Targeting PDGF/PDGFR Signaling Pathway by microRNA, lncRNA, and circRNA for Therapy of Vascular Diseases: A Narrow Review
Abstract
:1. Introduction
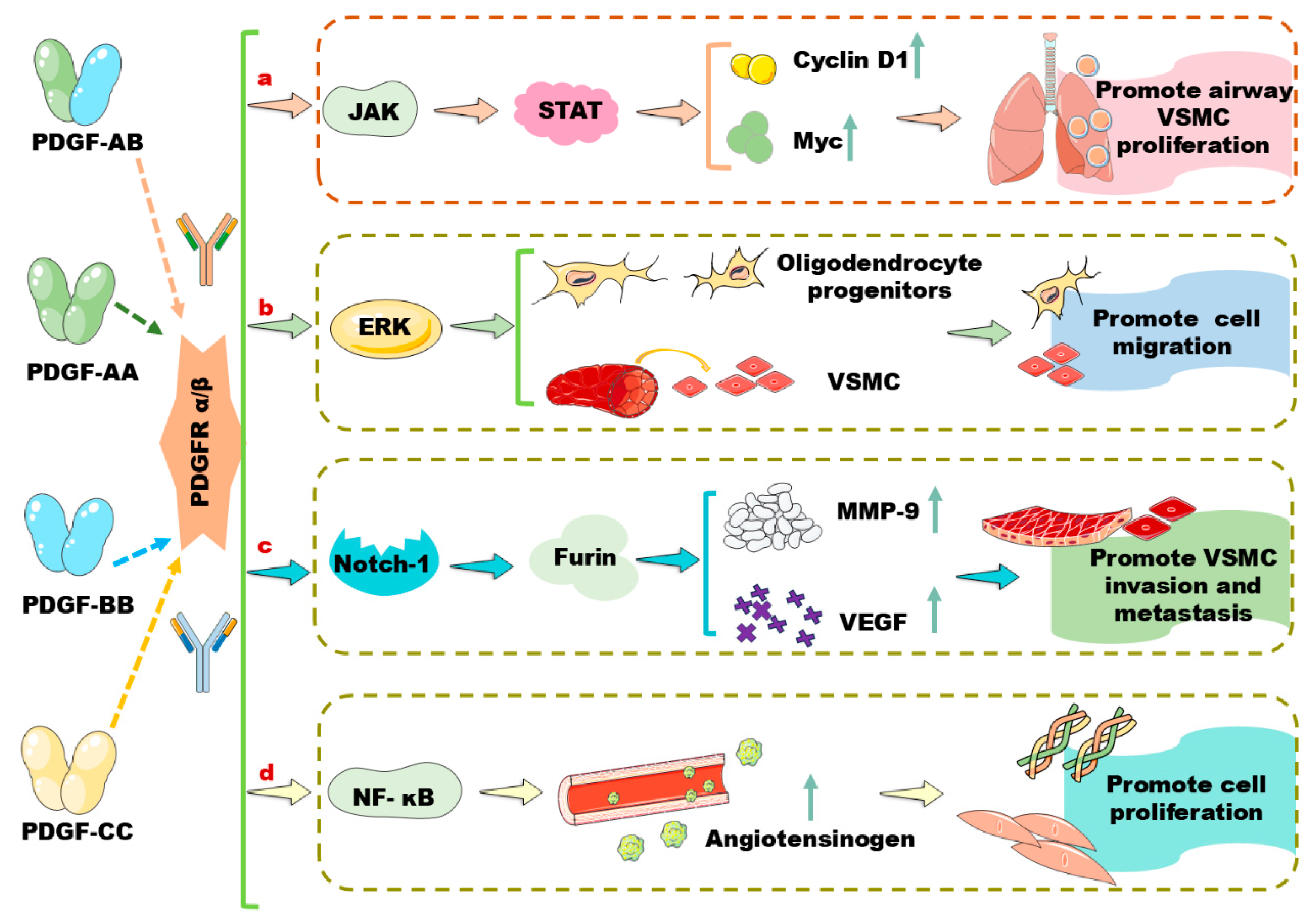
2. A Brief Review of PDGF/PDGFR Signaling Pathways in Vascular Cells
3. miRNA in Regulation of PDGF/PDGFR Signaling Pathway in Vascular Cells
3.1. Regulation of PDGF/PDGFR Signaling by miRNAs in VSMC
3.2. Regulation of PDGF/PDGFR Signaling Pathway by miRNAs in Aortic SMCs
3.3. Regulation of PDGF by miRNAs in Endothelial Cells
3.4. Regulation of PDGF/PDGFR Signaling by miRNAs in Primary PASMCs
3.5. Regulation of PDGF by miRNAs in Airway SMCs
| A | miRNA | Target | Signaling Pathway | Effects and References |
|---|---|---|---|---|
| VSMC | miR223 ↑ | PDGFRβ ↓ | miR223 ↑/PDGFRβ ↓ | Platelet-derived miR223 suppresses VSMC differentiation and restores Kawasaki-disease-induced vascular injury [29,30]. |
| miR145-5p ↑ | Smad4 ↓ | miR145-5p ↑/Smad4 ↓/PDGF ↓ | miR145-5p suppresses PDGF-induced VSMC proliferation and migration [36]. | |
| miR214 ↑ | Anti-MIG-6C ↓ | miR214 ↑/anti-MIG-6C ↓/TGF-β1 ↓ | miR214 promotes profibrotic gene expression [37]. | |
| miR147b ↑ | YY1 ↓ | miR147b ↑/YY1 ↓/PDGF-BB ↑ | Overexpression of miR147b increases the proliferative and migratory abilities of VSMCs [40]. | |
| miR663 ↑ | JunB ↓ | miR663 ↑/JunB ↓/MMPs ↓ | miR663 leads to inhibition of VSMC migration [41]. | |
| miR320 ↑ | Neuropilin 1 ↓ | miR320 ↑/Neuropilin 1 ↓/PDGF ↓ | miR320 inhibits the proliferation and migration of VSMCs in both basal and PDGF-stimulated conditions potentially [42]. | |
| miR340 ↑ | VHL ↓ | miR340 ↑/VHL ↓ | Overexpression of miR340 promotes VSMC proliferation and invasion potentially [43]. | |
| miR125a-5p ↑ | EGFR ↓ | miR125a-5p ↑/EGFR ↓/PDGF-BB ↑ | miR125a-5p suppresses the growth, migration, and invasion of VSMCs [30]. | |
| miR92 ↑ | KLF4 ↓ | miR92 ↑/KLF4 ↓ | miR92 enhances VSMC proliferation and migration [48]. | |
| miR378a-5p ↑ | CDK1 ↓ | PDGF-BB ↑/miR378a-5p ↑ /CDK1 ↓/p21 ↓ | miR378a-5p promotes VSMC proliferation [49]. | |
| miR146b-3p ↑ | PI3KCG ↓ | PDGF-BB ↓/miR146b-3p ↑/PI3KCG ↓ | miR146b-3p reverses the phenotype transition of VSMCs [50]. | |
| miR520c-3p agomiRNA ↑ | RelA ↓ | miR520c-3p ↑/RelA/p65NF-κB ↓ | miR520c-3p agomiRNA decreases atherosclerotic plaque size and collagen content [51]. | |
| Let-7 ↑ | Lin-28 HomologB ↓ | Let-7 ↑/Lin-28 Homolog B ↓ /TNF-α/IL-6/PDGFR ↑ | Let-7 suppresses vascular inflammation mediators, thereby attenuating atherosclerosis as well as diabetes [52]. | |
| Let-7g ↑ | MEKK1 ↓, PDGFB ↓ | Let-7g ↑/PDGFB ↓, Let-7g ↑/PDGF-BB/MEKK1/ERK/KLF4 ↓ | Let-7g can directly suppress PDGF-BB-activated MEKK1/ERK/KLF4 signaling pathway in VSMCs [53]. | |
| miR638 ↑ | LDA ↓ | miR638 ↑/LDA ↓ | miR638 plays a pivotal role in regulating PDGF-BB-induced proliferation and migration of human VSMCs via targeting lactate dehydrogenase A [41,56]. | |
| miR149-5p ↑ | HDAC4 ↓ | miR149-5p ↑/HDAC4/PDGF ↓ | miR149-5p suppresses VSMC proliferation, invasion, and migration [57]. | |
| miR365 ↑ | Cyclin D1 ↓ | miR365 ↑/cyclin D1 ↓ | Overexpression of miR365 suppresses VSMC proliferation [59]. | |
| miR451 ↑ | Ywhaz ↓ | miR451 ↑/Ywhaz ↓/p38/MAPK ↓ | miR451 protects VSMC injury [60]. | |
| miR212-5p ↑ | Ywhaz ↓ | DLEU2 ↓/miR212-5p ↑/Ywhaz ↓ | Overexpression of DLEU2 accelerates PDGF-BB-induced VSMC viability, migration, and invasion [61]. | |
| miR1274b ↑ | CNN1 ↓ | XBP1 ↑/miR1274B ↑/CNN1 ↓ | miR1274B activates VSMC proliferation partially [62]. | |
| miR612 ↑ | AKT2 ↓ | miR612 ↑/AKT2 ↓/PDGF-BB ↓ | miR612 inhibits PDGF-BB-induced migration and invasion of VSMCs [70]. | |
| miR17-5p ↑ | MMP-2 ↓, MMP-9 ↓ | PDGF-BB ↑/miR17-5p ↑/MMP-2/9 ↓ | miR17-5p mimics significantly inhibit the proliferation and migration of VSMCs [73]. | |
| miR30a-5p ↑ | PDGF-BB ↓ | miR30a-5p ↑/PDGF-BB ↓ | miR30a-5p inhibits VSMC proliferation in the arterial walls [75]. | |
| miR379 ↑ | IGF-1 ↓ | PDGF-BB ↑/miR379 ↑/IGF-1 ↓ | miR379 inhibits cell proliferation, invasion, and migration of VSMCs [78]. | |
| miR665 ↑ | FGF 9 ↓ | miR665 ↑/FGF 9 ↓/PDGF-BB ↑ | miR665 inhibits cell proliferation, invasion, and migration in PDGF-BB-induced VSMCs potentially [79]. | |
| miR182 ↑ | FGF 9 ↓ | miR182 ↑/FGF 9/PDGFRβ ↓ | miR182 induces the differentiation, proliferation, and migration of rat-derived VSMCs [80]. | |
| B | miRNA | Target | Signaling Pathway | Effects and References |
| Aortic SMCs | miR29a ↑ | PDGFRβ ↓ | miR29a ↑/PDGFRβ ↓ | miR29a inhibitors increase the expression of PDGFRβ in human aortic SMCs [81]. |
| miR34c ↑ | PDGFRβ ↓ | miR34c ↑/PDGFRβ ↓/SIRT1 ↓ | miR34c protects PDGF-BB-induced human aortic SMCs [85]. | |
| Let-7g ↑ | MEKK1 ↓ | Let-7g ↑/MEKK1/ERK/KLF4 ↓ /PDGFB ↓/PDGF-BB ↓ | Let-7g inhibiting MEKK1/ERK/KLF4 in human aortic SMCs [53]. | |
| miR520c-3p ↑ | RelA ↓ | miR520c-3p ↑/RelA/p65NF-κB ↓ | miR520c-3p inhibits PDGF-BB-mediated proliferation and migration of human aortic SMCs [51]. | |
| C | miRNA | Target | Signaling Pathway | Effects and References |
| Endothelial cells | miR214 ↑ | Pim-1 ↓ | miR214 ↑/PDGF/Pim-1 ↓ | Overexpression of miR214 inhibits SMC migration [102]. |
| D | miRNA | Target | Signaling Pathway | Effects and References |
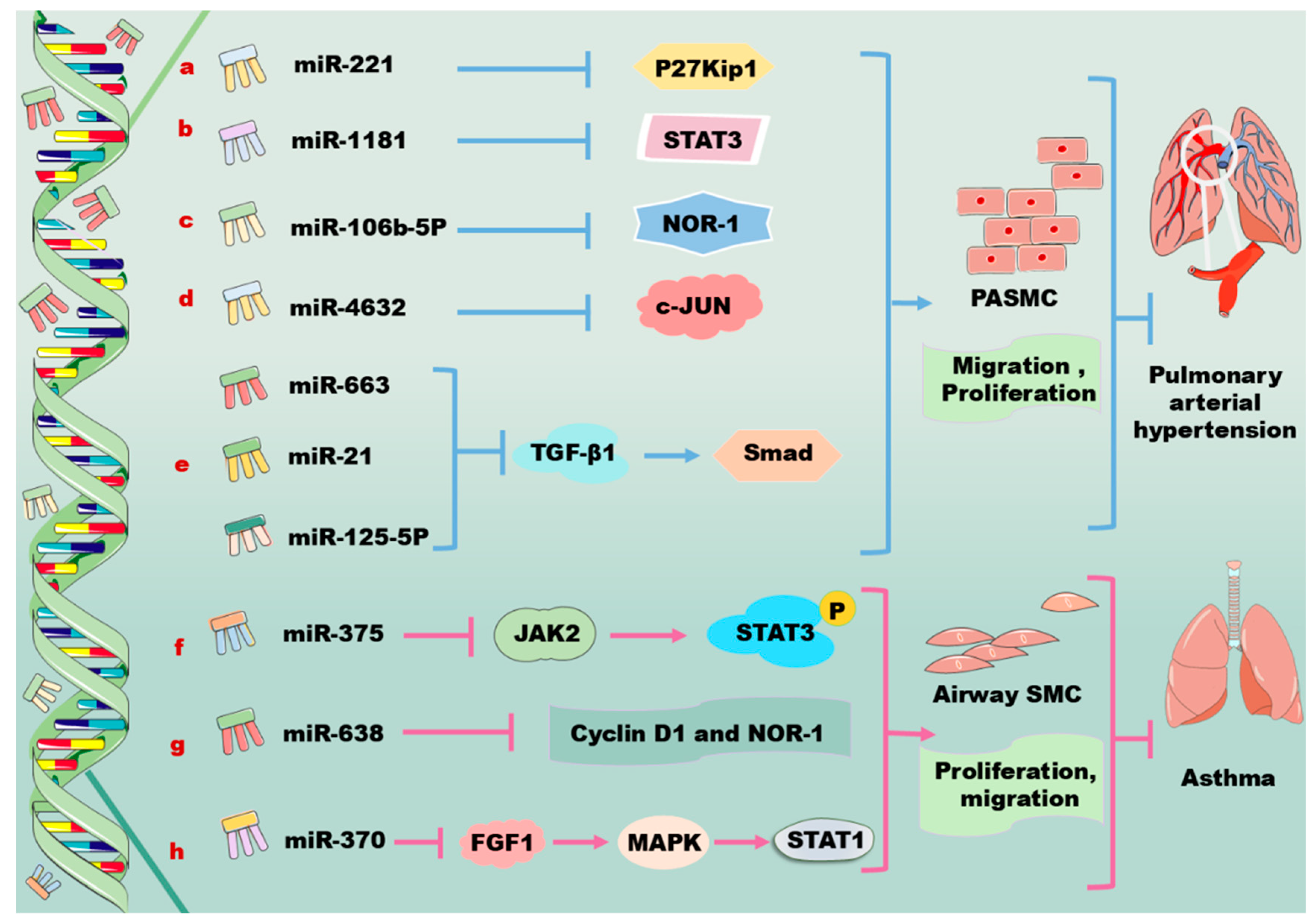
| PASMCs | miR221 ↑ | C-kit ↓, P27Kip1 ↓ | miR221 ↑/c-kit ↓, miR221 ↑/p27Kip1 ↓ | miR221 promotes the proliferation and migration PASMCs [103]. |
| miR106b-5p ↑ | NOR-1 ↓ | miR106b-5p ↑/NOR-1 ↓ | miR106b-5p decreases excessive cell proliferation and migration in PDGF-induced PASMCs [107,108,109]. | |
| miR4632 ↑ | C-JUN ↓ | miR4632 ↑/c-JUN ↓ | miR4632 promotes cell apoptosis in human PASMCs [110]. | |
| miR663 ↑ | TGF-β1 ↓ | miR663 ↑/TGF-β1/Smad 2/3 ↓ | miR663 decreases PDGF-BB-induced PASMC proliferation, migration, and collagen synthesis and prevents pulmonary vascular remodeling [111,112]. | |
| E | miRNA | Target | Signaling Pathway | Effects and References |
| Airway SMCs | miR375 ↑ | JAK2 ↓ | miR375 ↑/JAK2/STAT3 ↓ | miR375 suppresses PDGF-induced airway SMC proliferation [115,117,118]. |
| miR9-5p ↑ | SLC26A2 ↓ | miR9-5p ↑/SLC26A2 ↓/PDGF ↓ | miR9-5p inhibits the PDGF-induced proliferation and production of inflammatory factors in HASMCs [119]. | |
| miR638 ↑ | Cyclin D1 ↓, NOR-1 ↓ | miR638 ↑/cyclin D1 ↓, miR638 ↑/NOR-1 ↓ | miR638 inhibits airway SMC proliferation and migration [120]. | |
| miR30b-5p ↑ | PTEN ↓ | miR30b-5p ↑/PTEN ↓/PI3K–AKT ↑ | Overexpression of miR30b-5p upregulates PDGF-induced airway SMC dysfunction [124]. | |
| miR370 ↑ | LncRNA XIST ↓ | miR370 ↑/lncRNA XIST ↓ | miR370 reduces cell apoptosis and inflammation injury in acute pneumonia and in PDGF-BB-treated airway SMCs [127,128]. |
4. lncRNAs in Regulation of PDGF/PDGFR Signaling Pathway in Vascular Cells
| Cell Type | LncRNA | Target | Signaling Pathway | Effects and References |
|---|---|---|---|---|
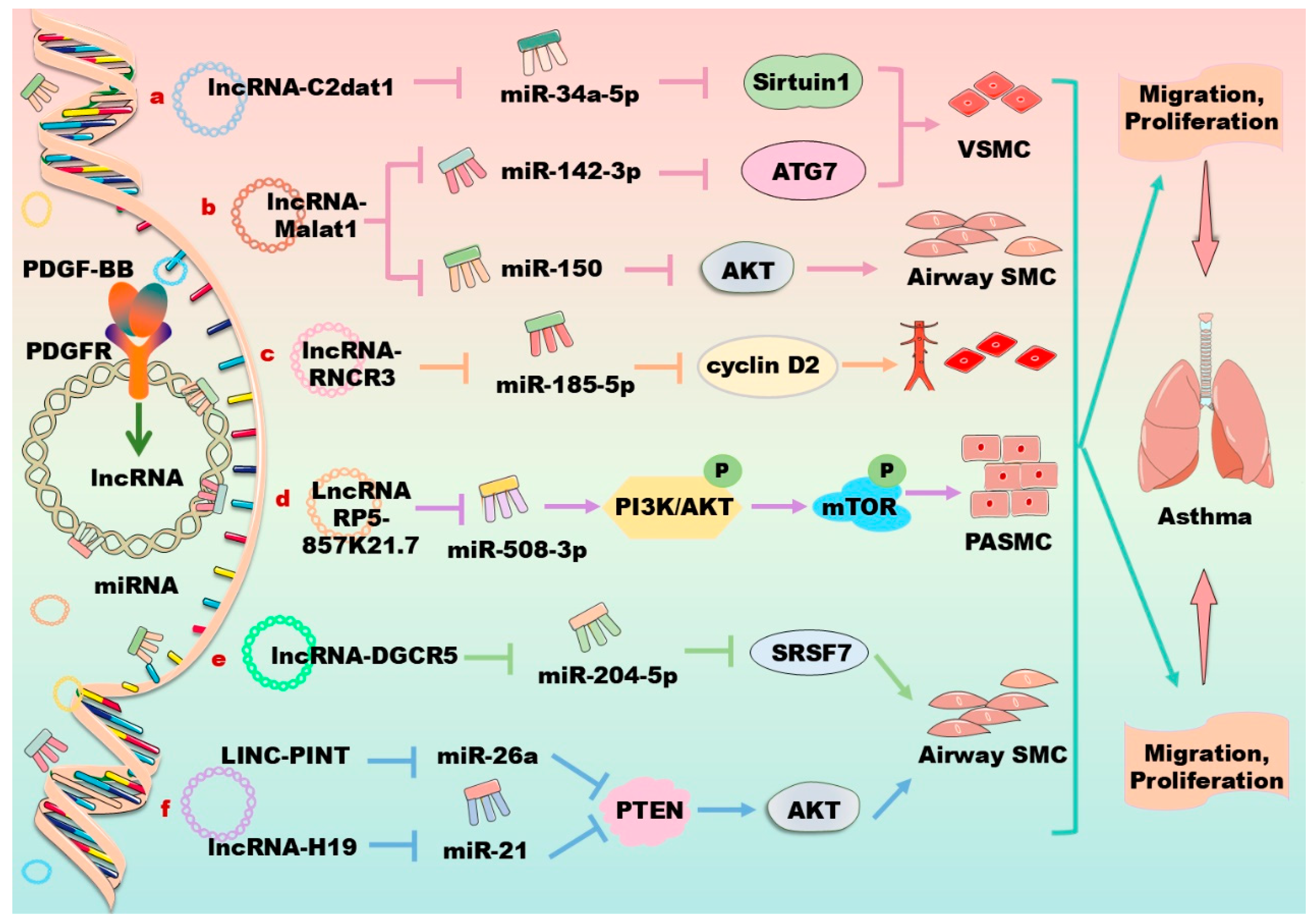
| VSMC | C2dat1 ↑ | miR34a-5p ↓ | PDGF-BB ↑/lncRNA C2dat1 ↑/ miR34a-5p ↓/SIRT1 ↑ | PDGF-BB promotes proliferation and migration of VSMCs [130]. |
| VSMC | KCNQ1OT1 ↑ | miR221 ↓ | KCNQ1OT1 ↑/miR221 ↓/IκBα ↑ | Overexpression of KCNQ1OT1 suppresses PDFG-BB-induced VSMC proliferation and inflammation [135]. |
