Tryptophan Metabolites in the Progression of Liver Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Metabolomic Analysis
2.2. Statistical Analysis
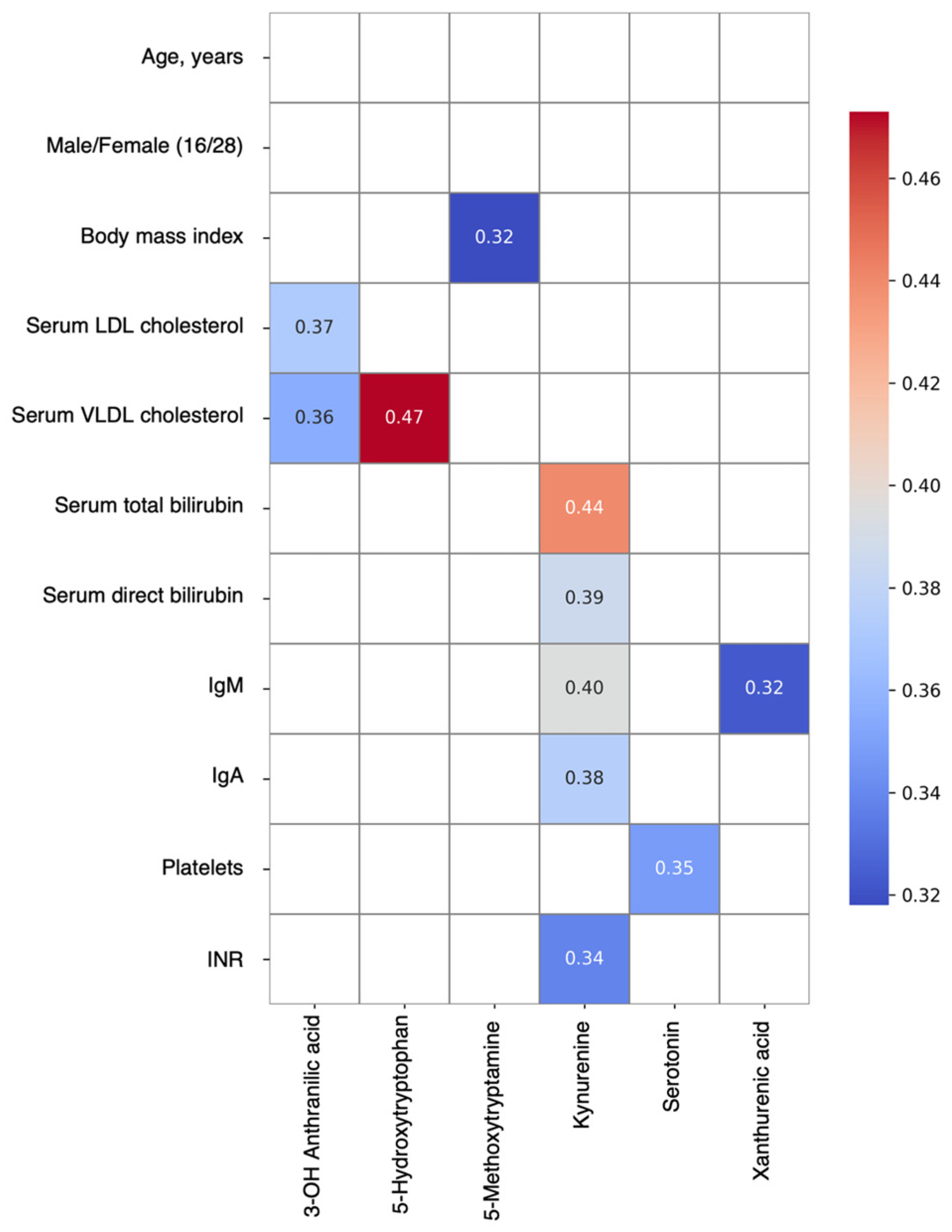
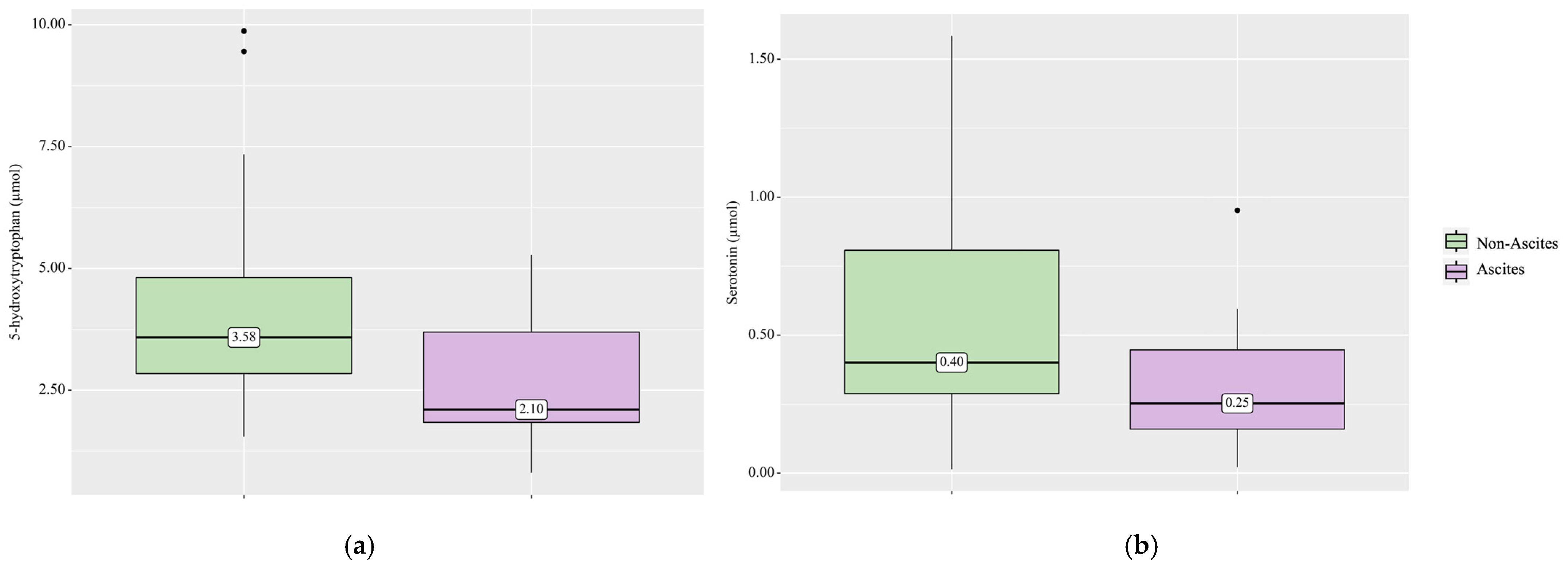
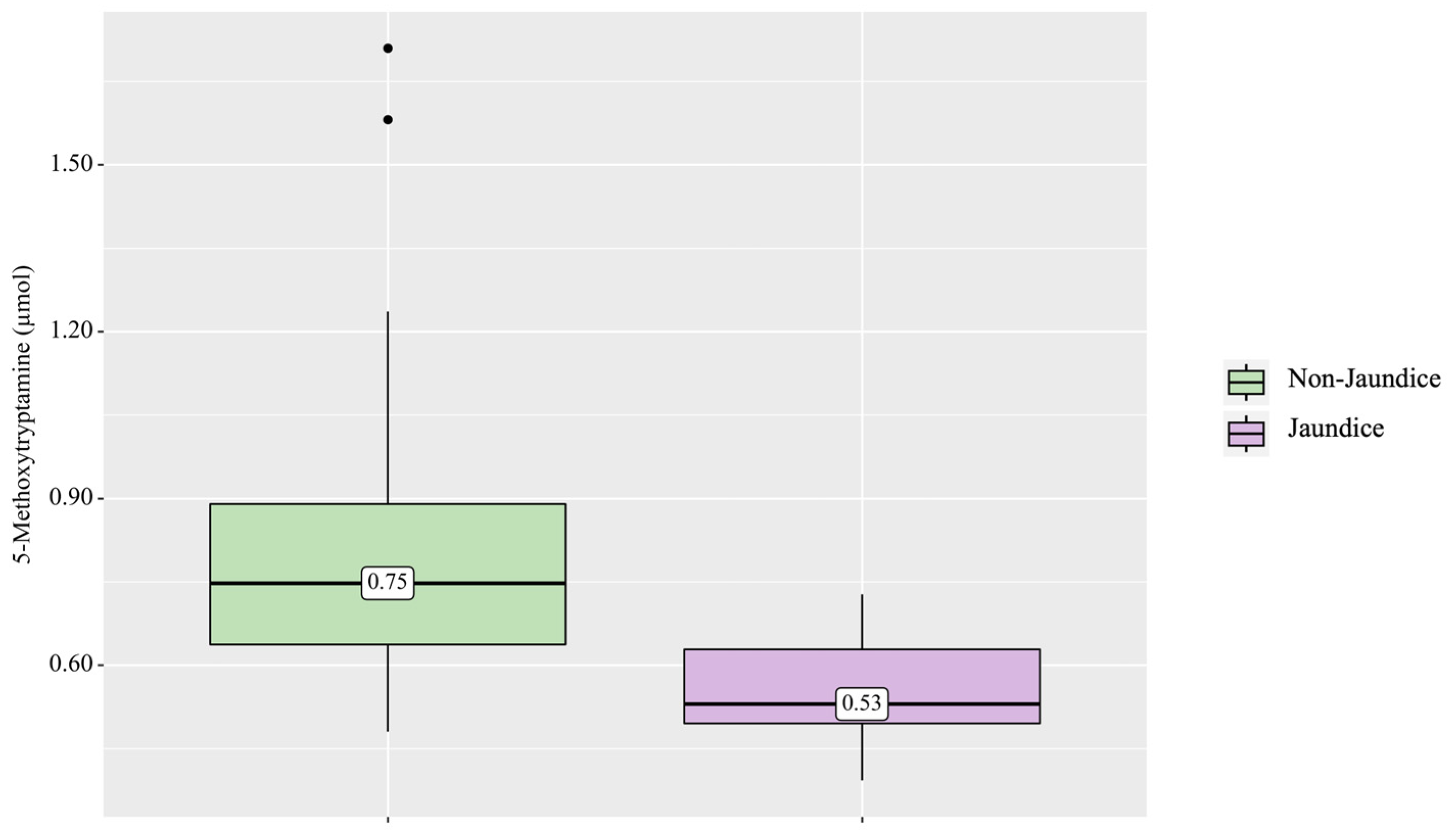
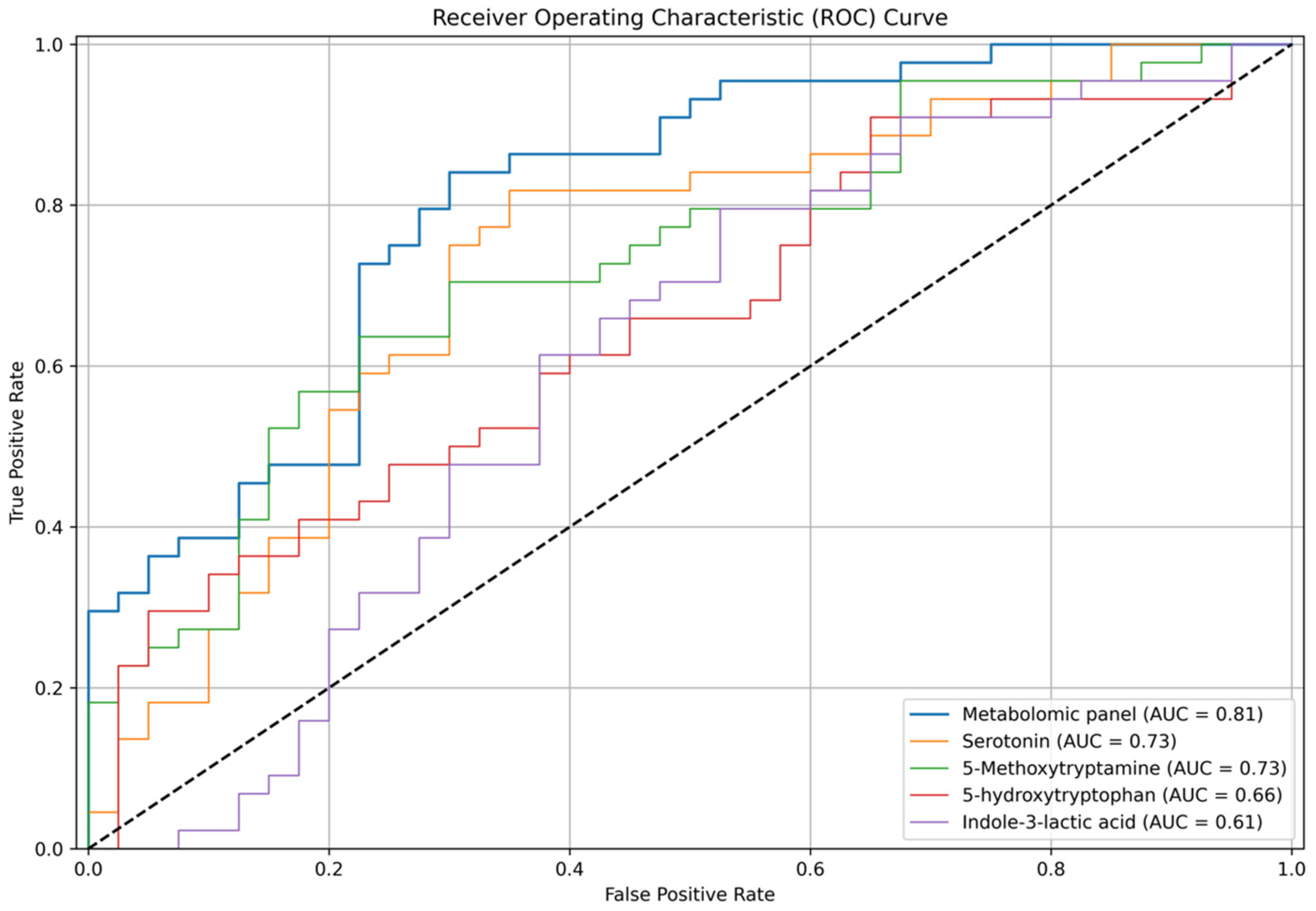
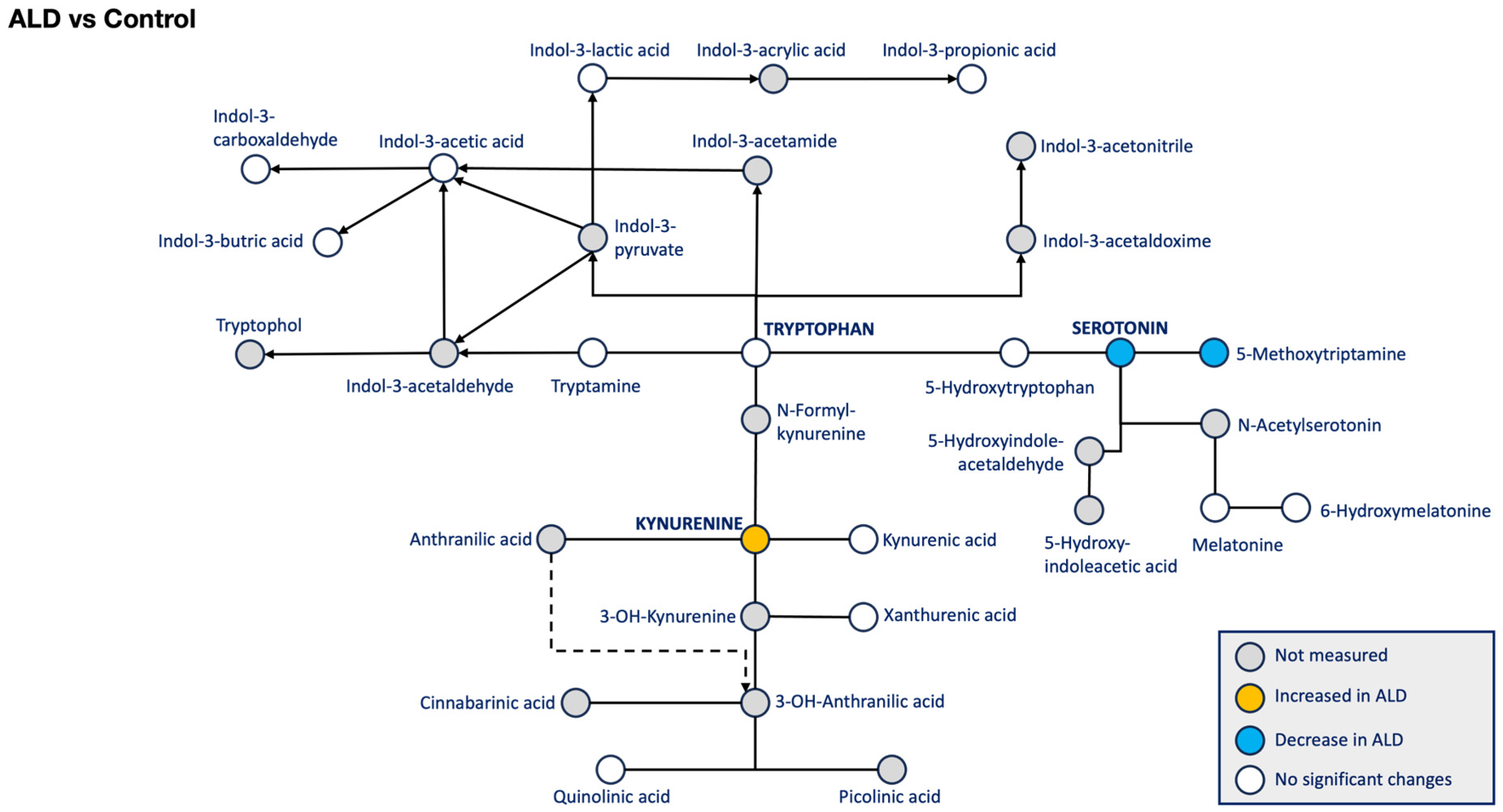
3. Results
4. Discussion
4.1. Serotonin
4.2. Kynurenine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 25 May 2024).
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Lberts, C.J.; Clifford, G.M.; Georges, D.; Negro, F.; Lesi, O.A.; Hutin, Y.J.; de Martel, C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 724–735. [Google Scholar] [CrossRef]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis—Aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ivashkin, V.T.; Zharkova, M.S.; Korochanskaya, N.V.; Khlynov, I.B.; Uspensky, Y.P. Phenotypes of Non-Alcoholic Fatty Liver Disease in Different Regions of the Russian Federation, Diagnostic and Therapeutic Approach in Clinical Practice. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2023, 33, 7–18. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Fan, X.; Luo, X. Metabolomics profiles in acute-on-chronic liver failure: Unveiling pathogenesis and predicting progression. Front. Pharmacol. 2022, 13, 953297. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhang, X.; Kuang, M.; Yu, J. The gut-liver axis in immune remodeling of hepatic cirrhosis. Front. Immunol. 2022, 13, 946628. [Google Scholar] [CrossRef]
