Fasting as an Adjuvant Therapy for Cancer: Mechanism of Action and Clinical Practice
Abstract
:1. Introduction
2. Types of Fasting
2.1. Calorie Restriction
2.2. Indirect Fasting
2.3. Time-Restricted Feeding
2.4. FMD
3. Mechanisms of Fasting in Cancer
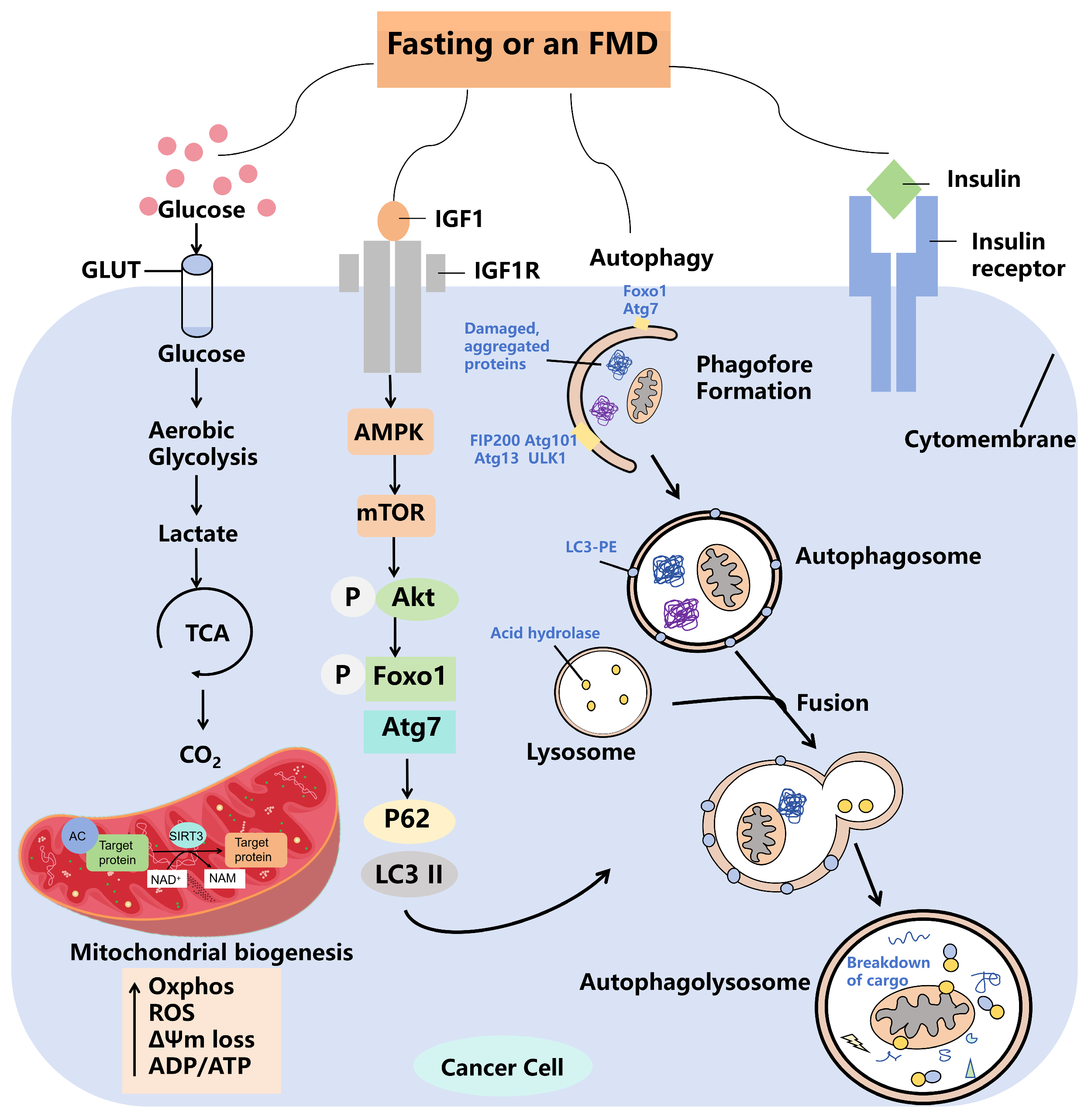
3.1. Fasting Suppresses Tumor Progression by Affecting the Body’s Metabolism
3.2. Fasting Promotes Cellular Autophagy, Thereby Inhibiting Tumor Progression
3.3. Mutations in the Genes of Cancer Cells Result in a Decreased Resistance to Stress Under Fasting
3.4. Fasting Suppresses the Tumor Process by Affecting Both the Composition and Quantity of Immune Cells Present in the Body
4. Role of Fasting in Cancer Clinical Therapy
4.1. Combined Radiotherapy and Chemotherapy with Fasting
4.2. Combined Treatment with Anticancer Drugs and Fasting
4.3. Considerations for the Clinical Use of Fasting
| Author | Cancer Model | Sample Feature | Dietary Regimen | Main Findings |
|---|---|---|---|---|
| Irene Caffa et al., 2020 [116] | Breast Cancer | The combination of periodic FMD and ET in 36 patients and MCF7 xenograft mice with HR BC | In the NCT03595540 trial, patients received a five-day FMD every four weeks | When fulvestrant is combined with palbociclib, adding periodic cycles of a fasting-mimicking diet promotes long-lasting tumor regression and reverts acquired resistance to drug treatment. Moreover, both fasting and a fasting-mimicking diet prevent tamoxifen-induced endometrial hyperplasia |
| Giulia Salvadori et al., 2022 [52] | Trple-Negative Breast Cancer (TNBC) | In vitro human TNBC SUM159 model | A fasting/FMD cycle based on strict calorie restriction of 50% or more, low levels of protein and sugar, and relatively high fat content | FMD activates the starvation escape pathway in TNBC cells, which can be recognized and targeted by drugs. In CSC, FMD reduces glucose-dependent protein kinase A signaling and stemness markers, which reduces cell numbers and improves mouse survival |
| Mei-lin Weng et al., 2020 [129] | Colon Rectal Cancer (CRC) | CRC and paired non-cancerous tissues were obtained from 81 patients who underwent surgical resection at FUSCC without preoperative chemotherapy or radiotherapy | An FMD consists of three components, designated as a day 1 diet, a day 2–3 diet, and a day 4–7 diet, fed in this order | Therapeutic implications in CRC and potential crosstalk between a cholesterogenic gene and glycolysis |
| Stephan P. Bauersfeld et al., 2018 [45] | Breast or ovarian cancer patients | Patients started fasting in the first half of the chemotherapy cycle (group A), while the other patients (group B) started with a normal diet | Thirty-four patients were randomized to STF in the first half of chemotherapy followed by a normocaloric diet (group A; n = 18) or vice versa (group B; n = 16). Fasting started 36 h before and ended 24 h after chemotherapy (60 h fasting period) | STF during chemotherapy is well-tolerated and appears to improve QOL and fatigue during chemotherapy. Larger studies should prove the effect of STF as an adjunct to chemotherapy |
| Rieneke T. Lugtenberg et al., 2021 [130] | HER2-negative stage II/III breast cancer patients | 131 patients with HER2-negative stage II/III breast cancer were recruited | 129 were randomly assigned (1:1) to receive an FMD or a regular diet for the first 3 days of neoadjuvant chemotherapy | An FMD as an adjunct to neoadjuvant chemotherapy appears to improve certain QOL and illness perception domains in patients with HER2-negative breast cancer |
| Young Jin Kim et al., 2022 [131] | Pancreatic Cancer | 19,050 Participants without pancreatic cancer | Multifactorial Cox proportional risk models were used to calculate risk ratios and 95% confidence intervals for pancreatic cancer development | Fasting blood glucose, even within pre-diabetic ranges, was significantly associated with the incidence of pancreatic cancer in Korea |