| Aortic SMCs | SNHG16 ↑ | miR205 ↓ | LncRNA SNHG16 ↑/ miR205 ↓/Samd2 ↑ | LncRNA SNHG16 enhances human aortic SMCs proliferation and migration [144]. |
| Aortic SMCs | H19 ↑ | miR193b-3p ↓ | LncRNA H19 ↑/miR193b-3p ↓ | miR193b-3p induces cell proliferation, migration, and phenotypic differentiation in human aortic SMCs [146]. |
| Aortic SMCs | PVT1 ↑ | miR27b-3p ↓ | PVT1 ↑/miR27b-3p ↓ | miR27b-3p induces cell proliferation, migration, and phenotypic differentiation in human aortic SMCs [147]. |
| PASMCs | OTUD6B-AS1 ↑ | Cyclin D1 ↑ | PDGF ↓/lncRNAOTUD6B-AS1 ↑/cyclin D1 ↑ | Silencing lncRNA OTUD6B-AS1 decreases cell proliferation and apoptosis [149]. |
| Airway SMCs | RP5-857 K21.7 ↑ | miR508-3 ↓ | LncRNA RP5-857K21.7 ↑/ miR508-3 ↓/PI3K/AKT/mTOR ↓ | LncRNA RP5-857K21.7 inhibits PDGF-BB-induced cell proliferation, migration, and inducing apoptosis [155]. |
| Airway SMCs | H19 ↑ | MiR21 ↓ | H19 ↑/miR21 ↓/PTEN/AKT ↑ | Downregulation of human lncRNA-H19 enhances the PDGF-BB-stimulated abnormal growth of airway SMCs [160]. |
| Airway SMCs | LINC-PINT ↑ | PTEN ↑ | LINC-PINT ↑/PTEN/AKT ↑ | Downregulation of human rat LINC-PINT enhances the PDGF-BB-stimulated abnormal growth of airway SMCs [161]. |
| Endothelial cell | HOTTIP ↑ | Wnt/β-catenin ↑ | LncRNAHOTTIP ↑/Wnt/β-catenin ↑ | LncRNA HOTTIP increases proliferation, migration, and inflammatory cytokine secretion [150]. |
| Endothelial cell | RNCR3 ↑ | miR185-5p ↓ | RNCR3 ↑/miR185-5p ↓/cyclin D2 ↑ | LncRNA RNCR3 promotes proliferation, migration, and inflammatory cytokine secretion [151]. |
| Endothelial cell | HOTAIR ↑ | PDGFRβ ↑ | LncRNA HOTAIR ↑/ PDGFRβ ↑/PI3K/AKT ↑, ERK ↑ | LncRNA HOTAIR inhibits the formation of endothelial cell [163]. |
5. CircRNAs in Regulation of PDGF/PDGFR Signaling Pathway in Vascular Cells
| Cell Type | CircRNA | Target | Signaling Pathway | Effects and References |
|---|---|---|---|---|
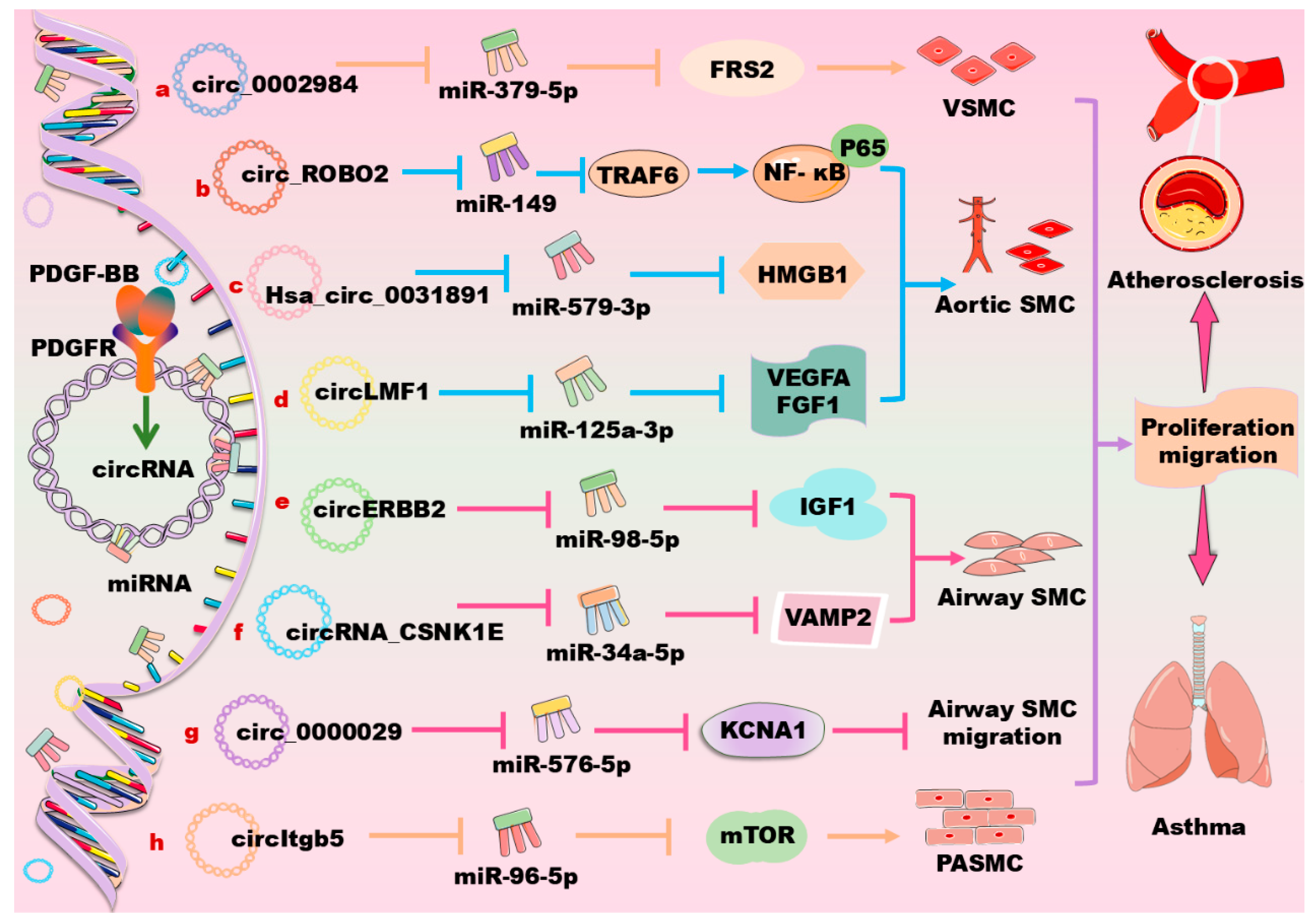
| VSMC | Circ_0002984 ↑ | miR379-5p ↓ | Circ_0002984 ↑/miR379-5p ↓/FRS2 ↑ | Circ_0002984 promotes PDGF-BB-induced VSMC proliferation, migration, and invasion [167]. |
| VSMC | Circ_0006251 ↑ | miR361-3p ↓ | Circ_0006251 ↑/miR361-3p ↓/TET3 and PPM1B ↑ | Circ_0006251 increases VSMC proliferation and decreases cell apoptosis [168]. |
| VSMC | Circ HAT1 ↑ | SFRS1 ↓ | PDGF ↑/Circ HAT1 ↑/SFRS1 ↓ | CircHAT1 inhibits the proliferation and migration of VSMCs [169]. |
| VSMC | CircSFMBT2 ↑ | miR331-3p ↓ | CircSFMBT2 ↑/miR331-3p ↓/HDAC5 ↑ | CircSFMBT2 targets angiogenic factors to regulate phenotypic regulation of VSMC [170]. |
| Aortic SMCs | CircDHCR24 ↑ | miR149-5p ↓ | PDGF-BB ↑/circDHCR24 ↑ /miR149-5p ↓/MMP-9 ↑ | CircDHCR24 promotes proliferation and migration of human aortic SMCs [171]. |
| Aortic SMCs | CircSOD2 ↑ | miR206 ↓ | CircSOD2 ↑/miR206 ↓Notch3 /cyclinD1/CDK4/6 ↑ | CircSOD2 promotes human aortic SMC proliferation and migration [172]. |
| Aortic SMCs | Circ PCNX ↑ | miR1278 ↓ | Circ PCNX ↑/miR1278 ↓/DNMT1 ↑ | CircPCNX promotes PDGF-BB-induced human aortic VSMC proliferation and migration [174]. |
| Aortic SMCs | Hsa_circ_0031891 ↑ | miR579-3p ↓ | Hsa_circ_0031891 ↑/miR579-3p ↓/HMGB1 ↑ | Hsa_circ_0031891 promotes PDGF-BB-induced human aortic VSMC proliferation, migration, and dedifferentiation partly [175]. |
| Aortic SMCs | Hsa_circ_0032389 ↑ | miR513a-5p ↓ | Hsa_circ_0032389 ↑/miR513a-5p ↓/FRS2 ↑ | Hsa_circ_0032389 enhances PDGF-BB-induced human aortic VSMC proliferation and migration [176]. |
| Aortic SMCs | CIrc_0004872 ↑ | miR513a-5p ↓ | Circ_0004872 ↑/miR513a-5p ↓/TXNIP ↑ | Circ_0004872 promotes PDGF-BB-induced cell proliferation, migration, and dedifferentiation in human aortic SMCs [177]. |
| Aortic SMCs | CircLMF1 ↑ | miR125A-3p ↓ | CircLMF1 ↑/miR125A-3p ↓/VEGFA or FGF1 ↑ | CircLMF1 accelerates atherosclerosis [178]. |
| Airway SMCs | CircERBB2 ↑ | miR98-5p ↓ | CircERBB2 ↑/miR98-5p ↓/IGF-1 ↑ | Knockdown of circERBB2 suppresses PDGF-BB-induced cell proliferation, migration, and inflammatory response [179]. |
| Airway SMCs | CircRNA_CSNK1E ↑ | miR34a-5p ↓ | CircRNA_CSNK1E ↑/miR34a-5p ↓/VAMP2 ↑ | CircRNA_CSNK1E promotes proliferation and migration of airway SMCs [181]. |
| PASMCs | CircItgb5 ↑ | miR96-5p ↓ | CircItgb5 ↑/miR96-5p ↓/mTOR ↑ | CircItgb5 results in an abnormal proliferation of PASMCs [186]. |
| Endothelial cells | Circ_0005941 ↑ | miR128-3p ↓ | Circ_0005941 ↑/miRNA-128-3p ↓/TXNIP ↑ | Circ_0005941 promotes PDGF expression [187]. |
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alshanwani, A.R.; Riches-Suman, K.; O’Regan, D.J.; Wood, I.C.; Turner, N.A.; Porter, K.E. MicroRNA-21 drives the switch to a synthetic phenotype in human saphenous vein smooth muscle cells. IUBMB Life 2018, 70, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Pawar, S.V.; Chopra, K.; Jain, M. Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases. Biochim. Biophys. Acta. Mol. Basis Dis. 2024, 1870, 167021. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar]
- Banach, M.; Surma, S.; Toth, P.P. 2023: The year in cardiovascular disease—The year of new and prospective lipid lowering therapies. Can we render dyslipidemia a rare disease by 2024? Arch. Med. Sci. 2023, 19, 1602–1615. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Zou, X.; Tang, X.Y.; Qu, Z.Y.; Sun, Z.W.; Ji, C.F.; Li, Y.J.; Guo, S.D. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int. J. Biol. Macromol. 2022, 202, 539–557. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Lennartsson, J. The PDGF/PDGFR pathway as a drug target. Mol. Aspects Med. 2018, 62, 75–88. [Google Scholar] [CrossRef]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arter. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef]
- Rönnstrand, L.; Heldin, C.H. Mechanisms of platelet-derived growth factor-induced chemotaxis. Int. J. Cancer 2001, 91, 757–762. [Google Scholar] [CrossRef]
- Heldin, C.H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal 2013, 11, 97. [Google Scholar] [CrossRef]
- Simon, A.R.; Takahashi, S.; Severgnini, M.; Fanburg, B.L.; Cochran, B.H. Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L1296–L1304. [Google Scholar] [CrossRef] [PubMed]
- Dardik, A.; Yamashita, A.; Aziz, F.; Asada, H.; Sumpio, B.E. Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1alpha. J. Vasc. Surg. 2005, 41, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, K.; Frost, E.E.; Pillai, P.P. Role of PDGF-A-Activated ERK Signaling Mediated FAK-Paxillin Interaction in Oligodendrocyte Progenitor Cell Migration. J. Mol. Neurosci. 2019, 67, 564–573. [Google Scholar] [CrossRef]
- Kingsley, K.; Huff, J.L.; Rust, W.L.; Carroll, K.; Martinez, A.M.; Fitchmun, M.; Plopper, G.E. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem. Biophys. Res. Commun. 2002, 293, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- van Roeyen, C.R.; Ostendorf, T.; Denecke, B.; Bokemeyer, D.; Behrmann, I.; Strutz, F.; Lichenstein, H.S.; LaRochelle, W.J.; Pena, C.E.; Chaudhuri, A.; et al. Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int. 2006, 69, 1393–1402. [Google Scholar] [CrossRef]
- Gao, S.Y.; Zheng, G.S.; Wang, L.; Liang, Y.J.; Zhang, S.E.; Lao, X.M.; Li, K.; Liao, G.Q. Zoledronate suppressed angiogenesis and osteogenesis by inhibiting osteoclasts formation and secretion of PDGF-BB. PLoS ONE 2017, 12, e0179248. [Google Scholar] [CrossRef]