- Zolnikova, O.Y.; Reshetova, M.S.; Ivanova, M.N.; Ivashkin, V.T. Metabolomic profiles as a new understanding of disease processes. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2022, 32, 46–52. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef]
- Bloom, P.P.; Tapper, E.B.; Young, V.B.; Lok, A.S. Microbiome therapeutics for hepatic encephalopathy. J. Hepatol. 2021, 75, 1452–1464. [Google Scholar] [CrossRef]
- Cao, X.; Zolnikova, O.; Maslennikov, R.; Reshetova, M.; Poluektova, E.; Bogacheva, A.; Zharkova, M.; Ivashkin, V. Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis. Metabolites 2023, 13, 859. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, H.; Anjum, K.; Zhong, X.; Miao, S.; Zheng, G.; Liu, W.; Li, L. Dual Role of Indoles Derived From Intestinal Microbiota on Human Health. Front. Immunol. 2022, 13, 903526. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Ivashkin, V.T.; Medvedev, O.S.; Poluektova, E.A.; Kudryavtseva, A.V.; Bakhtogarimov, I.R.; Karchevskaya, A.E. Direct and Indirect Methods for Studying Human Gut Microbiota. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2022, 32, 19–34. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef]
- Cerrito, F.; Lazzaro, M.P.; Gaudio, E.; Arminio, P.; Aloisi, G. 5HT2-receptors and serotonin release: Their role in human platelet aggregation. Life Sci. 1993, 53, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Laffi, G.; Marra, F.; Gresele, P.; Romagnoli, P.; Palermo, A.; Bartolini, O.; Simoni, A.; Orlandi, L.; Selli, M.L.; Nenci, G.G.; et