| Priya Rangan et al., 2022 [109] | TNBC | 4T1 or TS/A cells were injected orthotopically into the mammary fat pad of 6–8-week-old BALB/c or NSG mice | Mice bearing 4T1 breast cancer cells were subjected to two cycles of a 4-day FMD and treated with three doses of anti-OX40 and anti-PD-L1, alone or in combination | Regular fasting-mimicking diets can act on the tumor microenvironment and increase the efficacy of immunotherapies (anti-PD-L1 and anti-OX40) in poorly immunogenic TNBCs by expanding the early depletion of effector T-cells, switching cancer metabolism from glycolysis to the respiratory system, and reducing collagen deposition |
| Ziwen Zhong et al., 2023 [132] | CRC | IgA-deficient (Iga−/−) mice were constructed by deleting the Igha gene; Azoxymethane plus dextran sodium sulfate mouse colorectal cancer; Colorectal cancer in situ in mice | A 50% CR diet was fed on day 1. A 10% CR diet was fed on days 2–3. AIN-93G was administered on days 4–7 | Both MC38-induced orthotopic and AOM plus DSS mouse colorectal cancer models were constructed, followed by regular or FMD treatment |
| S. Cortellino et al., 2023 [113] | Melanoma (type of skin cancer) | Tumors were implanted into C57BL/6J mice by subcutaneous injection. LLC1 cells per mouse were inserted into the right side | One FMD cycle consists of alternating four consecutive days of a fasting-mimicking diet and three days of refeeding with a standard diet. FMD components are described in Brandhorst et al. and Di Biase et al. | FMD cycles in combination with immunotherapy can delay cancer growth while reducing side effects including cardiotoxicity |
| Stefano Di Biase et al., 2017 [133] | Breast Cancer and Melanoma | BALB/c mice or C57BL/6 mice were injected with 4T1 mammary adenocarcinoma cells or B16 melanoma cells, respectively | Animals underwent complete food deprivation with free access to water for a total of 48 to 60 h to allow a 20% bodyweight loss | FMD cycles combined with chemotherapy can enhance T-cell-dependent targeted killing of cancer cells both by stimulating the hematopoietic system and by enhancing CD8-dependent tumor-cytotoxicity |
| Catherine R. Marinac et al., 2017 [134] | Breast Cancer | 3088 patients with recent early invasive breast cancer | Mean (SD) duration of fasting 12.5 h per night | Prolonging the length of the nightly fasting interval may be a simple, nonpharmacologic strategy for reducing the risk of breast cancer recurrence. Improvements in glucoregulation and sleep may be mechanisms linking nightly fasting with breast cancer prognosis |
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Weng, M.L.; Chen, W.K.; Chen, X.Y.; Lu, H.; Sun, Z.R.; Yu, Q.; Sun, P.F.; Xu, Y.J.; Zhu, M.M.; Jiang, N.; et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1alpha pathway suppression. Nat. Commun. 2020, 11, 1869. [Google Scholar] [CrossRef] [PubMed]
- Lessan, N.; Ali, T. Energy Metabolism and Intermittent Fasting: The Ramadan Perspective. Nutrients 2019, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Morales-Suarez-Varela, M.; Collado Sanchez, E.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef]
- Ruddick-Collins, L.C.; Morgan, P.J.; Fyfe, C.L.; Filipe, J.A.N.; Horgan, G.W.; Westerterp, K.R.; Johnston, J.D.; Johnstone, A.M. Timing of daily calorie loading affects appetite and hunger responses without changes in energy metabolism in healthy subjects with obesity. Cell Metab. 2022, 34, 1472–1485.e6. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Tang, D.; Tang, Q.; Huang, W.; Zhang, Y.; Tian, Y.; Fu, X. Fasting: From Physiology to Pathology. Adv. Sci. 2023, 10, e2204487. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Kading, J.; Finck, B.N.; DeBosch, B.J. Targeting hepatocyte carbohydrate transport to mimic fasting and calorie restriction. FEBS J. 2020, 288, 3784–3798. [Google Scholar] [CrossRef] [PubMed]
