Parental Social Isolation during Adolescence Alters Gut Microbiome in Rat Male Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Generation of Offspring from Socially Isolated Parents
2.3. Sample Collection, Storage, and DNA Extraction
2.4. Analysis of Bacterial Communities by Next-Generation Sequencing (NGS) of 16S rRNA Gene
2.5. Interleukins and Corticosterone Assays
2.6. Statistical Analysis
3. Results
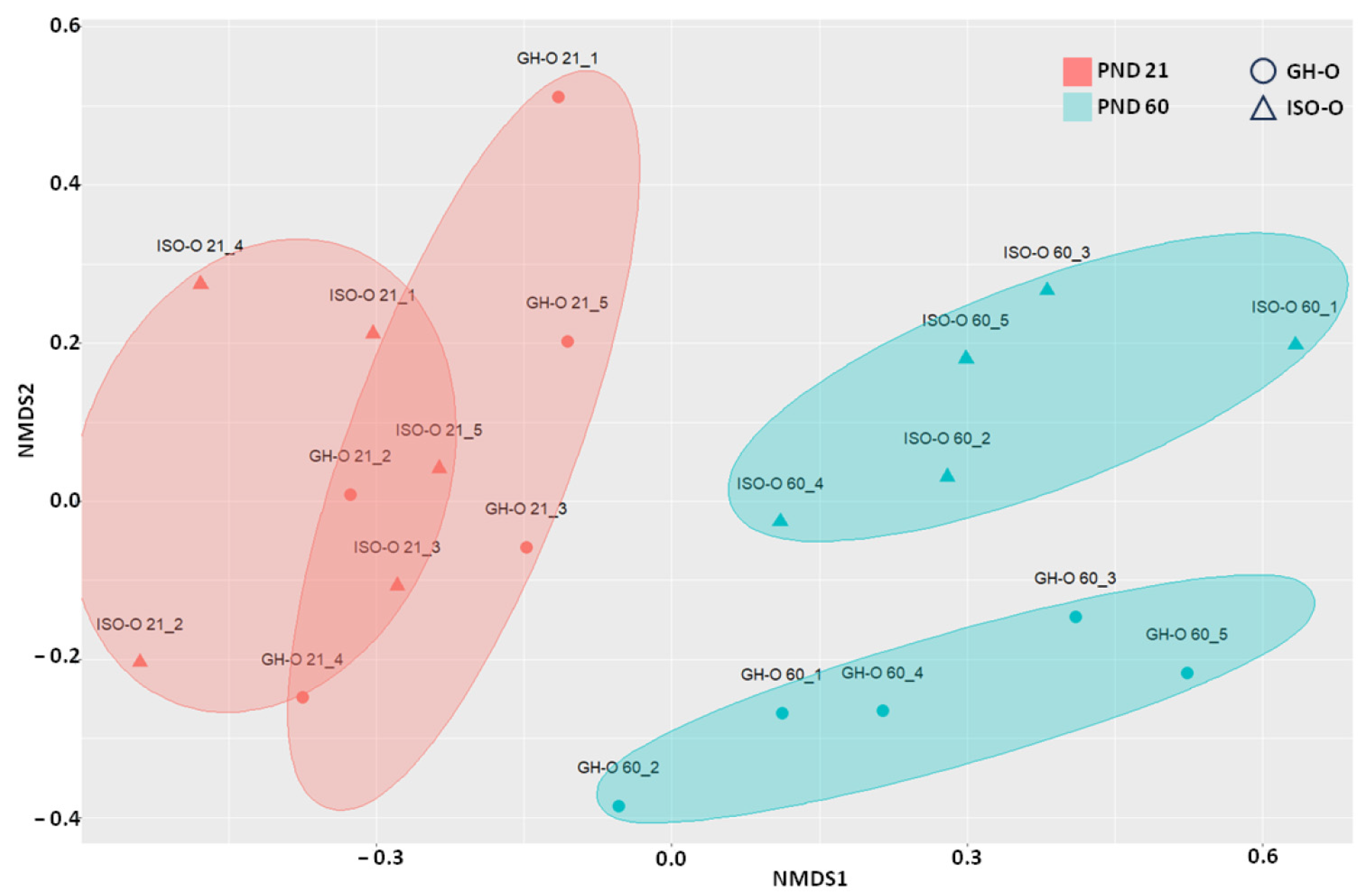
3.1. Structure of Bacterial Communities
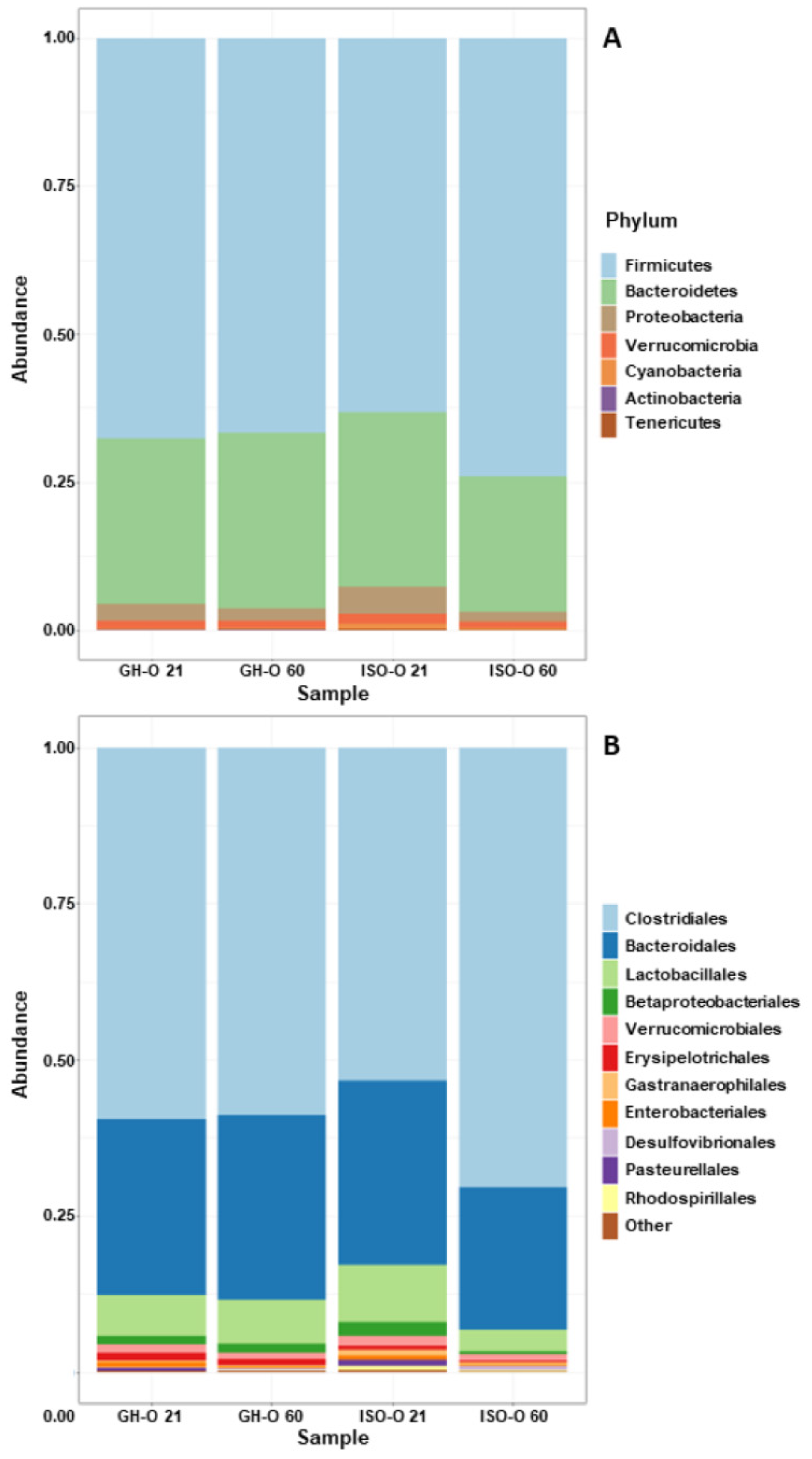
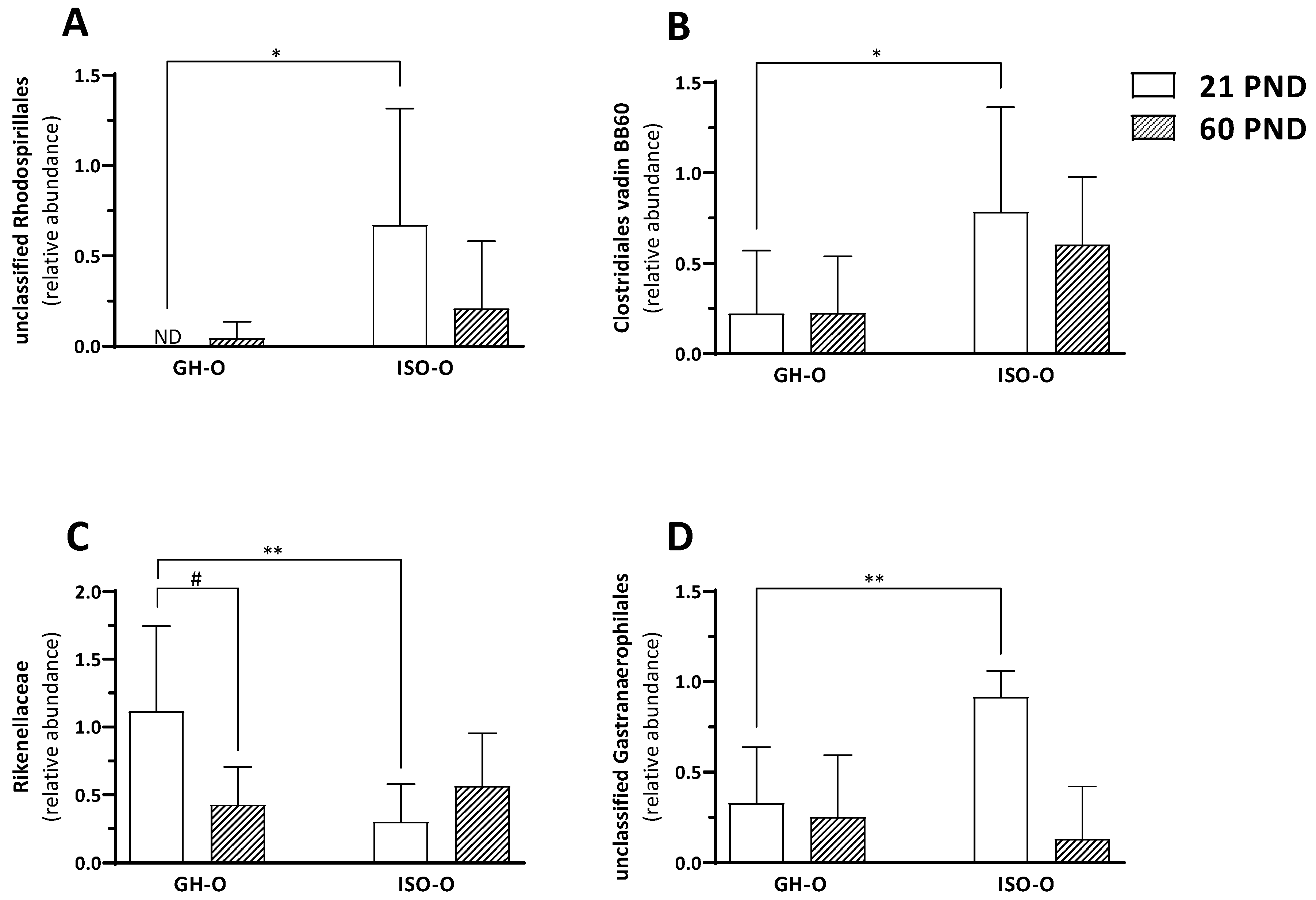
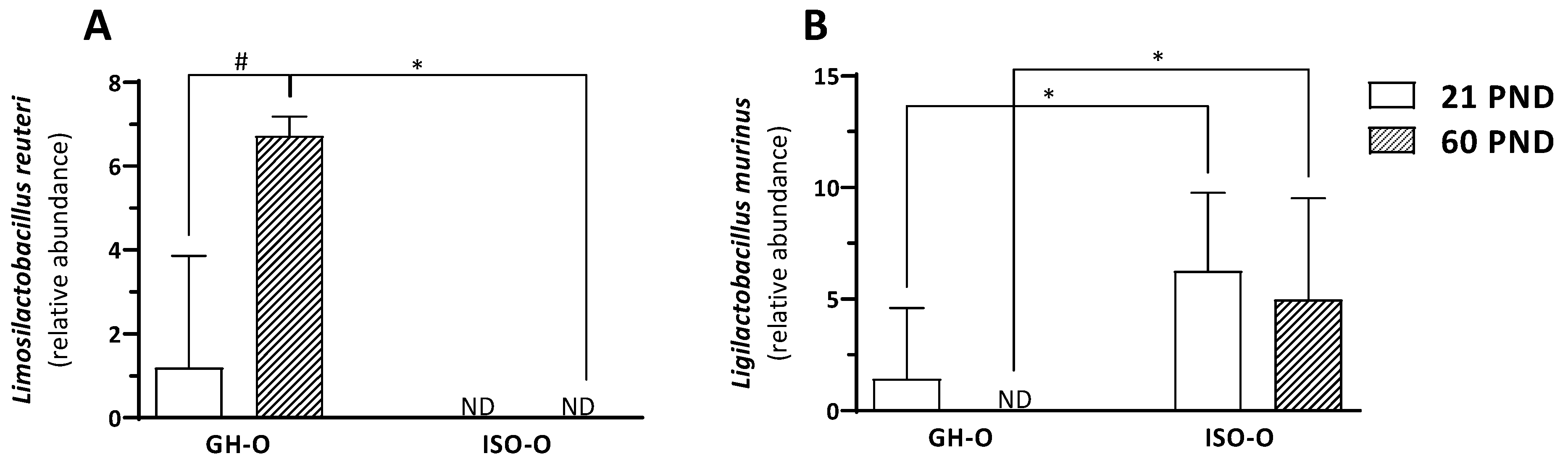
3.2. Shift in Composition of Bacterial Communities in ISO-O Rats
3.3. Inflammatory Pattern and Corticosterone Levels in Plasma of Socially Isolated Dams and Male ISO-O at PND 21
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, S. Maternal and Environmental Influences on the Adrenocortical Response to Stress in Weanling Rats. Science 1967, 156, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Nemeroff, C.B. Neurobiology of Early Life Stress: Clinical Studies. Semin. Clin. Neuropsychiatry 2002, 7, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.G.; Phillips, D.I.W. Minireview: Transgenerational Inheritance of the Stress Response: A New Frontier in Stress Research. Endocrinology 2010, 151, 7–13. [Google Scholar] [CrossRef]
- McCormick, G.L.; Robbins, T.R.; Cavigelli, S.A.; Langkilde, T. Ancestry Trumps Experience: Transgenerational but Not Early Life Stress Affects the Adult Physiological Stress Response. Horm. Behav. 2017, 87, 115–121. [Google Scholar] [CrossRef]
- Pisu, M.G.; Garau, A.; Olla, P.; Biggio, F.; Utzeri, C.; Dore, R.; Serra, M. Altered Stress Responsiveness and Hypothalamic-Pituitary-Adrenal Axis Function in Male Rat Offspring of Socially Isolated Parents. J. Neurochem. 2013, 126, 493–502. [Google Scholar] [CrossRef]
