Inside the Biology of the β3-Adrenoceptor
Abstract
1. Introduction
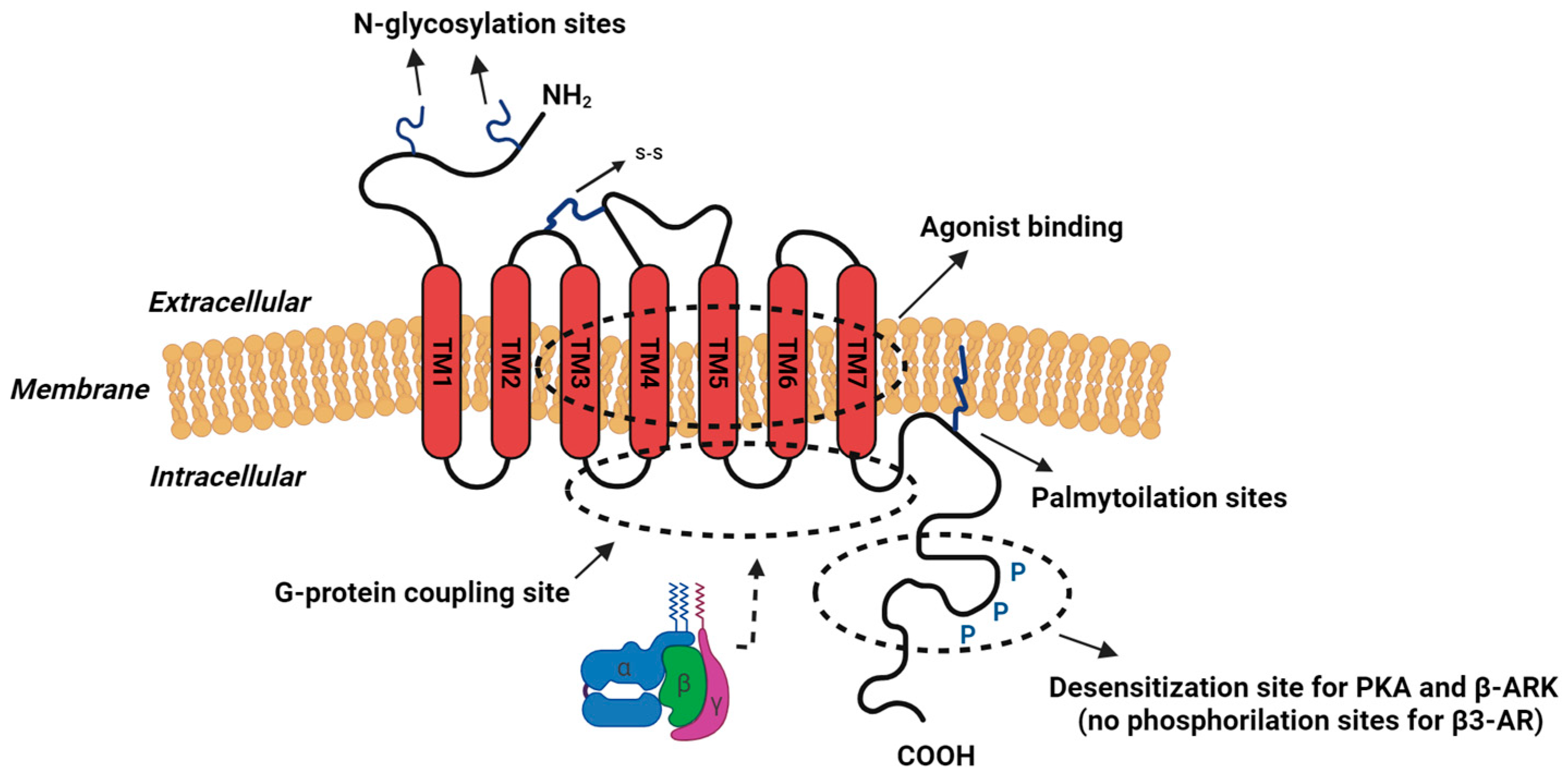
2. β-Adrenoceptors
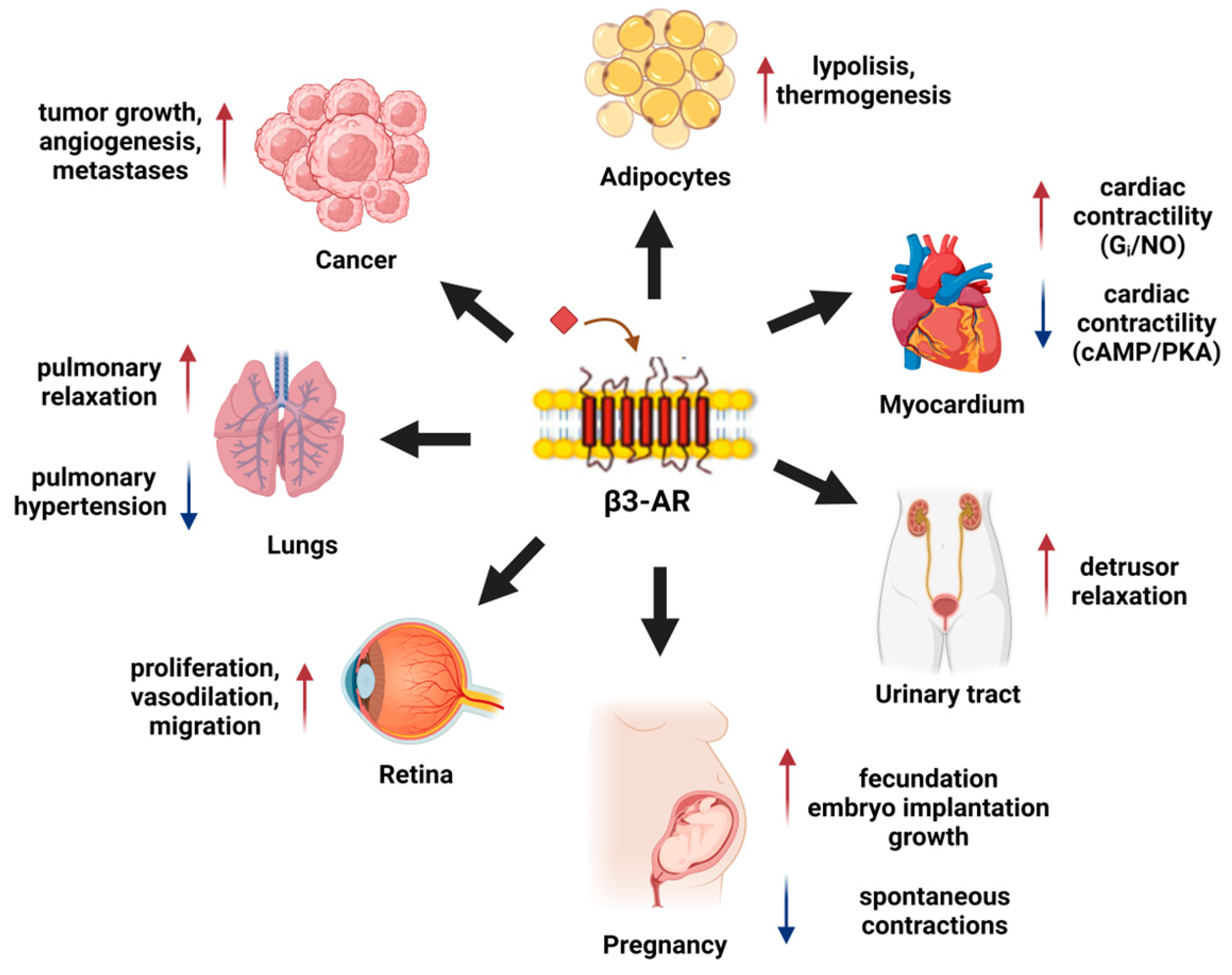
3. β3-AR Physiology
3.1. β3-AR in Adipose Tissue
3.2. β3-AR in the Myocardium
3.3. β3-AR in Urinary Bladder
3.4. β3-AR in the Myometrium and Pregnancy
4. β3-AR in Pathology
4.1. β3-AR in Melanoma
4.2. β3-AR in Breast Cancer
4.3. β3-AR in Retinopathy
4.4. β3-AR in Prostate Cancer
4.5. β3-AR in Neuroblastoma
4.6. β3-AR in Ewing Sarcoma
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, J.W. Ahlquist and the Development of Beta-Adrenoceptor Antagonists. Postgrad. Med. J. 1976, 52 (Suppl. S4), 11–13. [Google Scholar]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.-M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular Characterization of the Human β3-Adrenergic Receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular Pathways: Beta-Adrenergic Signaling in Cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic Nerve Development Contributes to Prostate Cancer Progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Entschladen, F.; Drell, T.L.; Lang, K.; Joseph, J.; Zaenker, K.S. Tumour-Cell Migration, Invasion, and Metastasis: Navigation by Neurotransmitters. Lancet Oncol. 2004, 5, 254–258. [Google Scholar] [CrossRef]
- Armaiz-Pena, G.N.; Allen, J.K.; Cruz, A.; Stone, R.L.; Nick, A.M.; Lin, Y.G.; Han, L.Y.; Mangala, L.S.; Villares, G.J.; Vivas-Mejia, P.; et al. Src Activation by β-Adrenoreceptors Is a Key Switch for Tumour Metastasis. Nat. Commun. 2013, 4, 1403. [Google Scholar] [CrossRef]
- Cheng, Y.; Gao, X.-H.; Li, X.-J.; Cao, Q.-H.; Zhao, D.-D.; Zhou, J.-R.; Wu, H.-X.; Wang, Y.; You, L.-J.; Yang, H.-B.; et al. Depression Promotes Prostate Cancer Invasion and Metastasis via a Sympathetic-cAMP-FAK Signaling Pathway. Oncogene 2018, 37, 2953–2966. [Google Scholar] [CrossRef]
- Eng, J.W.-L.; Kokolus, K.M.; Reed, C.B.; Hylander, B.L.; Ma, W.W.; Repasky, E.A. A Nervous Tumor Microenvironment: The Impact of Adrenergic Stress on Cancer Cells, Immunosuppression, and Immunotherapeutic Response. Cancer Immunol. Immunother. CII 2014, 63, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Simard, P.-M.; Atgié, C.; Mauriège, P.; D’Allaire, F.; Bukowiecki, L.J. Comparison of the Lipolytic Effects of Norepinephrine and BRL 37344 in Rat Brown and White Adipocytes. Obes. Res. 1994, 2, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Moreira, S.; Costa, A.F.; Silva, D.; Vieira, C.; Silva-Ramos, M.; Correia-de-Sá, P. Β3 Adrenoceptor-Induced Cholinergic Inhibition in Human and Rat Urinary Bladders Involves the Exchange Protein Directly Activated by Cyclic AMP 1 Favoring Adenosine Release. Br. J. Pharmacol. 2020, 177, 1589–1608. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.J.; Zhang, Z.S.; Onishi, K.; Ukai, T.; Sane, D.C.; Cheng, C.P. Upregulation of Functional Beta(3)-Adrenergic Receptor in the Failing Canine Myocardium. Circ. Res. 2001, 89, 599–606. [Google Scholar] [CrossRef]
- Rouget, C.; Bardou, M.; Breuiller-Fouché, M.; Loustalot, C.; Qi, H.; Naline, E.; Croci, T.; Cabrol, D.; Advenier, C.; Leroy, M.J. Β3-Adrenoceptor Is the Predominant β-Adrenoceptor Subtype in Human Myometrium and Its Expression Is Up-Regulated in Pregnancy. J. Clin. Endocrinol. Metab. 2005, 90, 1644–1650. [Google Scholar] [CrossRef]
- Fujinaga, M.; Scott, J.C. Gene Expression of Catecholamine Synthesizing Enzymes and Beta Adrenoceptor Subtypes during Rat Embryogenesis. Neurosci. Lett. 1997, 231, 108–112. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Seidler, F.J. Developmental Exposure to Terbutaline and Chlorpyrifos, Separately or Sequentially, Elicits Presynaptic Serotonergic Hyperactivity in Juvenile and Adolescent Rats. Brain Res. Bull. 2007, 73, 301–309. [Google Scholar] [CrossRef]
- Chiarugi, P.; Filippi, L. β3-adrenoreceptor and tumor microenvironment: A new hub. Oncoimmunology 2015, 4, e1026532. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Pelon, F.; Comito, G.; Taddei, M.L.; Moretti, S.; Innocenti, S.; Nassini, R.; Gerlini, G.; Borgognoni, L.; Bambi, F.; et al. Norepinephrine Promotes Tumor Microenvironment Reactivity through Β3-Adrenoreceptors during Melanoma Progression. Oncotarget 2014, 6, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Civantos Calzada, B.; Aleixandre de Artiñano, A. Alpha-Adrenoceptor Subtypes. Pharmacol. Res. 2001, 44, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Tilan, J.; Kitlinska, J. Sympathetic Neurotransmitters and Tumor Angiogenesis—Link between Stress and Cancer Progression. J. Oncol. 2010, 2010, 539706. [Google Scholar] [CrossRef] [PubMed]
- Strader, C.D.; Candelore, M.R.; Hill, W.S.; Sigal, I.S.; Dixon, R.A. Identification of Two Serine Residues Involved in Agonist Activation of the Beta-Adrenergic Receptor. J. Biol. Chem. 1989, 264, 13572–13578. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G. The Beta-Adrenergic Receptors. Herz 2002, 27, 683–690. [Google Scholar] [CrossRef]
- Lefkowitz, R.J.; Protein-Coupled Receptors, G., III. New Roles for Receptor Kinases and Beta-Arrestins in Receptor Signaling and Desensitization. J. Biol. Chem. 1998, 273, 18677–18680. [Google Scholar] [CrossRef]
- Wittpoth, C.; Scholich, K.; Bilyeu, J.D.; Patel, T.B. Adenylyl Cyclase Regulates Signal Onset via the Inhibitory GTP-Binding Protein, Gi. J. Biol. Chem. 2000, 275, 25915–25919. [Google Scholar] [CrossRef]
- Chuang, T.T.; LeVine, H.; De Blasi, A. Phosphorylation and Activation of Beta-Adrenergic Receptor Kinase by Protein Kinase C. J. Biol. Chem. 1995, 270, 18660–18665. [Google Scholar] [CrossRef] [PubMed]
- Bylund, D.B.; Eikenberg, D.C.; Hieble, J.P.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Molinoff, P.B.; Ruffolo, R.R.; Trendelenburg, U. International Union of Pharmacology Nomenclature of Adrenoceptors. Pharmacol. Rev. 1994, 46, 121–136. [Google Scholar] [PubMed]
- McGraw, D.W.; Liggett, S.B. Molecular Mechanisms of Β2-Adrenergic Receptor Function and Regulation. Proc. Am. Thorac. Soc. 2005, 2, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Padro, C.J.; Sanders, V.M. Neuroendocrine Regulation of Inflammation. Semin. Immunol. 2014, 26, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Molecular Mechanisms of Beta(2)-Adrenergic Receptor Function, Response, and Regulation. J. Allergy Clin. Immunol. 2006, 117, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, B.; Chen, X.; Zhao, N.; Cheng, G.; Jiang, Y.; Liu, Y.; Lin, C.; Tan, W.; Lu, D.; et al. Beta-2 Adrenergic Receptor Gene (ADRB2) Polymorphism and Risk for Lung Adenocarcinoma: A Case-Control Study in a Chinese Population. Cancer Lett. 2006, 240, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, A.; Koch, W.J. Targeting Β3-Adrenergic Receptors in the Heart: Selective Agonism and β-Blockade. J. Cardiovasc. Pharmacol. 2017, 69, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Gravas, S. Safety and Tolerability of Β3-Adrenoceptor Agonists in the Treatment of Overactive Bladder Syndrome—Insight from Transcriptosome and Experimental Studies. Expert Opin. Drug Saf. 2016, 15, 647–657. [Google Scholar] [CrossRef]
- Granneman, J.G.; Lahners, K.N.; Chaudhry, A. Characterization of the Human Beta 3-Adrenergic Receptor Gene. Mol. Pharmacol. 1993, 44, 264–270. [Google Scholar] [PubMed]
- Nahmias, C.; Blin, N.; Elalouf, J.M.; Mattei, M.G.; Strosberg, A.D.; Emorine, L.J. Molecular Characterization of the Mouse Beta 3-Adrenergic Receptor: Relationship with the Atypical Receptor of Adipocytes. EMBO J. 1991, 10, 3721–3727. [Google Scholar] [CrossRef]
- Forrest, R.H.; Hickford, J.G. Rapid Communication: Nucleotide Sequences of the Bovine, Caprine, and Ovine Β3-Adrenergic Receptor Genes. J. Anim. Sci. 2000, 78, 1397–1398. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Uchida, E.; Niiyama, M.; Yoshida, T.; Saito, M. Anti-Obesity Effects of Selective Agonists to the Beta 3-Adrenergic Receptor in Dogs. I. The Presence of Canine Beta 3-Adrenergic Receptor and in Vivo Lipomobilization by Its Agonists. J. Vet. Med. Sci. 1998, 60, 459–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jockers, R.; Da Silva, A.; Strosberg, A.D.; Bouvier, M.; Marullo, S. New Molecular and Structural Determinants Involved in Β2-Adrenergic Receptor Desensitization and Sequestration: Delineation Using Chimeric Β3/Β2-Adrenergic Receptors. J. Biol. Chem. 1996, 271, 9355–9362. [Google Scholar] [CrossRef] [PubMed]
- Nantel, F.; Bonin, H.; Emorine, L.J.; Zilberfarb, V.; Strosberg, A.D.; Bouvier, M.; Marullo, S. The Human Beta 3-Adrenergic Receptor Is Resistant to Short Term Agonist-Promoted Desensitization. Mol. Pharmacol. 1993, 43, 548–555. [Google Scholar] [PubMed]
- Dessy, C.; Balligand, J.-L. Beta3-Adrenergic Receptors in Cardiac and Vascular Tissues Emerging Concepts and Therapeutic Perspectives. Adv. Pharmacol. 2010, 59, 135–163. [Google Scholar] [CrossRef] [PubMed]
