Disruption of Sex-Linked Sox3 Causes ZW Female-to-Male Sex Reversal in the Japanese Frog Glandirana rugosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Frogs
2.2. Gene Expression Analysis
2.3. Histochemical Staining with Anti-Frog Sox3 Antibody
2.3.1. Preparation of Antibody and Specificity Analysis
2.3.2. Whole Mount Immunostaining
2.3.3. Transversal Section Immunostaining
2.4. TALEN Knockout and Transgenesis
2.4.1. Sox3 TALEN Constructs
2.4.2. Ar Transgenesis Construct
2.4.3. Microinjection
2.4.4. Identification of Sox3 Mutations
3. Results
3.1. W-Borne Sox3 Is Dominantly Expressed in ZW Gonad of Early Tadpole
3.2. Sox3 Is Expressed in Somatic Cells of ZW Gonad
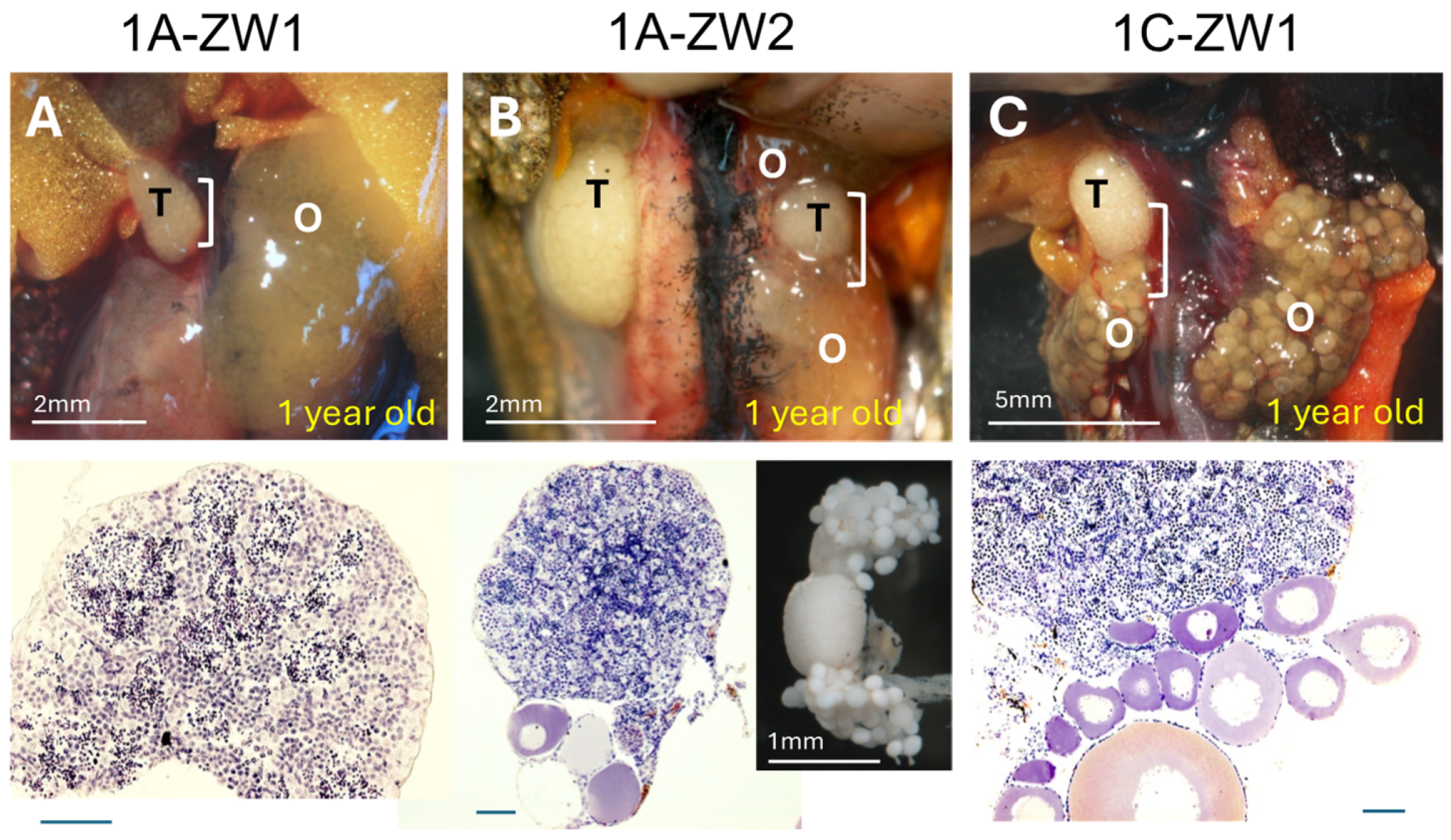
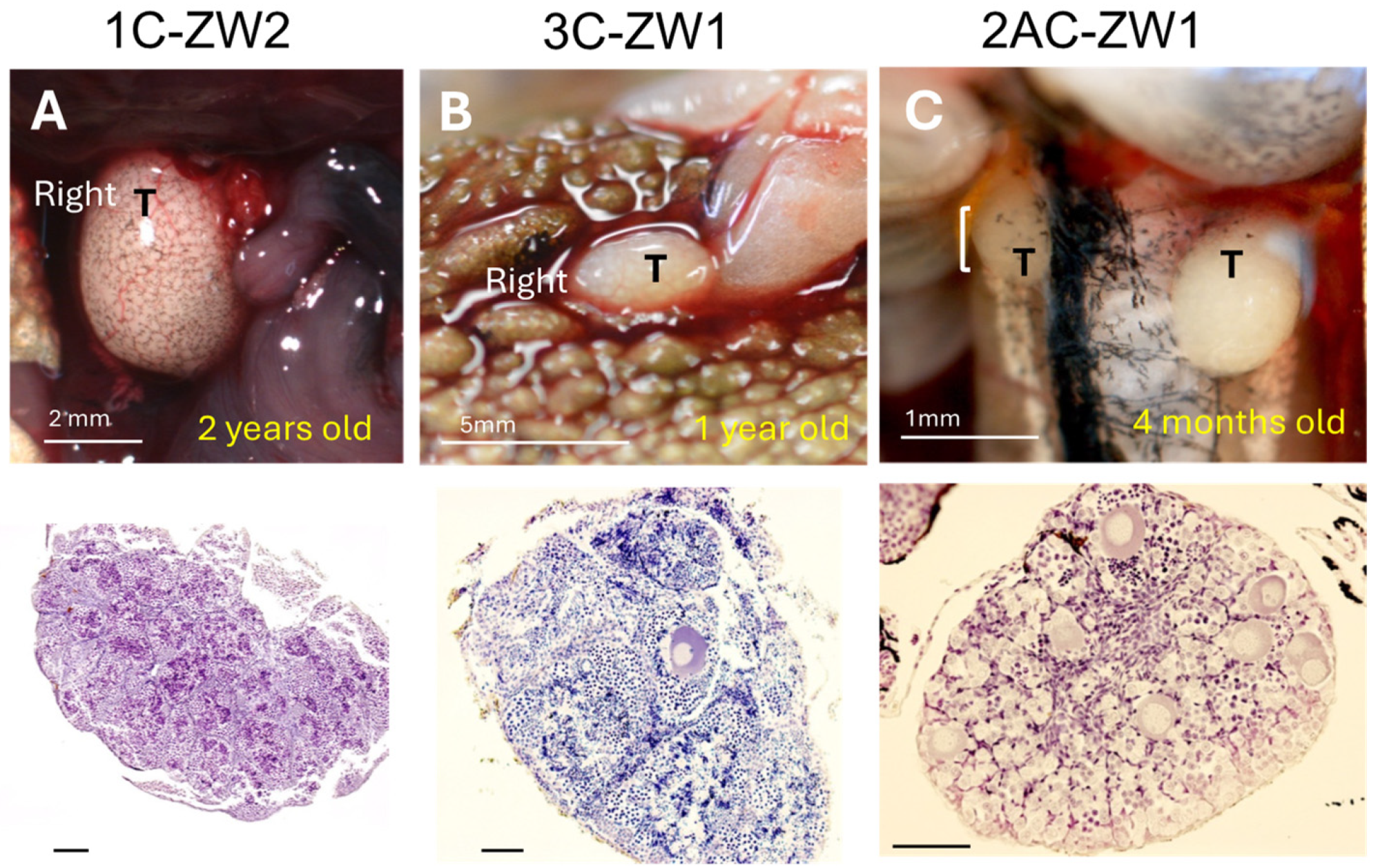
3.3. Disruption of Sox3 Causes ZW Female-to-Male Sex Reversal
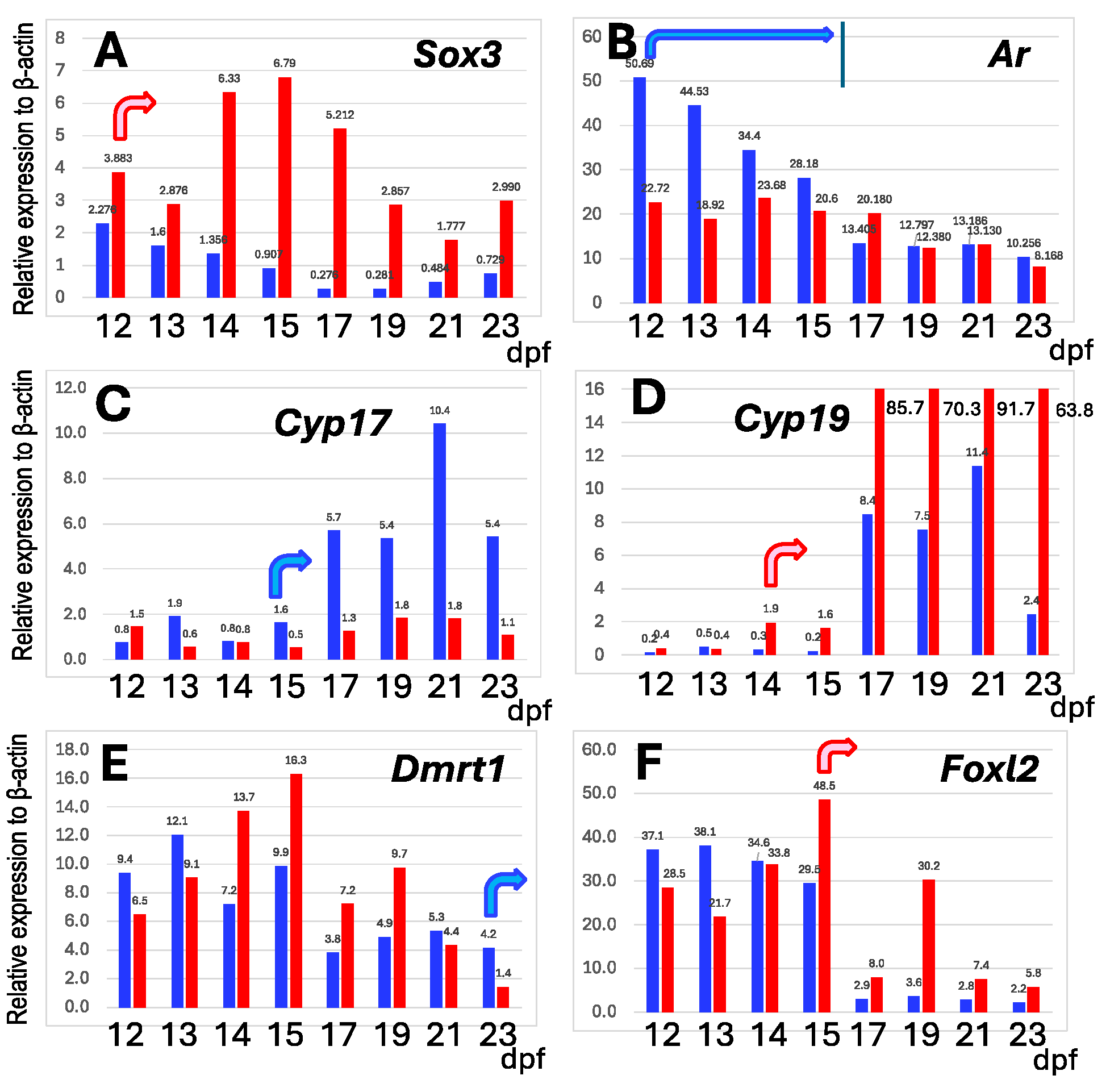
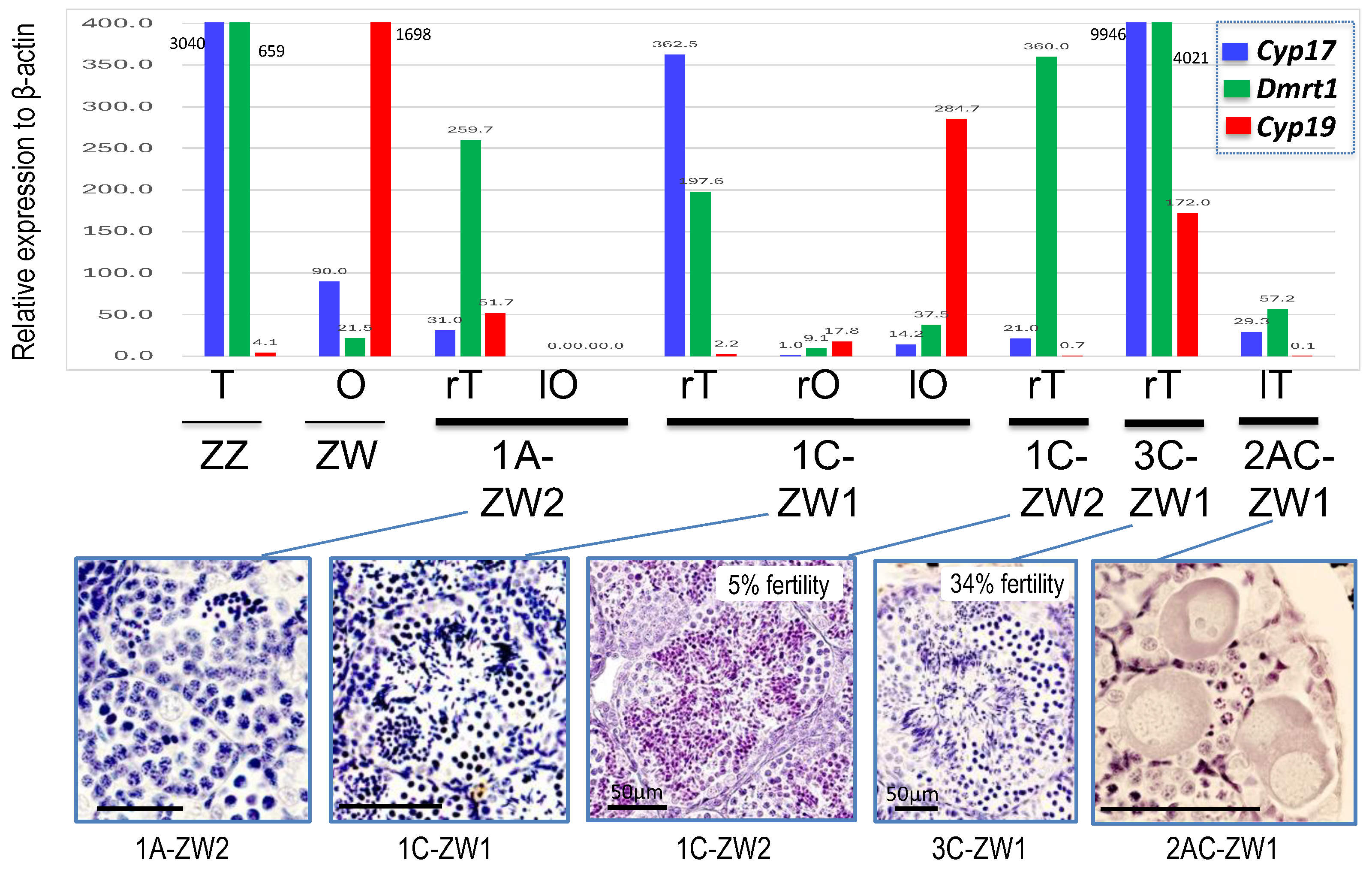
3.4. Change of Gene Expressions in ZW Testes by Disrupting Sox3
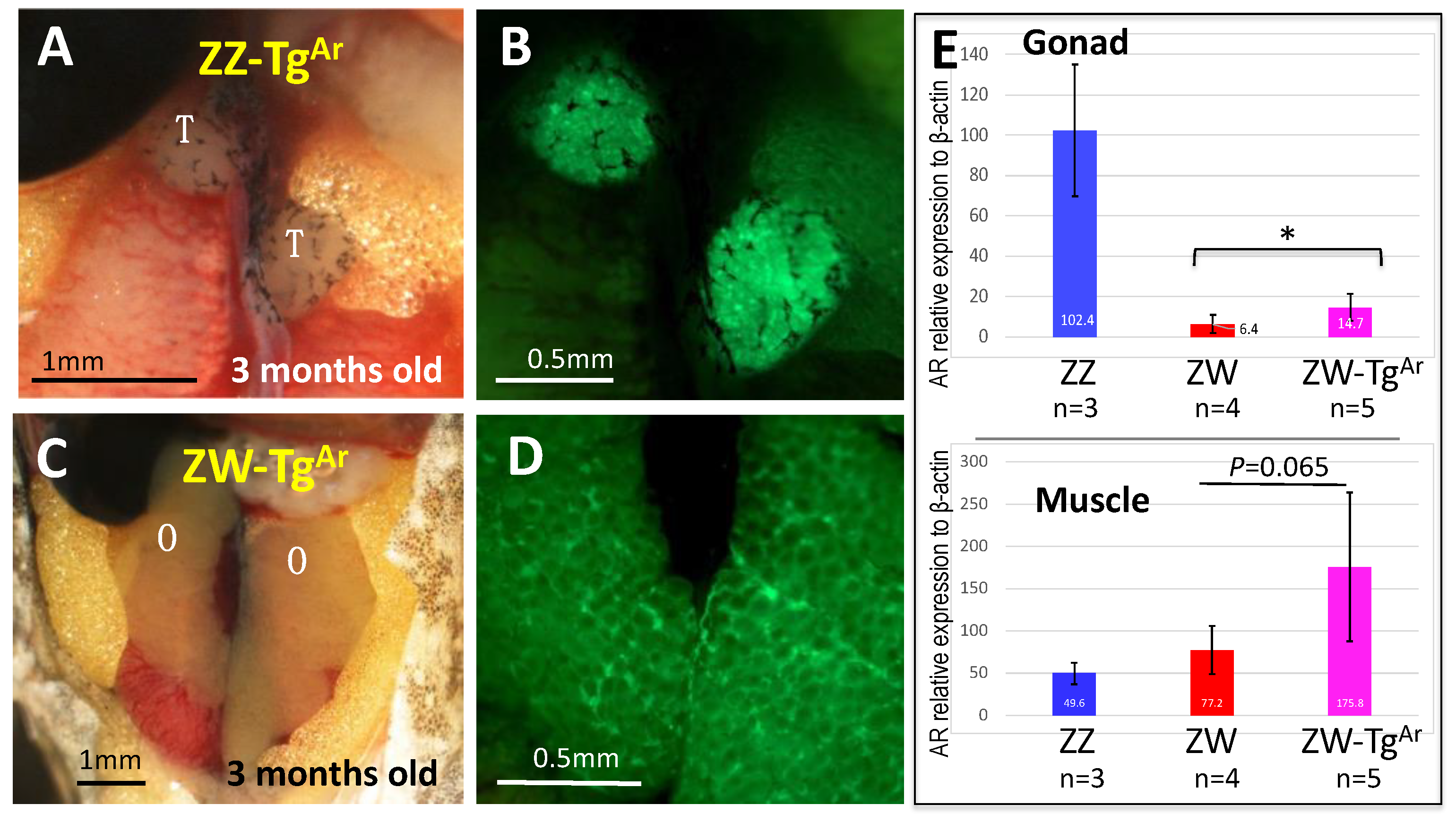
3.5. ZW Female Is Not Sex-Reversed by Up-Regulating Sex-Linked Androgen Receptor Gene
4. Discussion
4.1. Sex-Linked Sox3 Determines Femaleness in G. rugosa
4.2. Sex-Hormone Independent Sex-Determination in the Frog ZZ-ZW System
4.3. How Does Sox3 Determine an Ovary Differentiation in ZW Bipotential Gonad?
4.4. Sex-Linked Androgen Receptor Gene Is Not Involved in Sex Determination
4.5. Molecular Mechanisms of Female Heterogametic Sex Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bull, J.J. Evolution of Sex Determining Mechanisms; Benjamin/Cummings Pub. Co.: Menlo Park, CA, USA, 1983. [Google Scholar]
