Osteogenic Potential of a Biomaterial Enriched with Osteostatin and Mesenchymal Stem Cells in Osteoporotic Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Scaffolds
2.2. Osteostatin Loading and Mesenchymal Cell Seeding
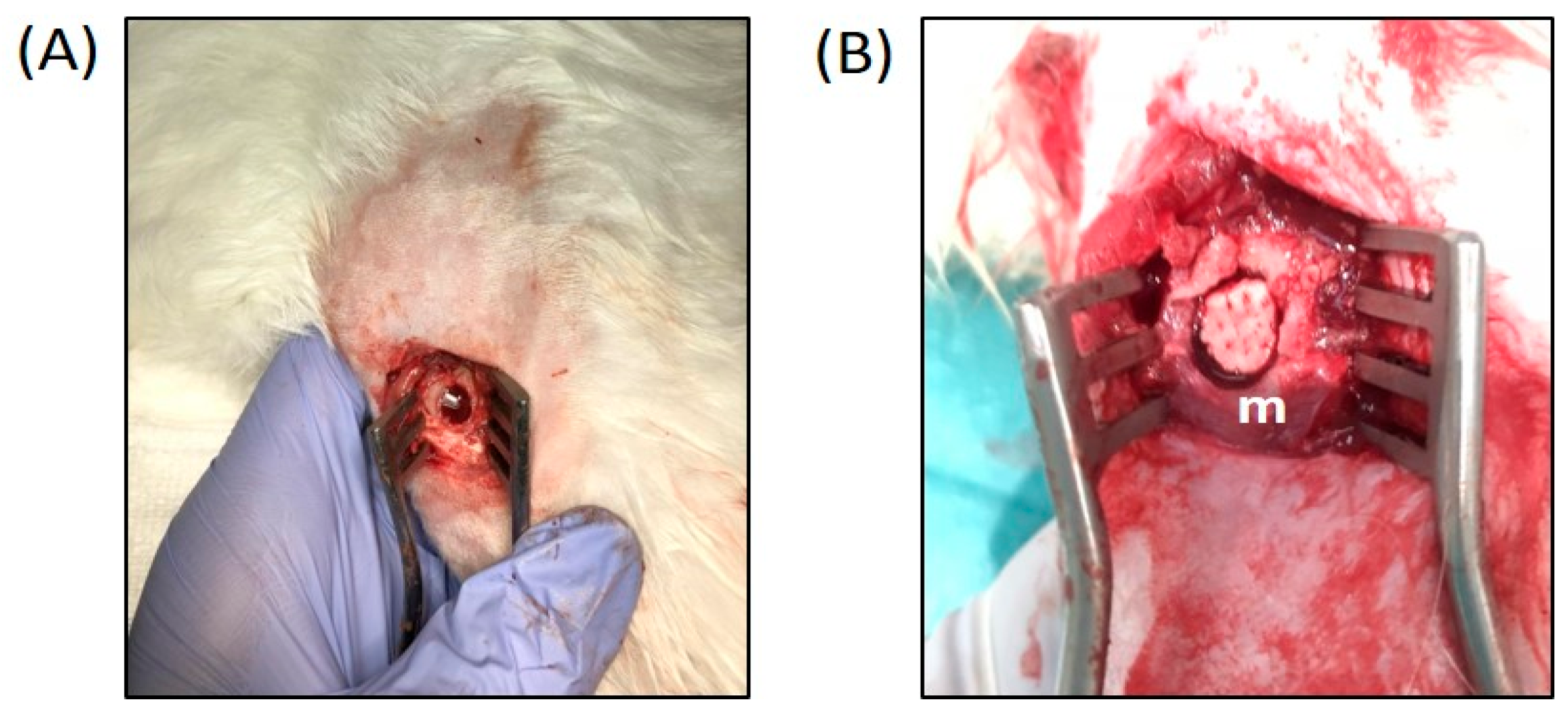
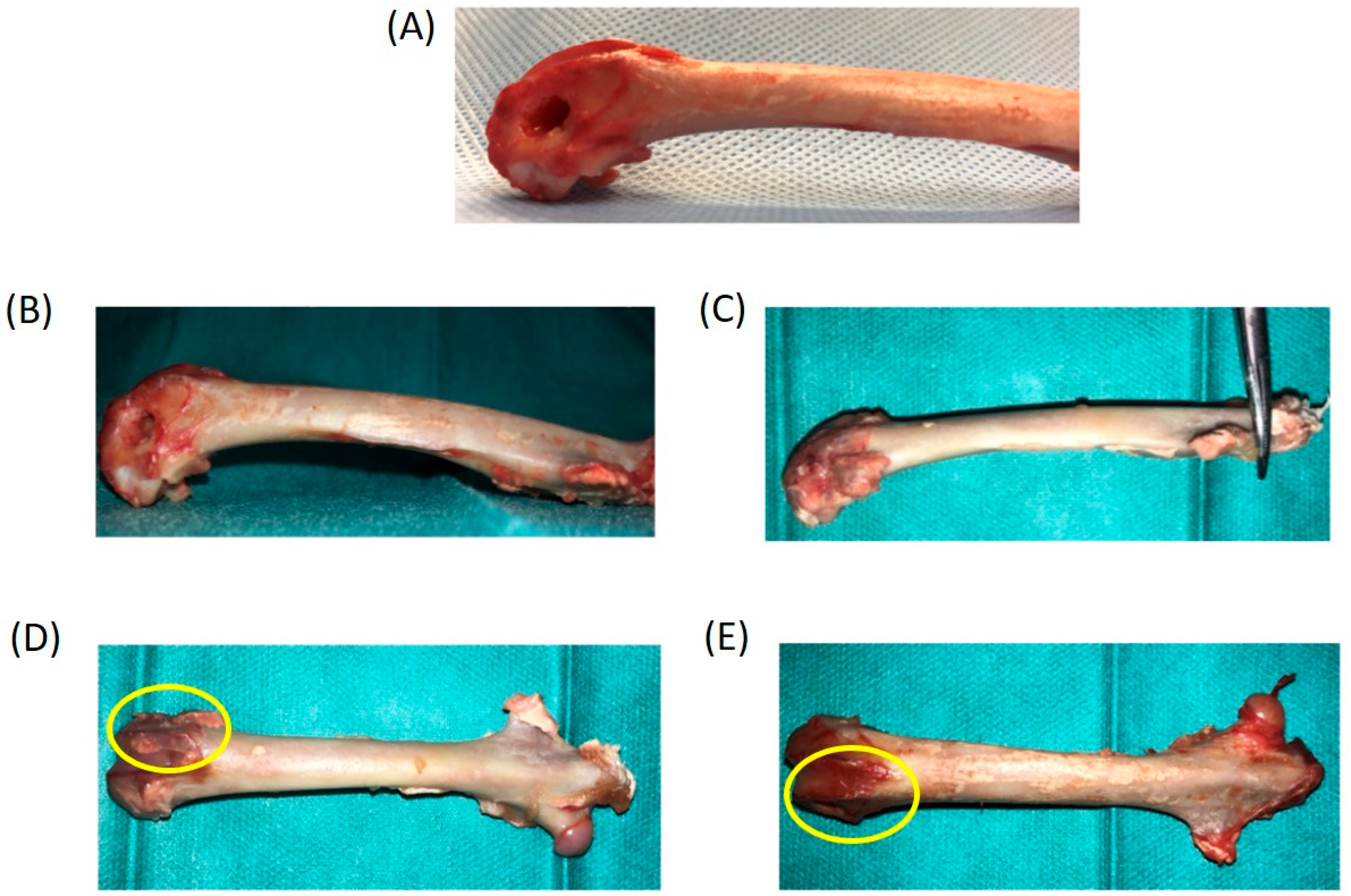
2.3. Surgical Procedure
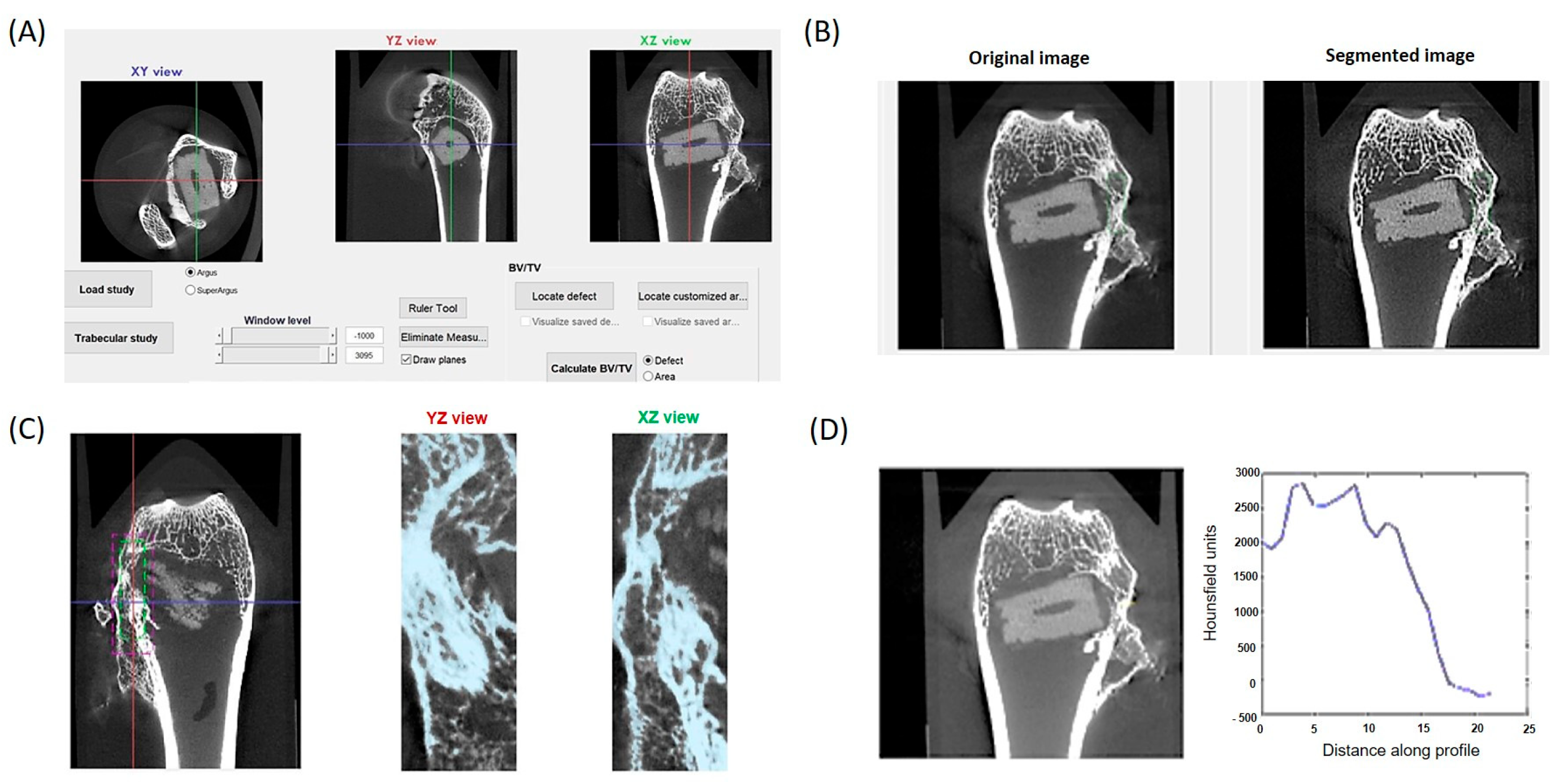
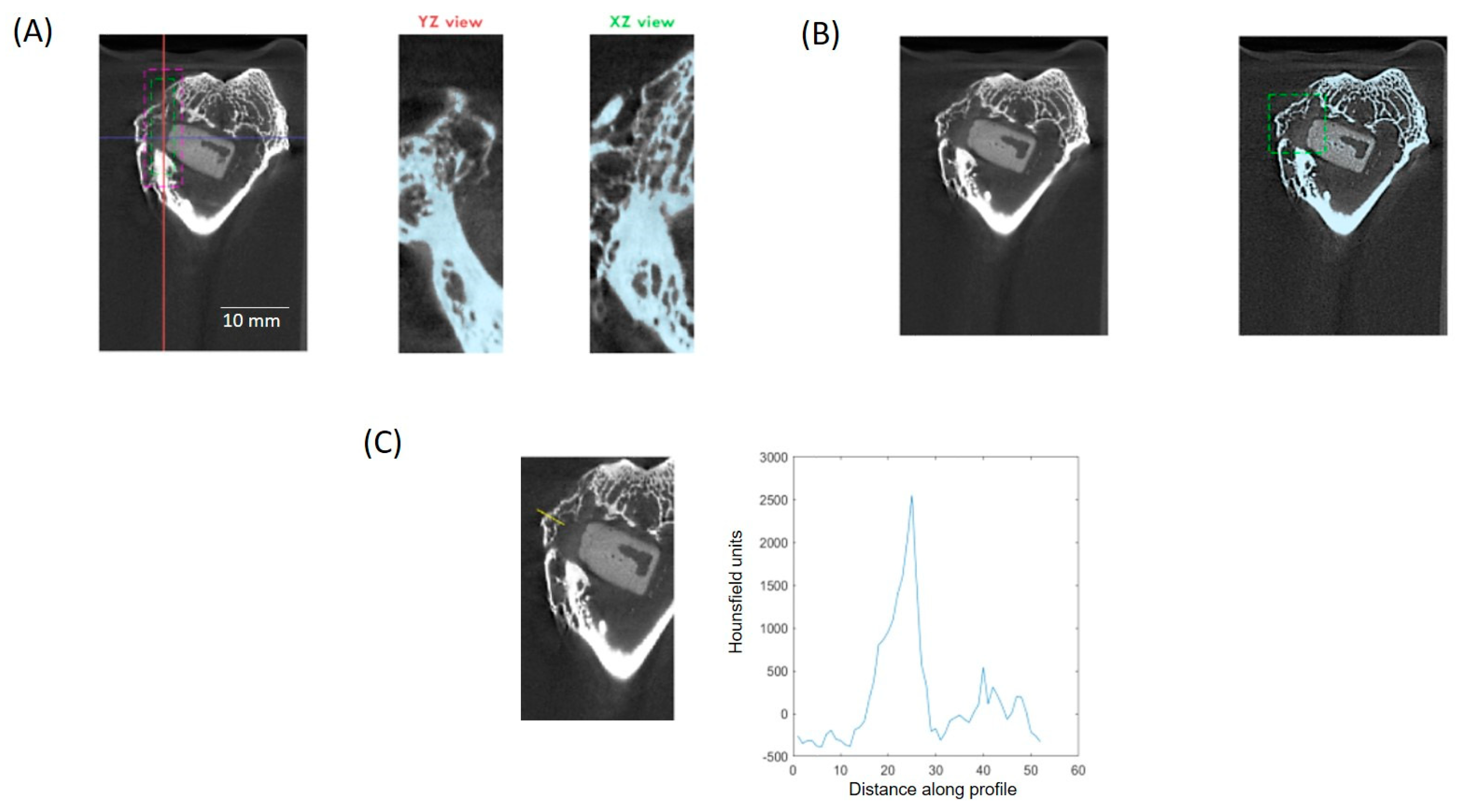
2.4. Histological and µCT Analysis
2.5. Study Limitations
2.6. Statistical Analysis
3. Results
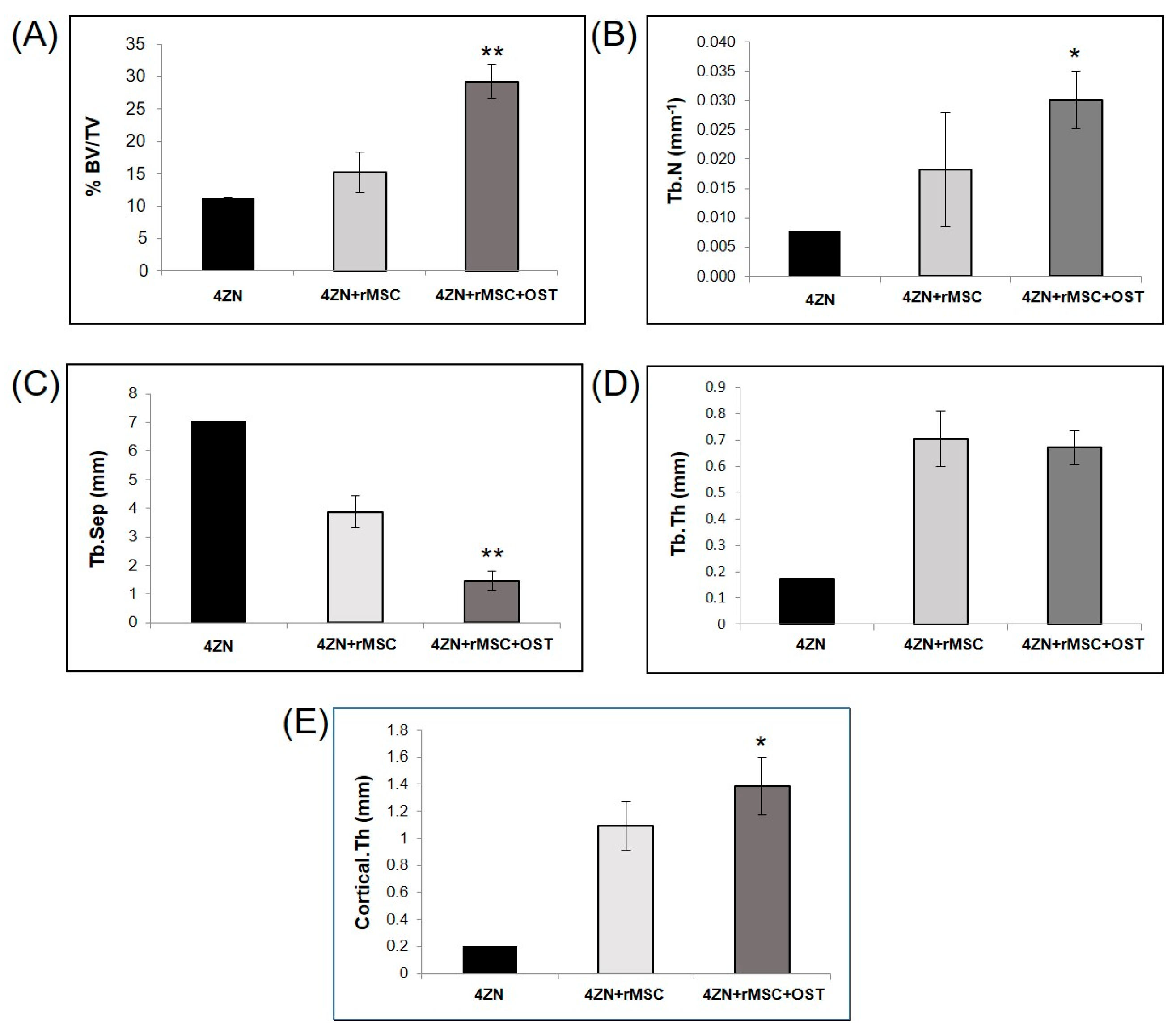
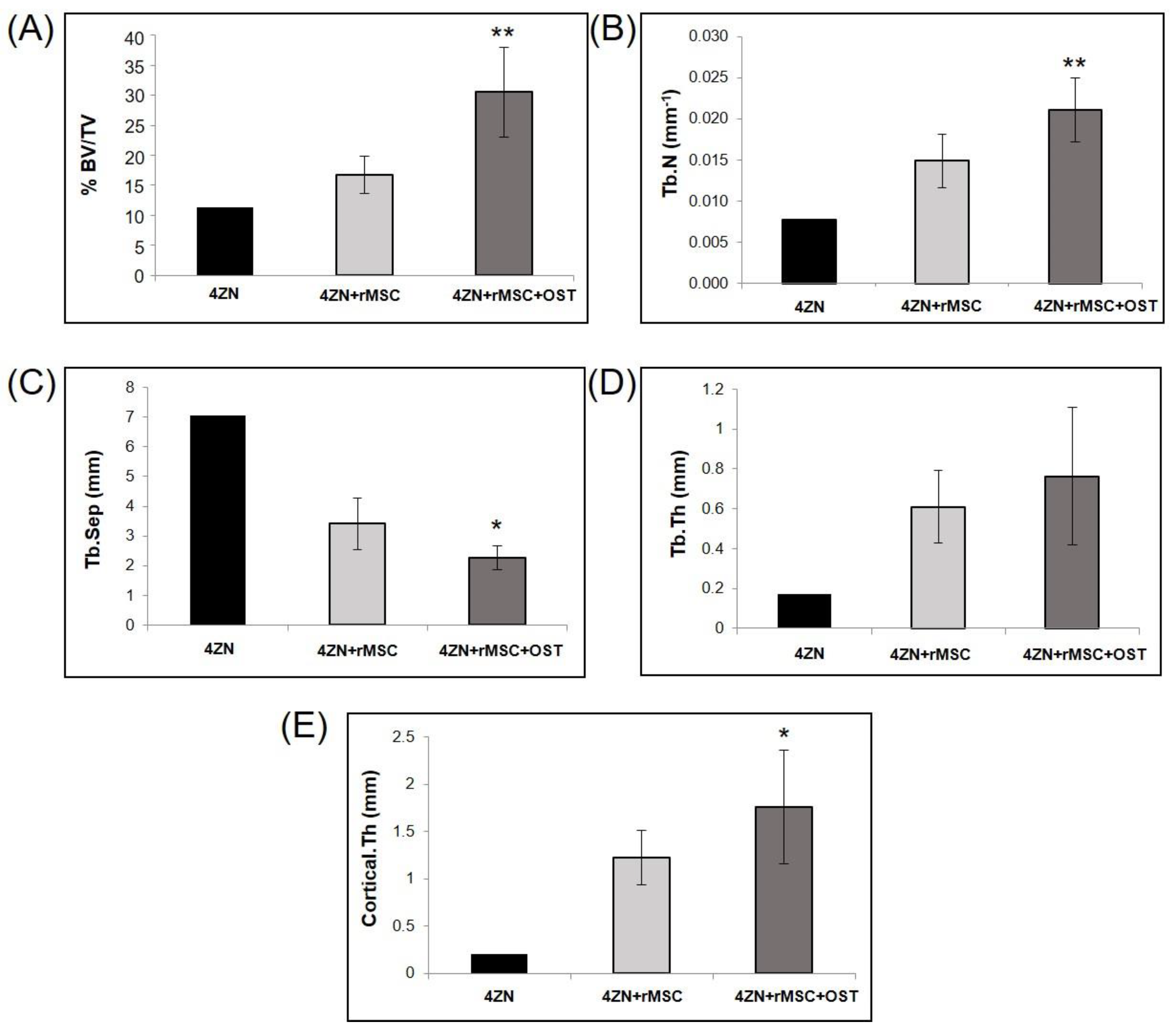
3.1. µCT Analysis
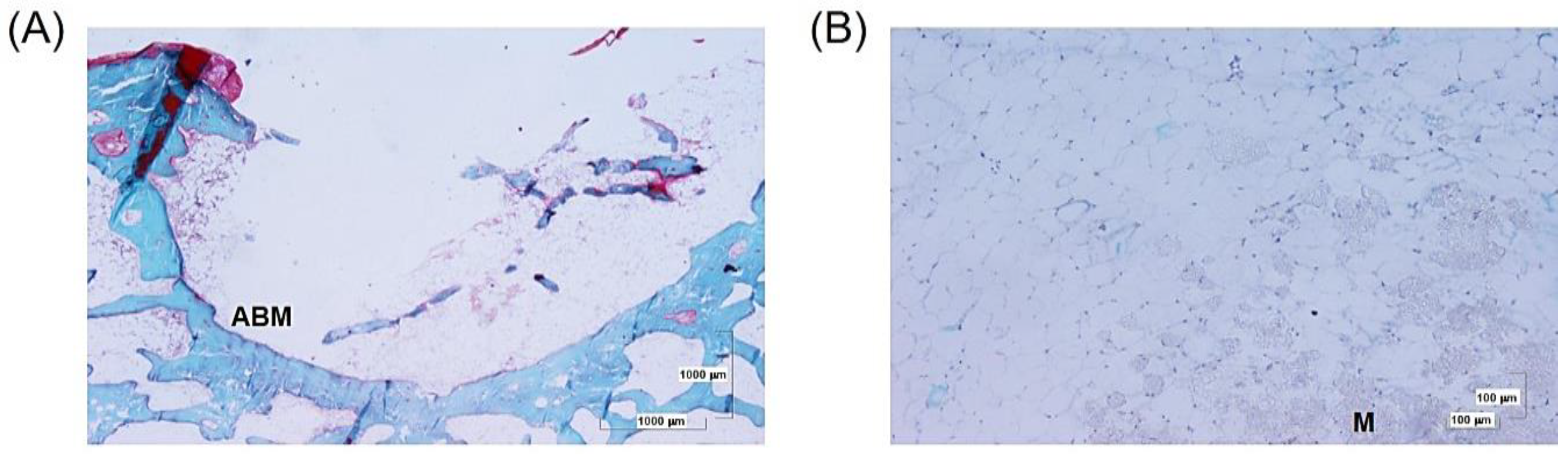
3.2. Histological Analysis
3.2.1. Perforation and 4ZN Samples
3.2.2. 4ZN+rMSCs Samples
3.2.3. 4ZN+rMSCs+OST Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013, 1, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Browner, W.; Cummings, S.R.; Black, D.M.; Nevitt, M.C.; Browner, W.; Genant, H.K.; Cauley, J.; Ensrud, K.; Scott, J.; et al. Bone density at various sites for prediction of hip fractures. Lancet 1993, 341, 72–75. [Google Scholar] [CrossRef]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A. Update on osteoporosis in men. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Talbot, C.; Cross, C.; Hinduja, K. NHSLA litigation in hip fractures: Lessons learnt from NHSLA data. Injury 2017, 48, 1853–1857. [Google Scholar] [CrossRef]
- Melton, L.J. Hip fractures: A worldwide problem today and tomorrow. Bone 1993, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Gittard, S.D.; Perfect, J.R.; Monteiro-Riviere, N.A.; Wei, W.; Jin, C.; Narayan, R.J. Assessing the antimicrobial activity of zinc oxide thin films using disk diffusion and biofilm reactor. Appl. Surf. Sci. 2009, 255, 5806–5811. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef]
- Heras, C.; Sanchez-Salcedo, S.; Lozano, D.; Peña, J.; Esbrit, P.; Vallet-Regi, M.; Salinas, A.J. Osteostatin potentiates the bioactivity of mesoporous glass scaffolds containing Zn2+ ions in human mesenchymal stem cells. Acta Biomater. 2019, 89, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, Y.; Cuniberti, G.; Xiao, Y.; Gelinsky, M. Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater. 2011, 7, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regí, M.; Gómez-Barrena, E.; Esbrit, P. Osteostatin-loaded bioceramics stimulate osteoblastic growth and differentiation. Acta Biomater. 2010, 6, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, A.R.; Alonso, V.; Alvarez-Arroyo, M.V.; Esbrit, P. Transient exposure to PTHrP (107-139) exerts anabolic effects through vascular endothelial growth factor receptor 2 in human osteoblastic cells in vitro. Calcif. Tissue Int. 2006, 79, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.J.; Martin, T.J.; Nicholson, G.C. Carboxyl-terminal parathyroid hormone-related protein inhibits bone resorption by isolated chicken osteoclasts. J. Bone Miner. Res. 1994, 9, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.J.; Kemp, B.E.; Hammonds, R.G., Jr.; Mitchelhill, K.; Moseley, J.M.; Martin, T.J.; Nicholson, G.C. A potent inhibitor of osteoclastic bone resorption within a highly conserved pentapeptide region of parathyroid hormone-related protein; PTHrP[107–lll]. Endocrinology 1991, 129, 3424–3426. [Google Scholar] [CrossRef]
- Ibáñez, L.; Nácher-Juan, J.; Terencio, M.C.; Ferrándiz, M.L.; Alcaraz, M.J. Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. Int. J. Mol. Sci. 2022, 23, 8551. [Google Scholar] [CrossRef]