- Cernecka, H.; Sand, C.; Michel, M.C. The Odd Sibling: Features of Β3-Adrenoceptor Pharmacology. Mol. Pharmacol. 2014, 86, 479–484. [Google Scholar] [CrossRef]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about Β3-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef]
- Koike, K.; Takayanagi, I.; Muramatsu, M.; Ohki, S.; Horinouchi, T. Involvement of Beta 3-Adrenoceptor in the Relaxation Response in Guinea Pig Taenia Caecum. Jpn. J. Pharmacol. 1994, 66, 213–220. [Google Scholar] [CrossRef][Green Version]
- Knox, A.J.; Tattersfield, A.E. Airway Smooth Muscle Relaxation. Thorax 1995, 50, 894–901. [Google Scholar] [CrossRef]
- Atgié, C.; D’Allaire, F.; Bukowiecki, L.J. Role of Beta1- and Beta3-Adrenoceptors in the Regulation of Lipolysis and Thermogenesis in Rat Brown Adipocytes. Am. J. Physiol. 1997, 273, C1136–C1142. [Google Scholar] [CrossRef]
- Michel, M.C.; Vrydag, W. Alpha1-, Alpha2- and Beta-Adrenoceptors in the Urinary Bladder, Urethra and Prostate. Br. J. Pharmacol. 2006, 147 (Suppl. S2), S88–S119. [Google Scholar] [CrossRef] [PubMed]
- Sanofi: Global Healthcare and Pharmaceutical Company. Available online: https://www.sanofi.com/en (accessed on 15 December 2023).
- Rothwell, N.J.; Stock, M.J. A Role for Brown Adipose Tissue in Diet-Induced Thermogenesis. Nature 1979, 281, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, E.P.; Balasubramanian, P.; Lee, Y.-H.; Weng, C.; Kershaw, E.E.; Granneman, J.G. Coupling of Lipolysis and de Novo Lipogenesis in Brown, Beige, and White Adipose Tissues during Chronic Β3-Adrenergic Receptor Activation. J. Lipid Res. 2014, 55, 2276–2286. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, S.-N.; Kwon, H.-J.; Granneman, J.G. Metabolic Heterogeneity of Activated Beige/Brite Adipocytes in Inguinal Adipose Tissue. Sci. Rep. 2017, 7, 39794. [Google Scholar] [CrossRef]
- Orava, J.; Nuutila, P.; Noponen, T.; Parkkola, R.; Viljanen, T.; Enerbäck, S.; Rissanen, A.; Pietiläinen, K.H.; Virtanen, K.A. Blunted Metabolic Responses to Cold and Insulin Stimulation in Brown Adipose Tissue of Obese Humans. Obesity 2013, 21, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Bagci, U.; Hussein, S.; Kelly, P.V.; Muzaffar, R.; Neuschwander-Tetri, B.A.; Osman, M.M. Brown Adipose Tissue Detected by PET/CT Imaging Is Associated with Less Central Obesity. Nucl. Med. Commun. 2017, 38, 629–635. [Google Scholar] [CrossRef]
- Summers, R.J.; Papaioannou, M.; Harris, S.; Evans, B.A. Expression of Β3-Adrenoceptor mRNA in Rat Brain. Br. J. Pharmacol. 1995, 116, 2547–2548. [Google Scholar] [CrossRef]
- Castillo-Meléndez, M.; McKinley, M.J.; Summers, R.J. Intracerebroventricular Administration of the Beta(3)-Adrenoceptor Agonist CL 316243 Causes Fos Immunoreactivity in Discrete Regions of Rat Hypothalamus. Neurosci. Lett. 2000, 290, 161–164. [Google Scholar] [CrossRef]
- Harrold, J.A. Hypothalamic Control of Energy Balance. Curr. Drug Targets 2004, 5, 207–219. [Google Scholar] [CrossRef]
- Tsujii, S.; Bray, G.A. Food Intake of Lean and Obese Zucker Rats Following Ventricular Infusions of Adrenergic Agonists. Brain Res. 1992, 587, 226–232. [Google Scholar] [CrossRef]
- Grujic, D.; Susulic, V.S.; Harper, M.E.; Himms-Hagen, J.; Cunningham, B.A.; Corkey, B.E.; Lowell, B.B. Beta3-Adrenergic Receptors on White and Brown Adipocytes Mediate Beta3-Selective Agonist-Induced Effects on Energy Expenditure, Insulin Secretion, and Food Intake. A Study Using Transgenic and Gene Knockout Mice. J. Biol. Chem. 1997, 272, 17686–17693. [Google Scholar] [CrossRef]
- Tsujii, S.; Bray, G.A. A Beta-3 Adrenergic Agonist (BRL-37,344) Decreases Food Intake. Physiol. Behav. 1998, 63, 723–728. [Google Scholar] [CrossRef]
- Himms-Hagen, J.; Cui, J.; Danforth, E.; Taatjes, D.J.; Lang, S.S.; Waters, B.L.; Claus, T.H. Effect of CL-316,243, a Thermogenic Beta 3-Agonist, on Energy Balance and Brown and White Adipose Tissues in Rats. Am. J. Physiol. 1994, 266, R1371–R1382. [Google Scholar] [CrossRef] [PubMed]
- Soeder, K.J.; Snedden, S.K.; Cao, W.; Della Rocca, G.J.; Daniel, K.W.; Luttrell, L.M.; Collins, S. The Beta3-Adrenergic Receptor Activates Mitogen-Activated Protein Kinase in Adipocytes through a Gi-Dependent Mechanism. J. Biol. Chem. 1999, 274, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Unelius, L.; Bengtsson, T.; Cannon, B.; Nedergaard, J. Coexisting Beta-Adrenoceptor Subtypes: Significance for Thermogenic Process in Brown Fat Cells. Am. J. Physiol. 1994, 267, C969–C979. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.S.; Dhillon, H.; Zhang, C.-Y.; Cinti, S.; Bianco, A.C.; Kobilka, B.K.; Lowell, B.B. betaAR Signaling Required for Diet-Induced Thermogenesis and Obesity Resistance. Science 2002, 297, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS--Lipases and Lipolysis in Lipid Metabolism and Signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Shrestha, Y.B.; Chen, M.; Chanturiya, T.; Gavrilova, O.; Weinstein, L.S. Gsα Deficiency in Adipose Tissue Improves Glucose Metabolism and Insulin Sensitivity without an Effect on Body Weight. Proc. Natl. Acad. Sci. USA 2016, 113, 446–451. [Google Scholar] [CrossRef]
- Paulo, E.; Wu, D.; Hecker, P.; Zhang, Y.; Wang, B. Adipocyte HDAC4 Activation Leads to Beige Adipocyte Expansion and Reduced Adiposity. J. Endocrinol. 2018, 239, 153–165. [Google Scholar] [CrossRef]
- Kuznetsova, L.A.; Sharova, T.S.; Pertseva, M.N.; Shpakov, A.O. Beta-adrenergic regulation of the adenylyl cyclase signaling system in myocardium and brain of rats with obesity and types 2 diabetes mellitus and the effect of long-term intranasal insulin treatment. Zh. Evol. Biokhim. Fiziol. 2015, 51, 170–180. [Google Scholar] [CrossRef]
- Keylin, S.; Kajimura, S. Mitochondrial Homeostasis in Adipose Tissue Remodeling. Sci. Signal. 2017, 10, eaai9248. [Google Scholar] [CrossRef]
- Hodis, J.; Vaclavíková, R.; Farghali, H. Beta-3 Agonist-Induced Lipolysis and Nitric Oxide Production: Relationship to PPARgamma Agonist/Antagonist and AMP Kinase Modulation. Gen. Physiol. Biophys. 2011, 30, 90–99. [Google Scholar] [CrossRef]
- Cypess, A.M.; White, A.P.; Vernochet, C.; Schulz, T.J.; Xue, R.; Sass, C.A.; Huang, T.L.; Roberts-Toler, C.; Weiner, L.S.; Sze, C.; et al. Anatomical Localization, Gene Expression Profiling and Functional Characterization of Adult Human Neck Brown Fat. Nat. Med. 2013, 19, 635–639. [Google Scholar] [CrossRef]
- Jespersen, N.Z.; Larsen, T.J.; Peijs, L.; Daugaard, S.; Homøe, P.; Loft, A.; de Jong, J.; Mathur, N.; Cannon, B.; Nedergaard, J.; et al. A Classical Brown Adipose Tissue mRNA Signature Partly Overlaps with Brite in the Supraclavicular Region of Adult Humans. Cell Metab. 2013, 17, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Memetimin, H.; Confides, A.L.; Kasza, I.; Zhu, B.; Vekaria, H.J.; Harfmann, B.; Jones, K.A.; Johnson, Z.R.; Westgate, P.M.; et al. Human Adipose Beiging in Response to Cold and Mirabegron. JCI Insight 2018, 3, e121510. [Google Scholar] [CrossRef]
- Clément, K.; Vaisse, C.; Manning, B.S.; Basdevant, A.; Guy-Grand, B.; Ruiz, J.; Silver, K.D.; Shuldiner, A.R.; Froguel, P.; Strosberg, A.D. Genetic Variation in the Beta 3-Adrenergic Receptor and an Increased Capacity to Gain Weight in Patients with Morbid Obesity. N. Engl. J. Med. 1995, 333, 352–354. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Oniki, K.; Kumagae, N.; Morita, K.; Otake, K.; Ogata, Y.; Saruwatari, J. Beta-3-Adrenergic Receptor Rs4994 Polymorphism Is a Potential Biomarker for the Development of Nonalcoholic Fatty Liver Disease in Overweight/Obese Individuals. Dis. Markers 2019, 2019, 4065327. [Google Scholar] [CrossRef] [PubMed]
- Kaumann, A.J.; Molenaar, P. Modulation of Human Cardiac Function through 4 Beta-Adrenoceptor Populations. Naunyn. Schmiedebergs Arch. Pharmacol. 1997, 355, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.-H.; Cypess, A.M. Β3-Adrenergic Receptors Regulate Human Brown/Beige Adipocyte Lipolysis and Thermogenesis. JCI Insight 2021, 6, e139160. [Google Scholar] [CrossRef]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De Matteis, R.; Cinti, S. The Emergence of Cold-Induced Brown Adipocytes in Mouse White Fat Depots Is Determined Predominantly by White to Brown Adipocyte Transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1244–E1253. [Google Scholar] [CrossRef] [PubMed]