- Stöck, M.; Dedukh, D.; Reifová, R.; Lamatsch, D.K.; Starostová, Z.; Janko, K. Sex chromosomes in meiotic, hemiclonal, clonal and polyploid hybrid vertebrates: Along the ‘extended speciation continuum’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200103. [Google Scholar] [CrossRef] [PubMed]
- Kratochvíl, L.; Stöck, M.; Rovatsos, M.; Bullejos, M.; Herpin, A.; Jeffries, D.L.; Peichel, C.L.; Perrin, N.; Valenzuela, N.; Pokorná, M.J. Expanding the classical paradigm: What we have learnt from vertebrates about sex chromosome evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200097. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Veltsos, P. The Diversity and Evolution of Sex Chromosomes in Frogs. Genes 2021, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Chakraborty, T.; Paul-Prasanth, B.; Ohta, K.; Nakamur, M. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 2021, 101, 1237–1308. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hamaguchi, S. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 2013, 242, 339–353. [Google Scholar] [CrossRef]
- Kitano, J.; Ansai, S.; Takehana, Y.; Yamamoto, Y. Diversity and Convergence of Sex-Determination Mechanisms in Teleost Fish. Annu. Rev. Anim. Biosci. 2024, 12, 233–259. [Google Scholar] [CrossRef]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef]
- Lambeth, L.S.; Raymond, C.S.; Roeszler, K.N.; Kuroiwa, A.; Nakata, T.; Zarkower, D.; Smith, C.A. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 2014, 389, 160–172. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Okada, E.; Umemoto, H.; Tamura, K.; Uno, Y.; Nishida-Umehara, C.; Matsuda, Y.; Takamatsu, N.; Shiba, T.; Ito, M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA 2008, 10, 2469–2674. [Google Scholar] [CrossRef]