- Tsao, P.N.; Matsuoka, C.; Wei, S.C.; Sato, A.; Sato, S.; Hasegawa, K.; Chen, H.K.; Ling, T.Y.; Mori, M.; Cardoso, W.V.; et al. Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc. Natl. Acad. Sci. USA 2016, 113, 8242–8247. [Google Scholar] [CrossRef]
- Ma, Y.C.; Shi, C.; Zhang, Y.N.; Wang, L.G.; Liu, H.; Jia, H.T.; Zhang, Y.X.; Sarkar, F.H.; Wang, Z.S. The tyrosine kinase c-Src directly mediates growth factor-induced Notch-1 and Furin interaction and Notch-1 activation in pancreatic cancer cells. PLoS ONE 2012, 7, e33414. [Google Scholar] [CrossRef]
- Jin, S.; Hansson, E.M.; Tikka, S.; Lanner, F.; Sahlgren, C.; Farnebo, F.; Baumann, M.; Kalimo, H.; Lendahl, U. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ. Res. 2008, 102, 1483–1491. [Google Scholar] [CrossRef]
- Uutela, M.; Laurén, J.; Bergsten, E.; Li, X.; Horelli-Kuitunen, N.; Eriksson, U.; Alitalo, K. Chromosomal location, exon structure, and vascular expression patterns of the human PDGFC and PDGFD genes. Circulation 2001, 103, 2242–2247. [Google Scholar] [CrossRef]
- Bowen-Pope, D.F.; Ross, R.; Seifert, R.A. Locally acting growth factors for vascular smooth muscle cells: Endogenous synthesis and release from platelets. Circulation 1985, 72, 735–740. [Google Scholar] [CrossRef] [PubMed]
- van Roeyen, C.R.C.; Martin, I.V.; Drescher, A.; Schuett, K.A.; Hermert, D.; Raffetseder, U.; Otten, S.; Buhl, E.M.; Braun, G.S.; Kuppe, C.; et al. Identification of platelet-derived growth factor C as a mediator of both renal fibrosis and hypertension. Kidney Int. 2019, 95, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Glim, J.E.; Niessen, F.B.; Everts, V.; van Egmond, M.; Beelen, R.H. Platelet derived growth factor-CC secreted by M2 macrophages induces alpha-smooth muscle actin expression by dermal and gingival fibroblasts. Immunobiology 2013, 218, 924–929. [Google Scholar] [CrossRef]
- Lin, P.; Ji, H.H.; Li, Y.J.; Guo, S.D. Macrophage Plasticity and Atherosclerosis Therapy. Front Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, C.; Du, Y.; Lin, X.; Jiang, Y.; Lee, C.; Tian, G.; Mi, J.; Li, X.; Chen, Q.; et al. PDGF-CC underlies resistance to VEGF-A inhibition and combinatorial targeting of both suppresses pathological angiogenesis more efficiently. Oncotarget 2016, 7, 77902–77915. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, T.; Guo, S.; Song, K.; Gong, C.; Huang, N.; Pang, D.; Xiao, H. CVD phenotyping in oncologic disorders: Cardio-miRNAs as a potential target to improve individual outcomes in revers cardio-oncology. J. Transl. Med. 2024, 22, 50. [Google Scholar] [CrossRef]
- van den Berge, M.; Tasena, H. Role of microRNAs and exosomes in asthma. Curr. Opin. Pulm. Med. 2019, 25, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xia, L.; Fan, X.; Ostriker, A.C.; Yarovinsky, T.; Su, M.; Zhang, Y.; Peng, X.; Xie, Y.; Pi, L.; et al. Platelet-derived miR223 promotes a phenotypic switch in arterial injury repair. J. Clin. Investig. 2019, 129, 1372–1386. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, S.; Hu, Y.; Guo, D.; Wang, Y.; Li, X. miR-125a-5p and miR-7 inhibits the proliferation, migration and invasion of vascular smooth muscle cell by targeting EGFR. Mol. Med. Rep. 2021, 24, 708. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Qi, J.; Yu, X.; Ren, H.; Zhao, X.; Xin, W.; He, S.; Zheng, X.; Ma, C.; et al. Circ-calm4 Serves as an miR337-3p Sponge to Regulate Myo10 (Myosin 10) and Promote Pulmonary Artery Smooth Muscle Proliferation. Hypertension 2020, 75, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, M.; Elia, L.; Condorelli, G.; Courtneidge, S.A. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J. Cell Biol. 2010, 189, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Wang, J.X.; Jiao, J.Q.; Tu, X.; Wang, Q.; Liu, F.; Li, Q.; Gao, J.; Zhou, Q.Y.; Gu, D.F.; et al. A pre-microRNA-149 (miR149) genetic variation affects miR149 maturation and its ability to regulate the Puma protein in apoptosis. J. Biol. Chem. 2013, 288, 26865–26877. [Google Scholar] [CrossRef]
- Fan, S.J.; Li, H.B.; Cui, G.; Kong, X.L.; Sun, L.L.; Zhao, Y.Q.; Li, Y.H.; Zhou, J. miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leuk. Res. 2016, 41, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Li, W.; Yang, J. MicroRNA-145 alleviates high glucose-induced proliferation and migration of vascular smooth muscle cells through targeting ROCK1. Biomed. Pharmacother. 2018, 99, 81–86. [Google Scholar] [CrossRef]
- Li, L.; Mao, D.; Li, C.; Li, M. miR145-5p Inhibits Vascular Smooth Muscle Cells (VSMCs) Proliferation and Migration by Dysregulating the Transforming Growth Factor-b Signaling Cascade. Med. Sci. Monit. 2018, 24, 4894–4904. [Google Scholar] [CrossRef]
- Okada, H.; Honda, M.; Campbell, J.S.; Takegoshi, K.; Sakai, Y.; Yamashita, T.; Shirasaki, T.; Takabatake, R.; Nakamura, M.; Tanaka, T.; et al. Inhibition of microRNA-214 ameliorates hepatic fibrosis and tumor incidence in platelet-derived growth factor C transgenic mice. Cancer Sci. 2015, 106, 1143–1152. [Google Scholar] [CrossRef]
- Santiago, F.S.; Ishii, H.; Shafi, S.; Khurana, R.; Kanellakis, P.; Bhindi, R.; Ramirez, M.J.; Bobik, A.; Martin, J.F.; Chesterman, C.N.; et al. Yin Yang-1 inhibits vascular smooth muscle cell growth and intimal thickening by repressing p21WAF1/Cip1 transcription and p21WAF1/Cip1-Cdk4-cyclin D1 assembly. Circ. Res. 2007, 101, 146–155. [Google Scholar] [CrossRef]
- Jin, M.; Wu, Y.; Wang, Y.; Yu, D.; Yang, M.; Yang, F.; Feng, C.; Chen, T. MicroRNA-29a promotes smooth muscle cell differentiation from stem cells by targeting YY1. Stem Cell Res. 2016, 17, 277–284. [Google Scholar] [CrossRef]
- Yue, Y.; Lv, W.; Zhang, L.; Kang, W. MiR147b influences vascular smooth muscle cell proliferation and migration via targeting YY1 and modulating Wnt/β-catenin activities. Acta. Biochim. Biophys. Sin. 2018, 50, 905–913. [Google Scholar] [CrossRef]
- Li, P.; Zhu, N.; Yi, B.; Wang, N.; Chen, M.; You, X.; Zhao, X.; Solomides, C.C.; Qin, Y.; Sun, J. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ. Res. 2013, 113, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, J.; Liu, B.; Luo, J.; Li, Z.; Qin, X.; Wei, Y. MicroRNA-320 targeting neuropilin 1 inhibits proliferation and migration of vascular smooth muscle cells and neointimal formation. Int. J. Med. Sci. 2019, 16, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, W.; Min, H.; Xu, Y. MiR340 Promotes the Proliferation of Vascular Smooth Muscle Cells by Targeting von Hippel-Lindau Tumor Suppressor Gene. J. Cardiovasc. Pharmacol. 2021, 77, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, L.Y.; Cheng, S.J.; Zhang, A.M.; Jin, X.S.; Li, Y. P53-induced microRNA-1246 inhibits the cell growth of human hepatocellular carcinoma cells by targeting NFIB. Oncol. Rep. 2015, 33, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Liu, G.; Li, B.; Jiang, J.; Chen, W.; Li, W.; Zhang, L.; Hu, Y.; Xie, S.; Yang, H. MicroRNA-1246 regulates proliferation, invasion, and differentiation in human vascular smooth muscle cells by targeting cystic fibrosis transmembrane conductance regulator (CFTR). Pflügers Arch. Eur. J. Physiol. 2021, 473, 231–240. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Getachew, R.; Ballinger, M.L.; Burch, M.L.; Reid, J.J.; Khachigian, L.M.; Wight, T.N.; Little, P.J.; Osman, N. PDGF beta-receptor kinase activity and ERK1/2 mediate glycosaminoglycan elongation on biglycan and increases binding to LDL. Endocrinology 2010, 151, 4356–4367. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, Y.; Wang, Y.; Lu, X.; Jiang, Q. MicroRNA-92 regulates vascular smooth muscle cell function by targeting KLF4 during vascular restenosis and injury. Int. J. Clin. Exp. Pathol. 2019, 12, 4253–4262. [Google Scholar]
