Serum T2-High Inflammation Mediators in Eosinophilic COPD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design and Population
2.3. Pulmonary Function Tests
2.3.1. Spirometry
2.3.2. FeNO Test
2.3.3. Skin Prick Testing
2.4. Blood Tests
2.4.1. Complete Blood Count and Total IgE
2.4.2. Measurement of Serum Levels of Selected Analytes by Enzyme-Linked Immunosorbent Assay
2.5. Statistical Analysis
3. Results
3.1. Study Subject Characteristics
3.2. Serum Levels of Chronic Inflammation, Blood Oxidative Stress, and Collagen Degradation Markers in the Eosinophilic Type of Chronic Lung Diseases
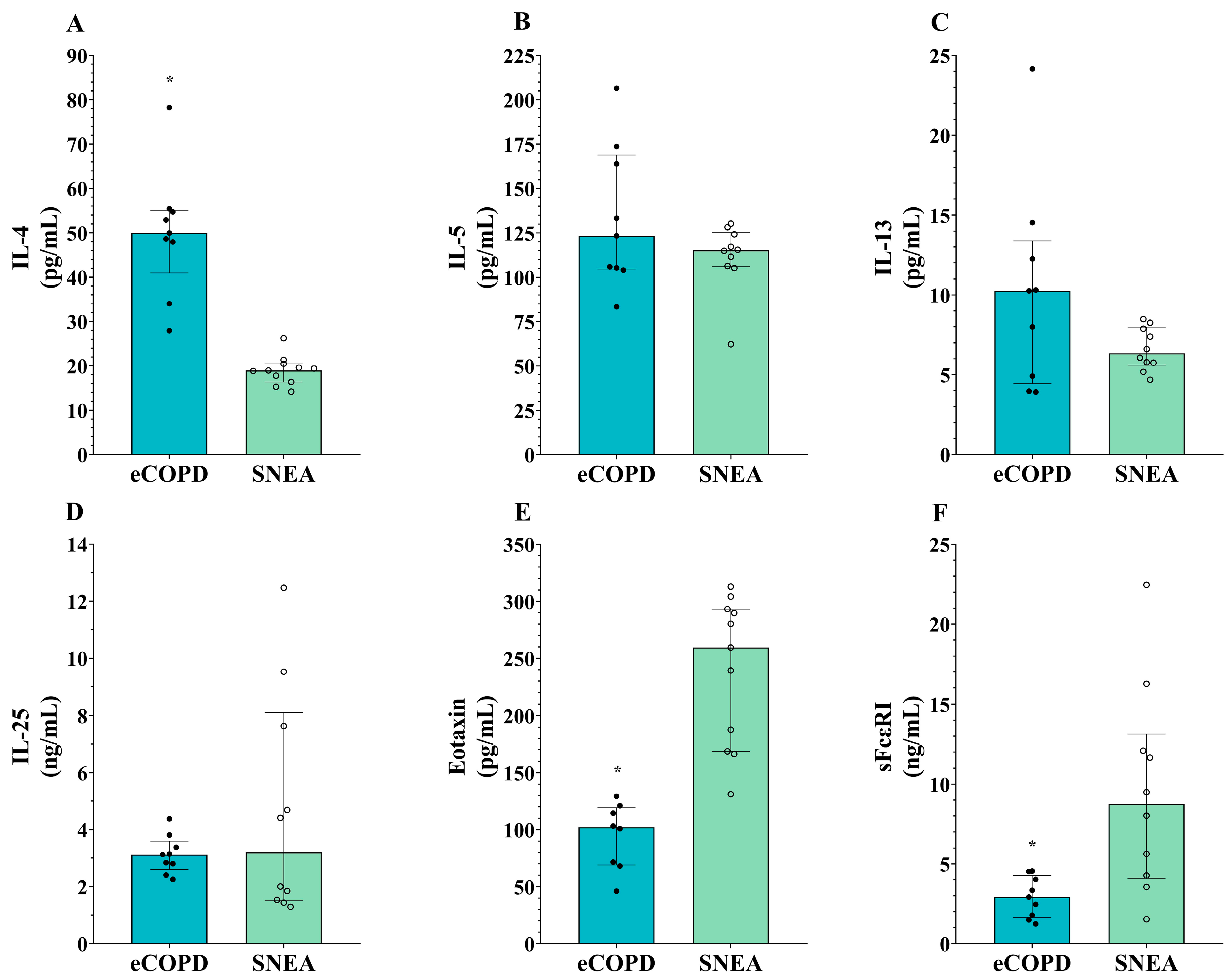
3.3. Heterogeneity in Serum Levels of T2-High Inflammation Mediators in eCOPD and Severe Eosinophilic Asthma Patients
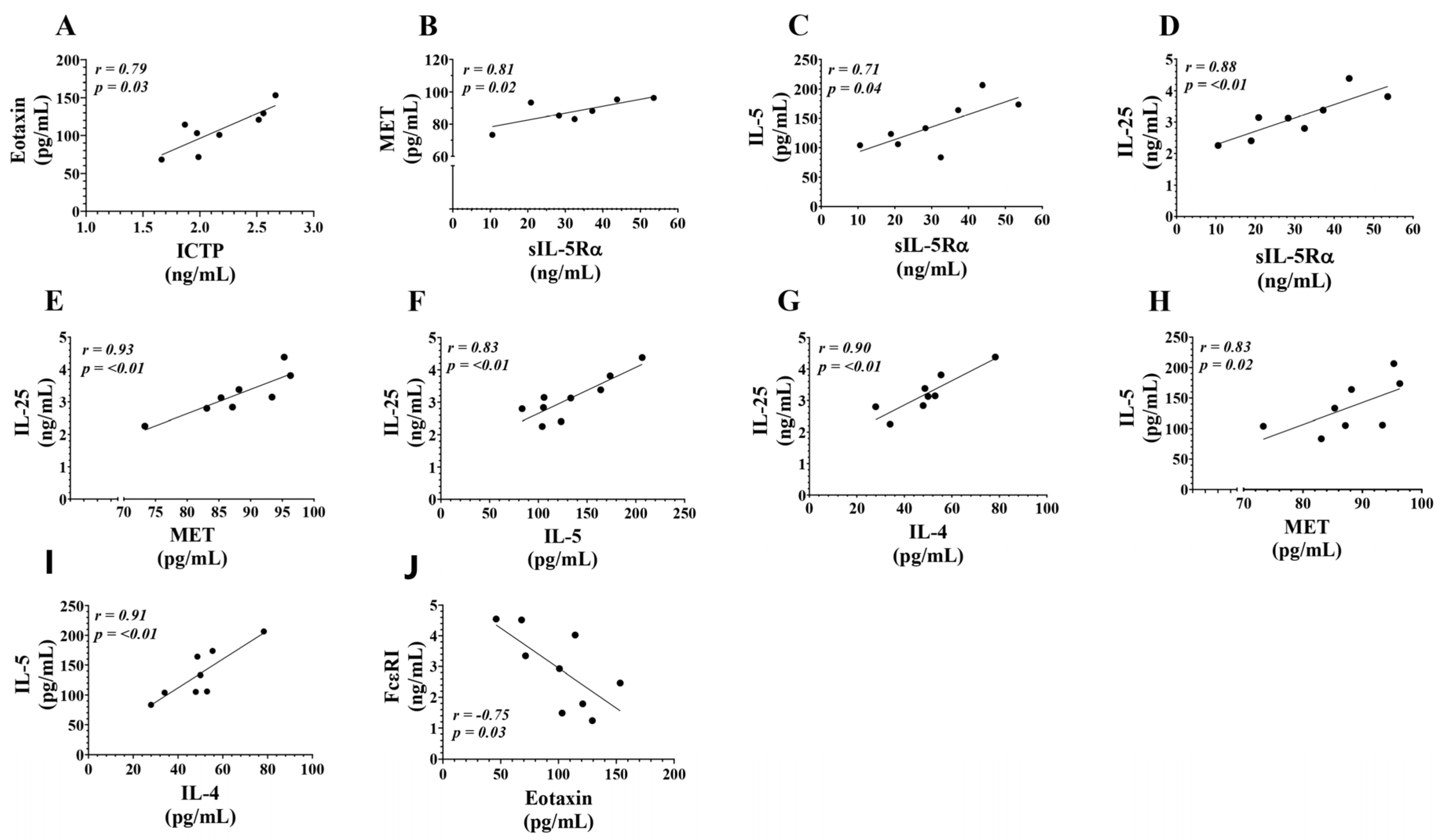
3.4. Correlations Between Serum Levels of T2-High Inflammation Mediators in eCOPD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R. Global burden of chronic obstructive pulmonary disease through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef] [PubMed]
- Varmaghani, M.; Dehghani, M.; Heidari, E.; Sharifi, F.; Moghaddam, S.S.; Farzadfar, F. Global prevalence of chronic obstructive pulmonary disease: Systematic review and meta-analysis. East. Mediterr. Health J. 2019, 25, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Cukic, V.; Lovre, V.; Dragisic, D.; Ustamujic, A. Asthma and chronic obstructive pulmonary disease (COPD)–differences and similarities. Mater. Socio-Medica 2012, 24, 100. [Google Scholar] [CrossRef]
- Saha, S.; Brightling, C.E. Eosinophilic airway inflammation in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2006, 1, 39–47. [Google Scholar] [CrossRef]
- Yun, J.H.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.M.; Saferali, A.; Vestbo, J.; Tal-Singer, R.; Castaldi, P.J.; Silverman, E.K. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047.e10. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kolsum, U.; Brightling, C.E.; Locantore, N.; Agusti, A.; Tal-Singer, R. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Bafadhel, M.; Pavord, I.D.; Russell, R.E. Eosinophils in COPD: Just another biomarker? Lancet Respir. Med. 2017, 5, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Ashdown, H.F.; Smith, M.; McFadden, E.; Pavord, I.D.; Butler, C.C.; Bafadhel, M. Blood eosinophils to guide inhaled maintenance therapy in a primary care COPD population. ERJ Open Res. 2022, 8, 606–2021. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Brightling, C.E. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016, 7, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Yii, A.; Tay, T.R.; Choo, X.; Koh, M.; Tee, A.; Wang, D.Y. Precision medicine in united airways disease: A “treatable traits” approach. Allergy 2018, 73, 1964–1978. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, S.; Timens, W.; Kaufmann, H.; van der Mark, T.W.; Koeter, G.; Postma, D. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur. Respir. J. 2000, 15, 109–115. [Google Scholar] [CrossRef]
- Higham, A.; Beech, A.; Wolosianka, S.; Jackson, N.; Long, G.; Kolsum, U.; Southworth, T. Type 2 inflammation in eosinophilic chronic obstructive pulmonary disease. Allergy 2021, 76, 1861–1864. [Google Scholar] [CrossRef]
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M.; Jones, P.; Gibson, P.G.; Vestbo, J. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.; Korn, S.; Lugogo, N.; Martinot, J.-B.; Sagara, H.; Albers, F.C.; Bradford, E.S. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Januskevicius, A.; Jurkeviciute, E.; Janulaityte, I.; Kalinauskaite-Zukauske, V.; Miliauskas, S.; Malakauskas, K. Blood eosinophils subtypes and their survivability in asthma patients. Cells 2020, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar]
- Palacionyte, J.; Januskevicius, A.; Vasyle, E.; Rimkunas, A.; Bajoriuniene, I.; Vitkauskiene, A.; Miliauskas, S.; Malakauskas, K. Novel Serum Biomarkers for Patients with Allergic Asthma Phenotype. Biomedicines 2024, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.; Criner, G.J.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.F. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, T.; Miravitlles, M.; Kocks, J.W. COPD management: Role of symptom assessment in routine clinical practice. Int. J. Chronic Obstr. Pulm. Dis. 2013, 8, 461–471. [Google Scholar] [CrossRef]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.-M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway remodeling in asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Celli, B.R.; Locantore, N.; Yates, J.; Tal-Singer, R.; Miller, B.E.; Bakke, P.; Calverley, P.; Coxson, H.; Crim, C.; Edwards, L.D. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Peterson, S.; De Blas, M.A.; Calverley, P.M.; Rennard, S.I.; Richter, K.; Fagerås, M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: A post-hoc analysis of three randomised trials. Lancet Respir. Med. 2018, 6, 117–126. [Google Scholar] [CrossRef]