- Preite, N.Z.; do Nascimento, B.P.P.; Muller, C.R.; Américo, A.L.V.; Higa, T.S.; Evangelista, F.S.; Lancellotti, C.L.; Henriques, F.d.S.; Batista, M.L.; Bianco, A.C.; et al. Disruption of Beta3 Adrenergic Receptor Increases Susceptibility to DIO in Mouse. J. Endocrinol. 2016, 231, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, I.; Gettys, T.W.; de Jonge, L.; Nguyen, T.; Smith, J.M.; Xie, H.; Greenway, F.; Smith, S.R. The Effect of Beta-Adrenergic and Peroxisome Proliferator-Activated Receptor-Gamma Stimulation on Target Genes Related to Lipid Metabolism in Human Subcutaneous Adipose Tissue. Diabetes Care 2007, 30, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Di Marzio, D. Peroxisome Proliferator-Activated Receptor-Gamma Agonists and Diabetes: Current Evidence and Future Perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Girón, J.V.; Palacios, R.; Martín, A.; Hernanz, R.; Aguado, A.; Martínez-Revelles, S.; Barrús, M.T.; Salaices, M.; Alonso, M.J. Pioglitazone Reduces Angiotensin II-Induced COX-2 Expression through Inhibition of ROS Production and ET-1 Transcription in Vascular Cells from Spontaneously Hypertensive Rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1582–H1593. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, A.; Nakamura, S.; Mitsunaga, F.; Inoue-Murayama, M.; Udono, T.; Suryobroto, B. Human-Specific SNP in Obesity Genes, Adrenergic Receptor Beta2 (ADRB2), Beta3 (ADRB3), and PPAR Γ2 (PPARG), during Primate Evolution. PLoS ONE 2012, 7, e43461. [Google Scholar] [CrossRef]
- Yoshihara, A.; Sugita, N.; Iwasaki, M.; Wang, Y.; Miyazaki, H.; Yoshie, H.; Nakamura, K. The Interaction Between β-3 Adrenergic Receptor and Peroxisome Proliferator-Activated Receptor Gamma Gene Polymorphism to Periodontal Disease in Community-Dwelling Elderly Japanese. J. Periodontol. 2015, 86, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional Control of Brown Fat Determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ishibashi, J.; Trefely, S.; Shao, M.; Cowan, A.J.; Sakers, A.; Lim, H.-W.; O’Connor, S.; Doan, M.T.; Cohen, P.; et al. A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate. Cell Metab. 2019, 30, 174–189.e5. [Google Scholar] [CrossRef]
- Cibi, D.M.; Bi-Lin, K.W.; Shekeran, S.G.; Sandireddy, R.; Tee, N.; Singh, A.; Wu, Y.; Srinivasan, D.K.; Kovalik, J.-P.; Ghosh, S.; et al. Prdm16 Deficiency Leads to Age-Dependent Cardiac Hypertrophy, Adverse Remodeling, Mitochondrial Dysfunction, and Heart Failure. Cell Rep. 2020, 33, 108288. [Google Scholar] [CrossRef]
- Liu, P.; Huang, S.; Ling, S.; Xu, S.; Wang, F.; Zhang, W.; Zhou, R.; He, L.; Xia, X.; Yao, Z.; et al. Foxp1 Controls Brown/Beige Adipocyte Differentiation and Thermogenesis through Regulating Β3-AR Desensitization. Nat. Commun. 2019, 10, 5070. [Google Scholar] [CrossRef]
- Krief, S.; Lönnqvist, F.; Raimbault, S.; Baude, B.; Van Spronsen, A.; Arner, P.; Strosberg, A.D.; Ricquier, D.; Emorine, L.J. Tissue Distribution of Beta 3-Adrenergic Receptor mRNA in Man. J. Clin. Investig. 1993, 91, 344–349. [Google Scholar] [CrossRef]
- Chamberlain, P.D.; Jennings, K.H.; Paul, F.; Cordell, J.; Berry, A.; Holmes, S.D.; Park, J.; Chambers, J.; Sennitt, M.V.; Stock, M.J.; et al. The Tissue Distribution of the Human Β3-Adrenoceptor Studied Using a Monoclonal Antibody: Direct Evidence of the Β3-Adrenoceptor in Human Adipose Tissue, Atrium and Skeletal Muscle. Int. J. Obes. 1999, 23, 1057–1065. [Google Scholar] [CrossRef]
- De Matteis, R.; Arch, J.R.S.; Petroni, M.L.; Ferrari, D.; Cinti, S.; Stock, M.J. Immunohistochemical Identification of the Beta(3)-Adrenoceptor in Intact Human Adipocytes and Ventricular Myocardium: Effect of Obesity and Treatment with Ephedrine and Caffeine. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2002, 26, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Tavernier, G.; Charpentier, F.; Langin, D.; Le Marec, H. Functional Beta3-Adrenoceptor in the Human Heart. J. Clin. Investig. 1996, 98, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Rozec, B.; Gauthier, C. Beta3-Adrenoceptors in the Cardiovascular System: Putative Roles in Human Pathologies. Pharmacol. Ther. 2006, 111, 652–673. [Google Scholar] [CrossRef]
- Moniotte, S.; Kobzik, L.; Feron, O.; Trochu, J.N.; Gauthier, C.; Balligand, J.L. Upregulation of Beta(3)-Adrenoceptors and Altered Contractile Response to Inotropic Amines in Human Failing Myocardium. Circulation 2001, 103, 1649–1655. [Google Scholar] [CrossRef]
- Trappanese, D.M.; Liu, Y.; McCormick, R.C.; Cannavo, A.; Nanayakkara, G.; Baskharoun, M.M.; Jarrett, H.; Woitek, F.J.; Tillson, D.M.; Dillon, A.R.; et al. Chronic Β1-Adrenergic Blockade Enhances Myocardial Β3-Adrenergic Coupling with Nitric Oxide-cGMP Signaling in a Canine Model of Chronic Volume Overload: New Insight into Mechanisms of Cardiac Benefit with Selective Β1-Blocker Therapy. Basic Res. Cardiol. 2015, 110, 456. [Google Scholar] [CrossRef]
- Balligand, J.-L. Cardiac Salvage by Tweaking with Beta-3-Adrenergic Receptors. Cardiovasc. Res. 2016, 111, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, E.; Tamaoki, J.; Takemura, H.; Isono, K.; Nagai, A. Atypical Adrenoceptor-Mediated Relaxation of Canine Pulmonary Artery through a Cyclic Adenosine Monophosphate-Dependent Pathway. Lung 1999, 177, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.Y.M.; Farah, C.; Balligand, J.-L. The Beta3 Adrenergic Receptor in Healthy and Pathological Cardiovascular Tissues. Cells 2020, 9, 2584. [Google Scholar] [CrossRef] [PubMed]
- Bubb, K.J.; Ravindran, D.; Cartland, S.P.; Finemore, M.; Clayton, Z.E.; Tsang, M.; Tang, O.; Kavurma, M.M.; Patel, S.; Figtree, G.A. Β3 Adrenergic Receptor Stimulation Promotes Reperfusion in Ischemic Limbs in a Murine Diabetic Model. Front. Pharmacol. 2021, 12, 666334. [Google Scholar] [CrossRef] [PubMed]
- Skeberdis, V.A.; Gendvilienė, V.; Zablockaitė, D.; Treinys, R.; Mačianskienė, R.; Bogdelis, A.; Jurevičius, J.; Fischmeister, R. β3-Adrenergic Receptor Activation Increases Human Atrial Tissue Contractility and Stimulates the L-Type Ca2+ Current. J. Clin. Investig. 2008, 118, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Gabauer, I.; Tillinger, A.; Novakova, M.; Pechan, I.; Krizanova, O.; Kvetnanský, R.; Myslivecek, J. Heart Adrenoceptor Gene Expression and Binding Sites in the Human Failing Heart. Ann. N. Y. Acad. Sci. 2008, 1148, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Port, J.D.; Bristow, M.R. Altered Beta-Adrenergic Receptor Gene Regulation and Signaling in Chronic Heart Failure. J. Mol. Cell. Cardiol. 2001, 33, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Moniotte, S.; Balligand, J.-L. Potential Use of Beta(3)-Adrenoceptor Antagonists in Heart Failure Therapy. Cardiovasc. Drug Rev. 2002, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, J.; García-Ruiz, J.M.; Sanz-Rosa, D.; Pun, A.; García-Alvarez, A.; Davidson, S.M.; Fernández-Friera, L.; Nuno-Ayala, M.; Fernández-Jiménez, R.; Bernal, J.A.; et al. Β3 Adrenergic Receptor Selective Stimulation during Ischemia/Reperfusion Improves Cardiac Function in Translational Models through Inhibition of mPTP Opening in Cardiomyocytes. Basic Res. Cardiol. 2014, 109, 422. [Google Scholar] [CrossRef]
- Rengo, G.; Parisi, V.; Femminella, G.D.; Pagano, G.; de Lucia, C.; Cannavo, A.; Liccardo, D.; Giallauria, F.; Scala, O.; Zincarelli, C.; et al. Molecular Aspects of the Cardioprotective Effect of Exercise in the Elderly. Aging Clin. Exp. Res. 2013, 25, 487–497. [Google Scholar] [CrossRef]
- Calvert, J.W.; Elston, M.; Aragón, J.P.; Nicholson, C.K.; Moody, B.F.; Hood, R.L.; Sindler, A.; Gundewar, S.; Seals, D.R.; Barouch, L.A.; et al. Exercise Protects Against Myocardial Ischemia-Reperfusion Injury via Stimulation of Β3-Adrenergic Receptors and Increased Nitric Oxide Signaling: Role of Nitrite and Nitrosothiols. Circ. Res. 2011, 108, 1448. [Google Scholar] [CrossRef]
- Salazar, N.C.; Vallejos, X.; Siryk, A.; Rengo, G.; Cannavo, A.; Liccardo, D.; De Lucia, C.; Gao, E.; Leosco, D.; Koch, W.J.; et al. GRK2 Blockade with βARKct Is Essential for Cardiac Β2-Adrenergic Receptor Signaling towards Increased Contractility. Cell Commun. Signal. CCS 2013, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Hasegawa, H.; Cheng, H.-J.; Little, W.C.; Cheng, C.-P. Endogenous Beta3-Adrenoreceptor Activation Contributes to Left Ventricular and Cardiomyocyte Dysfunction in Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2425–H2433. [Google Scholar] [CrossRef] [PubMed]
- Dinçer, U.D.; Bidasee, K.R.; Güner, S.; Tay, A.; Ozçelikay, A.T.; Altan, V.M. The Effect of Diabetes on Expression of Beta1-, Beta2-, and Beta3-Adrenoreceptors in Rat Hearts. Diabetes 2001, 50, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Amour, J.; Loyer, X.; Le Guen, M.; Mabrouk, N.; David, J.-S.; Camors, E.; Carusio, N.; Vivien, B.; Andriantsitohaina, R.; Heymes, C.; et al. Altered Contractile Response Due to Increased Beta3-Adrenoceptor Stimulation in Diabetic Cardiomyopathy: The Role of Nitric Oxide Synthase 1-Derived Nitric Oxide. Anesthesiology 2007, 107, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.L.; Leyton-Mange, J.S.; Niu, X.; Yang, R.; Cingolani, O.; Arkenbout, E.K.; Champion, H.C.; Bedja, D.; Gabrielson, K.L.; Chen, J.; et al. Adverse Ventricular Remodeling and Exacerbated NOS Uncoupling from Pressure-Overload in Mice Lacking the Beta3-Adrenoreceptor. J. Mol. Cell. Cardiol. 2009, 47, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhao, L.; Li, X.; Xue, Y.; Wang, B.; Lv, Z.; Chen, J.; Sun, D.; Zheng, Q. Β3-Adrenoreceptor Stimulation Protects against Myocardial Infarction Injury via eNOS and nNOS Activation. PLoS ONE 2014, 9, e98713. [Google Scholar] [CrossRef] [PubMed]