- Liu, S.; Yang, Y.; Jiang, S.; Xu, H.; Tang, N.; Lobo, A.; Zhang, R.; Liu, S.; Yu, T.; Xin, H. Corrigendum: MiR378a-5p Regulates Proliferation and Migration in Vascular Smooth Muscle Cell by Targeting CDK1. Front. Genet. 2019, 10, 193. [Google Scholar] [CrossRef]
- Zhuang, X.; Gao, F.; Shi, L.; Liu, W.; Wang, W.; He, X.; Gao, Y. MicroRNA-146b-3p regulates the dysfunction of vascular smooth muscle cells via repressing phosphoinositide-3 kinase catalytic subunit gamma. Bioengineered 2021, 12, 2627–2638. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Hu, X.; Gao, F.; Li, M.; Cui, Y.; Gao, Y. MicroRNA-520c-3p targeting of RelA/p65 suppresses atherosclerotic plaque formation. Int. J. Biochem. Cell Biol. 2021, 131, 105873. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective Effect of let-7 miRNA Family in Regulating Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 2017, 66, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Chen, K.C.; Hsu, P.Y.; Lin, H.F.; Wang, Y.S.; Chen, C.Y.; Liao, Y.C.; Juo, S.H. microRNA let-7g suppresses PDGF-induced conversion of vascular smooth muscle cell into the synthetic phenotype. J. Cell Mol. Med. 2017, 21, 3592–3601. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Hsieh, I.C.; Hsi, E.; Wang, Y.S.; Dai, C.Y.; Chou, W.W.; Juo, S.H. Negative feedback regulation between microRNA let-7g and the oxLDL receptor LOX-1. J. Cell Sci. 2011, 124, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bae, K.H.; Byun, J.K.; Lee, S.; Kim, J.G.; Lee, I.K.; Jung, G.S.; Lee, Y.M.; Park, K.G. Lactate dehydrogenase-A is indispensable for vascular smooth muscle cell proliferation and migration. Biochem. Biophys. Res. Commun. 2017, 492, 41–47. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Yu, C.; Lu, R.; Song, T.; Wang, X.; Tang, W.; Gao, Y. MiR638 Repressed Vascular Smooth Muscle Cell Glycolysis by Targeting LDHA. Open Med. 2019, 14, 663–672. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Y.; Liu, M.; Yang, L.; Zhao, Z. miR149-5p Inhibits Vascular Smooth Muscle Cells Proliferation, Invasion, and Migration by Targeting Histone Deacetylase 4 (HDAC4). Med. Sci. Monit. 2019, 25, 7581–7590. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, C.; Ye, H.; Teng, Y.; Zheng, B.; Yang, X.; Zhang, J. MicroRNA-365 inhibits vascular smooth muscle cell proliferation through targeting cyclin D1. Int. J. Med. Sci. 2014, 11, 765–770. [Google Scholar] [CrossRef]
- Kim, M.H.; Ham, O.; Lee, S.Y.; Choi, E.; Lee, C.Y.; Park, J.H.; Lee, J.; Seo, H.H.; Seung, M.; Choi, E. MicroRNA-365 inhibits the proliferation of vascular smooth muscle cells by targeting cyclin D1. J. Cell. Biochem. 2014, 115, 1752–1761. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Han, X.; Ren, J.; Zhou, P.; Ding, P. MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway. Exp. Cell Res. 2019, 379, 214–224. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, G.; Yang, J.; Lu, R.; Hu, H. DLEU2 modulates proliferation, migration and invasion of platelet-derived growth factor-BB (PDGF-BB)-induced vascular smooth muscle cells (VSMCs) via miR212-5p/YWHAZ axis. Cell Cycle 2022, 21, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, Y.; Yang, J.; Wang, G.; Margariti, A.; Xiao, Q.; Zampetaki, A.; Yin, X.; Mayr, M.; Mori, K.; et al. XBP 1-deficiency abrogates neointimal lesion of injured vessels via cross talk with the PDGF signaling. Arter. Thromb. Vasc. Biol. 2015, 35, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Masumoto, H.; Sakamoto, K.; Yamazaki, K.; Ikeda, T.; Minatoya, K. MicroRNA-145-loaded poly(lactic-co-glycolic acid) nanoparticles attenuate venous intimal hyperplasia in a rabbit model. J. Thorac. Cardiovasc. Surg. 2019, 157, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Wang, X.; Gong, J.; Xian, Y.; Wang, G.; Zheng, Z.; Shang, C.; Wang, B.; He, Y.; et al. MiR-145 alleviates Hcy-induced VSMC proliferation, migration, and phenotypic switch through repression of the PI3K/Akt/mTOR pathway. Histochem. Cell Biol. 2020, 153, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Holvoet, P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc. Res. 2013, 100, 7–18. [Google Scholar] [CrossRef]
- Sun, D.; Wu, Y.; Zhang, S.; Han, Y.; Shen, J.; Zheng, W.; Wei, L.; Liu, Y.; Ren, L.; Gu, Z. Distinct roles of miR34 family members on suppression of lung squamous cell carcinoma. Biomed. Pharmacother. 2021, 142, 111967. [Google Scholar] [CrossRef]
- Choe, N.; Kwon, J.S.; Kim, Y.S.; Eom, G.H.; Ahn, Y.K.; Baik, Y.H.; Park, H.Y.; Kook, H. The microRNA miR34c inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by targeting stem cell factor. Cell Signal. 2015, 27, 1056–1065. [Google Scholar] [CrossRef]
- Lee, J.; Lim, S.; Song, B.W.; Cha, M.J.; Ham, O.; Lee, S.Y.; Lee, C.; Park, J.H.; Bae, Y.; Seo, H.H.; et al. MicroRNA-29b inhibits migration and proliferation of vascular smooth muscle cells in neointimal formation. J. Cell. Biochem. 2015, 116, 598–608. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Li, X.; Lv, C.; Zhang, X.; Yu, H.; Zhang, M.; Fu, Y.; Meng, H.; Zhou, J. Platelet-derived growth factor-B (PDGF-B) induced by hypoxia promotes the survival of pulmonary arterial endothelial cells through the PI3K/Akt/Stat3 pathway. Cell. Physiol. Biochem. 2015, 35, 441–451. [Google Scholar] [CrossRef]
- Chen, C.; Yan, Y.; Liu, X. microRNA-612 is downregulated by platelet-derived growth factor-BB treatment and has inhibitory effects on vascular smooth muscle cell proliferation and migration via directly targeting AKT2. Exp. Ther. Med. 2018, 15, 159–165. [Google Scholar] [CrossRef]
- Holland, P.W. Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Aspuria, P.J.; Cheon, D.J.; Gozo, M.C.; Beach, J.A.; Recouvreux, M.S.; Walts, A.E.; Karlan, B.Y.; Orsulic, S. HOXB13 controls cell state through super-enhancers. Exp. Cell Res. 2020, 393, 112039. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, T.; Kuang, S.; Zhao, G.; Zhou, K.; Zhang, H. A microRNA-17-5p/homeobox B13 axis participates in the phenotypic modulation of vascular smooth muscle cells. Mol. Med. Rep. 2021, 24, 731. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Y.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. A necessary role of miR221 and miR222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 2009, 104, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Ding, J.; Xie, S.; Huang, L.; Zhang, H.; Chen, X.; Ren, X.; Zhou, S.; He, H.; Ma, W.; et al. Myocardin/microRNA-30a/Beclin1 signaling controls the phenotypic modulation of vascular smooth muscle cells by regulating autophagy. Cell Death Dis. 2022, 13, 121. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Wang, M.; Liu, G.; Yang, Z.; Wang, L. miR654-5p Suppresses Migration and Proliferation of Vascular Smooth Muscle Cells by Targeting ADAMTS-7. Cells Tissues Organs 2023, 212, 285–292. [Google Scholar]