- Quinlan, E.; Thompson, E.M.; Matsiko, A.; O’Brien, F.J.; López-Noriega, A. Functionalization of a Collagen-Hydroxyapatite Scaffold with Osteostatin to Facilitate Enhanced Bone Regeneration. Adv. Healthc. Mater. 2015, 4, 2649–2656. [Google Scholar] [CrossRef]
- Lozano, D.; Gil-Albarova, J.; Heras, C.; Sánchez-Salcedo, S.; Gómez-Palacio, V.E.; Gómez-Blasco, A.; Doadrio, J.C.; Vallet-Regí, M.; Salinas, A.J. ZnO-mesoporous glass scaffolds loaded with osteostatin and mesenchymal cells improve bone healing in a rabbit bone defect. J. Mater. Sci. Mater. Med. 2020, 31, 100. [Google Scholar] [CrossRef]
- Wu, D.; Liu, L.; Fu, S.; Zhang, J. Osteostatin improves the Osteogenic differentiation of mesenchymal stem cells and enhances angiogenesis through HIF-1α under hypoxia conditions in vitro. Biochem. Biophys. Res. Commun. 2022, 606, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regí, M.; García-Honduvilla, N.; Buján, J.; et al. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Sanchez-Salcedo, S.; Lozano, D.; Heras, C.; Esbrit, P.; Vallet-Regí, M.; Salinas, A.J. Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin. Nanomaterials 2018, 8, 592. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Giménez, B.; Heras, C.; Benito-Garzon, L.; Lozano, D.; Luengo-Alonso, G.; Sanchez-Salcedo, S.; Salinas, A.J.; Vallet-Regi, M. Bone regeneration capacity of bioactive glasses containing zinc, osteostatin and mesenchymal cells for the treatment of critical defects in rabbit ulna. Eur. Soc. Biomater. 2019, ESB 2019 A, 1065–1066. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Calvo, E.; Largo, R.; González-González, R.; De La Piedra, C.; Díaz-Curiel, M.; Herrero-Beaumont, G. Characterization of a new experimental model of osteoporosis in rabbits. J. Bone Miner. Metab. 2008, 26, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bemenderfer, T.B.; Harris, J.S.; Condon, K.W.; Li, J.; Kacena, M.A. Processing and Sectioning Undecalcified Murine Bone Specimens. Methods Mol. Biol. 2021, 2230, 231–257. [Google Scholar] [CrossRef] [PubMed]
- Berdón, P.; Tenrero, N.B.; Giménez, B.B.; Lozano, D.; Rubio, C.H.; Salinas, A.; Desco, M.; García, M.A. BoneAnalytics: Software para cuantificación ósea en imágenes de TAC. In Proceedings of the XXXVII Congreso Anual de la Sociedad Española de Ingeniería Biomédica: Actas del Congreso CASEIB 2019, Santander, Spain, 27–29 November 2019; pp. 1–4. [Google Scholar]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous biphasic calcium phosphate ceramics: Influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Glasses in bone regeneration: A multiscale issue. J. Non-Cryst. Sol. 2016, 432, 9–14. [Google Scholar] [CrossRef]
- Miar, S.; Pearson, J.; Montelongo, S.; Zamilpa, R.; Betancourt, A.M.; Ram, B.; Navara, C.; Appleford, M.R.; Ong, J.L.; Griffey, S.; et al. Regeneration enhanced in critical-sized bone defects using bone-specific extracellular matrix protein. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2021, 109, 538–547. [Google Scholar] [CrossRef]
- Mito, K.; Lachnish, J.; Le, W.; Chan, C.; Chang, Y.-L.; Yao, J. Scaffold-Free Bone Marrow-Derived Mesenchymal Stem Cell Sheets Enhance Bone Formation in a Weight-Bearing Rat Critical Bone Defect Model. Tissue Eng. Part A, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Wai-Ching, L.; Irina, S.R.; Rikin, P.; Ming, C.L.; Velez, M.; Chu, T.M. The