- Kayki-Mutlu, G.; Karaomerlioglu, I.; Arioglu-Inan, E.; Altan, V.M. Beta-3 Adrenoceptors: A Potential Therapeutic Target for Heart Disease. Eur. J. Pharmacol. 2019, 858, 172468. [Google Scholar] [CrossRef]
- Fry, N.A.S.; Liu, C.-C.; Garcia, A.; Hamilton, E.J.; Karimi Galougahi, K.; Kim, Y.J.; Whalley, D.W.; Bundgaard, H.; Rasmussen, H.H. Targeting Cardiac Myocyte Na+-K+ Pump Function with Β3 Adrenergic Agonist in Rabbit Model of Severe Congestive Heart Failure. Circ. Heart Fail. 2020, 13, e006753. [Google Scholar] [CrossRef]
- Karimi Galougahi, K.; Liu, C.-C.; Garcia, A.; Fry, N.A.; Hamilton, E.J.; Figtree, G.A.; Rasmussen, H.H. Β3-Adrenoceptor Activation Relieves Oxidative Inhibition of the Cardiac Na+-K+ Pump in Hyperglycemia Induced by Insulin Receptor Blockade. Am. J. Physiol. Cell Physiol. 2015, 309, C286–C295. [Google Scholar] [CrossRef]
- Bosch, R.F.; Nattel, S. Cellular Electrophysiology of Atrial Fibrillation. Cardiovasc. Res. 2002, 54, 259–269. [Google Scholar] [CrossRef]
- Kitamura, T.; Onishi, K.; Dohi, K.; Okinaka, T.; Isaka, N.; Nakano, T. The Negative Inotropic Effect of Β3-Adrenoceptor Stimulation in the Beating Guinea Pig Heart. J. Cardiovasc. Pharmacol. 2000, 35, 786. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.; Dumas, J.P.; Bardou, M.; Rochette, L.; Advenier, C.; Giudicelli, J.F. Influence of Beta-Adrenoceptor Agonists on the Pulmonary Circulation. Effects of a Beta3-Adrenoceptor Antagonist, SR 59230A. Eur. J. Pharmacol. 1998, 348, 223–228. [Google Scholar] [CrossRef] [PubMed]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, P.; Sarsero, D.; Kaumann, A.J. Proposal for the Interaction of Non-Conventional Partial Agonists and Catecholamines with the “putative Beta 4-Adrenoceptor” in Mammalian Heart. Clin. Exp. Pharmacol. Physiol. 1997, 24, 647–656. [Google Scholar] [CrossRef]
- Granneman, J.G. The Putative Beta4-Adrenergic Receptor Is a Novel State of the Beta1-Adrenergic Receptor. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E199–E202. [Google Scholar] [CrossRef]
- Staehelin, M.; Simons, P.; Jaeggi, K.; Wigger, N. CGP-12177. A Hydrophilic Beta-Adrenergic Receptor Radioligand Reveals High Affinity Binding of Agonists to Intact Cells. J. Biol. Chem. 1983, 258, 3496–3502. [Google Scholar] [CrossRef]
- Ito, M.; Grujic, D.; Abel, E.D.; Vidal-Puig, A.; Susulic, V.S.; Lawitts, J.; Harper, M.E.; Himms-Hagen, J.; Strosberg, A.D.; Lowell, B.B. Mice Expressing Human but Not Murine Beta3-Adrenergic Receptors under the Control of Human Gene Regulatory Elements. Diabetes 1998, 47, 1464–1471. [Google Scholar] [CrossRef]
- Galitzky, J.; Langin, D.; Verwaerde, P.; Montastruc, J.-L.; Lafontan, M.; Berlan, M. Lipolytic Effects of Conventional Β3-Adrenoceptor Agonists and of CGP 12,177 in Rat and Human Fat Cells: Preliminary Pharmacological Evidence for a Putative Β4-Adrenoceptor. Br. J. Pharmacol. 1997, 122, 1244–1250. [Google Scholar] [CrossRef]
- Nergårdh, A.; Boréus, L.O.; Naglo, A.S. Characterization of the Adrenergic Beta-Receptor in the Urinary Bladder of Man and Cat. Acta Pharmacol. Toxicol. 1977, 40, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.J. Alpha And Beta-Adrenoceptors in the Detrusor Muscle and Bladder Base of the Pig and Beta-Adrenoceptors in the Detrusor Muscle of Man. Br. J. Pharmacol. 1979, 65, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.E.; Fonseca, A.M.; Silva, I.D.; Girão, M.J.; Sartori, M.G.; Castro, R.A. The Relationship between the Trp 64 Arg Polymorphism of the Beta 3-Adrenoceptor Gene and Idiopathic Overactive Bladder. Am. J. Obstet. Gynecol. 2011, 205, 82.e10–82.e14. [Google Scholar] [CrossRef]
- Igawa, Y.; Michel, M.C. Pharmacological Profile of Β3-Adrenoceptor Agonists in Clinical Development for the Treatment of Overactive Bladder Syndrome. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 386, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Igawa, Y.; Yamazaki, Y.; Takeda, H.; Hayakawa, K.; Akahane, M.; Ajisawa, Y.; Yoneyama, T.; Nishizawa, O.; Andersson, K.E. Functional and Molecular Biological Evidence for a Possible Beta3-Adrenoceptor in the Human Detrusor Muscle. Br. J. Pharmacol. 1999, 126, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Igawa, Y.; Yamazaki, Y.; Takeda, H.; Kaidoh, K.; Akahane, M.; Ajisawa, Y.; Yoneyama, T.; Nishizawa, O.; Andersson, K.E. Relaxant Effects of Isoproterenol and Selective Beta3-Adrenoceptor Agonists on Normal, Low Compliant and Hyperreflexic Human Bladders. J. Urol. 2001, 165, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, O. Beta3-Adrenoceptors in Human Detrusor Muscle. Urology 2002, 59, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Chancellor, M.B. Neurophysiology of Lower Urinary Tract Function and Dysfunction. Rev. Urol. 2003, 5 (Suppl. S8), S3–S10. [Google Scholar] [PubMed]
- Limberg, B.J.; Andersson, K.-E.; Aura Kullmann, F.; Burmer, G.; de Groat, W.C.; Rosenbaum, J.S. β-Adrenergic Receptor Subtype Expression in Myocyte and Non-Myocyte Cells in Human Female Bladder. Cell Tissue Res. 2010, 342, 295–306. [Google Scholar] [CrossRef]
- Masunaga, K.; Chapple, C.R.; McKay, N.G.; Yoshida, M.; Sellers, D.J. The Β3-Adrenoceptor Mediates the Inhibitory Effects of β-Adrenoceptor Agonists via the Urothelium in Pig Bladder Dome. Neurourol. Urodyn. 2010, 29, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Calmasini, F.B.; Candido, T.Z.; Alexandre, E.C.; D’Ancona, C.A.; Silva, D.; de Oliveira, M.A.; De Nucci, G.; Antunes, E.; Mónica, F.Z. The Beta-3 Adrenoceptor Agonist, Mirabegron Relaxes Isolated Prostate from Human and Rabbit: New Therapeutic Indication? Prostate 2015, 75, 440–447. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Chapple, C.R. Beta3-Adrenoceptors in Urinary Bladder. Neurourol. Urodyn. 2007, 26, 752–756. [Google Scholar] [CrossRef]
- Vij, M.; Drake, M.J. Clinical Use of the Β3 Adrenoceptor Agonist Mirabegron in Patients with Overactive Bladder Syndrome. Ther. Adv. Urol. 2015, 7, 241–248. [Google Scholar] [CrossRef]
- Ohlstein, E.H.; von Keitz, A.; Michel, M.C. A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial of the Β3-Adrenoceptor Agonist Solabegron for Overactive Bladder. Eur. Urol. 2012, 62, 834–840. [Google Scholar] [CrossRef]
- Otsuka, A.; Shinbo, H.; Matsumoto, R.; Kurita, Y.; Ozono, S. Expression and Functional Role of Beta-Adrenoceptors in the Human Urinary Bladder Urothelium. Naunyn. Schmiedebergs Arch. Pharmacol. 2008, 377, 473–481. [Google Scholar] [CrossRef]
- Kullmann, F.A.; Downs, T.R.; Artim, D.E.; Limberg, B.J.; Shah, M.; Contract, D.; de Groat, W.C.; Rosenbaum, J.S. Urothelial Beta-3 Adrenergic Receptors in the Rat Bladder. Neurourol. Urodyn. 2011, 30, 144–150. [Google Scholar] [CrossRef]
- Tanaka, Y.; Horinouchi, T.; Koike, K. New Insights into Beta-Adrenoceptors in Smooth Muscle: Distribution of Receptor Subtypes and Molecular Mechanisms Triggering Muscle Relaxation. Clin. Exp. Pharmacol. Physiol. 2005, 32, 503–514. [Google Scholar] [CrossRef]
- Michel, M.C.; Korstanje, C. Β3-Adrenoceptor Agonists for Overactive Bladder Syndrome: Role of Translational Pharmacology in a Repositioning Clinical Drug Development Project. Pharmacol. Ther. 2016, 159, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Antunes-Lopes, T.; Gillespie, J.; Cruz, F. Beta-3 Adrenergic Receptor Is Expressed in Acetylcholine-Containing Nerve Fibers of the Human Urinary Bladder: An Immunohistochemical Study. Neurourol. Urodyn. 2017, 36, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Igawa, Y.; Nishizawa, O.; Wyndaele, J.-J. Effects of CL316,243, a Beta 3-Adrenoceptor Agonist, and Intravesical Prostaglandin E2 on the Primary Bladder Afferent Activity of the Rat. Neurourol. Urodyn. 2010, 29, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R.; Cardozo, L.; Nitti, V.W.; Siddiqui, E.; Michel, M.C. Mirabegron in Overactive Bladder: A Review of Efficacy, Safety, and Tolerability. Neurourol. Urodyn. 2014, 33, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Baka-Ostrowska, M.; Bolong, D.T.; Persu, C.; Tøndel, C.; Steup, A.; Lademacher, C.; Martin, N. Efficacy and Safety of Mirabegron in Children and Adolescents with Neurogenic Detrusor Overactivity: An Open-Label, Phase 3, Dose-Titration Study. Neurourol. Urodyn. 2021, 40, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takeda, M.; Gotoh, M.; Nagai, S.; Kurose, T. Vibegron, a Novel Potent and Selective Β3-Adrenoreceptor Agonist, for the Treatment of Patients with Overactive Bladder: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Eur. Urol. 2018, 73, 783–790. [Google Scholar] [CrossRef]