- Bayes-Genis, A.; Conover, C.A.; Schwartz, R.S. The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ. Res. 2000, 86, 125–130. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Zhang, A.; Liu, B.; Jia, L. miR379 Inhibits Cell Proliferation, Invasion, and Migration of Vascular Smooth Muscle Cells by Targeting Insulin-Like Factor-1. Yonsei Med. J. 2017, 58, 234–240. [Google Scholar] [CrossRef]
- Li, K.; Pan, J.; Wang, J.; Liu, F.; Wang, L. MiR665 regulates VSMCs proliferation via targeting FGF9 and MEF2D and modulating activities of Wnt/β-catenin signaling. Am. J. Transl. Res. 2017, 9, 4402–4414. [Google Scholar]
- Dong, N.; Wang, W.; Tian, J.; Xie, Z.; Lv, B.; Dai, J.; Jiang, R.; Huang, D.; Fang, S.; Tian, J.; et al. MicroRNA-182 prevents vascular smooth muscle cell dedifferentiation via FGF9/PDGFRβ signaling. Int. J. Mol. Med. 2017, 39, 791–798. [Google Scholar] [CrossRef]
- Wang, K.; Yu, J.; Wang, B.; Wang, H.; Shi, Z.; Li, G. miR29a Regulates the Proliferation and Migration of Human Arterial Smooth Muscle Cells in Arteriosclerosis Obliterans of the Lower Extremities. Kidney Blood Press. Res. 2019, 44, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zhou, Y.; Chen, L.; Wang, X.; Long, C.Y.; Pi, Y.; Gao, C.Y.; Li, J.C.; Zhang, L.L. SIRT1 improves VSMC functions in atherosclerosis. Prog. Biophys. Mol. Biol. 2016, 121, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Pereira-Smith, O.M. P53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006, 66, 8356–8360. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Kramer, M.P.; Maurer, M.; Wandl, S.; Wesierska-Gadek, J. Cellular and organismal ageing: Role of the p53 tumor suppressor protein in the induction of transient and terminal senescence. J. Cell. Biochem. 2007, 101, 1355–1369. [Google Scholar] [CrossRef]
- Wan, W.F.; Zhang, X.; Huang, C.R.; Chen, L.G.; Yang, X.B.; Bao, K.Y.; Peng, T.M. miR34c inhibits PDGF-BB-induced HAVSMCs phenotypic transformation and proliferation via PDGFR-β/SIRT1 pathway. Mol. Biol. Rep. 2021, 48, 4137–4151. [Google Scholar] [CrossRef]
- Bi, R.; Ding, F.; He, Y.; Jiang, L.; Jiang, Z.; Mei, J.; Liu, H. miR503 inhibits platelet-derived growth factor-induced human aortic vascular smooth muscle cell proliferation and migration through targeting the insulin receptor. Biomed. Pharmacother. 2016, 84, 1711–1716. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Liu, H.; Jiang, S.; Wang, G.; Sun, L.; Li, J.; Wang, X.; Yu, S.; Huang, J.; et al. MicroRNA-30a targets BECLIN-1 to inactivate autophagy and sensitizes gastrointestinal stromal tumor cells to imatinib. Cell Death Dis. 2020, 11, 198. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, M.; An, J.; Sun, H.; Li, T.; Li, D. A positive feedback loop of miR30a-5p-WWP1-NF-κB in the regulation of glioma development. Int. J. Biochem. Cell Biol. 2019, 112, 39–49. [Google Scholar] [CrossRef]
- Sakurai, Y. Autoimmune Aspects of Kawasaki Disease. J. Investig. Allergol. Clin. Immunol. 2019, 29, 251–261. [Google Scholar] [CrossRef]
- Shi, N.; Chen, S.Y. Smooth Muscle Cell Differentiation: Model Systems, Regulatory Mechanisms, and Vascular Diseases. J. Cell. Physiol. 2016, 231, 777–787. [Google Scholar] [CrossRef]
- Hernandez, G.E.; Iruela-Arispe, M.L. The many flavors of monocyte/macrophage—Endothelial cell interactions. Curr. Opin. Hematol. 2020, 27, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, B.; Fejes, Z.; Rusznyák, Á.; Fenyvesi, F.; Pócsi, M.; Halmi, S.; Griger, Z.; Kunapuli, S.P.; Kappelmayer, J.; Nagy, B. Platelet Microparticles Enriched in miR223 Reduce ICAM-1-Dependent Vascular Inflammation in Septic Conditions. Front. Physiol. 2021, 12, 658524. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liang, H.; Liu, H.; Li, D.; Chen, X.; Li, L.; Zhang, C.Y.; Zen, K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J. Immunol. 2014, 192, 437–446. [Google Scholar] [CrossRef]
- Wei, Q.; Tu, Y.; Zuo, L.; Zhao, J.; Chang, Z.; Zou, Y.; Qiu, J. MiR345-3p attenuates apoptosis and inflammation caused by oxidized low-density lipoprotein by targeting TRAF6 via TAK1/p38/NF-kB signaling in endothelial cells. Life Sci. 2020, 241, 117142. [Google Scholar] [CrossRef]
- Liao, Y.C.; Wang, Y.S.; Guo, Y.C.; Lin, W.L.; Chang, M.H.; Juo, S.H. Let-7g improves multiple endothelial functions through targeting transforming growth factor-beta and SIRT-1 signaling. J. Am. Coll. Cardiol. 2014, 63, 1685–1694. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Felli, N.; Fontana, L.; Pelosi, E.; Botta, R.; Bonci, D.; Facchiano, F.; Liuzzi, F.; Lulli, V.; Morsilli, O.; Santoro, S.; et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc. Natl. Acad. Sci. USA 2005, 102, 18081–18086. [Google Scholar] [CrossRef]
- Li, Y.; Liang, J.; Hu, J.; Ren, X.; Sheng, Y. Down-regulation of exosomal miR106b-5p derived from cholesteatoma perimatrix fibroblasts promotes angiogenesis in endothelial cells by overexpression of Angiopoietin 2. Cell Biol. Int. 2018, 42, 1300–1310. [Google Scholar] [CrossRef]
- van Balkom, B.W.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial cells require miR214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Rippe, C.; Blimline, M.; Magerko, K.A.; Lawson, B.R.; LaRocca, T.J.; Donato, A.J.; Seals, D.R. MicroRNA changes in human arterial endothelial cells with senescence: Relation to apoptosis, eNOS and inflammation. Exp. Gerontol. 2012, 47, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shao, L.; Yu, J.; Huang, J.; Feng, Q. PDGF-BB promotes vascular smooth muscle cell migration by enhancing Pim-1 expression via inhibiting miR214. Ann. Transl. Med. 2021, 9, 1728. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.N.; Hilyard, A.C.; Nguyen, P.H.; Lagna, G.; Hata, A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J. Biol. Chem. 2009, 284, 3728–3738. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Li, Y.; Yang, H.; Chen, J.; Li, X.; Gou, D. PDGFBB promotes proliferation and migration via regulating miR1181/STAT3 axis in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L1965–L1976. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, J.; Rius, J.; Castelló, A.; Cases-Langhoff, C.; Badimon, L. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ. Res. 2003, 92, 96–103. [Google Scholar] [CrossRef]
- Rius, J.; Martínez-González, J.; Crespo, J.; Badimon, L. Involvement of neuron-derived orphan receptor-1 (NOR-1) in LDL-induced mitogenic stimulus in vascular smooth muscle cells: Role of CREB. Arter. Thromb. Vasc. Biol. 2004, 24, 697–702. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Q.; Zhang, J.; Meng, Y.; Pan, L.; Tian, H. miR-106b-5p modulates acute pulmonary embolism via NOR1 in pulmonary artery smooth muscle cells. Int. J. Mol. Med. 2020, 45, 1525–1533. [Google Scholar] [CrossRef]
- Zhao, Y.; Bruemmer, D. NR4A orphan nuclear receptors: Transcriptional regulators of gene expression in metabolism and vascular biology. Arter. Thromb. Vasc. Biol. 2010, 30, 1535–1541. [Google Scholar] [CrossRef]