- Pisu, M.G.; Boero, G.; Garau, A.; Casula, C.; Cisci, S.; Biggio, F.; Concas, A.; Follesa, P.; Maciocco, E.; Porcu, P.; et al. Are Preconceptional Stressful Experiences Crucial Elements for the Aetiology of Autism Spectrum Disorder? Insights from an Animal Model. Neuropharmacology 2019, 157, 107686. [Google Scholar] [CrossRef]
- Fone, K.C.F.; Porkess, M.V. Behavioural and Neurochemical Effects of Post-Weaning Social Isolation in Rodents-Relevance to Developmental Neuropsychiatric Disorders. Neurosci. Biobehav. Rev. 2008, 32, 1087–1102. [Google Scholar] [CrossRef]
- Serra, M.; Pisu, M.G.; Littera, M.; Papi, G.; Sanna, E.; Tuveri, F.; Usala, L.; Purdy, R.H.; Biggio, G. Social Isolation-Induced Decreases in Both the Abundance of Neuroactive Steroids and GABA(A) Receptor Function in Rat Brain. J. Neurochem. 2000, 75, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Pisu, M.G.; Floris, I.; Cara, V.; Purdy, R.H.; Biggio, G. Social Isolation-Induced Increase in the Sensitivity of Rats to the Steroidogenic Effect of Ethanol. J. Neurochem. 2003, 85, 257–263. [Google Scholar] [CrossRef]
- Serra, M.; Pisu, M.G.; Floris, I.; Biggio, G. Social Isolation-Induced Changes in the Hypothalamic-Pituitary-Adrenal Axis in the Rat. Stress 2005, 8, 259–264. [Google Scholar] [CrossRef]
- Pisu, M.G.; Dore, R.; Mostallino, M.C.; Loi, M.; Pibiri, F.; Mameli, R.; Cadeddu, R.; Secci, P.P.; Serra, M. Down-Regulation of Hippocampal BDNF and Arc Associated with Improvement in Aversive Spatial Memory Performance in Socially Isolated Rats. Behav. Brain Res. 2011, 222, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pisu, M.G.; Boero, G.; Biggio, F.; Garau, A.; Corda, D.; Congiu, M.; Concas, A.; Porcu, P.; Serra, M. Juvenile Social Isolation Affects Maternal Care in Rats: Involvement of Allopregnanolone. Psychopharmacology 2017, 234, 2587–2596. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Lutz, B.; Ruiz de Azua, I. The Microbiome and Gut Endocannabinoid System in the Regulation of Stress Responses and Metabolism. Front. Cell. Neurosci. 2022, 16, 867267. [Google Scholar] [CrossRef] [PubMed]