- Attinà, A.; Leggeri, C.; Paroni, R.; Pivari, F.; Dei Cas, M.; Mingione, A.; Dri, M.; Marchetti, M.; Di Renzo, L. Fasting: How to Guide. Nutrients 2021, 13, 1570. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Rohling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- Duregon, E.; Pomatto-Watson, L.; Bernier, M.; Price, N.L.; de Cabo, R. Intermittent fasting: From calories to time restriction. Geroscience 2021, 43, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. A High Protein Calorie Restriction Diet Alters the Gut Microbiome in Obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Fuca, G.; Ligorio, F.; Huber, V.; Vingiani, A.; Iannelli, F.; Raimondi, A.; Rinchai, D.; Frige, G.; Belfiore, A.; et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022, 12, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Ferraresi, A.; Esposito, A.; Maheshwari, C.; Dhanasekaran, D.N.; Mollace, V.; Isidoro, C. Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy. J. Cancer Prev. 2021, 26, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Ratiner, K.; Shapiro, H.; Goldenberg, K.; Elinav, E. Time-limited diets and the gut microbiota in cardiometabolic disease. J. Diabetes 2022, 14, 377–393. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Protein Quantity and Source, Fasting-Mimicking Diets, and Longevity. Adv. Nutr. 2019, 10, S340–S350. [Google Scholar] [CrossRef]
- Tacad, D.K.M.; Tovar, A.P.; Richardson, C.E.; Horn, W.F.; Krishnan, G.P.; Keim, N.L.; Krishnan, S. Satiety Associated with Calorie Restriction and Time-Restricted Feeding: Peripheral Hormones. Adv. Nutr. 2022, 13, 792–820. [Google Scholar] [CrossRef]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; de Jonge, L.; Frisard, M.I.; DeLany, J.P.; Larson-Meyer, D.E.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; Most, M.M.; et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef]
- Camp, K.K.; Coleman, M.F.; McFarlane, T.L.; Doerstling, S.S.; Khatib, S.A.; Rezeli, E.T.; Lewis, A.G.; Pfeil, A.J.; Smith, L.A.; Bowers, L.W.; et al. Calorie restriction outperforms bariatric surgery in a murine model of obesity and triple-negative breast cancer. JCI Insight 2023, 8, e172868. [Google Scholar] [CrossRef] [PubMed]
- Clifton, K.K.; Ma, C.X.; Fontana, L.; Peterson, L.L. Intermittent fasting in the prevention and treatment of cancer. CA A Cancer J. Clin. 2021, 71, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S. Fasting and fasting-mimicking diets for chemotherapy augmentation. GeroScience 2021, 43, 1201–1216. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Chow, L.S.; Taub, P.R.; Laferrere, B.; Panda, S. Time-restricted Eating for the Prevention and Management of Metabolic Diseases. Endocr. Rev. 2022, 43, 405–436. [Google Scholar] [CrossRef]
- Hofer, S.J.; Carmona-Gutierrez, D.; Mueller, M.I.; Madeo, F. The ups and downs of caloric restriction and fasting: From molecular effects to clinical application. EMBO Mol. Med. 2022, 14, e14418. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Cienfuegos, S.; Lin, S.; Pavlou, V.; Gabel, K.; Tussing-Humphreys, L.; Varady, K.A. Time-restricted eating: Watching the clock to treat obesity. Cell Metab. 2024, 36, 301–314. [Google Scholar] [CrossRef]