- Keam, S.J. Vibegron: First Global Approval. Drugs 2018, 78, 1835–1839. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, V.; Chancellor, M. Mirabegron: A Safety Review. Expert Opin. Drug Saf. 2011, 10, 287–294. [Google Scholar] [CrossRef]
- Kanie, S.; Otsuka, A.; Yoshikawa, S.; Morimoto, T.; Hareyama, N.; Okazaki, S.; Kobayashi, R.; Hasebe, K.; Nakao, K.; Hayashi, R.; et al. Pharmacological Effect of TRK-380, a Novel Selective Human Β3-Adrenoceptor Agonist, on Mammalian Detrusor Strips. Urology 2012, 79, 744.e1–744.e7. [Google Scholar] [CrossRef] [PubMed]
- Liggett, S.B. Functional Properties of the Rat and Human Beta 3-Adrenergic Receptors: Differential Agonist Activation of Recombinant Receptors in Chinese Hamster Ovary Cells. Mol. Pharmacol. 1992, 42, 634–637. [Google Scholar] [PubMed]
- Bardou, M.; Loustalot, C.; Cortijo, J.; Simon, B.; Naline, E.; Dumas, M.; Esteve, S.; Croci, T.; Chalon, P.; Frydman, R.; et al. Functional, Biochemical and Molecular Biological Evidence for a Possible Beta(3)-Adrenoceptor in Human near-Term Myometrium. Br. J. Pharmacol. 2000, 130, 1960–1966. [Google Scholar] [CrossRef]
- Tyson, E.K.; MacIntyre, D.A.; Smith, R.; Chan, E.-C.; Read, M. Evidence That a Protein Kinase A Substrate, Small Heat-Shock Protein 20, Modulates Myometrial Relaxation in Human Pregnancy. Endocrinology 2008, 149, 6157–6165. [Google Scholar] [CrossRef]
- Hynes, P.G.; Friel, A.M.; Smith, T.J.; Morrison, J.J. Beta-Adrenoceptor Subtype Expression in Human Placenta and Umbilical Arteries in Normal and Preeclamptic Pregnancies. Hypertens. Pregnancy 2008, 27, 169–181. [Google Scholar] [CrossRef]
- Resch, B.E.; Ducza, E.; Gáspár, R.; Falkay, G. Role of Adrenergic Receptor Subtypes in the Control of Human Placental Blood Vessels. Mol. Reprod. Dev. 2003, 66, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Fazi, C.; Nardini, P.; Calvani, M.; Fabbri, S.; Guerrini, A.; Forni, G.; La Marca, G.; Rosa, A.C.; Filippi, L. Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus. Cells 2020, 9, 2625. [Google Scholar] [CrossRef]
- Manzo, G. Similarities Between Embryo Development and Cancer Process Suggest New Strategies for Research and Therapy of Tumors: A New Point of View. Front. Cell Dev. Biol. 2019, 7, 20. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.; Burton, G. Evaluation of Respiratory Gases and Acid-Base Gradients in Human Fetal Fluids and Uteroplacental Tissue between 7 and 16 Weeks’ Gestation. Am. J. Obstet. Gynecol. 2001, 184, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Nardini, P.; Zizi, V.; Molino, M.; Fazi, C.; Calvani, M.; Carrozzo, F.; Cavallaro, G.; Giuseppetti, G.; Calosi, L.; et al. Β3 Adrenoceptor Agonism Prevents Hyperoxia-Induced Colonic Alterations. Biomolecules 2023, 13, 1755. [Google Scholar] [CrossRef]
- Filippi, L.; Pini, A.; Cammalleri, M.; Bagnoli, P.; Dal Monte, M. Β3-Adrenoceptor, a Novel Player in the Round-Trip from Neonatal Diseases to Cancer: Suggestive Clues from Embryo. Med. Res. Rev. 2022, 42, 1179–1201. [Google Scholar] [CrossRef]
- Calvani, M.; Bruno, G.; Dal Monte, M.; Nassini, R.; Fontani, F.; Casini, A.; Cavallini, L.; Becatti, M.; Bianchini, F.; De Logu, F.; et al. Β3 -Adrenoceptor as a Potential Immuno-Suppressor Agent in Melanoma. Br. J. Pharmacol. 2019, 176, 2509–2524. [Google Scholar] [CrossRef]
- Breuiller, M.; Rouot, B.; Leroy, M.J.; Blot, P.; Kaplan, L.; Ferré, F. Adrenergic Receptors in Inner and Outer Layers of Human Myometrium near Term: Characterization of Beta-Adrenergic Receptor Sites by [125I]-Iodocyanopindolol Binding. Gynecol. Obstet. Investig. 1987, 24, 28–37. [Google Scholar] [CrossRef]
- Cikos, S.; Veselá, J.; Il’ková, G.; Rehák, P.; Czikková, S.; Koppel, J. Expression of Beta Adrenergic Receptors in Mouse Oocytes and Preimplantation Embryos. Mol. Reprod. Dev. 2005, 71, 145–153. [Google Scholar] [CrossRef]
- Adeoya-Osiguwa, S.A.; Gibbons, R.; Fraser, L.R. Identification of Functional Alpha2- and Beta-Adrenergic Receptors in Mammalian Spermatozoa. Hum. Reprod. Oxf. Engl. 2006, 21, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Asif, H.; Barnett, S.D.; Buxton, I.L.O. Β3 Adrenergic Receptor Signaling in the Human Myometrium. Reprod. Sci. 2023, 30, 124–134. [Google Scholar] [CrossRef]
- Buxton, I.L.O.; Asif, H.; Barnett, S.D. Β3 Receptor Signaling in Pregnant Human Myometrium Suggests a Role for Β3 Agonists as Tocolytics. Biomolecules 2023, 13, 1005. [Google Scholar] [CrossRef] [PubMed]
- Legrand, C.; Maltier, J.P.; Benghan-Eyene, Y. Rat Myometrial Adrenergic Receptors in Late Pregnancy. Biol. Reprod. 1987, 37, 641–650. [Google Scholar] [CrossRef]
- Maltier, J.P.; Benghan-Eyene, Y.; Legrand, C. Regulation of Myometrial Β2-Adrenergic Receptors by Progesterone and Estradiol-17β in Late Pregnant Rats1. Biol. Reprod. 1989, 40, 531–540. [Google Scholar] [CrossRef]
- Vivat, V.; Cohen-Tannoudji, J.; Revelli, J.P.; Muzzin, P.; Giacobino, J.P.; Maltier, J.P.; Legrand, C. Progesterone Transcriptionally Regulates the Beta 2-Adrenergic Receptor Gene in Pregnant Rat Myometrium. J. Biol. Chem. 1992, 267, 7975–7978. [Google Scholar] [CrossRef]
- Hatjis, C.G.; Grogan, D.M. Pregnancy-Induced Changes in the Interaction of Guinea Pig Myometrial β-Adrenergic Receptors with l-Isoproterenol. Am. J. Obstet. Gynecol. 1989, 161, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Ferre, F.; Uzan, M.; Janssens, Y.; Tanguy, G.; Jolivet, A.; Breuiller, M.; Sureau, C.; Cedard, L. Oral Administration of Micronized Natural Progesterone in Late Human Pregnancy. Effects on Progesterone and Estrogen Concentrations in the Plasma, Placenta, and Myometrium. Am. J. Obstet. Gynecol. 1984, 148, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Malo, A.; Puerta, M. Oestradiol and Progesterone Change Beta3-Adrenergic Receptor Affinity and Density in Brown Adipocytes. Eur. J. Endocrinol. 2001, 145, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Čikoš, Š.; Czikková, S.; Chrenek, P.; Makarevich, A.V.; Burkuš, J.; Janštová, Ž.; Fabian, D.; Koppel, J. Expression of Adrenergic Receptors in Bovine and Rabbit Oocytes and Preimplantation Embryos. Reprod. Domest. Anim. Zuchthyg. 2014, 49, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Rouget, C.; Breuiller-Fouché, M.; Loustalot, C.; Naline, E.; Sagot, P.; Frydman, R.; Morcillo, E.J.; Advenier, C.; Leroy, M.-J.; et al. Is the Beta3-Adrenoceptor (ADRB3) a Potential Target for Uterorelaxant Drugs? BMC Pregnancy Childbirth 2007, 7, S14. [Google Scholar] [CrossRef] [PubMed]
- Dennedy, M.C.; Houlihan, D.D.; McMillan, H.; Morrison, J.J. Beta2- and Beta3-Adrenoreceptor Agonists: Human Myometrial Selectivity and Effects on Umbilical Artery Tone. Am. J. Obstet. Gynecol. 2002, 187, 641–647. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The Hypoxic Tumour Microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Dal Monte, M.; Casini, G.; Daniotti, M.; Sereni, F.; Bagnoli, P. Infantile Hemangiomas, Retinopathy of Prematurity and Cancer: A Common Pathogenetic Role of the β-Adrenergic System. Med. Res. Rev. 2015, 35, 619–652. [Google Scholar] [CrossRef]
- Lirussi, F.; Rakotoniaina, Z.; Madani, S.; Goirand, F.; Breuiller-Fouché, M.; Leroy, M.-J.; Sagot, P.; Morrison, J.J.; Dumas, M.; Bardou, M. ADRB3 Adrenergic Receptor Is a Key Regulator of Human Myometrial Apoptosis and Inflammation during Chorioamnionitis. Biol. Reprod. 2008, 78, 497–505. [Google Scholar] [CrossRef][Green Version]
- Hadi, T.; Barrichon, M.; Mourtialon, P.; Wendremaire, M.; Garrido, C.; Sagot, P.; Bardou, M.; Lirussi, F. Biphasic Erk1/2 Activation Sequentially Involving Gs and Gi Signaling Is Required in Beta3-Adrenergic Receptor-Induced Primary Smooth Muscle Cell Proliferation. Biochim. Biophys. Acta 2013, 1833, 1041–1051. [Google Scholar] [CrossRef]
- Hadi, T.; Douhard, R.; Dias, A.M.M.; Wendremaire, M.; Pezzè, M.; Bardou, M.; Sagot, P.; Garrido, C.; Lirussi, F. Beta3 Adrenergic Receptor Stimulation in Human Macrophages Inhibits NADPHoxidase Activity and Induces Catalase Expression via PPARγ Activation. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1769–1784. [Google Scholar] [CrossRef]
- Doheny, H.C.; Lynch, C.M.; Smith, T.J.; Morrison, J.J. Functional Coupling of Beta3-Adrenoceptors and Large Conductance Calcium-Activated Potassium Channels in Human Uterine Myocytes. J. Clin. Endocrinol. Metab. 2005, 90, 5786–5796. [Google Scholar] [CrossRef]
- Anwer, K.; Oberti, C.; Perez, G.J.; Perez-Reyes, N.; McDougall, J.K.; Monga, M.; Sanborn, B.M.; Stefani, E.; Toro, L. Calcium-Activated K+ Channels as Modulators of Human Myometrial Contractile Activity. Am. J. Physiol. 1993, 265, C976–C985. [Google Scholar] [CrossRef]