- Dunphy-Doherty, F.; O’Mahony, S.M.; Peterson, V.L.; O’Sullivan, O.; Crispie, F.; Cotter, P.D.; Wigmore, P.; King, M.V.; Cryan, J.F.; Fone, K.C.F. Post-Weaning Social Isolation of Rats Leads to Long-Term Disruption of the Gut Microbiota-Immune-Brain Axis. Brain Behav. Immun. 2018, 68, 261–273. [Google Scholar] [CrossRef]
- Lopizzo, N.; Marizzoni, M.; Begni, V.; Mazzelli, M.; Provasi, S.; Borruso, L.; Riva, M.A.; Cattaneo, A. Social Isolation in Adolescence and Long-Term Changes in the Gut Microbiota Composition and in the Hippocampal Inflammation: Implications for Psychiatric Disorders—Dirk Hellhammer Award Paper 2021. Psychoneuroendocrinology 2021, 133, 105416. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Thriene, K.; Michels, K.B. Human Gut Microbiota Plasticity throughout the Life Course. Int. J. Environ. Res. Public Health 2023, 20, 1463. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress Hormones, Proinflammatory and Antiinflammatory Cytokines, and Autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Korgan, A.C.; Foxx, C.L.; Hashmi, H.; Sago, S.A.; Stamper, C.E.; Heinze, J.D.; O’Leary, E.; King, J.L.; Perrot, T.S.; Lowry, C.A.; et al. Effects of Paternal High-Fat Diet and Maternal Rearing Environment on the Gut Microbiota and Behavior. Sci. Rep. 2022, 12, 10179. [Google Scholar] [CrossRef]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef]
- Masís-Calvo, M.; Sequeira-Cordero, A.; Mora-Gallegos, A.; Fornaguera-Trías, J. Behavioral and Neurochemical Characterization of Maternal Care Effects on Juvenile Sprague-Dawley Rats. Physiol. Behav. 2013, 118, 212–217. [Google Scholar] [CrossRef]
- Curley, J.P.; Champagne, F.A. Influence of Maternal Care on the Developing Brain: Mechanisms, Temporal Dynamics and Sensitive Periods. Front. Neuroendocrinol. 2016, 40, 52–66. [Google Scholar] [CrossRef]
- Walter, J.; Britton, R.A.; Roos, S. Host-Microbial Symbiosis in the Vertebrate Gastrointestinal Tract and the Lactobacillus reuteri Paradigm. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4645–4652. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef]