- Tiwari, S.; Sapkota, N.; Han, Z. Effect of fasting on cancer: A narrative review of scientific evidence. Cancer Sci. 2022, 113, 3291–3302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Foheidi, M.H.; Al-Mansour, M.M. Energy and caloric restriction, and fasting and cancer: A narrative review. Support. Care Cancer 2021, 29, 2299–2304. [Google Scholar] [CrossRef]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, D.H.; Lee, S.H.; Kwak, H.B.; Kang, J.H. Contribution of High-Intensity Interval Exercise in the Fasted State to Fat Browning: Potential Roles of Lactate and β-Hydroxybutyrate. Med. Sci. Sports Exerc. 2023, 55, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Gudden, J.; Arias Vasquez, A.; Bloemendaal, M. The Effects of Intermittent Fasting on Brain and Cognitive Function. Nutrients 2021, 13, 3166. [Google Scholar] [CrossRef]
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw. Open 2021, 4, e2139558. [Google Scholar] [CrossRef]
- Bauersfeld, S.P.; Kessler, C.S.; Wischnewsky, M.; Jaensch, A.; Steckhan, N.; Stange, R.; Kunz, B.; Brückner, B.; Sehouli, J.; Michalsen, A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer 2018, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xie, J.; Wu, G.; Shen, J.; Collins, R.; Chen, W.; Kang, X.; Luo, M.; Zou, Y.; Huang, L.J.; et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat. Med. 2017, 23, 79–90. [Google Scholar] [CrossRef]
- Petersen, M.C.; Gallop, M.R.; Flores Ramos, S.; Zarrinpar, A.; Broussard, J.L.; Chondronikola, M.; Chaix, A.; Klein, S. Complex physiology and clinical implications of time-restricted eating. Physiol. Rev. 2022, 102, 1991–2034. [Google Scholar] [CrossRef]
- Kopeina, G.S.; Senichkin, V.V.; Zhivotovsky, B. Caloric restriction—A promising anti-cancer approach: From molecular mechanisms to clinical trials. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 29–41. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Huang, R.; Guo, M.; Zhou, Y.; Xu, L. The role and its mechanism of intermittent fasting in tumors: Friend or foe? Cancer Biol. Med. 2021, 18, 63–73. [Google Scholar] [CrossRef]
- Schreck, K.C.; Hsu, F.C.; Berrington, A.; Henry-Barron, B.; Vizthum, D.; Blair, L.; Kossoff, E.H.; Easter, L.; Whitlow, C.T.; Barker, P.B.; et al. Feasibility and Biological Activity of a Ketogenic/Intermittent-Fasting Diet in Patients With Glioma. Neurology 2021, 97, e953–e963. [Google Scholar] [CrossRef]
- Brandhorst, S.; Harputlugil, E.; Mitchell, J.R.; Longo, V.D. Protective effects of short-term dietary restriction in surgical stress and chemotherapy. Ageing Res. Rev. 2017, 39, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, G.; Zanardi, F.; Iannelli, F.; Lobefaro, R.; Vernieri, C.; Longo, V.D. Fasting-mimicking diet blocks triple-negative breast cancer and cancer stem cell escape. Cell Metab. 2021, 33, 2247–2259.e6. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Ochiya, T. Possible connection between diet and microRNA in cancer scenario. Semin. Cancer Biol. 2021, 73, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le, Z.; Yanxiang Guo, J.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Q.; Wan, H.; Hu, Y.; Xing, S.; Yang, H.; Gao, Y.; He, Z. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab. Investig. 2020, 100, 801–811. [Google Scholar] [CrossRef]
- Xu, K.; Yin, N.; Peng, M.; Stamatiades, E.G.; Shyu, A.; Li, P.; Zhang, X.; Do, M.H.; Wang, Z.; Capistrano, K.J.; et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 2021, 371, 405–410. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Liu, L.; Yang, H.; Zhou, P.; Song, F.; Dong, G.; Chen, J.; Wang, G.; Dong, X. Effect of Heat Stress on Bovine Mammary Cellular Metabolites and Gene Transcription Related to Amino Acid Metabolism, Amino Acid Transportation and Mammalian Target of Rapamycin (mTOR) Signaling. Animals 2021, 11, 3153. [Google Scholar] [CrossRef] [PubMed]
- Loftus, R.M.; Assmann, N.; Kedia-Mehta, N.; O’Brien, K.L.; Garcia, A.; Gillespie, C.; Hukelmann, J.L.; Oefner, P.J.; Lamond, A.I.; Gardiner, C.M.; et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 2018, 9, 2341. [Google Scholar] [CrossRef]