- Fieldes, M.; Bourguignon, C.; Assou, S.; Nasri, A.; Fort, A.; Vachier, I.; De Vos, J.; Ahmed, E.; Bourdin, A. Targeted therapy in eosinophilic chronic obstructive pulmonary disease. ERJ Open Res. 2021, 7, 00437-2020. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.M.; Maric, I.; Shukla, J.; Brown, M.; Santos, C.; Simakova, O.; Khoury, P.; Fay, M.P.; Kozhich, A.; Kolbeck, R. IL-5 receptor α levels in patients with marked eosinophilia or mastocytosis. J. Allergy Clin. Immunol. 2011, 128, 1086–1092.e3. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, J.; Tuypens, T.; Plaetinck, G.; Verhee, A.; Fiers, W.; Devos, R. Molecular basis of the membrane-anchored and two soluble isoforms of the human interleukin 5 receptor alpha subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 7041–7045. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Sedgwick, J.B.; Bates, M.E.; Vrtis, R.F.; Gern, J.E.; Kita, H.; Jarjour, N.N.; Busse, W.W.; Kelly, E.A. Decreased expression of membrane IL-5 receptor α on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J. Immunol. 2002, 169, 6459–6466. [Google Scholar] [CrossRef]
- Kalinauskaite-Zukauske, V.; Januskevicius, A.; Janulaityte, I.; Miliauskas, S.; Malakauskas, K. Expression of eosinophil β chain-signaling cytokines receptors, outer-membrane integrins, and type 2 inflammation biomarkers in severe non-allergic eosinophilic asthma. BMC Pulm. Med. 2019, 19, 158. [Google Scholar] [CrossRef]
- Brightling, C.E.; Bleecker, E.R.; Panettieri, R.A.; Bafadhel, M.; She, D.; Ward, C.K.; Xu, X.; Birrell, C.; van der Merwe, R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir. Med. 2014, 2, 891–901. [Google Scholar] [CrossRef]
- Dasgupta, A.; Kjarsgaard, M.; Capaldi, D.; Radford, K.; Aleman, F.; Boylan, C.; Altman, L.C.; Wight, T.N.; Parraga, G.; O’Byrne, P.M. A pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur. Respir. J. 2017, 49, 1602486. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, M.; Zhan, D.; Zhang, Y.; Liu, S. Roles of the HGF/Met signaling in head and neck squamous cell carcinoma: Focus on tumor immunity. Oncol. Rep. 2020, 44, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Correll, K.; Zemans, R.L.; Leslie, C.C.; Murphy, R.C.; Mason, R.J. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L1178–L1188. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ueki, S.; Konno, Y.; Ito, W.; Takeda, M.; Nakamura, Y.; Nishikawa, J.; Moritoki, Y.; Omokawa, A.; Saga, T. The effect of hepatocyte growth factor on secretory functions in human eosinophils. Cytokine 2016, 88, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Organ, S.L.; Tsao, M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011, 3, S7–S19. [Google Scholar] [CrossRef]
