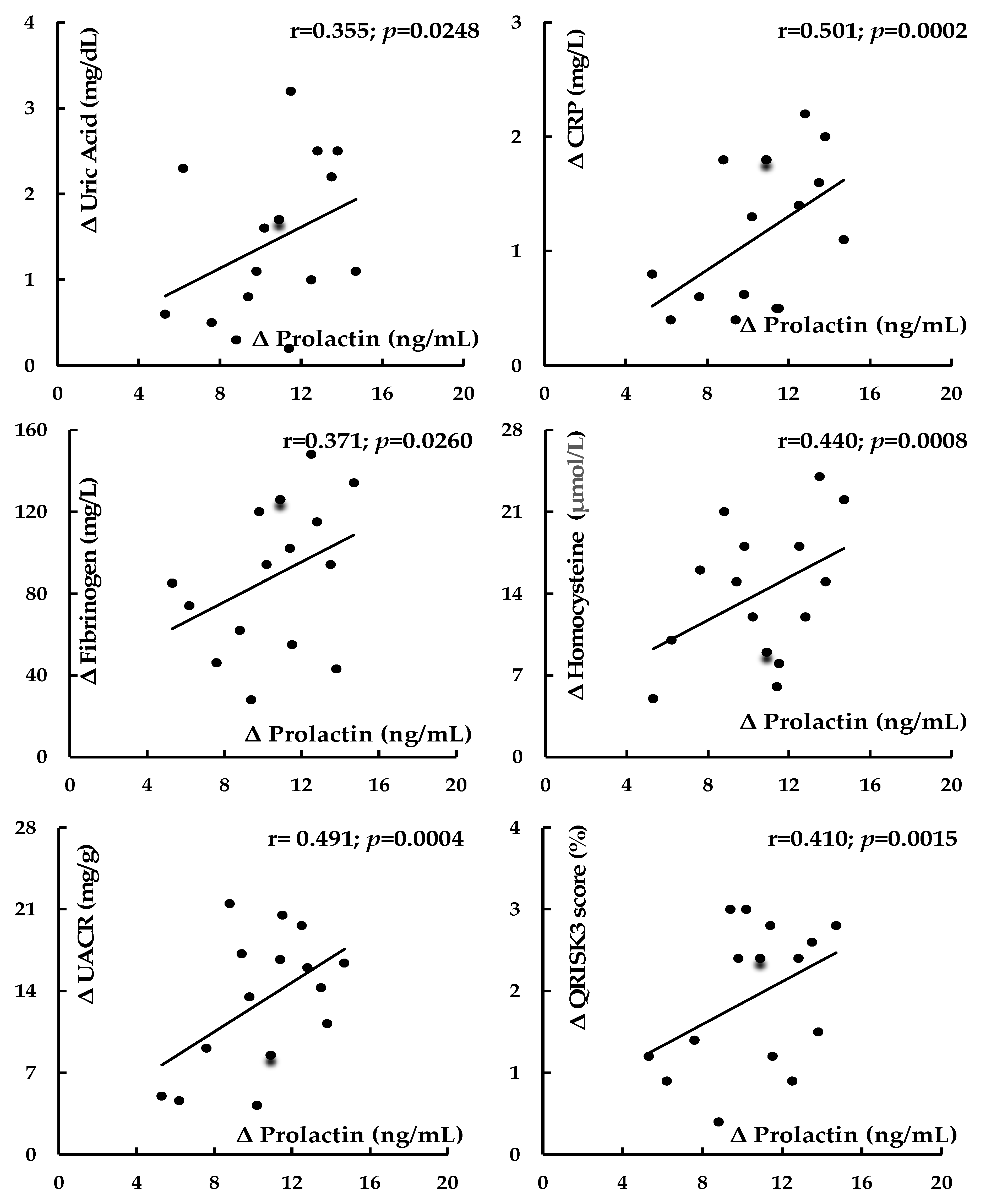
| Prolactin | Uric acid | −0.386 | 0.0065 | 0.355 | 0.0248 |
| Prolactin | hsCRP | −0.468 | 0.0008 | 0.501 | 0.0002 |
| Prolactin | Fibrinogen | −0.326 | 0.0406 | 0.371 | 0.0260 |
| Prolactin | Homocysteine | −0.419 | 0.0014 | 0.440 | 0.0008 |
| Prolactin | UACR | −0.456 | 0.0010 | 0.491 | 0.0004 |
| Prolactin | Testosterone | 0.482 | 0.0004 | 0.465 | 0.0004 |
| Prolactin | Intima–media thickness | −0.388 | 0.0062 | 0.460 | 0.0005 |
| Prolactin | QRISK3 score | −0.402 | 0.0018 | 0.410 | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Increased Cardiometabolic Risk in Men with Hypoprolactinemia: A Pilot Study. Biomolecules 2024, 14, 1335. https://doi.org/10.3390/biom14101335
Krysiak R, Kowalcze K, Szkróbka W, Okopień B. Increased Cardiometabolic Risk in Men with Hypoprolactinemia: A Pilot Study. Biomolecules. 2024; 14(10):1335. https://doi.org/10.3390/biom14101335
Chicago/Turabian StyleKrysiak, Robert, Karolina Kowalcze, Witold Szkróbka, and Bogusław Okopień. 2024. "Increased Cardiometabolic Risk in Men with Hypoprolactinemia: A Pilot Study" Biomolecules 14, no. 10: 1335. https://doi.org/10.3390/biom14101335
APA StyleKrysiak, R., Kowalcze, K., Szkróbka, W., & Okopień, B. (2024). Increased Cardiometabolic Risk in Men with Hypoprolactinemia: A Pilot Study. Biomolecules, 14(10), 1335. https://doi.org/10.3390/biom14101335