- Sivanand, S.; Vander Heiden, M.G. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: Essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef]
- Villa, E.; Sahu, U.; O’Hara, B.P.; Ali, E.S.; Helmin, K.A.; Asara, J.M.; Gao, P.; Singer, B.D.; Ben-Sahra, I. mTORC1 stimulates cell growth through SAM synthesis and m(6)A mRNA-dependent control of protein synthesis. Mol. Cell 2021, 81, 2076–2093.e9. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Ebner, M.; Puchkov, D.; López-Ortega, O.; Muthukottiappan, P.; Su, Y.; Schmied, C.; Zillmann, S.; Nikonenko, I.; Koddebusch, J.; Dornan, G.L.; et al. Nutrient-regulated control of lysosome function by signaling lipid conversion. Cell 2023, 186, 5328–5346.e26. [Google Scholar] [CrossRef]
- Newsholme, P. Cellular and metabolic mechanisms of nutrient actions in immune function. Nutr. Diabetes 2021, 11, 22. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Kojima, Y.; Sakamoto, T.; Igarashi, K.; Umetsu, K.; Ishikawa, T.; Hirayama, A.; Kajino-Sakamoto, R.; Sakamoto, N.; Yasumoto, K.I.; et al. L-2hydroxyglutaric acid rewires amino acid metabolism in colorectal cancer via the mTOR-ATF4 axis. Oncogene 2023, 42, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Ferro, F.; Servais, S.; Besson, P.; Roger, S.; Dumas, J.F.; Brisson, L. Autophagy and mitophagy in cancer metabolic remodelling. Semin. Cell Dev. Biol. 2020, 98, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.; Cirò, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3β-MCL-1 Axis. Cancer Cell 2019, 35, 798–815.e5. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE(-/-) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. An overview of the molecular mechanism of autophagy. In Autophagy in Infection and Immunity; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 335, pp. 1–32. [Google Scholar]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.; Longo, V.D. Starvation, Stress Resistance, and Cancer. Trends Endocrinol. Metab. 2018, 29, 271–280. [Google Scholar] [CrossRef]
- Antunes, F.; Erustes, A.G.; Costa, A.J.; Nascimento, A.C.; Bincoletto, C.; Ureshino, R.P.; Pereira, G.J.S.; Smaili, S.S. Autophagy and intermittent fasting: The connection for cancer therapy? Clinics 2018, 73, e814s. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.F.; Jiang, Z.R.; Huang, X.; Wang, S.S.; Li, H.B.; Peng, Y.J.; Zhang, J. [Long-term intermittent fasting induces abnormal lipid accumulation in mouse liver]. Sheng Li Xue Bao 2022, 74, 962–969. [Google Scholar]
- May, S.; Bird, T.G. How the liver keeps itself in shape. eLife 2023, 12, e85606. [Google Scholar] [CrossRef]
- Ma, Y.N.; Jiang, X.; Tang, W.; Song, P. Influence of intermittent fasting on autophagy in the liver. Biosci. Trends 2023, 17, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Seok, S.; Kim, Y.C.; Zhang, Y.; Yau, P.; Iwamori, N.; Xu, H.E.; Ma, J.; Kemper, B.; Kemper, J.K. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat. Commun. 2020, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- López-Soldado, I.; Bertini, A.; Adrover, A.; Duran, J.; Guinovart, J.J. Maintenance of liver glycogen during long-term fasting preserves energy state in mice. FEBS Lett. 2020, 594, 1698–1710. [Google Scholar] [CrossRef] [PubMed]
- Libman, I.M.; Barinas-Mitchell, E.; Bartucci, A.; Chaves-Gnecco, D.; Robertson, R.; Arslanian, S. Fasting and 2-Hour Plasma Glucose and Insulin. Diabetes Care 2010, 33, 2674–2676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Gyamfi, J.; Kim, J.; Choi, J. Cancer as a Metabolic Disorder. Int. J. Mol. Sci. 2022, 23, 1155. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—The grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef]