- Mo, H.N.; Liu, P. Targeting MET in cancer therapy. Chronic Dis. Transl. Med. 2017, 3, 148–153. [Google Scholar] [CrossRef]
- Nath, D.; Williamson, N.J.; Jarvis, R.; Murphy, G. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J. Cell Sci. 2001, 114, 1213–1220. [Google Scholar] [CrossRef]
- Pryor, W.A.; Stone, K. Oxidants in cigarette smoke radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite A. Ann. N. Y. Acad. Sci. 1993, 686, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Modi, T.; Huihui, J.; Ghosh, K.; Ozkan, B. Ancient thioredoxins evolved to modern day stability-function requirement by altering native state ensemble. Biophys. J. 2018, 114, 231a. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Kishimoto, C.; Nakamura, H.; Makita, T.; Ishii, K.; Okuda, N.; Yodoi, J.; Sasayama, S. Serum thioredoxin and α-tocopherol concentrations in patients with major risk factors. Circ. J. 2005, 69, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.; Wang, C.; Fukunaga, A.; Li, S.; Yodoi, J.; Tian, H. Thioredoxin-1: A promising target for the treatment of allergic diseases. Front. Immunol. 2022, 13, 883116. [Google Scholar] [CrossRef]
- Kobayashi, N.; Yamada, Y.; Ito, W.; Ueki, S.; Kayaba, H.; Nakamura, H.; Yodoi, J.; Chihara, J. Thioredoxin reduces C-C chemokine-induced chemotaxis of human eosinophils. Allergy 2009, 64, 1130–1135. [Google Scholar] [CrossRef]
- Ichiki, H.; Hoshino, T.; Kinoshita, T.; Imaoka, H.; Kato, S.; Inoue, H.; Nakamura, H.; Yodoi, J.; Young, H.A.; Aizawa, H. Thioredoxin suppresses airway hyperresponsiveness and airway inflammation in asthma. Biochem. Biophys. Res. Commun. 2005, 334, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Herzenberg, L.A.; Bai, J.; Araya, S.; Kondo, N.; Nishinaka, Y.; Herzenberg, L.A.; Yodoi, J. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA 2001, 98, 15143–15148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, C.; Wu, J.; Fukunaga, A.; Cheng, Z.; Wang, J.; Yamauchi, A.; Yodoi, J.; Tian, H. Anti-allergic and anti-inflammatory effects and molecular mechanisms of thioredoxin on respiratory system diseases. Antioxid. Redox Signal. 