- Martínez, P.; Robledo, D.; Taboada, X.; Blanco, A.; Moser, M.; Maroso, F.; Hermida, M.; Gómez-Tato, A.; Álvarez-Blázquez, B.; Cabaleiro, S.; et al. A genome-wide association study, supported by a new chromosome-level genome assembly, suggests sox2 as a main driver of the undifferentiatiated ZZ/ZW sex determination of turbot (Scophthalmus maximus). Genomics 2021, 113, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wagner, S.; Holleley, C.E.; Deakin, J.E.; Matsubara, K.; Deveson, I.W.; O’Meally, D.; Patel, H.R.; Ezaz, T.; Li, Z.; et al. Sex-specific splicing of Z- and W-borne nr5a1 alleles suggests sex determination is controlled by chromosome conformation. Proc. Natl. Acad. Sci. USA 2022, 119, e2116475119. [Google Scholar] [CrossRef] [PubMed]
- Miura, I.; Shams, F.; Jeffries, D.L.; Katsura, Y.; Mawaribuchi, S.; Perrin, N.; Ito, M.; Ogata, M.; Ezaz, T. Identification of ancestral sex chromosomes in the frog Glandirana rugosa bearing XX-XY and ZZ-ZW sex-determining systems. Mol. Ecol. 2022, 1, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Mawaribuchi, S.; Ito, M.; Ogata, M.; Oota, H.; Katsumura, T.; Takamatsu, N.; Miura, I. Meiotic recombination counteracts male-biased mutation (male-driven evolution). Proc. Biol. Sci. 2016, 283, 20152691. [Google Scholar] [CrossRef]
- Oike, A.; Watanabe, K.; Min, M.S.; Tojo, K.; Kumagai, M.; Kimoto, Y.; Yamashiro, T.; Matsuo, T.; Kodama, M.; Nakamura, Y.; et al. Origin of sex chromosomes in six groups of Rana rugosa frogs inferred from a sex-linked DNA marker. J. Exp. Zool. A Ecol. Integr. Physiol. 2017, 327, 444–452. [Google Scholar] [CrossRef]