- Lebovitz, Y.; Theus, M.H. Molecular Phenotyping and Genomic Characterization of a Novel Neuroactive Bacterium Strain, Lactobacillus murinus HU-1. Front. Pharmacol. 2019, 10, 1162. [Google Scholar] [CrossRef]
- Liu, F.; Horton-Sparks, K.; Hull, V.; Li, R.W.; Martínez-Cerdeño, V. The Valproic Acid Rat Model of Autism Presents with Gut Bacterial Dysbiosis Similar to That in Human Autism. Mol. Autism 2018, 9, 61. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short Chain Fatty Acids in Human Large Intestine, Portal, Hepatic and Venous Blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.-P. Neurobiological Effects of Intraventricular Propionic Acid in Rats: Possible Role of Short Chain Fatty Acids on the Pathogenesis and Characteristics of Autism Spectrum Disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of Histone Deacetylase Activity by Butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef]
- Zaidan, H.; Wnuk, A.; Aderka, I.M.; Kajta, M.; Gaisler-Salomon, I. Pre-Reproductive Stress in Adolescent Female Rats Alters Maternal Care and DNA Methylation Patterns across Generations. Stress 2023, 26, 2201325. [Google Scholar] [CrossRef]
- Kratsman, N.; Getselter, D.; Elliott, E. Sodium Butyrate Attenuates Social Behavior Deficits and Modifies the Transcription of Inhibitory/Excitatory Genes in the Frontal Cortex of an Autism Model. Neuropharmacology 2016, 102, 136–145. [Google Scholar] [CrossRef]
- Xie, A.; Ensink, E.; Li, P.; Gordevičius, J.; Marshall, L.L.; George, S.; Pospisilik, J.A.; Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov. Disord. 2022, 37, 1644–1653. [Google Scholar] [CrossRef]
- Gillespie, C.F.; Nemeroff, C.B. Hypercortisolemia and Depression. Psychosom. Med. 2005, 67 (Suppl. S1), S26–S28. [Google Scholar] [CrossRef]
- Abelson, J.L.; Khan, S.; Liberzon, I.; Young, E.A. HPA Axis Activity in Patients with Panic Disorder: Review and Synthesis of Four Studies. Depress. Anxiety 2007, 24, 66–76. [Google Scholar] [CrossRef]