2020, 32, 785–801. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, H.; Adachi, T.; Sannohe, S.; Oyamada, H.; Kayaba, H.; Yodoi, J.; Chihara, J. Elevated serum levels of thioredoxin in patients with acute exacerbation of asthma. Immunol. Lett. 2003, 86, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.M.; Leeming, D.; Papakonstantinou, E.; Blasi, F.; Kostikas, K.; Boersma, W.; Louis, R.; Milenkovic, B.; Aerts, J.; Sand, J.M. Collagen degradation and formation are elevated in exacerbated COPD compared with stable disease. Chest 2018, 154, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Timens, W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006, 3, 434–439. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Heriyanto, D.S.; Yuliani, F.S.; Laiman, V.; Choridah, L.; Lee, K.-Y.; Chang, J.-H.; Chung, K.F.; Chang, L.-T.; Chang, T.-Y. Eosinophilic inflammation: A key player in COPD pathogenesis and progression. Ann. Med. 2024, 56, 2408466. [Google Scholar] [CrossRef]
- Pelaia, C.; Heffler, E.; Crimi, C.; Maglio, A.; Vatrella, A.; Pelaia, G.; Canonica, G.W. Interleukins 4 and 13 in asthma: Key pathophysiologic cytokines and druggable molecular targets. Front. Pharmacol. 2022, 13, 851940. [Google Scholar] [CrossRef]
- Kokubo, K.; Onodera, A.; Kiuchi, M.; Tsuji, K.; Hirahara, K.; Nakayama, T. Conventional and pathogenic Th2 cells in inflammation, tissue repair, and fibrosis. Front. Immunol. 2022, 13, 945063. [Google Scholar] [CrossRef]
- Gevaert, P.; Wong, K.; Millette, L.A.; Carr, T.F. The role of IgE in upper and lower airway disease: More than just allergy! Clin. Rev. Allergy Immunol. 2022, 62, 200–215. [Google Scholar] [CrossRef]
- Criner, G.J.; Celli, B.R.; Brightling, C.E.; Agusti, A.; Papi, A.; Singh, D.; Sin, D.D.; Vogelmeier, C.F.; Sciurba, F.C.; Bafadhel, M. Benralizumab for the prevention of COPD exacerbations. N. Engl. J. Med. 2019, 381, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, T.-H.; Kang, S.-Y.; Park, H.J.; Lim, S.Y.; Kim, S.H.; Jung, K.-S.; Yoo, K.H.; Yoon, H.K.; Rhee, C.K. Association between Serum Levels of Interleukin-25/Thymic Stromal Lymphopoietin and the Risk of Exacerbation of Chronic Obstructive Pulmonary Disease. Biomolecules 2023, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xue, Z.; Yi, L.; Shi, H.; Zhang, K.; Huo, X.; Bonser, L.R.; Zhao, J.; Xu, Y.; Erle, D.J. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am. J. Respir. Crit. Care Med. 2014, 190, 639–648. [Google Scholar] [CrossRef]