- Fujii, J.; Kodama, M.; Oike, A.; Matsuo, Y.; Min, M.S.; Hasebe, T.; Ishizuya-Oka, A.; Kawakami, K.; Nakamura, M. Involvement of androgen receptor in sex determination in an amphibian species. PLoS ONE 2014, 14, e93655. [Google Scholar] [CrossRef]
- Oike, A.; Kodama, M.; Yasumasu, S.; Yamamoto, T.; Nakamura, Y.; Ito, E.; Nakamura, M. Participation of androgen and its receptor in sex determination of an amphibian species. PLoS ONE 2017, 12, e0178067. [Google Scholar] [CrossRef]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef]
- Koopman, P.; Gubbay, J.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 1991, 351, 117–121. [Google Scholar] [CrossRef]
- Foster, J.W.; Graves, J.A. An SRY-related sequence on the marsupial X chromosome: Implications for the evolution of the mammalian testis-determining gene. Proc. Natl. Acad. Sci. USA 1994, 91, 1927–1931. [Google Scholar] [CrossRef]
- Katoh, K.; Miyata, T. A heuristic approach of maximum likelihood method for inferring phylogenetic tree and an application to the mammalian SOX-3 origin of the testis-determining gene SRY. FEBS Lett. 1999, 463, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin-I, T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A.; et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 2014, 5, 4157. [Google Scholar] [CrossRef] [PubMed]
- Myosho, T.; Takehana, Y.; Hamaguchi, S.; Sakaizumi, M. Turnover of Sex Chromosomes in Celebensis Group Medaka Fishes. G3 2015, 5, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M. Sex determination in amphibians. Semin. Cell Dev. Biol. 2009, 20, 271–282. [Google Scholar] [CrossRef]
- Oshima, Y.; Naruse, K.; Nakamura, Y.; Nakamura, M. Sox3: A transcription factor for Cyp19 expression in the frog Rana rugosa. Gene 2009, 445, 38–48. [Google Scholar] [CrossRef]
