A Set of Possible Markers for Monitoring Heart Failure and Cognitive Impairment Associated: A Review of Literature from the Past 5 Years
Abstract
:1. Introduction
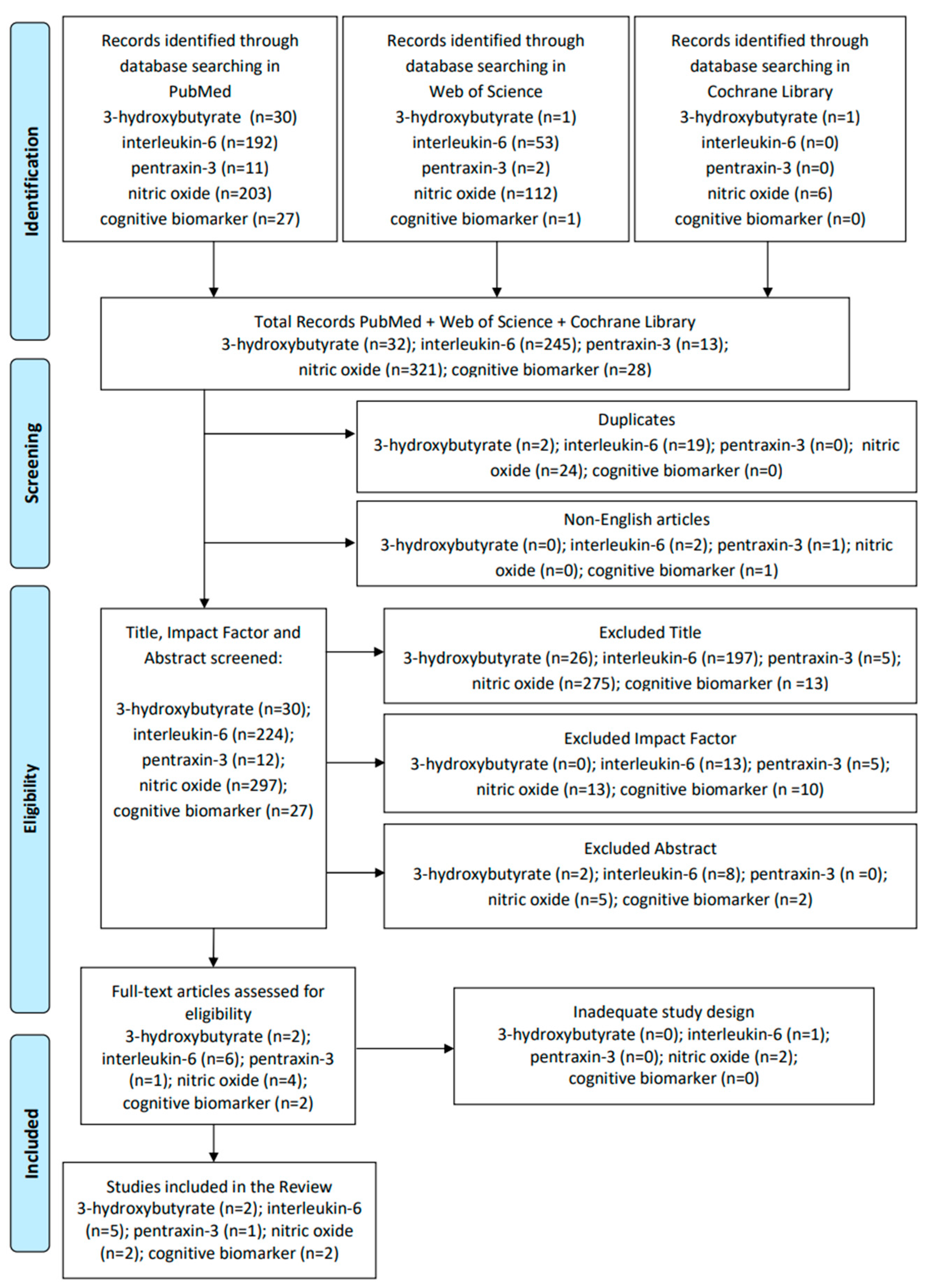
2. Materials and Methods
3. Results
3.1. 3-Hydroxybutyrate or Beta-Hydroxybutyrate
3.2. Pentraxin-3
3.3. Interleukin-6
3.4. Nitric Oxide (NO)
3.5. Cognitive Biomarker
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Vittorini, S.; Passino, C.; Clerico, A. Old and new biomarkers of heart failure. Eur. J. Heart Fail. 2009, 11, 331–335. [Google Scholar] [CrossRef]
- Snipelisky, D.; Chaudhry, S.-P.; Stewart, G.C. The Many Faces of Heart Failure. Card. Electrophysiol. Clin. 2019, 11, 11–20. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Clark, M.D.L.; Desai, N.R.; Owens, G.M.; Stemple, D.C.A. Rethinking heart failure: Patient classification and treatment. Am. J. Manag. Care 2022, 28, S255–S267. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Lam, C.S.; McMurray, J.J.; Redfield, M.M. How to Manage Heart Failure with Preserved Ejection Fraction: Practical Guidance for Clinicians. JACC Heart Fail. 2023, 11, 619–636. [Google Scholar] [CrossRef]
- Sinnenberg, L.; Givertz, M.M. Acute heart failure. Trends Cardiovasc. Med. 2020, 30, 104–112. [Google Scholar] [CrossRef]
- Mascolo, A.; di Mauro, G.; Cappetta, D.; De Angelis, A.; Torella, D.; Urbanek, K.; Berrino, L.; Nicoletti, G.F.; Capuano, A.; Rossi, F. Current and future therapeutic perspective in chronic heart failure. Pharmacol. Res. 2022, 175, 106035. [Google Scholar] [CrossRef]
- Cannon, J.A.; Moffitt, P.; Perez-Moreno, A.C.; Walters, M.R.; Broomfield, N.M.; McMurray, J.J.; Quinn, T.J. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J. Card. Fail. 2017, 23, 464–475. [Google Scholar] [CrossRef]
- Pressler, S.J. Cognitive Functioning and chronic heart failure: A review of the literature (2002–July 2007). J. Cardiovasc. Nurs. 2008, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Jonides, J.; Northouse, L.; Berman, M.G.; Koelling, T.M.; Pressler, S.J. Randomized Crossover Study of the Natural Restorative Environment Intervention to Improve Attention and Mood in Heart Failure. J. Cardiovasc. Nurs. 2017, 32, 464–479. [Google Scholar] [CrossRef]
- World Health Organization. Biomarkers in Risk Assessment: Validity and Validation; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Ibrahim, N.E.; Januzzi, J.L. Sodium-Glucose Co-Transporter 2 Inhibitors and Insights from Biomarker Measurement in Heart Failure Patients. Clin. Chem. 2021, 67, 79–86. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Biomarkers in Heart Failure: From Research to Clinical Practice. Ann. Lab. Med. 2023, 43, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Emani, S. Remote Monitoring to Reduce Heart Failure Readmissions. Curr. Heart Fail. Rep. 2017, 14, 40–47. [Google Scholar] [CrossRef]