- Martí-Pàmies, I.; Cañes, L.; Alonso, J.; Rodríguez, C.; Martínez-González, J. The nuclear receptor NOR-1/NR4A3 regulates the multifunctional glycoprotein vitronectin in human vascular smooth muscle cells. Faseb. J. 2017, 31, 4588–4599. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Chen, J.; Li, X.; Gou, D. miR4632 mediates PDGF-BB-induced proliferation and antiapoptosis of human pulmonary artery smooth muscle cells via targeting cJUN. Am. J. Physiol. Cell Physiol. 2017, 313, C380–C391. [Google Scholar] [CrossRef]
- Li, P.; Song, J.; Du, H.; Lu, Y.; Dong, S.; Zhou, S.; Guo, Z.; Wu, H.; Zhao, X.; Qin, Y.; et al. MicroRNA-663 prevents monocrotaline-induced pulmonary arterial hypertension by targeting TGF-β1/smad2/3 signaling. J. Mol. Cell Cardiol. 2021, 161, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Han, W.; Greer, P.A.; Tuder, R.M.; Toque, H.A.; Wang, K.K.; Caldwell, R.W.; Su, Y. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J. Clin. Investig. 2011, 121, 4548–4566. [Google Scholar] [CrossRef]
- Cai, Z.; Li, J.; Zhuang, Q.; Zhang, X.; Yuan, A.; Shen, L.; Kang, K.; Qu, B.; Tang, Y.; Pu, J.; et al. MiR125a-5p ameliorates monocrotaline-induced pulmonary arterial hypertension by targeting the TGF-β1 and IL-6/STAT3 signaling pathways. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Ding, F.; You, T.; Hou, X.D.; Yi, K.; Liu, X.G.; Zhang, P.; Wang, X.K. MiR21 regulates pulmonary hypertension in rats via TGF-β1/Smad2 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8625. [Google Scholar] [PubMed]
- Ji, Y.; Yang, X.; Su, H. Overexpression of microRNA-375 impedes platelet-derived growth factor-induced proliferation and migration of human fetal airway smooth muscle cells by targeting Janus kinase 2. Biomed. Pharmacother. 2018, 98, 69–75. [Google Scholar] [CrossRef]
- Simeone-Penney, M.C.; Severgnini, M.; Tu, P.; Homer, R.J.; Mariani, T.J.; Cohn, L.; Simon, A.R. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J. Immunol. 2007, 178, 6191–6199. [Google Scholar] [CrossRef]
- Gavino, A.C.; Nahmod, K.; Bharadwaj, U.; Makedonas, G.; Tweardy, D.J. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy 2016, 71, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Redhu, N.S.; Shan, L.; Al-Subait, D.; Ashdown, H.L.; Movassagh, H.; Lamkhioued, B.; Gounni, A.S. IgE induces proliferation in human airway smooth muscle cells: Role of MAPK and STAT3 pathways. Allergy Asthma Clin. Immunol. 2013, 9, 41. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Chi, D.; Dai, C.; Sheng, M. Role of NEAT1/MiR9-5p/SLC26A2 Pathway on Human Airway Smooth Muscle Cell. Yonsei Med. J. 2021, 62, 858–867. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Yi, B.; Kazama, K.; Liu, Y.; Deshpande, D.; Zhang, J.; Sun, J. MicroRNA-638 inhibits human airway smooth muscle cell proliferation and migration through targeting cyclin D1 and NOR1. J. Cell. Physiol. 2018, 234, 369–381. [Google Scholar] [CrossRef]
- Fan, M.; Xu, J.; Xiao, Q.; Chen, F.; Han, X. Long non-coding RNA TCF7 contributes to the growth and migration of airway smooth muscle cells in asthma through targeting TIMMDC1/Akt axis. Biochem. Biophys. Res. Commun. 2019, 508, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yan, K.; Chen, Y.; Zhang, W.; Ji, Z.; Dang, C. ABCA1 inhibits PDGF-induced proliferation and migration of rat airway smooth muscle cell through blocking TLR2/NF-κB/NFATc1 signaling. J. Cell. Biochem. 2018, 119, 7388–7396. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, J.; Chao, J.; Scheuerman, M.P.; Rahimi, S.A.; Lee, L.Y.; Li, S. PTEN suppresses epithelial-mesenchymal transition and cancer stem cell activity by downregulating Abi1. Sci. Rep. 2020, 10, 12685. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J.; Wang, Y. MicroRNA-30b-5p promotes the proliferation and migration of human airway smooth muscle cells induced by platelet-derived growth factor by targeting phosphatase and tensin homolog deleted on chromosome ten. Bioengineered 2021, 12, 3662–3673. [Google Scholar] [CrossRef]
- Fernandes-Freitas, I.; Owen, B.M. Metabolic roles of endocrine fibroblast growth factors. Curr. Opin. Pharmacol. 2015, 25, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Gasser, E.; Moutos, C.P.; Downes, M.; Evans, R.M. FGF1—A new weapon to control type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2017, 13, 599–609. [Google Scholar] [CrossRef]
- Li, C.; Deng, C.; Zhou, T.; Hu, J.; Dai, B.; Yi, F.; Tian, N.; Jiang, L.; Dong, X.; Zhu, Q.; et al. MicroRNA-370 carried by M2 macrophage-derived exosomes alleviates asthma progression through inhibiting the FGF1/MAPK/STAT1 axis. Int. J. Biol. Sci. 2021, 17, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Gao, G.; Zhou, Z. Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and inflammation injury via targeting miR370-3p/TLR4 in acute pneumonia. Cell Biochem. Funct. 2019, 37, 348–358. [Google Scholar] [CrossRef]
- Lin, P.; Yin, F.; Shen, N.; Liu, N.; Zhang, B.; Li, Y.; Guo, S. Integrated bioinformatics analysis of the anti-atherosclerotic mechanisms of the polysaccharide CM1 from Cordyceps militaris. Int. J. Biol. Macromol. 2021, 193, 1274–1285. [Google Scholar] [CrossRef]
- Shi, X.; Pan, S.; Li, L.; Li, Y.; Ma, W.; Wang, H.; Zhang, L.; Zhang, M.; Zhang, G. HIX003209 promotes vascular smooth muscle cell migration and proliferation through modulating miR6089. Aging 2020, 12, 8913–8922. [Google Scholar] [CrossRef]
- Wang, H.; Jin, Z.; Pei, T.; Song, W.; Gong, Y.; Chen, D.; Xu, C.; Li, L.; Wang, D. Long noncoding RNAs C2dat1 enhances vascular smooth muscle cell proliferation and migration by targeting MiR34a-5p. J. Cell. Biochem. 2019, 120, 3001–3008. [Google Scholar] [CrossRef]
- Song, T.F.; Huang, L.W.; Yuan, Y.; Wang, H.Q.; He, H.P.; Ma, W.J.; Huo, L.H.; Zhou, H.; Wang, N.; Zhang, T.C. LncRNA MALAT1 regulates smooth muscle cell phenotype switch via activation of autophagy. Oncotarget 2018, 9, 4411–4426. [Google Scholar] [CrossRef]
- Zhou, Y.; He, X.; Liu, R.; Qin, Y.; Wang, S.; Yao, X.; Li, C.; Hu, Z. LncRNA CRNDE regulates the proliferation and migration of vascular smooth muscle cells. J. Cell. Physiol. 2019, 234, 16205–16214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, J.; Zhu, S.; Zhang, H.; Zhu, Q.; Li, Y. LncRNA HCG18 is critical for vascular smooth muscle cell proliferation and phenotypic switching. Hum. Cell 2020, 33, 537–544. [Google Scholar] [CrossRef]
- Ye, B.; Wu, Z.H.; Tsui, T.Y.; Zhang, B.F.; Su, X.; Qiu, Y.H.; Zheng, X.T. lncRNA KCNQ1OT1 Suppresses the Inflammation and Proliferation of Vascular Smooth Muscle Cells through IκBa in Intimal Hyperplasia. Mol. Ther. Nucleic Acids 2020, 20, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.J.; Li, Z.Y.; Zhu, Y.T.; Li, C.C. Overexpression of long noncoding RNA ANRIL inhibits phenotypic switching of vascular smooth muscle cells to prevent atherosclerotic plaque development in vivo. Aging 2020, 13, 4299–4316. [Google Scholar] [CrossRef]
- Wu, Y.X.; Zhang, S.H.; Cui, J.; Liu, F.T. Long Noncoding RNA XR007793 Regulates Proliferation and Migration of Vascular Smooth Muscle Cell via Suppressing miR23b. Med. Sci. Monit. 2018, 24, 5895–5903. [Google Scholar] [CrossRef]
- Ni, Y.Q.; Lin, X.; Zhan, J.K.; Liu, Y.S. Roles and Functions of Exosomal Non-coding RNAs in Vascular Aging. Aging Dis. 2020, 11, 164–178. [Google Scholar]
- Yu, C.K.; Xu, T.; Assoian, R.K.; Rader, D.J. Mining the Stiffness-Sensitive Transcriptome in Human Vascular Smooth Muscle Cells Identifies Long Noncoding RNA Stiffness Regulators. Arter. Thromb. Vasc. Biol. 2018, 38, 164–173. [Google Scholar] [CrossRef]