- Ogata, M.; Shams, S.; Yoshimura, Y.; Ezaz, T.; Miura, I. W Chromosome Evolution by Repeated Recycling in the Frog Glandirana rugosa. DNA 2022, 2, 172–184. [Google Scholar] [CrossRef]
- Miura, I.; Ohtani, H.; Ogata, M.; Ezaz, T. Evolutionary Changes in Sensitivity to Hormonally Induced Gonadal Sex Reversal in a Frog Species. Sex. Dev. 2016, 10, 79–90. [Google Scholar] [CrossRef]
- Mussolino, C.; Morbitzer, R.; Lütge, F.; Dannemann, N.; Lahaye, T.; Cathomen, T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011, 39, 9283–9293. [Google Scholar] [CrossRef]
- Berger, I.; Ryback, M.; Hotz, H. Artificial fertilization of water frogs. Amphibia-Reptilia 1994, 15, 408–413. [Google Scholar] [CrossRef]
- Ohtani, H.; Miura, I.; Ichikawa, Y. Role of aromatase and androgen receptor expression in gonadal sex differentiation of ZW/ZZ-type frogs, Rana rugosa. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 134, 215–225. [Google Scholar] [CrossRef]
- Ogino, H.; McConnell, W.B.; Grainger, R.M. High-throughput transgenesis in Xenopus using I-SceI meganuclease. Nat. Protoc. 2006, 1, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Molecular Cloning a Laboratory Manual, 4th ed.; Coldspring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
- Miura, I.; Ohtani, H.; Ogata, M. Independent degeneration of the W and Y sex chromosomes in frog Rana rugosa. Chromosome Res. 2012, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Meeks, J.J.; Hurley, L.; Raverot, G.; Frassetto, A.; Jameson, J.L. Sox3 is required for gonadal function, but not sex determination, in males and females. Mol. Cell Biol. 2003, 23, 8084–8091. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.; Hughes, J.; White, S.; Sekido, R.; Tan, J.; Arboleda, V.; Rogers, N.; Knower, K.; Rowley, L.; Eyre, H.; et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J. Clin. Investig. 2011, 121, 328–341. [Google Scholar] [CrossRef]
- Tilmann, C.; Capel, B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development 1999, 126, 2883–2890. [Google Scholar] [CrossRef]
- Combes, A.N.; Wilhelm, D.; Davidson, T.; Dejana, E.; Harley, V.; Sinclair, A.; Koopman, P. Endothelial cell migration directs testis cord formation. Dev. Biol. 2009, 326, 112–120. [Google Scholar] [CrossRef]
- Yokoyama, S.; Oshima, Y.; Tokita, J.; Suda, M.; Shinozuka, T.; Nakamura, M. Androgen receptor of the frog Rana rugosa: Molecular cloning and its characterization. J. Exp. Zool. A Ecol. Genet. Physiol. 2009, 311, 796–812. [Google Scholar] [CrossRef]
- Oike, A.; Kodama, M.; Nakamura, Y.; Nakamura, M. A Threshold Dosage of Testosterone for Female-to-Male Sex Reversal in Rana rugosa Frogs. J. Exp. Zool. A Ecol. Genet. Physiol. 2016, 325, 532–538. [Google Scholar] [CrossRef]
- Koyama, T.; Nakamoto, M.; Morishima, K.; Yamashita, R.; Yamashita, T.; Sasaki, K.; Kuruma, Y.; Mizuno, N.; Suzuki, M.; Okada, Y.; et al. A SNP in a Steroidogenic Enzyme Is Associated with Phenotypic Sex in Seriola Fishes. Curr. Biol. 2019, 29, 1901–1909. [Google Scholar] [CrossRef]
- Williams, C.A.C.; Soufi, A.; Pollard, S.M. Post-translational modification of SOX family proteins: Key biochemical targets in cancer? Semin. Cancer Biol. 2020, 67, 30–38. [Google Scholar] [CrossRef]