- Schuller, H.M.; Cole, B. Regulation of Cell Proliferation by Beta-Adrenergic Receptors in a Human Lung Adenocarcinoma Cell Line. Carcinogenesis 1989, 10, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, Z.; Lu, L.; Cho, C.H. β-Adrenergic System, a Backstage Manipulator Regulating Tumour Progression and Drug Target in Cancer Therapy. Semin. Cancer Biol. 2013, 23, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Notarnicola, M.; Caruso, M.G.; Tutino, V.; Scilimati, A. Upregulation of Beta3-Adrenergic Receptor mRNA in Human Colon Cancer: A Preliminary Study. Oncology 2008, 75, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lamkin, D.M.; Sloan, E.K.; Patel, A.J.; Chiang, B.S.; Pimentel, M.A.; Ma, J.C.Y.; Arevalo, J.M.; Morizono, K.; Cole, S.W. Chronic Stress Enhances Progression of Acute Lymphoblastic Leukemia via β-Adrenergic Signaling. Brain Behav. Immun. 2012, 26, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, K.M.; Chang, K.W.; Truong, M.T.; Kwok, S.; West, R.B.; Heerema-McKenney, A.E. β-Adrenergic Receptor Expression in Vascular Tumors. Mod. Pathol. 2012, 25, 1446–1451. [Google Scholar] [CrossRef]
- Modzelewska, B.; Jóźwik, M.; Jóźwik, M.; Sulkowski, S.; Pędzińska-Betiuk, A.; Kleszczewski, T.; Kostrzewska, A. Altered Uterine Contractility in Response to β-Adrenoceptor Agonists in Ovarian Cancer. J. Physiol. Sci. 2017, 67, 711. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; De Logu, F.; Souza Monteiro de Araujo, D.; Subbiani, A.; Lunardi, F.; Rettori, S.; Nassini, R.; Favre, C.; Calvani, M. Β2-and Β3-Adrenergic Receptors Contribute to Cancer-Evoked Pain in a Mouse Model of Osteosarcoma via Modulation of Neural Macrophages. Front. Pharmacol. 2021, 12, 697912. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Dabraio, A.; Bruno, G.; De Gregorio, V.; Coronnello, M.; Bogani, C.; Ciullini, S.; Marca, G.L.; Vignoli, M.; Chiarugi, P.; et al. Β3-Adrenoreceptor Blockade Reduces Hypoxic Myeloid Leukemic Cells Survival and Chemoresistance. Int. J. Mol. Sci. 2020, 21, 4210. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Pisani, F.; Laudadio, E.; Cammalleri, M.; Lucchesi, M.; Marracci, S.; Filippi, L.; Galeazzi, R.; Svelto, M.; Dal Monte, M.; et al. HIF-1-Dependent Induction of Β3 Adrenoceptor: Evidence from the Mouse Retina. Cells 2022, 11, 1271. [Google Scholar] [CrossRef]
- Samudio, I.; Fiegl, M.; McQueen, T.; Clise-Dwyer, K.; Andreeff, M. The Warburg Effect in Leukemia-Stroma Cocultures Is Mediated by Mitochondrial Uncoupling Associated with Uncoupling Protein 2 Activation. Cancer Res. 2008, 68, 5198. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Grazzini, M.; Gandini, S.; Benemei, S.; Asbury, C.D.; Marchionni, N.; Geppetti, P. β-Adrenergic-Blocking Drugs and Melanoma: Current State of the Art. Expert Rev. Anticancer Ther. 2012, 12, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Lemeshow, S.; Sørensen, H.T.; Phillips, G.; Yang, E.V.; Antonsen, S.; Riis, A.H.; Lesinski, G.B.; Jackson, R.; Glaser, R. β-Blockers and Survival among Danish Patients with Malignant Melanoma: A Population-Based Cohort Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2273–2279. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Geppetti, P.; Gandini, S. β-Blocker Use and Reduced Disease Progression in Patients with Thick Melanoma: 8 Years of Follow-Up. Melanoma Res. 2017, 27, 268–270. [Google Scholar] [CrossRef]
- Hoffmann, C.; Leitz, M.R.; Oberdorf-Maass, S.; Lohse, M.J.; Klotz, K.-N. Comparative Pharmacology of Human Beta-Adrenergic Receptor Subtypes—Characterization of Stably Transfected Receptors in CHO Cells. Naunyn. Schmiedebergs Arch. Pharmacol. 2004, 369, 151–159. [Google Scholar] [CrossRef]
- Moretti, S.; Massi, D.; Farini, V.; Baroni, G.; Parri, M.; Innocenti, S.; Cecchi, R.; Chiarugi, P. β-Adrenoceptors Are Upregulated in Human Melanoma and Their Activation Releases pro-Tumorigenic Cytokines and Metalloproteases in Melanoma Cell Lines. Lab. Investig. J. Tech. Methods Pathol. 2013, 93, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Sereni, F.; Dal Monte, M.; Filippi, L.; Bagnoli, P. Role of Host Β1- and Β2-Adrenergic Receptors in a Murine Model of B16 Melanoma: Functional Involvement of Β3-Adrenergic Receptors. Naunyn. Schmiedebergs Arch. Pharmacol. 2015, 388, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Calvani, M.; Cammalleri, M.; Favre, C.; Filippi, L.; Bagnoli, P. β-Adrenoceptors as Drug Targets in Melanoma: Novel Preclinical Evidence for a Role of Β3-adrenoceptors. Br. J. Pharmacol. 2019, 176, 2496–2508. [Google Scholar] [CrossRef]
- Rains, S.L.; Amaya, C.N.; Bryan, B.A. Beta-Adrenergic Receptors Are Expressed across Diverse Cancers. Oncoscience 2017, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Bruno, G.; Domazetovic, V.; Favre, C.; Calvani, M. Current Therapies and New Targets to Fight Melanoma: A Promising Role for the Β3-Adrenoreceptor. Cancers 2020, 12, 1415. [Google Scholar] [CrossRef]
- Dal Monte, M.; Casini, G.; Filippi, L.; Nicchia, G.P.; Svelto, M.; Bagnoli, P. Functional Involvement of Β3-Adrenergic Receptors in Melanoma Growth and Vascularization. J. Mol. Med. Berl. Ger. 2013, 91, 1407–1419. [Google Scholar] [CrossRef]
- Calvani, M.; Cavallini, L.; Tondo, A.; Spinelli, V.; Ricci, L.; Pasha, A.; Bruno, G.; Buonvicino, D.; Bigagli, E.; Vignoli, M.; et al. Β3-Adrenoreceptors Control Mitochondrial Dormancy in Melanoma and Embryonic Stem Cells. Oxid. Med. Cell. Longev. 2018, 2018, 6816508. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Hirtz, C.; Carrera, G.; Cazenave, R.; Troly, M.; Salvayre, R.; Pénicaud, L.; Casteilla, L. A Role for Uncoupling Protein-2 as a Regulator of Mitochondrial Hydrogen Peroxide Generation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1997, 11, 809–815. [Google Scholar] [CrossRef]
- Laukova, M.; Vargovic, P.; Csaderova, L.; Chovanova, L.; Vlcek, M.; Imrich, R.; Krizanova, O.; Kvetnansky, R. Acute Stress Differently Modulates Β1, Β2 and Β3 Adrenoceptors in T Cells, but Not in B Cells, from the Rat Spleen. Neuroimmunomodulation 2012, 19, 69–78. [Google Scholar] [CrossRef]
- Maisel, A.S.; Fowler, P.; Rearden, A.; Motulsky, H.J.; Michel, M.C. A New Method for Isolation of Human Lymphocyte Subsets Reveals Differential Regulation of Beta-Adrenergic Receptors by Terbutaline Treatment. Clin. Pharmacol. Ther. 1989, 46, 429–439. [Google Scholar] [CrossRef]
- Estrada, L.D.; Ağaç, D.; Farrar, J.D. Sympathetic Neural Signaling via the Β2-Adrenergic Receptor Suppresses T-Cell Receptor-Mediated Human and Mouse CD8(+) T-Cell Effector Function. Eur. J. Immunol. 2016, 46, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Guereschi, M.G.; Araujo, L.P.; Maricato, J.T.; Takenaka, M.C.; Nascimento, V.M.; Vivanco, B.C.; Reis, V.O.; Keller, A.C.; Brum, P.C.; Basso, A.S. Beta2-Adrenergic Receptor Signaling in CD4+ Foxp3+ Regulatory T Cells Enhances Their Suppressive Function in a PKA-Dependent Manner. Eur. J. Immunol. 2013, 43, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Schouppe, E.; De Baetselier, P.; Van Ginderachter, J.A.; Sarukhan, A. Instruction of Myeloid Cells by the Tumor Microenvironment. Oncoimmunology 2012, 1, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Clegg, R.A.; Mullaney, I. Acute Change in the Cyclic AMP Content of Rat Mammary Acini in Vitro. Influence of Physiological and Pharmacological Agents. Biochem. J. 1985, 230, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lavandero, S.; Donoso, E.; Sapag-Hagar, M. Beta-Adrenergic Receptors in Rat Mammary Gland. Biochem. Pharmacol. 1985, 34, 2034–2036. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B.; Spinola, P.G.; Plante, M.; Poyet, P.; Folléa, N.; Pelletier, G.; Labrie, F. Beta-Adrenergic Receptors in DMBA-Induced Rat Mammary Tumors: Correlation with Progesterone Receptor and Tumor Growth. Breast Cancer Res. Treat. 1989, 13, 251–263. [Google Scholar] [CrossRef]
- Roets, E.; Peeters, G.; Leysen, J.E. Identification of Beta-Adrenoceptors in Bovine Teat Muscles by 3H-Dihydroalprenolol Binding. Arch. Int. Pharmacodyn. Ther. 1984, 270, 203–214. [Google Scholar]
- Roets, E.; Vandeputte-Van Messom, G.; Peeters, G. Relationship between Milkability and Adrenoceptor Concentrations in Teat Tissue in Primiparous Cows. J. Dairy Sci. 1986, 69, 3120–3130. [Google Scholar] [CrossRef]
- Hammon, H.M.; Bruckmaier, R.M.; Honegger, U.E.; Blum, J.W. Distribution and Density of Alpha- and Beta-Adrenergic Receptor Binding Sites in the Bovine Mammary Gland. J. Dairy Res. 1994, 61, 47–57. [Google Scholar] [CrossRef]
- Marchetti, B.; Fortier, M.A.; Poyet, P.; Folléa, N.; Pelletier, G.; Labrie, F. Beta-Adrenergic Receptors in the Rat Mammary Gland during Pregnancy and Lactation: Characterization, Distribution, and Coupling to Adenylate Cyclase. Endocrinology 1990, 126, 565–574. [Google Scholar] [CrossRef]