- Voorrips, S.; Boorsma, E.; Beusekamp, J.; De-Boer, R.; Connelly, M.; Dullaart, R.; Van-Der-Meer, P.; Van-Veldhuisen, D.; Voors, A.; Damman, K.; et al. Longitudinal changes in circulating ketone body levels in patients with acute heart failure: A post hoc analysis of the EMPA-response-AHF trial. J. Card. Fail. 2023, 29, 33–41. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Westenbrink, B.D.; Connelly, M.A.; Otvos, J.D.; Groothof, D.; Shalaurova, I.; Garcia, E.; Navis, G.; de Boer, R.A.; Bakker, S.J.L.; et al. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur. J. Clin. Investig. 2021, 51, e13468. [Google Scholar] [CrossRef]
- Yamamoto, M.; Seo, Y.; Ishizua, T.; Nakagawa, D.; Sato, K.; Machino-Ohtsuka, T.; Nishi, I.; Hamada-Harimura, Y.; Sai, S.; Sugano, A.; et al. Comparison of soluble ST2, pentraxin-3, galectin-3, and high-sensitivity troponin t of cardiovascular outcomes in patients with acute decompensated heart failure. J. Card. Fail. 2021, 27, 1240–1250. [Google Scholar] [CrossRef]
- Fish-Trotter, H.; Ferguson, J.F.; Patel, N.; Arora, P.; Allen, N.B.; Bachmann, K.N.; Daniels, L.B.; Reilly, M.P.; Lima, J.A.; Wang, T.J.; et al. Inflammation and circulating natriuretic peptide levels. Circ. Heart Fail. 2020, 13, e006570. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.C.; Kieneker, L.M.; van Hassel, G.; Binnenmars, S.H.; Nolte, I.M.; van Zanden, J.J.; van der Meer, P.; Navis, G.; Voors, A.A.; Bakker, S.J.L.; et al. Interleukin 6 and development of heart failure with preserved ejection fraction in the general population. J. Am. Heart Assoc. 2021, 10, e018549. [Google Scholar] [CrossRef]
- Perez, A.L.; Grodin, J.L.; Chaikijurajai, T.; Wu, Y.; Hernandez, A.F.; Butler, J.; Metra, M.; Felker, G.M.; Voors, A.A.; Mcmurray, J.J.; et al. Interleukin-6 and outcomes in acute heart failure: An ASCEND-HF substudy. J. Card. Fail. 2021, 27, 670–676. [Google Scholar] [CrossRef]
- Alogna, A.; Koepp, K.E.; Sabbah, M.; Netto, J.M.E.; Jensen, M.D.; Kirkland, J.L.; Lam, C.S.; Obokata, M.; Petrie, M.C.; Ridker, P.M.; et al. Interleukin-6 in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2023, 11, 1549–1561. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Tromp, J.; Mentz, R.J.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. The additive prognostic value of serial plasma interleukin-6 levels over changes in brain natriuretic peptide in patients with acute heart failure. J. Card. Fail. 2021, 27, 808–811. [Google Scholar] [CrossRef]
- Momot, K.; Krauz, K.; Czarzasta, K.; Zarębiński, M.; Puchalska, L.; Wojciechowska, M. Evaluation of Nitrosative/Oxidative Stress and Inflammation in Heart Failure with Preserved and Reduced Ejection Fraction. Int. J. Mol. Sci. 2023, 24, 15944. [Google Scholar] [CrossRef]
- Lewsey, S.C.; Hays, A.G.; Schär, M.; Bonanno, G.; Sharma, K.; Afework, Y.; Gerstenblith, G.; Weiss, R.G. Nitric Oxide–Mediated Coronary Endothelial Function Is Impaired in Patients With Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, e009582. [Google Scholar] [CrossRef] [PubMed]