- Stange, J.P.; Alloy, L.B.; Fresco, D.M. Inflexibility as a Vulnerability to Depression: A Systematic Qualitative Review. Clin. Psychol. 2017, 24, 245–276. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Fang, Z.; Lin, L.; Wu, C.; Huang, Q. Behavioral and Neurobiological Studies on the Male Progeny of Maternal Rats Exposed to Chronic Unpredictable Stress before Pregnancy. Neurosci. Lett. 2010, 469, 278–282. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut Microbiota and Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key Role of Gut Microbiota in Anhedonia-like Phenotype in Rodents with Neuropathic Pain. Transl. Psychiatry 2019, 9, 57. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Y.; Chen, H.; Jiang, H.; Zhou, F.; Lv, B.; Xu, M. Curcumin Alleviates DSS-Induced Anxiety-Like Behaviors via the Microbial-Brain-Gut Axis. Oxid. Med. Cell. Longev. 2022, 2022, 6244757. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
| Parameter | Effect |
|---|---|
| Allopregnanolone brain and plasma levels | Increased [5] |
| BDNF brain and plasma levels | Increased [6] |
| Oxytocin plasma levels | Decreased [6] |
| Basal HPA axis activity | Increased [5] |
| Acute stress responsiveness | Decreased [5] |
| Social behavior | Decreased [6] |
| Behavioral flexibility | Decreased [6] |
| Seizures sensitivity | Increased [6] |
| Anxiety-like behavior | No change [5] |
| Spatial learning and memory | No change [6] |
| Novelty-induced behavior | No change [6] |
| Aggressive behavior | No change [6] |
| Locomotor activity | No change [5] |
| Groups | S | H’ | J’ |
|---|---|---|---|
| GH-O PND 21 | |||
| 1 | 99 | 4.548031 | 0.9897524 |
| 2 | 114 | 4.680060 | 0.9881470 |
| 3 | 143 | 4.903395 | 0.9880212 |
| 4 | 110 | 4.653042 | 0.9899079 |
| 5 | 152 | 4.966281 | 0.9885349 |
| GH-O PND 60 | |||
| 1 | 117 | 4.704156 | 0.9878169 |
| 2 | 74 | 4.253327 | 0.9882116 |
| 3 | 173 | 5.092157 | 0.9881367 |
| 4 | 129 | 4.798470 | 0.9873776 |
| 5 | 138 | 4.872007 | 0.9887875 |
| ISO-O PND 21 | |||
| 1 | 110 | 4.642027 | 0.9875644 |
| 2 | 73 | 4.245236 | 0.9894596 |
| 3 | 101 | 4.559047 | 0.9878501 |