- Amaral, P.P.; Neyt, C.; Wilkins, S.J.; Askarian-Amiri, M.E.; Sunkin, S.M.; Perkins, A.C.; Mattick, J.S. Complex architecture and regulated expression of the Sox2ot locus during vertebrate development. RNA 2009, 15, 2013–2027. [Google Scholar] [CrossRef] [PubMed]
- Messemaker, T.C.; van Leeuwen, S.M.; van den Berg, P.R.; ‘t Jong, A.E.J.; Palstra, R.J.; Hoeben, R.C.; Semrau, S.; Mikkers, H.M.M. Allele-specific repression of Sox2 through the long non-coding RNA Sox2ot. Sci. Rep. 2018, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.D.; Harafuji, N.; Archer, T.; Cunningham, D.D.; Casey, E.S. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech. Dev. 2009, 126, 42–55. [Google Scholar] [CrossRef] [PubMed]
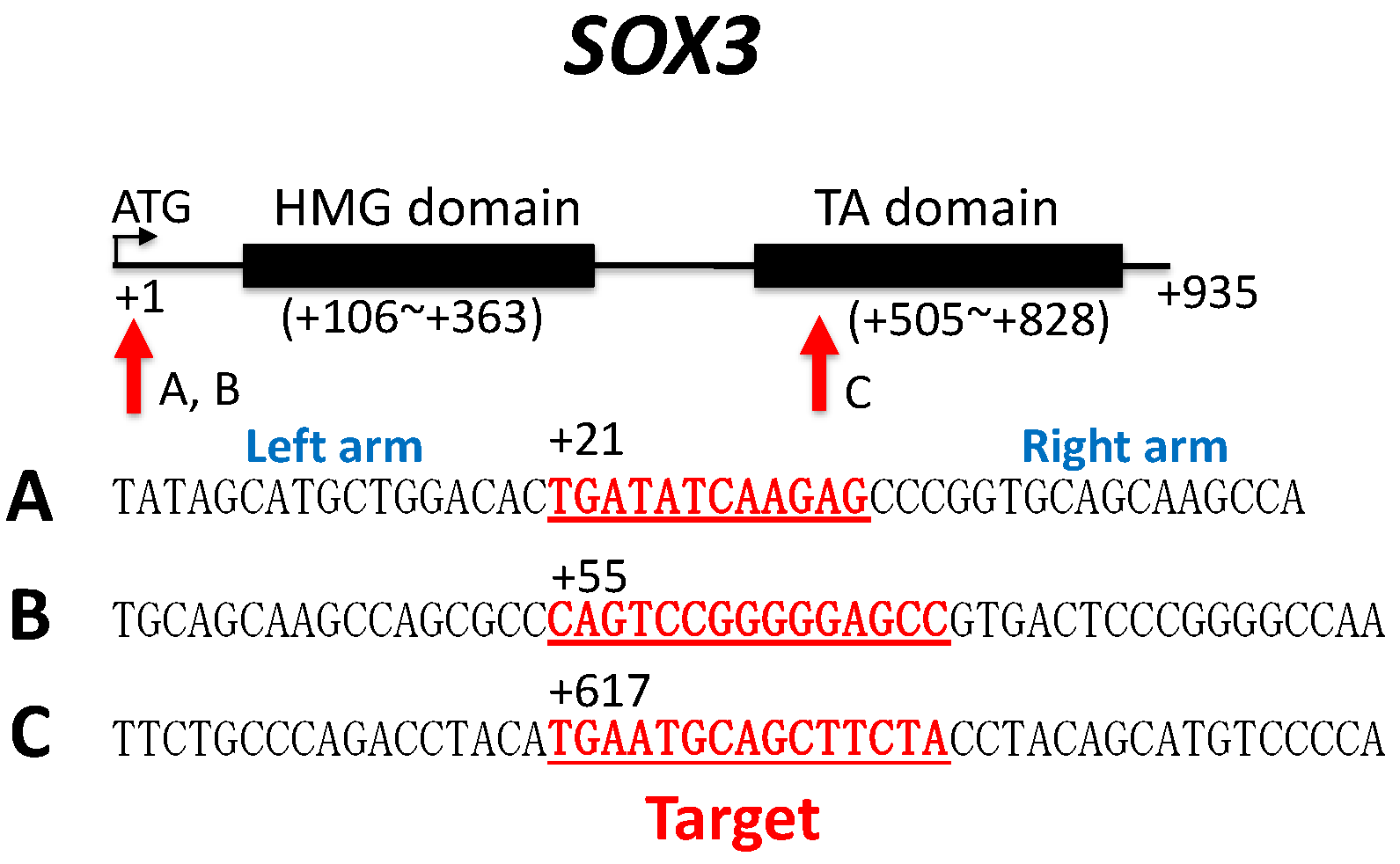



| Crossing | Target Sequence | Feeding Tadpole | Metamorphosed Frog | Genetic Sex | Phenotypic Sex | ||
|---|---|---|---|---|---|---|---|
| Male | Female | Total | |||||
| Na1 (1) | A | 25 | 15 | ZZ | 9 | 0 | 9 |
| ZW | 2 | 4 | 6 | ||||
| Na2 | B | 35 | 19 | ZZ | 14 | 0 | 14 |
| ZW | 0 | 5 | 5 | ||||
| Na1 | C | 36 | 26 | ZZ | 10 | 0 | 10 |
| ZW | 2 | 14 | 16 | ||||
| Na3 | C | 39 | 20 | ZZ | 8 | 0 | 8 |
| ZW | 1 | 11 | 12 | ||||
| Na2 | A and C | 19 | 19 | ZZ | 14 | 0 | 14 |
| ZW | 1 | 4 | 5 | ||||
| Na4 | B and C | 24 | 14 | ZZ | 6 | 0 | 6 |
| ZW | 0 | 8 | 8 | ||||
| Sex-Reversed ZW Males and Hermaphrodites | Gonad | |
|---|---|---|
| Right | Left | |
| 1A-ZW1 | Tests | Ovary |
| 1A-ZW2 | Testis | O-T-O (2) |
| 1C-ZW1 | T-O (1) | Ovary |
| 1C-ZW2 | Testis | Testis |
| 3C-ZW1 | Testis | Testis |
| 2AC-ZW1 | Testis | Testis |
| ZW Males and Hermaphrodites | Tissue DNA | No. of Clones Sequenced | Wild Type | Mutation (In-Frame) | Mutation Rate (%) |