al. Evidence for a storage pool defect in platelets from cirrhotic patients with defective aggregation. Gastroenterology 1992, 103, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Ruddell, R.G.; Oakley, F.; Hussain, Z.; Yeung, I.; Bryan-Lluka, L.J.; Ramm, G.A.; Mann, D.A. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am. J. Pathol. 2006, 169, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, S.; Pang, Q.; Zhang, R.; Zhou, L.; Liu, S.; Meng, F.; Wu, Q.; Liu, C. Serotonin Deficiency Exacerbates Acetaminophen-Induced Liver Toxicity In Mice. Sci. Rep. 2015, 5, 8098, Erratum in Sci. Rep. 2015, 5, 12184. [Google Scholar] [CrossRef]
- Lesurtel, M.; Graf, R.; Aleil, B.; Walther, D.J.; Tian, Y.; Jochum, W.; Gachet, C.; Bader, M.; Clavien, P.A. Platelet-derived serotonin mediates liver regeneration. Science 2006, 312, 104–107. [Google Scholar] [CrossRef]
- Furrer, K.; Rickenbacher, A.; Tian, Y.; Jochum, W.; Bittermann, A.G.; Käch, A.; Humar, B.; Graf, R.; Moritz, W.; Clavien, P.A. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 2945–2950. [Google Scholar] [CrossRef]
- Lang, P.A.; Contaldo, C.; Georgiev, P.; El-Badry, A.M.; Recher, M.; Kurrer, M.; Cervantes-Barragan, L.; Ludewig, B.; Calzascia, T.; Bolinger, B.; et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat. Med. 2008, 14, 756–761. [Google Scholar] [CrossRef]
- Lesurtel, M.; Soll, C.; Humar, B.; Clavien, P.A. Serotonin: A double-edged sword for the liver? Surgeon 2012, 10, 107–113. [Google Scholar] [CrossRef]