- Williams, T.J. Eotaxin-1 (CCL11). Front. Immunol. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, P.C.; Fischetti, C.A.; McBride, M.L.; Hassman, L.M.; Hogan, S.P.; Rothenberg, M.E. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc. Natl. Acad. Sci. USA 2006, 103, 16418–16423. [Google Scholar] [CrossRef]
- Hartl, D.; Krauss-Etschmann, S.; Koller, B.; Hordijk, P.L.; Kuijpers, T.W.; Hoffmann, F.; Hector, A.; Eber, E.; Marcos, V.; Bittmann, I. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J. Immunol. 2008, 181, 8053–8067. [Google Scholar] [CrossRef] [PubMed]
- Messingham, K.N.; Holahan, H.M.; Frydman, A.S.; Fullenkamp, C.; Srikantha, R.; Fairley, J.A. Human eosinophils express the high affinity IgE receptor, FcεRI, in bullous pemphigoid. PLoS ONE 2014, 9, e107725. [Google Scholar] [CrossRef] [PubMed]
- Sihra, B.S.; Kon, O.M.; Grant, J.A.; Kay, A.B. Expression of high-affinity IgE receptors (FcϵRI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: Relationship to total serum IgE concentrations. J. Allergy Clin. Immunol. 1997, 99, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Canonica, G.W.; Matucci, A.; Paolini, R.; Triggiani, M.; Paggiaro, P. Targeted therapy in severe asthma today: Focus on immunoglobulin E. Drug Des. Dev. Ther. 2017, 11, 1979–1987. [Google Scholar] [CrossRef]
- Foster, B.; Metcalfe, D.D.; Prussin, C. Human dendritic cell 1 and dendritic cell 2 subsets express FcεRI: Correlation with serum IgE and allergic asthma. J. Allergy Clin. Immunol. 2003, 112, 1132–1138. [Google Scholar] [CrossRef]
- Miller, M.A.; Sullivan, R.J.; Lauffenburger, D.A. Molecular pathways: Receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin. Cancer Res. 2017, 23, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Lu, L.; Cai, S.; Chen, J.; Lin, W.; Han, F. Alternative splicing: A new cause and potential therapeutic target in autoimmune disease. Front. Immunol. 2021, 12, 713540. [Google Scholar] [CrossRef]
- Moñino-Romero, S.; Lexmond, W.S.; Singer, J.; Bannert, C.; Amoah, A.S.; Yazdanbakhsh, M.; Boakye, D.A.; Jensen-Jarolim, E.; Fiebiger, E.; Szépfalusi, Z. Soluble FcεRI: A biomarker for IgE-mediated diseases. Allergy 2019, 74, 1381. [Google Scholar] [CrossRef]