- Antoni, M.H.; Lechner, S.; Diaz, A.; Vargas, S.; Holley, H.; Phillips, K.; McGregor, B.; Carver, C.S.; Blomberg, B. Cognitive Behavioral Stress Management Effects on Psychosocial and Physiological Adaptation in Women Undergoing Treatment for Breast Cancer. Brain. Behav. Immun. 2009, 23, 580–591. [Google Scholar] [CrossRef]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The Influence of Bio-Behavioural Factors on Tumour Biology: Pathways and Mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Lüthy, I.A.; Bruzzone, A.; Piñero, C.P.; Castillo, L.F.; Chiesa, I.J.; Vázquez, S.M.; Sarappa, M.G. Adrenoceptors: Non Conventional Target for Breast Cancer? Curr. Med. Chem. 2009, 16, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of Non-Selective β-Blockers Is Associated with Decreased Tumor Proliferative Indices in Early Stage Breast Cancer. Oncotarget 2016, 8, 6446–6460. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Zhang, J.; Dancel, R.; Garcia, S.J.; Willis, C.; Seidler, F.J. Beta-Adrenoceptor Signaling and Its Control of Cell Replication in MDA-MB-231 Human Breast Cancer Cells. Breast Cancer Res. Treat. 2000, 60, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Lorimer, E.; Tyburski, M.D.; Williams, C.L. β-Adrenergic Receptors Suppress Rap1B Prenylation and Promote the Metastatic Phenotype in Breast Cancer Cells. Cancer Biol. Ther. 2015, 16, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Rico, M.; Baglioni, M.; Bondarenko, M.; Laluce, N.C.; Rozados, V.; Nicolas, A.; Carré, M.; Scharovsky, O.G.; Márquez, M.M. Metformin and Propranolol Combination Prevents Cancer Progression and Metastasis in Different Breast Cancer Models. Oncotarget 2016, 8, 2874–2889. [Google Scholar] [CrossRef]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta Blockers and Breast Cancer Mortality: A Population- Based Study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef]
- Carie, A.E.; Sebti, S.M. A Chemical Biology Approach Identifies a Beta-2 Adrenergic Receptor Agonist That Causes Human Tumor Regression by Blocking the Raf-1/Mek-1/Erk1/2 Pathway. Oncogene 2007, 26, 3777–3788. [Google Scholar] [CrossRef]
- Pérez Piñero, C.; Bruzzone, A.; Sarappa, M.; Castillo, L.; Lüthy, I. Involvement of A2- and Β2-Adrenoceptors on Breast Cancer Cell Proliferation and Tumour Growth Regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef]
- Feigelson, H.S.; Teras, L.R.; Diver, W.R.; Tang, W.; Patel, A.V.; Stevens, V.L.; Calle, E.E.; Thun, M.J.; Bouzyk, M. Genetic Variation in Candidate Obesity Genes ADRB2, ADRB3, GHRL, HSD11B1, IRS1, IRS2, and SHC1 and Risk for Breast Cancer in the Cancer Prevention Study II. Breast Cancer Res. BCR 2008, 10, R57. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-E.; Hamajima, N.; Saito, T.; Matsuo, K.; Mizutani, M.; Iwata, H.; Iwase, T.; Miura, S.; Mizuno, T.; Tokudome, S.; et al. Possible Association of Β2- and Β3-Adrenergic Receptor Gene Polymorphisms with Susceptibility to Breast Cancer. Breast Cancer Res. BCR 2001, 3, 264–269. [Google Scholar] [CrossRef]
- Babol, K.; Przybylowska, K.; Lukaszek, M.; Pertynski, T.; Blasiak, J. An Association between the Trp64Arg Polymorphism in the Beta3-Adrenergic Receptor Gene and Endometrial Cancer and Obesity. J. Exp. Clin. Cancer Res. CR 2004, 23, 669–674. [Google Scholar]
- Powe, D.G.; Voss, M.J.; Habashy, H.O.; Zänker, K.S.; Green, A.R.; Ellis, I.O.; Entschladen, F. Alpha- and Beta-Adrenergic Receptor (AR) Protein Expression Is Associated with Poor Clinical Outcome in Breast Cancer: An Immunohistochemical Study. Breast Cancer Res. Treat. 2011, 130, 457–463. [Google Scholar] [CrossRef]
- Gargiulo, L.; Copsel, S.; Rivero, E.M.; Galés, C.; Sénard, J.-M.; Lüthy, I.A.; Davio, C.; Bruzzone, A. Differential Β2-Adrenergic Receptor Expression Defines the Phenotype of Non-Tumorigenic and Malignant Human Breast Cell Lines. Oncotarget 2014, 5, 10058–10069. [Google Scholar] [CrossRef] [PubMed]
- Creed, S.J.; Le, C.P.; Hassan, M.; Pon, C.K.; Albold, S.; Chan, K.T.; Berginski, M.E.; Huang, Z.; Bear, J.E.; Lane, J.R.; et al. Β2-Adrenoceptor Signaling Regulates Invadopodia Formation to Enhance Tumor Cell Invasion. Breast Cancer Res. BCR 2015, 17, 145. [Google Scholar] [CrossRef]
- Pon, C.K.; Lane, J.R.; Sloan, E.K.; Halls, M.L. The Β2-Adrenoceptor Activates a Positive cAMP-Calcium Feedforward Loop to Drive Breast Cancer Cell Invasion. FASEB J. 2016, 30, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wu, G.; Yang, Z.; Zhang, B.; Jiang, Y.; Han, Y.; He, G.; Zhuang, Q.; Wang, Y.; Huang, Z.; et al. Role of Beta1-Adrenoceptor in Increased Lipolysis in Cancer Cachexia. Cancer Sci. 2010, 101, 1639–1645. [Google Scholar] [CrossRef]
- McKean-Cowdin, R.; Li, X.; Bernstein, L.; McTiernan, A.; Ballard-Barbash, R.; Gauderman, W.J.; Gilliland, F. The ADRB3 Trp64Arg Variant and Obesity in African-American Breast Cancer Cases. Int. J. Obes. 2005 2007, 31, 1110–1118. [Google Scholar] [CrossRef][Green Version]
- Zhou, Z.; Zhan, J.; Luo, Q.; Hou, X.; Wang, S.; Xiao, D.; Xie, Z.; Liang, H.; Lin, S.; Zheng, M. ADRB3 Induces Mobilization and Inhibits Differentiation of Both Breast Cancer Cells and Myeloid-Derived Suppressor Cells. Cell Death Dis. 2022, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Casini, G.; Dal Monte, M.; Fornaciari, I.; Filippi, L.; Bagnoli, P. The β-Adrenergic System as a Possible New Target for Pharmacologic Treatment of Neovascular Retinal Diseases. Prog. Retin. Eye Res. 2014, 42, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Westenskow, P.D.; Murinello, S.; Dorrell, M.I.; Scheppke, L.; Bucher, F.; Sakimoto, S.; Paris, L.P.; Aguilar, E.; Friedlander, M. Angiogenesis and Eye Disease. Annu. Rev. Vis. Sci. 2015, 1, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.J.; Booz, G.W.; Meininger, C.J.; Day, J.N.E.; Granger, H.J. Β3-Adrenergic Receptors Regulate Retinal Endothelial Cell Migration and Proliferation. J. Biol. Chem. 2003, 278, 20681–20686. [Google Scholar] [CrossRef] [PubMed]
- Ristori, C.; Filippi, L.; Dal Monte, M.; Martini, D.; Cammalleri, M.; Fortunato, P.; la Marca, G.; Fiorini, P.; Bagnoli, P. Role of the Adrenergic System in a Mouse Model of Oxygen-Induced Retinopathy: Antiangiogenic Effects of β-Adrenoreceptor Blockade. Investig. Ophthalmol. Vis. Sci. 2011, 52, 155–170. [Google Scholar] [CrossRef]
- Steinle, J.J.; Chin, V.C.; Williams, K.P.; Panjala, S.R. Beta-Adrenergic Receptor Stimulation Modulates iNOS Protein Levels through P38 and ERK1/2 Signaling in Human Retinal Endothelial Cells. Exp. Eye Res. 2008, 87, 30–34. [Google Scholar] [CrossRef]
- Morbidelli, L.; Chang, C.H.; Douglas, J.G.; Granger, H.J.; Ledda, F.; Ziche, M. Nitric Oxide Mediates Mitogenic Effect of VEGF on Coronary Venular Endothelium. Am. J. Physiol. 1996, 270, H411–H415. [Google Scholar] [CrossRef]
- Cavallaro, G.; Filippi, L.; Bagnoli, P.; La Marca, G.; Cristofori, G.; Raffaeli, G.; Padrini, L.; Araimo, G.; Fumagalli, M.; Groppo, M.; et al. The Pathophysiology of Retinopathy of Prematurity: An Update of Previous and Recent Knowledge. Acta Ophthalmol. 2014, 92, 2–20. [Google Scholar] [CrossRef]
- Dal Monte, M.; Filippi, L.; Bagnoli, P. Beta3-Adrenergic Receptors Modulate Vascular Endothelial Growth Factor Release in Response to Hypoxia through the Nitric Oxide Pathway in Mouse Retinal Explants. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 386, 269–278. [Google Scholar] [CrossRef]
- Mori, A.; Miwa, T.; Sakamoto, K.; Nakahara, T.; Ishii, K. Pharmacological Evidence for the Presence of Functional Beta(3)-Adrenoceptors in Rat Retinal Blood Vessels. Naunyn. Schmiedebergs Arch. Pharmacol. 2010, 382, 119–126. [Google Scholar] [CrossRef]
- Chen, J.; Joyal, J.-S.; Hatton, C.J.; Juan, A.M.; Pei, D.T.; Hurst, C.G.; Xu, D.; Stahl, A.; Hellstrom, A.; Smith, L.E.H. Propranolol Inhibition of β-Adrenergic Receptor Does Not Suppress Pathologic Neovascularization in Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Kulik, G.A.; Hassan, S.; Karpova, Y.; Baurin, V. Abstract SY04-01: Behavioral Stress Protects Prostate Cancer Cells from Apoptosis. Cancer Res. 2011, 71, SY04-01. [Google Scholar] [CrossRef]
- Palm, D.; Lang, K.; Niggemann, B.; Drell, T.L.; Masur, K.; Zaenker, K.S.; Entschladen, F. The Norepinephrine-Driven Metastasis Development of PC-3 Human Prostate Cancer Cells in BALB/c Nude Mice Is Inhibited by Beta-Blockers. Int. J. Cancer 2006, 118, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.G.; Morizono, K.; Karanikolas, B.D.W.; Wu, L.; et al. The Sympathetic Nervous System Induces a Metastatic Switch in Primary Breast Cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Hulsurkar, M.; Li, Z.; Zhang, Y.; Li, X.; Zheng, D.; Li, W. Beta-Adrenergic Signaling Promotes Tumor Angiogenesis and Prostate Cancer Progression through HDAC2-Mediated Suppression of Thrombospondin-1. Oncogene 2017, 36, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Horvathova, L.; Hunakova, L. Neurobiology of Cancer: The Role of β-Adrenergic Receptor Signaling in Various Tumor Environments. Int. J. Mol. Sci. 2020, 21, 7958. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.M.; Hill, S.J. Beta-Adrenoceptor-Mediated Inhibition of Alpha 1-Adrenoceptor-Mediated and Field Stimulation-Induced Contractile Responses in the Prostate of the Guinea Pig. Br. J. Pharmacol. 1997, 122, 1067–1074. [Google Scholar] [CrossRef]