- Beaudry, P.; Hadengue, A.; Callebert, J.; Gaudin, C.; Soliman, H.; Moreau, R.; Launay, J.M.; Lebrec, D. Blood and plasma 5-hydroxytryptamine levels in patients with cirrhosis. Hepatology 1994, 20, 800–803. [Google Scholar] [CrossRef]
- Marasini, B.; Biondi, M.L.; Agostoni, A. Platelet and plasma serotonin in patients with liver cirrhosis. J. Clin. Chem. Clin. Biochem. 1989, 27, 419–421. [Google Scholar] [CrossRef]
- Marwa Gamaleldin, A.; Walid Ellakany, I.; Marwa Saad, A.; Reham Aboelwafa, A. Serum serotonin as a non-invasive marker of portal hypertensive gastropathy in Egyptian patients with HCV-related liver cirrhosis. Acta Gastroenterol. Belg. 2022, 85, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Moreau, R.; Fenaille, F.; Amorós, A.; Junot, C.; Gronbaek, H.; Coenraad, M.J.; Pruvost, A.; Ghettas, A.; Chu-Van, E.; et al. Orchestration of Tryptophan-Kynurenine Pathway, Acute Decompensation, and Acute-on-Chronic Liver Failure in Cirrhosis. Hepatology 2019, 69, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Alieva, A.; Kashuh, E.; Tsvetaeva, E.; Poluektova, E.; Shirokova, E.; Ivashkin, K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J. Hepatol. 2021, 13, 557–570. [Google Scholar] [CrossRef]
- Butterworth, R.F. The neurobiology of hepatic encephalopathy. Semin. Liver Dis. 1996, 16, 235–244. [Google Scholar] [CrossRef]
- Chojnacki, C.; Walecka-Kapica, E.; Klupińska, G.; Wachowska-Kelly, P.; Żylińska, K.; Winczyk, K.; Chojnacki, J. Serotonin and melatonin secretion and metabolism in patients with liver cirrhosis. Pol. Arch. Med. Wewn. 2012, 122, 392–397. [Google Scholar] [CrossRef]