- Dridi, H.; Liu, Y.; Reiken, S.; Liu, X.; Argyrousi, E.K.; Yuan, Q.; Miotto, M.C.; Sittenfeld, L.; Meddar, A.; Soni, R.K.; et al. Heart failure-induced cognitive dysfunction is mediated by intracellular Ca2+ leak through ryanodine receptor type 2. Nat. Neurosci. 2023, 26, 1365–1378. [Google Scholar] [CrossRef]
- Lyra, V.; Parissis, J.; Kallergi, M.; Rizos, E.; Filippatos, G.; Kremastinos, D.; Chatziioannou, S. 18F-FDG PET/CT brain glucose metabolism as a marker of different types of depression comorbidity in chronic heart failure patients with impaired systolic function. Eur. J. Heart Fail. 2020, 22, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Aimo, A.; Jhund, P.; Richards, M.; de Boer, R.A.; Arfsten, H.; Fabiani, I.; Lupón, J.; Anker, S.D.; González, A.; et al. Biomarkers in heart failure clinical trials. A review from the Biomarkers Working Group of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1767–1777. [Google Scholar] [CrossRef]
- Yan, C.L.; Grazette, L. A review of biomarker and imaging monitoring to predict heart failure recovery. Front. Cardiovasc. Med. 2023, 10, 1150336. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.J., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Gorelick, P.B. Blood and Cerebrospinal Fluid Biomarkers in Vascular Dementia and Alzheimer’s Disease. Clin. Geriatr. Med. 2023, 39, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kapasi, A.; Schneider, J. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Janota, C.; Lemere, C.A.; Brito, M.A. Dissecting the contribution of vascular alterations and aging to Alzheimer’s disease. Mol. Neurobiol. 2016, 53, 3793–3811. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Kimura, C.; Konhilas, J.P.; Mansour, H.M.; Polt, R.; Doyle, K.P.; Billheimer, D.; Hay, M. Neurofilament light: A possible prognostic biomarker for treatment of vascular contributions to cognitive impairment and dementia. J. Neuroinflamm. 2021, 18, 236. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.S.S.; Raffi, H.Q.M.; Dong, Y. The pathophysiology of cognitive impairment in individuals with heart failure: A systematic review. Front. Cardiovasc. Med. 2023, 10, 1181979. [Google Scholar] [CrossRef]
- Marinescu, M.; Oprea, V.D.; Nechita, A.; Tutunaru, D.; Nechita, L.-C.; Romila, A. The Use of Brain Natriuretic Peptide in the Evaluation of Heart Failure in Geriatric Patients. Diagnostics 2023, 13, 1512. [Google Scholar] [CrossRef]
- Bunch, T.J.; May, H.; Cutler, M.; Woller, S.C.; Jacobs, V.; Stevens, S.M.; Carlquist, J.; Knowlton, K.U.; Muhlestein, J.B.; Steinberg, B.A.; et al. Impact of anticoagulation therapy on the cognitive decline and dementia in patients with non-valvular atrial fibrillation (cognitive decline and dementia in patients with non-valvular atrial fibrillation [CAF] trial). J. Arrhythmia 2022, 38, 997–1008. [Google Scholar] [CrossRef]