effects of 3D bioactive glass scaffolds and BMP-2 on bone formation in rat femoral critical size defects and adjacent bones. Biomed. Mater. 2014, 9, 045013. [Google Scholar] [CrossRef]
- Gao, T.; Aro, H.T.; Ylänen, H.; Vuorio, E. Silica-based bioactive glasses modulate expression of bone morphogenetic protein-2 mRNA in Saos-2 osteoblasts in vitro. Biomaterials 2001, 22, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schütz, M.A.; Duda, G.N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef] [PubMed]
- Cacchioli, A.; Spaggiari, B.; Ravanetti, F.; Martini, F.M.; Borghetti, P.; Gabbi, C. The critical size bone defect: Morphological study of bone healing. Ann. Fac. Medic. Vet. Parma 2006, 26, 97–100. [Google Scholar]





| 4ZN+rMSC | 4ZN+rMSC+OST | 6 Weeks | 12 Weeks | |
|---|---|---|---|---|
| Number | 8 | 8 | 6 | 10 |
| Total | 16 | 16 |
| Group | 6 Weeks | 12 Weeks | Lost during Monitoring |
|---|---|---|---|
| 4ZN+rMSCs | 3 | 5 | 1 Induction 1 Femur fracture 1 Inmediate postoperation |
| 4ZN+rMSCs+OST | 3 | 5 | 1 Femur fracture |