- Maitre, M.; Taleb, O.; Jeltsch-David, H.; Klein, C.; Mensah-Nyagan, A.G. Xanthurenic acid: A role in brain intercellular signaling. J. Neurochem. 2024, 168, 2303–2315. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.; Zuo, S.; Cao, K.; Li, H. Integrative analysis of the gut microbiota and faecal and serum short-chain fatty acids and tryptophan metabolites in patients with cirrhosis and hepatic encephalopathy. J. Transl. Med. 2023, 21, 395. [Google Scholar] [CrossRef]
- Komrower, G.M.; Westall, R. Hydroxykynureninuria. Am. J. Dis. Child. 1967, 113, 77–80. [Google Scholar] [CrossRef]






| Control (n = 14) | Steatosis MAFLD (n = 22) | Steatohepatitis MAFLD (n = 13) | Cirrhosis MAFLD (n = 9) | Steatosis ALD (n = 2) | Hepatitis ALD (n = 7) | Cirrhosis ALD (n = 31) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age, years | 40.50 [29.75–44.75] | 57.50 ** [49.25–65.00] | 48.00 [39.00–58.00] | 59.00 [54.00–66.00] | 40.00 [36.50–43.50] | 56.00 [48.00–65.00] | 52.00 ** [41.50–60.00] | <0.001 |
| Male/Female | 3/11 | 9/13 | 6/7 | 1/8 | 2/0 | 4/3 | 16/15 | 0.251 |
| Body mass index, kg/m2 | 22.71 ± 2.99 [20.99–24.44] | 30.59 ± 5.30 ** [28.17–33.00] | 31.43 ± 7.67 ** [26.79–36.06] | 32.56 ± 4.43 ** [29.16–35.97] | 29.82 [28.21–31.42] | 27.44 [24.28–34.36] | 25.70 [22.02–31.63] | <0.001 |
| Serum HDL cholesterol, mmol/L | 1.47 [1.47–1.47] | 1.10 ** [0.92–1.14] | 1.14 [0.92–1.65] | 1.25 [0.86–1.35] | 1.15 [1.07–1.22] | 1.47 [0.99–1.74] | 1.05 ** [0.82–1.33] | 0.004 |