- Ni, H.; Haemmig, S.; Deng, Y.; Chen, J.; Simion, V.; Yang, D.; Sukhova, G.; Shvartz, E.; Wara, A.K.M.K.; Cheng, H.S.; et al. A Smooth Muscle Cell-Enriched Long Noncoding RNA Regulates Cell Plasticity and Atherosclerosis by Interacting with Serum Response Factor. Arter. Thromb. Vasc. Biol. 2021, 41, 2399–2416. [Google Scholar] [CrossRef]
- Dong, K.; Shen, J.; He, X.; Hu, G.; Wang, L.; Osman, I.; Bunting, K.M.; Dixon-Melvin, R.; Zheng, Z.; Xin, H.; et al. CARMN Is an Evolutionarily Conserved Smooth Muscle Cell-Specific LncRNA That Maintains Contractile Phenotype by Binding Myocardin. Circulation 2021, 144, 1856–1875. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, J.; Yin, Q.; Dong, M.; Zhang, Y.; Chen, M.; Chen, X.; Min, J.; He, X.; Tan, Y.; et al. lncRNA JPX-Enriched Chromatin Microenvironment Mediates Vascular Smooth Muscle Cell Senescence and Promotes Atherosclerosis. Arter. Thromb. Vasc. Biol. 2024, 44, 156–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hao, Y.; Zhang, J. The lncRNA DANCR promotes development of atherosclerosis by regulating the miR-214-5p/COX20 signaling pathway. Cell. Mol. Biol. Lett. 2022, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tian, G.; Zhang, H.; Yuan, W.; Xie, Y.; Yang, Y.; Wang, J.; Liang, Y. Long non-coding RNA SNHG16 regulates human aortic smooth muscle cell proliferation and migration via sponging miR205 and modulating Smad2. J. Cell. Mol. Med. 2019, 23, 6919–6929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qi, Y.; Wang, M.; Chen, Q. Long non-coding RNA HIF1A-AS2 modulates the proliferation, migration, and phenotypic switch of aortic smooth muscle cells in aortic dissection via sponging microRNA-33b. Bioengineered 2022, 13, 6383–6395. [Google Scholar] [CrossRef]
- Ren, M.; Wang, T.; Wei, X.; Wang, Y.; Ouyang, C.; Xie, Y.; Ye, X.; Han, Z. LncRNA H19 regulates smooth muscle cell functions and participates in the development of aortic dissection through sponging miR193b-3p. Biosci. Rep. 2021, 41, BSR20202298. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Cheng, S.; Li, J.; Bai, X.; Meng, X. Downregulating long non-coding RNA PVT1 expression inhibited the viability, migration and phenotypic switch of PDGF-BB-treated human aortic smooth muscle cells via targeting miR27b-3p. Hum. Cell 2021, 34, 335–348. [Google Scholar] [CrossRef]
- Fasolo, F.; Jin, H.; Winski, G.; Chernogubova, E.; Pauli, J.; Winter, H.; Li, D.Y.; Glukha, N.; Bauer, S.; Metschl, S.; et al. Long Noncoding RNA MIAT Controls Advanced Atherosclerotic Lesion Formation and Plaque Destabilization. Circulation 2021, 144, 1567–1583. [Google Scholar] [CrossRef]
- Takata, M.; Pachera, E.; Frank-Bertoncelj, M.; Kozlova, A.; Jüngel, A.; Whitfield, M.L.; Assassi, S.; Calcagni, M.; de Vries-Bouwstra, J.; Huizinga, T.W.; et al. OTUD6B-AS1 Might Be a Novel Regulator of Apoptosis in Systemic Sclerosis. Front. Immunol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Liao, B.; Chen, R.; Lin, F.; Mai, A.; Chen, J.; Li, H.; Xu, Z.; Dong, S. Long noncoding RNA HOTTIP promotes endothelial cell proliferation and migration via activation of the Wnt/β-catenin pathway. J. Cell. Biochem. 2018, 119, 2797–2805. [Google Scholar] [CrossRef]
- Hong, Q.; Ling, L.; Huang, W.; Liu, Y.; Zhuo, Y.; Hong, Z.; Wu, B.; Zhang, Y. LncRNA RNCR3 promotes endothelial cell proliferation and inflammatory cytokine secretion via regulating miR185-5p/cyclin D2 axis. Environ. Sci. Pollut. Res. Int. 2021, 28, 27025–27032. [Google Scholar] [CrossRef] [PubMed]
- Pelisek, J.; Reutersberg, B.; Greber, U.F.; Zimmermann, A. Vascular dysfunction in COVID-19 patients: Update on SARS-CoV-2 infection of endothelial cells and the role of long non-coding RNAs. Clin. Sci. 2022, 136, 1571–1590. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Q.; Hao, W.; Zhang, Y.; Zhao, L.; Han, W. Upregulation of LncRNA Malat1 Induced Proliferation and Migration of Airway Smooth Muscle Cells via miR150-eIF4E/Akt Signaling. Front. Physiol. 2019, 10, 1337. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, G.; Zhuo, S.; Zhuang, H.; Chen, S. lncRNA FTX promotes asthma progression by sponging miR590-5p and upregulating JAK2. Am. J. Transl. Res. 2021, 13, 8833–8846. [Google Scholar] [PubMed]
- Wang, X.; Xu, L.; Yu, Y.; Fu, Y. LncRNA RP5-857K21.7 inhibits PDGF-BB-induced proliferation and migration of airway smooth muscle cells through the miR508-3p/PI3K/AKT/mTOR axis. Autoimmunity 2022, 55, 65–73. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, X.; Feng, X. LncRNA DGCR5/miR204-5p/SRSF7 axis regulates PDGF-BB-induced proliferation and migration of airway smooth muscle cells with potential role in asthma. Autoimmunity 2023, 56, 2193678. [Google Scholar] [CrossRef]
- Zhou, H.; Long, C.; Liu, P.; Chen, Y.; Luo, L.; Xiao, Z. Long non-coding RNA TUG1 accelerates abnormal growth of airway smooth muscle cells in asthma by targeting the miR138-5p/E2F3 axis. Exp. Ther. Med. 2021, 22, 1229. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, X.Y.; Li, N.; Zhao, L.M.; Guo, Y.L.; Li, X.S.; Tian, C.J.; Cheng, D.J.; Chen, Z.C.; Zhang, L.X. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR10a/BDNF signaling pathway. Life Sci. 2018, 212, 93–101. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, L.X.; Tian, C.J.; Tang, X.Y.; Zhao, L.M.; Guo, Y.L.; Cheng, D.J.; Chen, X.L.; Ma, L.J.; Chen, Z.C. LncRNAs BCYRN1 promoted the proliferation and migration of rat airway smooth muscle cells in asthma via upregulating the expression of transient receptor potential 1. Am. J. Transl. Res. 2016, 8, 3409–3418. [Google Scholar]
- Yu, H.; Qi, N.; Zhou, Q. LncRNA H19 Inhibits Proliferation and Migration of Airway Smooth Muscle Cells Induced by PDGF-BB Through miR21/PTEN/Akt Axis. J. Asthma Allergy 2021, 14, 71–80. [Google Scholar] [CrossRef]
- Gao, P.; Ding, Y.; Yin, B.; Gu, H. Long noncoding RNA LINC-PINT retards the abnormal growth of airway smooth muscle cells via regulating the microRNA-26a-5p/PTEN axis in asthma. Int. Immunopharmacol. 2021, 99, 107997. [Google Scholar] [CrossRef]
- Dong, Z.; Li, S.; Si, L.; Ma, R.; Bao, L.; Bo, A. Identification lncRNA LOC102551149/miR23a-5p pathway in hepatic fibrosis. Eur. J. Clin. Investig. 2020, 50, e13243. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Q.; Chen, M.; Wang, B.; Yang, X.; Yang, M.; Wang, Q.; Cui, Z.; Ge, F. Long noncoding RNA HOTAIR interacts with Y-Box Protein-1 (YBX1) to regulate cell proliferation. Life Sci. Alliance 2021, 4, e202101139. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Shi, C.; Wang, D.; Cheng, J.; Wang, Q.; Wang, L.; Yang, G. Long non-coding RNA-non-coding RNA activated by DNA damage inhibition suppresses hepatic stellate cell activation via microRNA-495-3p/sphingosine 1-phosphate receptor 3 axis. Bioengineered 2022, 13, 6150–6162. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, Y.; Guo, T.; Li, G. Identification, biogenesis, function, and mechanism of action of circular RNAs in plants. Plant Commun. 2023, 4, 100430. [Google Scholar] [CrossRef]