| 4 | 87 | 4.420237 | 0.9897734 |
| 5 | 132 | 4.831808 | 0.9895565 |
| ISO-O PND 60 | |||
| 1 | 131 | 4.822543 | 0.9891996 |
| 2 | 147 | 4.924567 | 0.9868017 |
| 3 | 120 | 4.735935 | 0.9892309 |
| 4 | 122 | 4.747505 | 0.9882356 |
| 5 | 138 | 4.874141 | 0.9892206 |
| Taxonomy | Examines Age Groups (across All Type Groups) | Examines Type Groups (across All Age Groups) | Two-Way ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Order | Family | Genus | PND 21 | PND 60 | GH-O | ISO-O | Type | Age | Interaction | |||
| F(1,16) | p | F(1,16) | p | F(1,16) | p | |||||||
| Bacteroidales | Muribaculaceae | 1.16 ± 0.59 | 0.97 ± 0.62 | 1.35 ± 0.42 | 0.77 ± 0.63 | 6.37 | 0.02 | 0.67 | 0.42 | 2.70 | 0.12 | |
| Rikenellaceae | Alistipes | 0.34 ± 0.39 | 0.40 ± 0.24 | 0.51 ± 0.32 | 0.23 ± 0.25 | 5.33 | 0.03 | 0.26 | 0.62 | 3.55 | 0.08 | |
| Clostridiales | Lachnospiraceae | Acetatifactor | 0.00 ± 0.00 | 0.31 ± 0.42 | 0.00 ± 0.00 | 0.31 ± 0.42 | 11.50 | 0.004 | 11.50 | 0.004 | 11.50 | 0.004 |
| Ruminococcaceae | GCA-900066225 | 0.25 ± 0.37 | 0.24 ± 0.27 | 0.05 ± 0.11 | 0.44 ± 0.33 | 10.51 | 0.005 | 0.014 | 0.91 | 0.018 | 0.89 | |
| Ruminococcaceae | Pygmaiobacter | 0.13 ± 0.27 | 0.10 ± 0.21 | 0.00 ± 0.00 | 0.23 ± 0.30 | 5.13 | 0.04 | 0.10 | 0.75 | 0.10 | 0.75 | |
| Ruminococcaceae | Ruminiclostridium 5 | 0.46 ± 0.51 | 1.39 ± 0.99 | 0.47 ± 0.48 | 1.37 ± 1.02 | 14.20 | 0.002 | 15.23 | 0.001 | 9.12 | 0.008 | |
| Ruminococcaceae | UCG-005 | 1.80 ± 0.79 | 0.75 ± 0.59 | 1.69 ± 0.68 | 0.85 ± 0.87 | 10.48 | 0.005 | 16.75 | 0.0009 | 0.0029 | 0.96 | |
| Erysipelotrichales | Erysipelotrichaceae | Erysipelatoclostridium | 0.26 ± 0.42 | 0.04 ± 0.14 | 0.30 ± 0.42 | 0.00 ± 0.00 | 6.62 | 0.02 | 3.38 | 0.08 | 3.38 | 0.08 |
| Erysipelotrichaceae | Turicibacter | 0.24 ± 0.43 | 0.04 ± 0.14 | 0.28 ± 0.42 | 0.00 ± 0.00 | 5.40 | 0.03 | 2.63 | 0.12 | 2.63 | 0.12 | |
| Rhodospirillales | 0.31 ± 0.51 | 0.08 ± 0.15 | 0.02 ± 0.07 | 0.37 ± 0.49 | 6.15 | 0.02 | 2.51 | 0.13 | 3.55 | 0.08 | ||