- Elahi, F.M.; Alladi, S.; Black, S.E.; Claassen, J.A.; DeCarli, C.; Hughes, T.M.; Moonen, J.; Pajewski, N.M.; Price, B.R.; Satizabal, C.; et al. Clinical trials in vascular cognitive impairment following SPRINT-MIND: An international perspective. Cell Rep. Med. 2023, 4, 101089. [Google Scholar] [CrossRef]
- Wiberg, S.; Holmgaard, F.; Blennow, K.; Nilsson, J.C.; Kjaergaard, J.; Wanscher, M.; Langkilde, A.R.; Hassager, C.; Rasmussen, L.S.; Zetterberg, H.; et al. Associations between mean arterial pressure during cardiopulmonary bypass and biomarkers of cerebral injury in patients undergoing cardiac surgery: Secondary results from a randomized controlled trial. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 229–235. [Google Scholar] [CrossRef]
- Traub, J.; Otto, M.; Sell, R.; Göpfert, D.; Homola, G.; Steinacker, P.; Oeckl, P.; Morbach, C.; Frantz, S.; Pham, M.; et al. Serum phosphorylated tau protein 181 and neurofilament light chain in cognitively impaired heart failure patients. Alzheimer’s Res. Ther. 2022, 14, 149. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Z.; Teng, Y.; Pan, W.; Li, Y.; Su, S.; Chang, J.; Zhao, M. Cognitive impairment is associated with BDNF-TrkB signaling mediating synaptic damage and reduction of amino acid neurotransmitters in heart failure. FASEB J. 2024, 38, e23351. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cog-nitive function, and dysfunction. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 223–250. [Google Scholar] [CrossRef]
- Chen, S.-D.; Wu, C.-L.; Hwang, W.-C.; Yang, D.-I. More insight into BDNF against neurodegeneration: Anti-apoptosis, anti-oxidation, and suppression of autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef]
- Fioranelli, M.; Garo, M.L.; Roccia, M.G.; Prizbelek, B.; Sconci, F.R. Brain–Heart Axis: Brain-Derived Neurotrophic Factor and Cardiovascular Disease—A Review of Systematic Reviews. Life 2023, 13, 2252. [Google Scholar] [CrossRef]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: A bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014, 8, 430. [Google Scholar] [CrossRef]
- Netto, M.B.; de Oliveira Junior, A.N.; Goldim, M.; Mathias, K.; Fileti, M.E.; da Rosa, N.; Laurentino, A.O.; de Farias, B.X.; Costa, A.B.; Rezin, G.T.; et al. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav. Immun. 2018, 73, 661–669. [Google Scholar] [CrossRef]
- Brummel, N.E.; Hughes, C.G.; Thompson, J.L.; Jackson, J.C.; Pandharipande, P.; McNeil, J.B.; Raman, R.; Orun, O.M.; Ware, L.B.; Bernard, G.R.; et al. Inflammation and Coagulation during Critical Illness and Long-Term Cognitive Impairment and Disability. Am. J. Respir. Crit. Care Med. 2021, 203, 699–706. [Google Scholar] [CrossRef]
- Lian, Z.; Ma, Z.; Zhang, Z.-L.; Liu, P.-L.; Zhang, G.-Y.; Guo, C.-X. Association between polymorphisms in connexin 40 gene (Cx40) and risk of atrial fibrillation: A meta-analysis based on 3,452 subjects. Biomarkers 2023, 28, 519–530. [Google Scholar] [CrossRef]