- Gao, F.Y.; Li, X.T.; Xu, K.; Wang, R.T.; Guan, X.X. c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Commun. Signal. 2023, 21, 28. [Google Scholar] [CrossRef]
- Di Biase, S.; Lee, C.; Brandhorst, S.; Manes, B.; Buono, R.; Cheng, C.W.; Cacciottolo, M.; Martin-Montalvo, A.; de Cabo, R.; Wei, M.; et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30, 136–146. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, S.; Xie, Z.; Bao, W.; Xu, B.; Yang, W.; Zhou, L. Epigenetic Therapeutics Targeting NRF2/KEAP1 Signaling in Cancer Oxidative Stress. Front. Pharmacol. 2022, 13, 924817. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Szypowska, A.; Regulska-Ilow, B. Significance of low-carbohydrate diets and fasting in patients with cancer. Rocz. Panstw. Zakl. Hig. 2019, 70, 325–336. [Google Scholar]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef]
- Di Francesco, A.; Deighan, A.G.; Litichevskiy, L.; Chen, Z.; Luciano, A.; Robinson, L.; Garland, G.; Donato, H.; Vincent, M.; Schott, W.; et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 2024, 634, 684–692. [Google Scholar] [CrossRef]
- Paredes, F.; Williams, H.C.; San Martin, A. Metabolic adaptation in hypoxia and cancer. Cancer Lett. 2021, 502, 133–142. [Google Scholar] [CrossRef]
- Wu, L.; Yan, J.; Bai, Y.; Chen, F.; Zou, X.; Xu, J.; Huang, A.; Hou, L.; Zhong, Y.; Jing, Z.; et al. An invasive zone in human liver cancer identified by Stereo-seq promotes hepatocyte-tumor cell crosstalk, local immunosuppression and tumor progression. Cell Res. 2023, 33, 585–603. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Selective protection of normal cells from chemotherapy, while killing drug-resistant cancer cells. Oncotarget 2023, 14, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Cortellino, S.; Raveane, A.; Chiodoni, C.; Delfanti, G.; Pisati, F.; Spagnolo, V.; Visco, E.; Fragale, G.; Ferrante, F.; Magni, S.; et al. Fasting renders immunotherapy effective against low-immunogenic breast cancer while reducing side effects. Cell Rep. 2022, 40, 111256. [Google Scholar] [CrossRef]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Troncone, G.; Malapelle, U.; Scalvenzi, M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022, 23, 6388. [Google Scholar] [CrossRef]
- Liu, X.; Peng, S.; Tang, G.; Xu, G.; Xie, Y.; Shen, D.; Zhu, M.; Huang, Y.; Wang, X.; Yu, H.; et al. Fasting-mimicking diet synergizes with ferroptosis against quiescent, chemotherapy-resistant cells. EBioMedicine 2023, 90, 104496. [Google Scholar] [CrossRef] [PubMed]
- Di Tano, M.; Raucci, F.; Vernieri, C.; Caffa, I.; Buono, R.; Fanti, M.; Brandhorst, S.; Curigliano, G.; Nencioni, A.; de Braud, F.; et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 2020, 11, 2332. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz Bonilla, M.; Stemler, K.M.; Jeter-Jones, S.; Fujimoto, T.N.; Molkentine, J.; Asencio Torres, G.M.; Zhang, X.; Broaddus, R.R.; Taniguchi, C.M.; Piwnica-Worms, H. Fasting Reduces Intestinal Radiotoxicity, Enabling Dose-Escalated Radiation Therapy for Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 537–547. [Google Scholar] [CrossRef]
- Cortellino, S.; Quagliariello, V.; Delfanti, G.; Blaževitš, O.; Chiodoni, C.; Maurea, N.; Di Mauro, A.; Tatangelo, F.; Pisati, F.; Shmahala, A.; et al. Fasting mimicking diet in mice delays cancer growth and reduces immunotherapy-associated cardiovascular and systemic side effects. Nat. Commun. 2023, 14, 5529. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef]