| ASV | Taxonomy | Two-Way SIMPER | Two-Way ANOVA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Examines Age Groups (across All Type Groups) | Examines Type Groups (across All Age Groups) | Type | Age | Interaction | |||||||||||||
| No. | Family | Genus | Species | PND 21 (ab ± SD) | % | PND 60 (ab ± SD) | % | GH-O (ab ± SD) | % | ISO-O (ab ± SD) | % | F(1,16) | p | F(1,16) | p | F(1,16) | p |
| ASV05 | Bacteroidaceae | Bacteroides | 5.1 ± 3.5 | 2.1 | 3.0 ± 3.9 | 0.8 | 5.1 ± 3.6 | 2.2 | 3.0 ± 3.9 | 0.9 | 1.555 | 0.23 | 1.499 | 0.24 | 0.437 | 0.52 | |
| ASV07 | Bacteroidaceae | Bacteroides | 5.5 ± 4.7 | 2.0 | 8.0 ± 3.0 | 4.7 | 8.2 ± 3.0 | 4.8 | 5.2 ± 4.6 | 1.8 | 2.928 | 0.11 | 2.178 | 0.16 | 0.016 | 0.90 | |
| ASV11 | Bacteroidaceae | Bacteroides | 4.1 ± 3.6 | 1.5 | 2.9 ± 3.8 | 0.8 | 4.9 ± 3.5 | 2.1 | 2.1 ± 3.4 | 0.3 | 3.009 | 0.10 | 0.567 | 0.46 | 0.051 | 0.82 | |
| ASV03 | Muribaculaceae | 5.2 ± 2.9 | 2.8 | 3.6 ± 3.8 | 1.0 | 3.4 ± 3.7 | 0.9 | 5.3 ± 3.0 | 2.9 | 1.613 | 0.22 | 1.123 | 0.31 | 0.491 | 0.49 | ||
| ASV04 | Muribaculaceae | 4.7 ± 3.3 | 2.2 | 2.3 ± 3.7 | 0.2 | 2.8 ± 3.6 | 0.7 | 4.2 ± 3.7 | 1.8 | 0.820 | 0.38 | 2.381 | 0.14 | 0.020 | 0.89 | ||
| ASV08 | Muribaculaceae | 2.5 ± 3.2 | 1.2 | 5.2 ± 2.9 | 2.9 | 6.0 ± 2.2 | 3.5 | 1.7 ± 2.8 | 0.5 | 20.270 | 0.0004 | 7.889 | 0.01 | 0.496 | 0.49 | ||
| ASV09 | Muribaculaceae | 2.5 ± 3.2 | 0.4 | 5.7 ± 3.2 | 3.0 | 5.1 ± 3.6 | 2.6 | 3.1 ± 3.3 | 0.7 | 2.206 | 0.16 | 5.670 | 0.03 | 1.468 | 0.24 | ||
| ASV14 | Muribaculaceae | 4.1 ± 2.8 | 1.8 | 4.2 ± 3.7 | 1.5 | 3.2 ± 3.4 | 0.8 | 5.1 ± 2.8 | 2.5 | 1.775 | 0.20 | 0.006 | 0.94 | 0.128 | 0.73 | ||
| ASV01 | Prevotellaceae | NK3B31 | 7.4 ± 3.9 | 4.4 | 5.5 ± 4.4 | 2.7 | 3.9 ± 4.6 | 1.0 | 9.0 ± 1.1 | 6.2 | 11.100 | 0.004 | 1.548 | 0.23 | 0.250 | 0.62 | |
| ASV10 | Lactobacillaceae | Limosilactobacillus | L. reuteri | 0.6 ± 1.9 | 0.0 | 3.4 ± 3.6 | 2.3 | 4.0 ± 3.4 | 2.2 | 0.0 ± 0.0 | 0.0 | 42.640 | 0.0001 | 20.800 | 0.0003 | 20.800 | 0.0003 |
| ASV13 | Lactobacillaceae | Ligilactobacillus | L. murinus | 3.8 ± 4.1 | 1.7 | 2.5 ± 4.0 | 0.8 | 0.7 ± 2.2 | 0.0 | 5.6 ± 3.9 | 2.5 | 11.160 | 0.004 | 0.844 | 0.37 | 0.002 | 0.96 |