- Givi, M.; Blokhuis, B.; Da Silva, C.; Adcock, I.; Garssen, J.; Folkerts, G.; Redegeld, F.; Mortaz, E. Cigarette smoke suppresses the surface expression of c-kit and FcεRI on mast cells. Mediat. Inflamm. 2013, 2013, 813091. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.; Adir, Y.; Al-Ahmad, M.; Celis-Preciado, C.A.; Colodenco, F.D.; Giavina-Bianchi, P.; Lababidi, H.; Ledanois, O.; Mahoub, B.; Perng, D.-W. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022, 8, 576. [Google Scholar] [CrossRef] [PubMed]
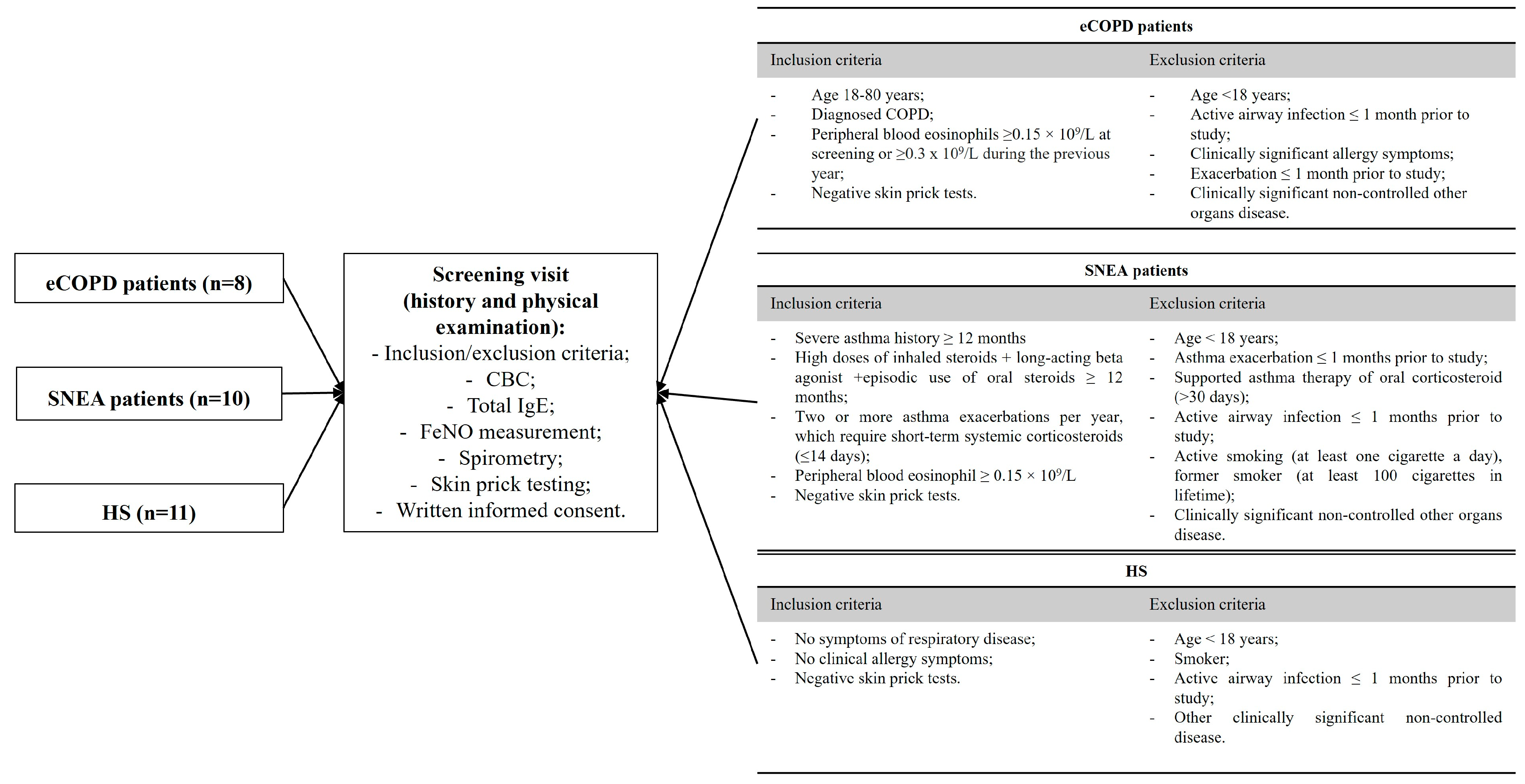



| Characteristic | eCOPD | SNEA | HS |
|---|---|---|---|
| Number, n | 8 | 10 | 11 |
| Sex, M/F | 6/2 | 4/6 | 4/7 |
| Age, years | 61.3 ± 3.4 # | 62.7 ± 1.0 # | 31.5 ± 2.8 |
| BMI, kg/m2 | 30.9 ± 1.4 # | 27.4 ± 2.0 | 25.0 ± 1.6 |
| FEV1, L | 1.8 ± 0.2 # | 1.8 ± 0.2 # | 3.8 ± 0.3 |
| FEV1, % of predicted | 47.9 ± 4.6 *# | 70.4 ± 8.2 # | 99.6 ± 6.3 |
| FeNO, ppb | 15.0 ± 4.1 * | 34.3 ± 6.5 # | 18.6 ± 6.2 |
| Blood EOS count, × 109/L | 0.27 ± 0.02 *# | 0.71 ± 0.16 # | 0.15 ± 0.04 |
| Total IgE, IU/mL | 101.8 ± 41.54 # | 168.3 ± 39.5 # | 16.7 ± 4.6 |
| eCOPD | SNEA | HS | |
|---|---|---|---|
| sIL-5Rα (ng/mL) | 28.3 * [14.7; 40.5] | 2.2 # [1.3; 6.7] | 25.2 [11.3; 33.2] |
| MET (pg/mL) | 87.1 *# [78.2; 94.3] | 58.7 [49.2; 66.5] | 51.5 [25.7; 83.8] |
| TRX1(ng/mL) | 85.5 * [69.4; 70.7] | 47.5 # [43.7; 50.8] | 98.0 [66.8; 123.9] |
| ICTP(ng/mL) | 2.1 *# [1.9; 2.5] | 1.4 # [1.0; 1.8] | 0.59 [0.54; 0.69] |
| eCOPD | SNEA | |
|---|---|---|
| IL-4 (pg/mL) | 50.0 * [41.0; 55.1] | 19.0 [16.4; 20.5] |
| IL-5 (pg/mL) | 123.4 [104.6; 168.8] | 115.1 [106.0; 125.3] |
| IL-13 (pg/mL) | 10.3 [4.4; 13.4] | 6.3 [5.6; 8.5] |
| IL-25 (ng/mL) | 3.1 [2.6; 3.6] | 3.2 [1.5; 8.1] |
| Eotaxin-1 (pg/mL) | 103.2 * [69.8; 125.1] | 259.4 [168.6; 293.1] |
| sFcεRI (ng/mL) | 2.9 * [1.6; 4.3] | 8.8 [4.1; 13.1] |
| ICTP | TRX1 | sIL-5Rα | MET | IL-4 | IL-5 | IL-13 | IL-25 | Eotaxin-1 | FcεRI | |
|---|---|---|---|---|---|---|---|---|---|---|
| ICTP | r = 0.28 (p = 0.46) | r = −0.03 (p = 0.95) | r = −0.20 (p = 0.61) | r = 0.40 (p = 0.29) | r = 0.17 (p = 0.68) | r = 0.22 (p = 0.58) | r = 0.03 (p = 0.95) | r = 0.79 (p = 0.03) | r = −0.55 (p = 0.13) | |
| TRX1 | r = 0.28 (p = 0.46) | r = 0.40 (p = 0.29) | r = 0.02 (p = 0.98) | r = 0.35 (p = 0.36) | r = 0.32 (p = 0.41) | r = 0.08 (p = 0.84) | r = 0.15 (p = 0.71) | r = −0.03 (p = 0.95) | r = 0.28 (p = 0.46) | |
| sIL-5Rα | r = −0.03 (p = 0.95) | r = 0.40 (p = 0.29) | r = 0.81 (p = 0.02) | r = 0.50 (p = 0.18) | r = 0.71 (p = 0.04) | r = 0.27 (p = 0.49) | r = 0.88 (p < 0.01) | r = −0.40 (p = 0.29) | r = 0.27 (p = 0.49) | |