- Zhuang, J.B.; Li, T.; Hu, X.M.; Ning, M.; Gao, W.Q.; Lang, Y.H.; Zheng, W.F.; Wei, J. Circ_CHFR expedites cell growth, migration and inflammation in ox-LDL-treated human vascular smooth muscle cells via the miR-214-3p/Wnt3/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3282–3292. [Google Scholar]
- Zheng, X.; Liu, J.; Gong, X.; Zhang, X.; Ma, S. Circ_0002984 Enhances Growth, Invasion, and Migration in PDGF-bb-Induced Vascular Smooth Muscle Cells Through miR379-5p/FRS2 Axis. J. Cardiovasc. Pharmacol. 2021, 78, 875–884. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, L.; Xiong, L. Circ_0006251 mediates the proliferation and apoptosis of vascular smooth muscle cells in CAD via enhancing TET3 and PPM1B expression. Cell. Mol. Biol. 2023, 69, 34–39. [Google Scholar] [CrossRef]
- Huang, X.Y.; Fu, F.Y.; Qian, K.; Feng, Q.L.; Cao, S.; Wu, W.Y.; Luo, Y.L.; Chen, W.J.; Zhang, Z.; Huang, S.C. CircHAT1 regulates the proliferation and phenotype switch of vascular smooth muscle cells in lower extremity arteriosclerosis obliterans through targeting SFRS1. Mol. Cell. Biochem. 2024, 24, 3282–3292. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, C. CircSFMBT2 facilitates vascular smooth muscle cell proliferation by targeting miR331-3p/HDAC5. Life Sci. 2021, 264, 118691. [Google Scholar] [CrossRef]
- Peng, W.; Li, T.; Pi, S.; Huang, L.; Liu, Y. Suppression of circular RNA circDHCR24 alleviates aortic smooth muscle cell proliferation and migration by targeting miR149-5p/MMP9 axis. Biochem. Biophys. Res. Commun. 2020, 529, 753–759. [Google Scholar] [CrossRef]
- Mei, X.; Cui, X.B.; Li, Y.; Chen, S.Y. CircSOD2: A Novel Regulator for Smooth Muscle Proliferation and Neointima Formation. Arter. Thromb. Vasc. Biol. 2021, 41, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Zhang, C.Y.; Li, L.; Ye, G.H.; Jiang, L.P.; Jin, Q. Circ_ROBO2/miR149 Axis Promotes the Proliferation and Migration of Human Aortic Smooth Muscle Cells by Activating NF-κB Signaling. Cytogenet. Genome Res. 2021, 161, 414–424. [Google Scholar] [CrossRef]
- Ma, W.; Wei, D.; Li, X.; Shan, L.; Fan, H.; Jin, H.; Song, B.; Zhang, B. CircPCNX Promotes PDGF-BB-Induced Proliferation and Migration of Human Aortic Vascular Smooth Muscle Cells Through Regulating miR1278/DNMT1 Axis. Cardiovasc. Drugs Ther. 2023, 37, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Zheng, Z.; Li, Y. Hsa_circ_0031891 targets miR579-3p to enhance HMGB1 expression and regulate PDGF-BB-induced human aortic vascular smooth muscle cell proliferation, migration, and dedifferentiation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Ma, S.; Zhou, Q.; Lei, C. Hsa_circ_0032389 Enhances Proliferation and Migration in PDGF-BB-Induced Human Aortic Vascular Smooth Muscle Cells. Cardiovasc. Toxicol. 2024, 24, 111–121. [Google Scholar] [CrossRef]
- Fan, K.; Ruan, X.; Wang, L.; Lu, W.; Shi, Q.; Xu, Y. Circ_0004872 promotes platelet-derived growth factor-BB-induced proliferation, migration and dedifferentiation in HA-VSMCs via miR513a-5p/TXNIP axis. Vascul. Pharmacol. 2021, 140, 106842. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, W.; Wang, L.; Lu, L.; Pang, Y. Circular RNA circLMF1 regulates PDGF-BB-induced proliferation and migration of human aortic smooth muscle cells by regulating the miR125a-3p/VEGFA or FGF1 axis. Clin. Hemorheol. Microcirc. 2022, 80, 167–183. [Google Scholar] [CrossRef]
- Huang, J.Q.; Wang, F.; Wang, L.T.; Li, Y.M.; Lu, J.L.; Chen, J.Y. Circular RNA ERBB2 Contributes to Proliferation and Migration of Airway Smooth Muscle Cells via miR98-5p/IGF1R Signaling in Asthma. J. Asthma Allergy 2021, 14, 1197–1207. [Google Scholar] [CrossRef]
- Quan, L.; Ren, G.; Liu, L.; Huang, W.; Li, M. Circular RNA circ_0002594 regulates PDGF-BB-induced proliferation and migration of human airway smooth muscle cells via sponging miR139-5p/TRIM8 in asthma. Autoimmunity 2022, 55, 339–350. [Google Scholar] [CrossRef]
- Ding, L.; Liu, G.L.; Lu, L.; Ge, L.; Wang, J.Y. circ_CSNK1E modulates airway smooth muscle cells proliferation and migration via miR34a-5p/VAMP2 axis in asthma. Cell. Signal. 2022, 95, 110340. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tan, Y.; Bao, X.; Xiong, S.; Liang, R.; Cai, M.; Bian, J. Circ_0000029 Interacts with the miR576-5p/KCNA1 Axis to Hamper the Development of Pediatric Asthma in an Asthma-Like in vitro Assessment. Ann. Clin. Lab. Sci. 2023, 53, 200–211. [Google Scholar]
- Lin, J.; Feng, X.; Zhang, J. Circular RNA circHIPK3 modulates the proliferation of airway smooth muscle cells by miR326/STIM1 axis. Life Sci. 2020, 255, 117835. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, X.; Qin, J. Silencing of circHIPK3 hampers platelet-derived growth factor-induced proliferation and migration in airway smooth muscle cells through the miR375/MMP-16 axis. Cytotechnology 2021, 73, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fu, Y.; Li, Q.; Xu, Y.; Xi, X.; Zheng, Y.; Yu, L.; Wang, Z.; Yu, B.; Tian, J. Differential Expression and Bioinformatics Analysis of CircRNA in PDGF-BB-Induced Vascular Smooth Muscle Cells. Front. Genet. 2020, 11, 530. [Google Scholar] [CrossRef]
- Su, H.; Zhu, H.; Wang, S.; Li, Y.; Yan, C.; Wang, J.; Ying, K. CircItgb5 promotes synthetic phenotype of pulmonary artery smooth muscle cells via interacting with miR96-5p and Uba1 in monocrotaline-induced pulmonary arterial hypertension. Respir. Res. 2023, 24, 165. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, F.; Ren, W.; Zhu, Y.; Du, Q.; Li, Q.; Li, X. Circular Ribonucleic Acid circFTO Promotes Angiogenesis and Impairs Blood-Retinal Barrier Via Targeting the miR128-3p/Thioredoxin Interacting Protein Axis in Diabetic Retinopathy. Front. Mol. Biosci. 2021, 8, 685466. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Fan, H.; Yu, Y.; Luo, Y.; Gu, F.; Yu, H.; Liao, X. Anthocyanins improve liver fibrosis in mice by regulating the autophagic flux level of hepatic stellate cells by mmu_circ_0000623. Food Sci. Nutr. 2023, 11, 3002–3018. [Google Scholar] [CrossRef]
- Huang, P.S.; Liao, C.J.; Huang, Y.H.; Yeh, C.T.; Chen, C.Y.; Tang, H.C.; Chang, C.C.; Lin, K.H. Functional and Clinical Significance of Dysregulated microRNAs in Liver Cancer. Cancers 2021, 13, 5361. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.-N.; Shi, S.-R.; Zhang, X.-Y.; Xin, G.-S.; Zou, X.; Li, W.-L.; Guo, S.-D. Targeting PDGF/PDGFR Signaling Pathway by microRNA, lncRNA, and circRNA for Therapy of Vascular Diseases: A Narrow Review. Biomolecules 2024, 14, 1446. https://doi.org/10.3390/biom14111446
Ma C-N, Shi S-R, Zhang X-Y, Xin G-S, Zou X, Li W-L, Guo S-D. Targeting PDGF/PDGFR Signaling Pathway by microRNA, lncRNA, and circRNA for Therapy of Vascular Diseases: A Narrow Review. Biomolecules. 2024; 14(11):1446. https://doi.org/10.3390/biom14111446
Chicago/Turabian StyleMa, Chao-Nan, Shan-Rui Shi, Xue-Ying Zhang, Guo-Song Xin, Xiang Zou, Wen-Lan Li, and Shou-Dong Guo. 2024. "Targeting PDGF/PDGFR Signaling Pathway by microRNA, lncRNA, and circRNA for Therapy of Vascular Diseases: A Narrow Review" Biomolecules 14, no. 11: 1446. https://doi.org/10.3390/biom14111446
APA StyleMa, C.-N., Shi, S.-R., Zhang, X.-Y., Xin, G.-S., Zou, X., Li, W.-L., & Guo, S.-D. (2024). Targeting PDGF/PDGFR Signaling Pathway by microRNA, lncRNA, and circRNA for Therapy of Vascular Diseases: A Narrow Review. Biomolecules, 14(11), 1446. https://doi.org/10.3390/biom14111446