| ASV02 | Lachnospiraceae | Blautia | 5.2 ± 3.7 | 2.8 | 0.5 ± 1.6 | 0.0 | 2.1 ± 3.5 | 0.2 | 3.6 ± 3.9 | 2.7 | 1.877 | 0.19 | 17.710 | 0.0007 | 5.078 | 0.04 | |
| ASV06 | Lachnospiraceae | 5.4 ± 4.7 | 2.0 | 2.7 ± 3.6 | 0.4 | 4.6 ± 4.3 | 2.0 | 3.4 ± 4.4 | 0.5 | 0.453 | 0.51 | 2.123 | 0.16 | 1.700 | 0.21 | ||
| ASV15 | Ruminococcaceae | Ruminococcus 1 | 4.2 ± 4.5 | 2.0 | 2.0 ± 3.4 | 0.3 | 1.0 ± 2.3 | 0.0 | 5.2 ± 4.5 | 2.3 | 7.419 | 0.01 | 2.120 | 0.16 | 1.304 | 0.27 | |
| ASV12 | Akkermansiaceae | Akkermansia | 5.1 ± 4.5 | 1.6 | 5.7 ± 4.1 | 0.2 | 5.2 ± 4.6 | 1.8 | 5.6 ± 4.0 | 2.2 | 0.032 | 0.86 | 0.078 | 0.78 | 0.062 | 0.81 | |
| IL-1β | TNF-α | Corticosterone | |
|---|---|---|---|
| (pg/mL) | (ng/mL) | ||
| Dams | |||
| GH | 629.4 ± 141.8 | 690.0 ± 235.2 | 130.0 ± 20.8 |
| ISO | 731.0 ± 243.7 | 709.7 ± 191.5 | 71.5 ± 21.4 ** |
| Male offspring PND 60 | |||
| GH-O | 539.8 ± 87.1 | 519.3 ± 106.9 | 96.2 ± 16.7 |
| ISO-O | 609.5 ± 181.2 | 538.4 ± 107.3 | 126.2 ± 22.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddi, C.; Cosentino, S.; Tamburini, E.; Concas, L.; Pisano, M.B.; Ardu, R.; Deplano, M.; Follesa, P.; Maciocco, E.; Porcu, P.; et al. Parental Social Isolation during Adolescence Alters Gut Microbiome in Rat Male Offspring. Biomolecules 2024, 14, 172. https://doi.org/10.3390/biom14020172
Siddi C, Cosentino S, Tamburini E, Concas L, Pisano MB, Ardu R, Deplano M, Follesa P, Maciocco E, Porcu P, et al. Parental Social Isolation during Adolescence Alters Gut Microbiome in Rat Male Offspring. Biomolecules. 2024; 14(2):172. https://doi.org/10.3390/biom14020172
Chicago/Turabian StyleSiddi, Carlotta, Sofia Cosentino, Elena Tamburini, Luca Concas, Maria Barbara Pisano, Riccardo Ardu, Maura Deplano, Paolo Follesa, Elisabetta Maciocco, Patrizia Porcu, and et al. 2024. "Parental Social Isolation during Adolescence Alters Gut Microbiome in Rat Male Offspring" Biomolecules 14, no. 2: 172. https://doi.org/10.3390/biom14020172