| MET | r = −0.20 (p = 0.61) | r = 0.02 (p = 0.98) | r = 0.81 (p = 0.02) | r = 0.57 (p = 0.12) | r = 0.83 (p = 0.02) | r = −0.12 (p = 0.78) | r = 0.93 (p < 0.01) | r = −0.18 (p = 0.64) | r = −0.22 (p = 0.58) | |
| IL-4 | r = 0.40 (p = 0.29) | r = 0.35 (p = 0.36) | r = 0.50 (p = 0.18) | r = 0.57 (p = 0.12) | r = 0.91 (p < 0.01) | r = 0.50 (p = 0.18) | r = 0.90 (p < 0.01) | r = 0.47 (p = 0.21) | r = −0.47 (p = 0.21) | |
| IL-5 | r = 0.17 (p = 0.68) | r = 0.32 (p = 0.41) | r = 0.71 (p = 0.04) | r = 0.83 (p = 0.02) | r = 0.91 (p < 0.01) | r = 0.48 (p = 0.19) | r = 0.83 (p < 0.01) | r = 0.15 (p = 0.71) | r = −0.32 (p = 0.41) | |
| IL-13 | r = 0.22 (p = 0.58) | r = 0.08 (p = 0.84) | r = 0.27 (p = 0.49) | r = −0.12 (p = 0.78) | r = 0.50 (p = 0.18) | r = 0.48 (p = 0.19) | r = 0.05 (p = 0.91) | r = 0.42 (p = 0.27) | r = −0.07 (p = 0.88) | |
| IL-25 | r = 0.03 (p = 0.95) | r = 0.15 (p = 0.71) | r = 0.88 (p < 0.01) | r = 0.93 (p < 0.01) | r = 0.90 (p < 0.01) | r = 0.83 (p < 0.01) | r = 0.05 (p = 0.91) | r = −0.12 (p = 0.78) | r = −0.28 (p = 0.46) | |
| Eotaxin-1 | r = 0.79 (p = 0.03) | r = −0.03 (p = 0.95) | r = −0.40 (p = 0.29) | r = −0.18 (p = 0.64) | r = 0.47 (p = 0.21) | r = 0.15 (p = 0.71) | r = 0.42 (p = 0.27) | r = −0.12 (p = 0.78) | r = −0.75 (p = 0.03) | |
| FcεRI | r = −0.55 (p = 0.13) | r = 0.28 (p = 0.46) | r = 0.27 (p = 0.49) | r = −0.22 (p = 0.58) | r = −0.47 (p = 0.21) | r = −0.32 (p = 0.41) | r = −0.07 (p = 0.88) | r = −0.28 (p = 0.46) | r = −0.75 (p = 0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januskevicius, A.; Vasyle, E.; Rimkunas, A.; Palacionyte, J.; Kalinauskaite-Zukauske, V.; Malakauskas, K. Serum T2-High Inflammation Mediators in Eosinophilic COPD. Biomolecules 2024, 14, 1648. https://doi.org/10.3390/biom14121648
Januskevicius A, Vasyle E, Rimkunas A, Palacionyte J, Kalinauskaite-Zukauske V, Malakauskas K. Serum T2-High Inflammation Mediators in Eosinophilic COPD. Biomolecules. 2024; 14(12):1648. https://doi.org/10.3390/biom14121648
Chicago/Turabian StyleJanuskevicius, Andrius, Egle Vasyle, Airidas Rimkunas, Jolita Palacionyte, Virginija Kalinauskaite-Zukauske, and Kestutis Malakauskas. 2024. "Serum T2-High Inflammation Mediators in Eosinophilic COPD" Biomolecules 14, no. 12: 1648. https://doi.org/10.3390/biom14121648
APA StyleJanuskevicius, A., Vasyle, E., Rimkunas, A., Palacionyte, J., Kalinauskaite-Zukauske, V., & Malakauskas, K. (2024). Serum T2-High Inflammation Mediators in Eosinophilic COPD. Biomolecules, 14(12), 1648. https://doi.org/10.3390/biom14121648