| Serum LDL cholesterol, mmol/L | 2.75 [2.75–2.75] | 3.61 ** [3.38–4.38] | 3.38 [2.89–3.41] | 3.41 [3.31–4.29] | 3.85 [3.74–3.96] | 4.21 ** [3.96–4.27] | 2.80 [2.10–4.21] | 0.004 |
| Serum VLDL cholesterol, mmol/L | 0.36 [0.36–0.36] | 0.85 * [0.67–1.19] | 0.87 * [0.68–0.89] | 0.94 * [0.87–1.18] | 0.54 [0.45–0.64] | 0.62 [0.53–0.80] | 0.46 [0.41–0.54] | <0.001 |
| Serum glucose, μmol/L | 4.80 [4.35–5.48] | 5.10 [4.81–5.70] | 5.70 [5.50–7.18] | 6.90 ** [5.36–7.50] | 5.02 [4.96–5.07] | 6.07 [5.39–6.45] | 5.30 [4.99–6.08] | 0.011 |
| Alanine Aminotransferase, U/L | 17.00 [11.00–21.00] | 31.00 ■ [18.00–32.00] | 76.00 * [50.00–84.00] | 35.00 ** [28.00–47.00] | 27.00 [20.50–33.50] | 72.00 * [60.00–75.00] | 23.00 ■■ [18.00–35.00] | <0.001 |
| Aspartate aminotransferase, U/L | 20.00 [17.00–23.00] | 24.00 ■■ [21.00–26.00] | 36.00 ** [30.00–51.00] | 44.00 ** [40.00–51.00] | 20.00 [16.50–23.50] | 79.00 * [48.00–90.50] | 55.00 * [34.50–61.50] | <0.001 |
| IgG, g/L | 11.10 [11.10–11.10] | 11.10 [10.27–11.60] | 11.10 [9.50–11.20] | 12.60 [11.10–15.80] | 10.69 [10.48–10.89] | 14.60 [14.28–15.05] | 16.10 * [14.38–19.16] | <0.001 |
| IgM, g/L | 1.40 [1.40–1.40] | 0.90 [0.72–1.25] | 0.87 ** [0.71–1.21] | 1.27 [0.90–1.68] | 1.43 [1.42–1.44] | 1.30 [1.29–1.76] | 2.10 [1.33–3.16] | <0.001 |
| IgA, g/L | 2.00 [2.00–2.00] | 2.50 [2.26–2.57] | 2.50 [2.27–2.52] | 2.50 [2.50–3.72] | 2.00 [2.00–2.00] | 3.70 ** [3.70–3.72] | 5.70 * □ [4.46–7.81] | <0.001 |