|---|---|---|---|---|---|
| 1A-ZW1 | Right testis gDNA | 28 | 15 | 13 (1) | 46.4 |
| Left Ovary gDNA | 23 | 9 | 14 (0) | 60.9 | |
| 1A-ZW2 | Right testis cDNA | 22 | 10 | 12 (1) | 54.5 |
| Left Ovary cDNA | 14 | 10 | 3 (1) | 21.4 | |
| 1C-ZW1 | Right testis gDNA | 18 | 2 | 16 (0) | 88.9 |
| cDNA | 17 | 1 | 16 (2) | 94.1 | |
| Right Ovary gDNA | 24 | 4 | 20 (7) | 79.2 | |
| cDNA | 13 | 2 | 11 (3) | 84.6 | |
| Left Ovary gDNA | 19 | 6 | 13 (1) | 68.4 | |
| 1C-ZW2 | NE | ||||
| 3C-ZW1 | Right testis gDNA | 21 | 11 | 11 (0) | 47.6 |
| cDNA | 27 | 23 | 4 (4) | 14.8 | |
| 2AC-ZW1 | Right test gDNA | 16 | 6 | 10 (2) | 62.5 |
| cDNA | 13 | 5 | 8 (0) | 61.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, I.; Hasegawa, Y.; Ito, M.; Ezaz, T.; Ogata, M. Disruption of Sex-Linked Sox3 Causes ZW Female-to-Male Sex Reversal in the Japanese Frog Glandirana rugosa. Biomolecules 2024, 14, 1566. https://doi.org/10.3390/biom14121566
Miura I, Hasegawa Y, Ito M, Ezaz T, Ogata M. Disruption of Sex-Linked Sox3 Causes ZW Female-to-Male Sex Reversal in the Japanese Frog Glandirana rugosa. Biomolecules. 2024; 14(12):1566. https://doi.org/10.3390/biom14121566
Chicago/Turabian StyleMiura, Ikuo, Yoshinori Hasegawa, Michihiko Ito, Tariq Ezaz, and Mitsuaki Ogata. 2024. "Disruption of Sex-Linked Sox3 Causes ZW Female-to-Male Sex Reversal in the Japanese Frog Glandirana rugosa" Biomolecules 14, no. 12: 1566. https://doi.org/10.3390/biom14121566
APA StyleMiura, I., Hasegawa, Y., Ito, M., Ezaz, T., & Ogata, M. (2024). Disruption of Sex-Linked Sox3 Causes ZW Female-to-Male Sex Reversal in the Japanese Frog Glandirana rugosa. Biomolecules, 14(12), 1566. https://doi.org/10.3390/biom14121566