| Total | 6 | 10 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luengo-Alonso, G.; Bravo-Gimenez, B.; Lozano, D.; Heras, C.; Sanchez-Salcedo, S.; Benito-Garzón, L.; Abella, M.; Vallet-Regi, M.; Cecilia-Lopez, D.; Salinas, A.J. Osteogenic Potential of a Biomaterial Enriched with Osteostatin and Mesenchymal Stem Cells in Osteoporotic Rabbits. Biomolecules 2024, 14, 143. https://doi.org/10.3390/biom14020143
Luengo-Alonso G, Bravo-Gimenez B, Lozano D, Heras C, Sanchez-Salcedo S, Benito-Garzón L, Abella M, Vallet-Regi M, Cecilia-Lopez D, Salinas AJ. Osteogenic Potential of a Biomaterial Enriched with Osteostatin and Mesenchymal Stem Cells in Osteoporotic Rabbits. Biomolecules. 2024; 14(2):143. https://doi.org/10.3390/biom14020143
Chicago/Turabian StyleLuengo-Alonso, Gonzalo, Beatriz Bravo-Gimenez, Daniel Lozano, Clara Heras, Sandra Sanchez-Salcedo, Lorena Benito-Garzón, Monica Abella, María Vallet-Regi, David Cecilia-Lopez, and Antonio J. Salinas. 2024. "Osteogenic Potential of a Biomaterial Enriched with Osteostatin and Mesenchymal Stem Cells in Osteoporotic Rabbits" Biomolecules 14, no. 2: 143. https://doi.org/10.3390/biom14020143