| Platelets, 109/L | 307.50 [253.25–369.50] | 247.50 [216.50–265.75] | 256.00 [226.00–272.00] | 162.00 * □ [113.00–178.00] | 265.50 [265.25–265.75] | 277.00 [255.50–303.50] | 139.00 * ■■ [81.00–195.00] | <0.001 |
| Red blood cells, 109/L | 4.54 [4.33–4.79] | 4.80 [4.56–5.05] | 4.67 [4.46–4.98] | 4.51 [4.22–4.69] | 5.24 [4.97–5.51] | 5.97 [5.64–8.28] | 4.99 ** □ ■■ [3.96–6.87] | <0.001 |
| INR | 1.06 ± 0.10 [1.00–1.12] | 1.01 ± 0.07 [0.98–1.05] | 1.02 ± 0.08 [0.97–1.07] | 1.13 ± 0.09 [1.06–1.20] | 1.08 [1.07–1.08] | 1.00 [0.93–1.06] | 1.36 * ■ [1.22–1.65] | <0.001 |
| Control (n = 14) | MAFLD (n = 44) | ALD (n = 40) | MAFLD vs. ALD | MAFLD vs. CON | ALD vs. CON | |
|---|---|---|---|---|---|---|
| 3-OH Anthranilic acid | 0.03 [0.02–0.03] | 0.06 [0.04–0.12] | 0.04 [0.02–0.07] | 0.047 * | 0.002 * | 0.066 |
| 5-Hydroxytryptophan | 3.25 [2.96–3,68] | 3.87 [3.06–5.07] | 3.33 [2.14–4.18] | 0.036 * | 0.161 | 0.970 |
| 5-Methoxytryptamine | 0.80 [0.64–0.92] | 0.82 [0.68–0.91] | 0.64 [0.52–0.75] | <0.001 * | 0.806 | 0.044 * |
| Kynurenine | 1.40 [1.10–1.60] | 1.70 [1.31–2.11] | 1.94 [1.44–2.49] | 0.402 | 0.038 * | 0.011 * |
| Serotonin | 1.80 [1.16–2.25] | 1.08 [0.79–1.62] | 0.45 [0.24–0.89] | <0.001 * | 0.049 * | <0.001 * |
| Control (n = 14) | Steatosis MAFLD (n = 22) | Steatohepatitis MAFLD (n = 13) | Cirrhosis MAFLD (n = 9) | Steatosis ALD (n = 2) | Hepatitis ALD (n = 7) | Cirrhosis ALD (n = 31) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Kynurenine | 1.40 [1.10–1.60] | 1.66 [1.08–2.02] | 1.49 [1.29–1.95] | 2.02 ** [1.83–2.61] | 1.61 [1.53–1.70] | 1.41 [0.93–1.70] | 2.26 ** [1.59–2.80] | 0.003 |