- Kalodimos, P.J.; Ventura, S. Beta2-Adrenoceptor-Mediated Inhibition of Field Stimulation Induced Contractile Responses of the Smooth Muscle of the Rat Prostate Gland. Eur. J. Pharmacol. 2001, 431, 81–89. [Google Scholar] [CrossRef]
- Kang, T.W.; Kim, S.J.; Kim, M.H.; Jung, J.H. Beta 3 Adrenoreceptor Agonist for the Management of Lower Urinary Tract Symptoms in Men With Benign Prostatic Hyperplasia: A Systematic Review. Int. Neurourol. J. 2021, 25, 182–191. [Google Scholar] [CrossRef]
- Haynes, J.M. Beta(2) and Beta(3)-Adrenoceptor Inhibition of Alpha(1)-Adrenoceptor-Stimulated Ca(2+) Elevation in Human Cultured Prostatic Stromal Cells. Eur. J. Pharmacol. 2007, 570, 18–26. [Google Scholar] [CrossRef]
- Ponzoni, M.; Bachetti, T.; Corrias, M.V.; Brignole, C.; Pastorino, F.; Calarco, E.; Bensa, V.; Giusto, E.; Ceccherini, I.; Perri, P. Recent Advances in the Developmental Origin of Neuroblastoma: An Overview. J. Exp. Clin. Cancer Res. 2022, 41, 92. [Google Scholar] [CrossRef] [PubMed]
- Strenger, V.; Kerbl, R.; Dornbusch, H.J.; Ladenstein, R.; Ambros, P.F.; Ambros, I.M.; Urban, C. Diagnostic and Prognostic Impact of Urinary Catecholamines in Neuroblastoma Patients. Pediatr. Blood Cancer 2007, 48, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.L.; Ng, L.W.C.; Poon, L.S.W.; Chan, W.W.Y.; Wong, Y.H. Dopaminergic and Adrenergic Toxicities on SK-N-MC Human Neuroblastoma Cells Are Mediated through G Protein Signaling and Oxidative Stress. Apoptosis Int. J. Program. Cell Death 2007, 12, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, S.; Pique-Regi, R.; Sposto, R.; Wang, H.; Yang, Y.; Shimada, H.; Matthay, K.; Buckley, J.; Ortega, A.; Seeger, R.C. Prognostic Significance of Gene Expression Profiles of Metastatic Neuroblastomas Lacking MYCN Gene Amplification. J. Natl. Cancer Inst. 2006, 98, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Street, J.; Pouchy, C.; Carre, M.; Gifford, A.J.; Murray, J.; Norris, M.D.; Trahair, T.; Andre, N.; Kavallaris, M. β-Blockers Increase Response to Chemotherapy via Direct Antitumour and Anti-Angiogenic Mechanisms in Neuroblastoma. Br. J. Cancer 2013, 108, 2485. [Google Scholar] [CrossRef] [PubMed]
- Wolter, J.K.; Wolter, N.E.; Blanch, A.; Partridge, T.; Cheng, L.; Morgenstern, D.A.; Podkowa, M.; Kaplan, D.R.; Irwin, M.S. Anti-Tumor Activity of the Beta-Adrenergic Receptor Antagonist Propranolol in Neuroblastoma. Oncotarget 2013, 5, 161–172. [Google Scholar] [CrossRef]
- Bruno, G.; Cencetti, F.; Pini, A.; Tondo, A.; Cuzzubbo, D.; Fontani, F.; Strinna, V.; Buccoliero, A.M.; Casazza, G.; Donati, C.; et al. Β3-Adrenoreceptor Blockade Reduces Tumor Growth and Increases Neuronal Differentiation in Neuroblastoma via SK2/S1P2 Modulation. Oncogene 2020, 39, 368–384. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, P.; Yang, T.; Huang, M.; Qi, W.; Zhou, T.; Yang, Z.; Zou, Y.; Gao, G.; Yang, X. Targeting Β3-Adrenergic Receptor Signaling Inhibits Neuroblastoma Cell Growth via Suppressing the mTOR Pathway. Biochem. Biophys. Res. Commun. 2019, 514, 295–300. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, W.; Han, Q.; Xiao, R.-P. Emerging Concepts and Therapeutic Implications of β-Adrenergic Receptor Subtype Signaling. Pharmacol. Ther. 2005, 108, 257–268. [Google Scholar] [CrossRef]
- Bruno, G.; Nastasi, N.; Subbiani, A.; Boaretto, A.; Ciullini Mannurita, S.; Mattei, G.; Nardini, P.; Della Bella, C.; Magi, A.; Pini, A.; et al. Β3-Adrenergic Receptor on Tumor-Infiltrating Lymphocytes Sustains IFN-γ-Dependent PD-L1 Expression and Impairs Anti-Tumor Immunity in Neuroblastoma. Cancer Gene Ther. 2023, 30, 890–904. [Google Scholar] [CrossRef]
- Melaiu, O.; Chierici, M.; Lucarini, V.; Jurman, G.; Conti, L.A.; De Vito, R.; Boldrini, R.; Cifaldi, L.; Castellano, A.; Furlanello, C.; et al. Cellular and Gene Signatures of Tumor-Infiltrating Dendritic Cells and Natural-Killer Cells Predict Prognosis of Neuroblastoma. Nat. Commun. 2020, 11, 5992. [Google Scholar] [CrossRef]
- Peterson, E.E.; Barry, K.C. The Natural Killer–Dendritic Cell Immune Axis in Anti-Cancer Immunity and Immunotherapy. Front. Immunol. 2021, 11, 621254. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Dabraio, A.; Subbiani, A.; Buonvicino, D.; De Gregorio, V.; Ciullini Mannurita, S.; Pini, A.; Nardini, P.; Favre, C.; Filippi, L. Β3-Adrenoceptors as Putative Regulator of Immune Tolerance in Cancer and Pregnancy. Front. Immunol. 2020, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Kovar, H. Ewing’s Sarcoma and Peripheral Primitive Neuroectodermal Tumors after Their Genetic Union. Curr. Opin. Oncol. 1998, 10, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Magwere, T.; Burchill, S.A. Oxidative Stress and Therapeutic Opportunities: Focus on the Ewing’s Sarcoma Family of Tumors. Expert Rev. Anticancer Ther. 2011, 11, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Burdsall, J.; Workman, L.; Hollinger, B.; Neely, J. β-Adrenergic Receptors in Pediatric Tumors: Uncoupled Β1-Adrenergic Receptor in Ewing’s Sarcoma23. JNCI J. Natl. Cancer Inst. 1983, 71, 779–786. [Google Scholar] [CrossRef] [PubMed]
- van Valen, F.; Jürgens, H.; Winkelmann, W.; Keck, E. Beta-Adrenergic Agonist- and Prostaglandin-Mediated Regulation of cAMP Levels in Ewing’s Sarcoma Cells in Culture. Biochem. Biophys. Res. Commun. 1987, 146, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Pasha, A.; Vignoli, M.; Subbiani, A.; Nocentini, A.; Selleri, S.; Gratteri, P.; Dabraio, A.; Casini, T.; Filippi, L.; Fotzi, I.; et al. Β3-Adrenoreceptor Activity Limits Apigenin Efficacy in Ewing Sarcoma Cells: A Dual Approach to Prevent Cell Survival. Int. J. Mol. Sci. 2019, 20, 2149. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kadoi, H.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Noradrenaline Increases Intracellular Glutathione in Human Astrocytoma U-251 MG Cells by Inducing Glutamate-Cysteine Ligase Protein via Β3-Adrenoceptor Stimulation. Eur. J. Pharmacol. 2016, 772, 51–61. [Google Scholar] [CrossRef]
- Calvani, M.; Favre, C. Antioxidant Nutraceutical Approach to Ewing Sarcoma: Where Is the Trap? Biomed. J. Sci. Tech. Res. 2019, 17, 12805–12814. [Google Scholar] [CrossRef]
- Benini, S.; Gamberi, G.; Cocchi, S.; Garbetta, J.; Alberti, L.; Righi, A.; Gambarotti, M.; Picci, P.; Ferrari, S. Detection of Circulating Tumor Cells in Liquid Biopsy from Ewing Sarcoma Patients. Cancer Manag. Res. 2018, 10, 49–60. [Google Scholar] [CrossRef]
- Calvani, M.; Vignoli, M.; Beltrami, G.; Pasha, A.; Scalini, P.; Mannurita, S.C.; Cardellicchio, S.; Coccoli, L.; Cecchi, C.; De Marco, E.; et al. Preliminary Study on Β3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients. Biomedicines 2020, 8, 413. [Google Scholar] [CrossRef]
- Hayashi, M.; Zhu, P.; McCarty, G.; Meyer, C.F.; Pratilas, C.A.; Levin, A.; Morris, C.D.; Albert, C.M.; Jackson, K.W.; Tang, C.-M.; et al. Size-Based Detection of Sarcoma Circulating Tumor Cells and Cell Clusters. Oncotarget 2017, 8, 78965–78977. [Google Scholar] [CrossRef]
- Lowell, B.B.; Flier, J.S. Brown Adipose Tissue, Beta 3-Adrenergic Receptors, and Obesity. Annu. Rev. Med. 1997, 48, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Chen, M.; Bucsek, M.J.; Repasky, E.A.; Hylander, B.L. Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response. Front. Immunol. 2018, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Bruno, G.; Dabraio, A.; Subbiani, A.; Bianchini, F.; Fontani, F.; Casazza, G.; Vignoli, M.; De Logu, F.; Frenos, S.; et al. Β3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment. Int. J. Mol. Sci. 2020, 21, 1420. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasha, A.; Tondo, A.; Favre, C.; Calvani, M. Inside the Biology of the β3-Adrenoceptor. Biomolecules 2024, 14, 159. https://doi.org/10.3390/biom14020159
Pasha A, Tondo A, Favre C, Calvani M. Inside the Biology of the β3-Adrenoceptor. Biomolecules. 2024; 14(2):159. https://doi.org/10.3390/biom14020159
Chicago/Turabian StylePasha, Amada, Annalisa Tondo, Claudio Favre, and Maura Calvani. 2024. "Inside the Biology of the β3-Adrenoceptor" Biomolecules 14, no. 2: 159. https://doi.org/10.3390/biom14020159
APA StylePasha, A., Tondo, A., Favre, C., & Calvani, M. (2024). Inside the Biology of the β3-Adrenoceptor. Biomolecules, 14(2), 159. https://doi.org/10.3390/biom14020159