- Wang, J.; Su, N.; Fang, Y.; Ma, S.; Zhang, Y.; Cai, J.; Zou, Q.; Tian, X.; Xia, Y.; Liu, P.; et al. Comparison of Chemotherapy Combined With Chidamide Versus Chemotherapy in the Frontline Treatment for Peripheral T-Cell Lymphoma. Front. Immunol. 2022, 13, 835103. [Google Scholar] [CrossRef] [PubMed]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; Vernieri, C.; Di Tano, M.; Ligorio, F.; Blaževitš, O.; Lazzeri, S.; Shmahala, A.; Fragale, G.; Salvadori, G.; Varano, G.; et al. Cyclic Fasting-Mimicking Diet Plus Bortezomib and Rituximab Is an Effective Treatment for Chronic Lymphocytic Leukemia. Cancer Res. 2024, 84, 1133–1148. [Google Scholar] [CrossRef]
- Khalifa, A.; Guijarro, A.; Ravera, S.; Bertola, N.; Adorni, M.P.; Papotti, B.; Raffaghello, L.; Benelli, R.; Becherini, P.; Namatalla, A.; et al. Cyclic fasting bolsters cholesterol biosynthesis inhibitors’ anticancer activity. Nat. Commun. 2023, 14, 6951. [Google Scholar] [CrossRef]
- Bogdanos, D.P.; Gao, B.; Gershwin, M.E. Liver immunology. Compr. Physiol. 2013, 3, 567–598. [Google Scholar]
- Gallage, S.; Ali, A.; Barragan Avila, J.E.; Seymen, N.; Ramadori, P.; Joerke, V.; Zizmare, L.; Aicher, D.; Gopalsamy, I.K.; Fong, W.; et al. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARα and PCK1. Cell Metab. 2024, 36, 1371–1393.e7. [Google Scholar] [CrossRef] [PubMed]
- Loft, A.; Schmidt, S.F.; Caratti, G.; Stifel, U.; Havelund, J.; Sekar, R.; Kwon, Y.; Sulaj, A.; Chow, K.K.; Alfaro, A.J.; et al. A macrophage-hepatocyte glucocorticoid receptor axis coordinates fasting ketogenesis. Cell Metab. 2022, 34, 473–486.e9. [Google Scholar] [CrossRef] [PubMed]
- Saran, A.R.; Dave, S.; Zarrinpar, A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology 2020, 158, 1948–1966.e1. [Google Scholar] [CrossRef]
- Pope, E.D., 3rd; Kimbrough, E.O.; Vemireddy, L.P.; Surapaneni, P.K.; Copland, J.A., 3rd; Mody, K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin. Ther. Targets 2019, 23, 473–483. [Google Scholar] [CrossRef]
- Becker, S.; Momoh, J.; Biancacci, I.; Möckel, D.; Wang, Q.; May, J.N.; Su, H.; Candels, L.S.; Berres, M.L.; Kiessling, F.; et al. Intermittent Fasting Primes the Tumor Microenvironment and Improves Nanomedicine Delivery in Hepatocellular Carcinoma. Small 2023, 19, e2208042. [Google Scholar] [CrossRef]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2011, 110, 236–245. [Google Scholar] [CrossRef]
- Page, A.J. Gastrointestinal Vagal Afferents and Food Intake: Relevance of Circadian Rhythms. Nutrients 2021, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shao, N.; Qiu, H.; Zhao, J.; Chen, C.; Wan, J.; He, Z.; Zhao, X.; Xu, L. Intestinal microbiota: A bridge between intermittent fasting and tumors. Biomed. Pharmacother. 2023, 167, 115484. [Google Scholar] [CrossRef]
- Imada, S.; Khawaled, S.; Shin, H.; Meckelmann, S.W.; Whittaker, C.A.; Corrêa, R.O.; Alquati, C.; Lu, Y.; Tie, G.; Pradhan, D.; et al. Short-term post-fast refeeding enhances intestinal stemness via polyamines. Nature 2024, 633, 895–904. [Google Scholar] [CrossRef]
- Lugtenberg, R.T.; de Groot, S.; Kaptein, A.A.; Fischer, M.J.; Kranenbarg, E.M.; Carpentier, M.D.; Cohen, D.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Quality of life and illness perceptions in patients with breast cancer using a fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in the phase 2 DIRECT (BOOG 2013-14) trial. Breast Cancer Res. Treat. 2021, 185, 741–758. [Google Scholar] [CrossRef]
- Kim, Y.J.; Oh, C.M.; Park, S.K.; Jung, J.Y.; Kim, M.H.; Ha, E.; Nam, D.J.; Kim, Y.; Yang, E.H.; Lee, H.C.; et al. Fasting blood glucose and risk of incident pancreatic cancer. PLoS ONE 2022, 17, e0274195. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zhang, H.; Nan, K.; Zhong, J.; Wu, Q.; Lu, L.; Yue, Y.; Zhang, Z.; Guo, M.; Wang, Z.; et al. Fasting-Mimicking Diet Drives Antitumor Immunity against Colorectal Cancer by Reducing IgA-Producing Cells. Cancer Res. 2023, 83, 3529–3543. [Google Scholar] [CrossRef] [PubMed]