| Serotonin | 1.80 [1.16–2.25] | 1.08 [0.84–1.47] | 1.73 [1.12–2.10] | 0.73 ** [0.28–1.01] | 2.32 [2.21–2.44] | 1.22 [0.86–1.60] | 0.34 * □ ■ [0.21–0.58] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshetova, M.; Markin, P.; Appolonova, S.; Yunusov, I.; Zolnikova, O.; Bueverova, E.; Dzhakhaya, N.; Zharkova, M.; Poluektova, E.; Maslennikov, R.; et al. Tryptophan Metabolites in the Progression of Liver Diseases. Biomolecules 2024, 14, 1449. https://doi.org/10.3390/biom14111449
Reshetova M, Markin P, Appolonova S, Yunusov I, Zolnikova O, Bueverova E, Dzhakhaya N, Zharkova M, Poluektova E, Maslennikov R, et al. Tryptophan Metabolites in the Progression of Liver Diseases. Biomolecules. 2024; 14(11):1449. https://doi.org/10.3390/biom14111449
Chicago/Turabian StyleReshetova, Maria, Pavel Markin, Svetlana Appolonova, Ismail Yunusov, Oksana Zolnikova, Elena Bueverova, Natiya Dzhakhaya, Maria Zharkova, Elena Poluektova, Roman Maslennikov, and et al. 2024. "Tryptophan Metabolites in the Progression of Liver Diseases" Biomolecules 14, no. 11: 1449. https://doi.org/10.3390/biom14111449
APA StyleReshetova, M., Markin, P., Appolonova, S., Yunusov, I., Zolnikova, O., Bueverova, E., Dzhakhaya, N., Zharkova, M., Poluektova, E., Maslennikov, R., & Ivashkin, V. (2024). Tryptophan Metabolites in the Progression of Liver Diseases. Biomolecules, 14(11), 1449. https://doi.org/10.3390/biom14111449