- Marinac, C.R.; Nelson, S.H.; Breen, C.I.; Hartman, S.J.; Natarajan, L.; Pierce, J.P.; Flatt, S.W.; Sears, D.D.; Patterson, R.E. Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncol. 2016, 2, 1049–1055. [Google Scholar] [CrossRef] [PubMed]


| English Name | Abbreviation | Definition Description | Reference |
|---|---|---|---|
| Calorie Restriction | CR | A reduction of 20–40% in the average daily caloric intake can be achieved without resulting in malnutrition or the deficiency of essential nutrients. | [23] |
| Indirect Fasting | IF | Method of energy deprivation during regular periods of very limited or no calorie intake, i.e., periods of voluntary fasting and fluid intake. Typically, it consists of 16 h of fasting per day, 24 h of fasting every other day, or 2 days of fasting per week on non-consecutive days. | [24] |
| Time-Restricted Feeding | TRF | A method of dietary restriction that involves confining food consumption to a 10 h window each day while maintaining a consistent total daily caloric intake. | [25] |
| Fasting-Mimicking Diet | FMD | A dietary regimen involving periodic intermittent fasting is designed to replicate the physiological alterations associated with continuous fasting. This approach effectively mimics a state of famine, thereby inducing the body to perceive itself as being in a state of starvation. | [26,27] |
| Fasting Program | Advantages | Disadvantages | Reference |
|---|---|---|---|
| CR | Long-term sustainability; Inhibiting tumor growth; Improving metabolic health. | Individualization varies widely; Slows recovery from surgery, radiation, and chemotherapy. | [48] |
| IF | Easier to adhere to than other fasting programs; Metabolic regulation; Protection of healthy cells. | Response may vary from patient to patient; Patients may consume insufficient nutrients to maintain a normal metabolism. | [49,50,51] |
| TRF | Promotes cellular repair and autophagy; Improves metabolic health; Potential anti-cancer effects; Weight loss; Improved cardiovascular health. | Prolonged fasting may stress the body, especially for those who are not used to this eating pattern. It may lead to symptoms such as dizziness, fatigue, and cognitive decline; Unsuitable for certain groups of people: pregnant women, lactating women, adolescents, the elderly, patients with chronic diseases, and other patients. | [45] |
| FMD | Promotes cellular autophagy repair and removal; Improves metabolic health; Weight loss and fat burning; Higher feasibility: FMD allows small amounts of food compared to complete fasting, making it more feasible for some people and reducing the psychological and physical stress associated with complete fasting. | Short-term discomfort; Nutritionally incomplete; Not suitable for all. | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Ye, H.; Liu, Z.; Liang, Z.; Zhu, J.; Zhang, R.; Li, Y. Fasting as an Adjuvant Therapy for Cancer: Mechanism of Action and Clinical Practice. Biomolecules 2024, 14, 1437. https://doi.org/10.3390/biom14111437
Xie Y, Ye H, Liu Z, Liang Z, Zhu J, Zhang R, Li Y. Fasting as an Adjuvant Therapy for Cancer: Mechanism of Action and Clinical Practice. Biomolecules. 2024; 14(11):1437. https://doi.org/10.3390/biom14111437
Chicago/Turabian StyleXie, Yichun, Huabin Ye, Zhongjun Liu, Zhiqing Liang, Jinrong Zhu, Rongxin Zhang, and Yan Li. 2024. "Fasting as an Adjuvant Therapy for Cancer: Mechanism of Action and Clinical Practice" Biomolecules 14, no. 11: 1437. https://doi.org/10.3390/biom14111437
APA StyleXie, Y., Ye, H., Liu, Z., Liang, Z., Zhu, J., Zhang, R., & Li, Y. (2024). Fasting as an Adjuvant Therapy for Cancer: Mechanism of Action and Clinical Practice. Biomolecules, 14(11), 1437. https://doi.org/10.3390/biom14111437