- Guizani, I.; Zidi, W.; Zayani, Y.; Nesrine, F.; Douik, H.; Sanhaji, H.; Mourali, M.S.; Feki, M.; Allal-Elasmi, M. Matrix metalloproteinase 3 and 9 as genetic biomarkers for the occurrence of cardiovascular complications in coronary artery disease: A prospective cohort study. Mol. Biol. Rep. 2022, 49, 9171–9179. [Google Scholar] [CrossRef]
- Luo, H.-Y.; Gao, L.-C.; Long, H.-Z.; Zhou, Z.-W.; Xu, S.-G.; Li, F.-J.; Li, H.-L.; Cheng, Y.; Li, C.-X.; Peng, X.-Y.; et al. Association between the NEP rs701109 polymorphism and the clinical efficacy and safety of sacubitril/valsartan in Chinese patients with heart failure. Eur. J. Clin. Pharmacol. 2023, 79, 663–670. [Google Scholar] [CrossRef]
- Feng, S.; Cai, K.; Lin, S.; Chen, X.; Luo, Y.; Wang, J.; Lian, G.; Lin, Z.; Xie, L. Exploring potential therapeutic agents for lipopolysaccharide-induced septic cardiomyopathy based on transcriptomics using bioinformatics. Sci. Rep. 2023, 13, 20589. [Google Scholar] [CrossRef]
- Schiano, C.; Costa, V.; Aprile, M.; Grimaldi, V.; Maiello, C.; Esposito, R.; Soricelli, A.; Colantuoni, V.; Donatelli, F.; Ciccodicola, A.; et al. Heart failure: Pilot transcriptomic analysis of cardiac tissue by RNA-sequencing. Cardiol. J. 2017, 24, 539–553. [Google Scholar] [CrossRef]
- Chair, S.-Y.; Chan, J.-Y.; Waye, M.-M.; Liu, T.; Law, B.-M.; Chien, W.-T. Exploration of potential genetic biomarkers for heart failure: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 5904. [Google Scholar] [CrossRef]
- Li, S.; Ge, T.; Xu, X.; Xie, L.; Song, S.; Li, R.; Li, H.; Tong, J. Integrating scRNA-seq to explore novel macrophage infiltration-associated biomarkers for diagnosis of heart failure. BMC Cardiovasc. Disord. 2023, 23, 560. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. J. Clin. Investig. 2019, 4, e124079. [Google Scholar] [CrossRef] [PubMed]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef]
- Janardhan, A.; Chen, J.; A Crawford, P. Altered systemic ketone body metabolism in advanced heart failure. Tex. Heart Inst. J. 2011, 38, 533–538. [Google Scholar]
- Jóhannsson, E.; Lunde, P.K.; Heddle, C.; Sjaastad, I.; Thomas, M.J.; Bergersen, L.; Halestrap, A.P.; Blackstad, T.W.; Ottersen, O.P.; Sejersted, O.M. Upregulation of the cardiac monocarboxylate transporter MCT1 in a rat model of congestive heart failure. Circulation 2001, 104, 729–734. [Google Scholar] [CrossRef]
- Yurista, S.R.; Nguyen, C.T.; Rosenzweig, A.; de Boer, R.A.; Westenbrink, B.D. Ketone bodies for the failing heart: Fuels that can fix the engine? Trends Endocrinol. Metab. 2021, 32, 814–826. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The failing heart relies on ketone bodies as a fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Kotooka, N.; Inoue, T.; Aoki, S.; Anan, M.; Komoda, H.; Node, K. Prognostic value of pentraxin 3 in patients with chronic heart failure. Int. J. Cardiol. 2008, 130, 19–22. [Google Scholar] [CrossRef]
- Introna, M.; Alles, V.; Castellano, M.; Picardi, G.; De Gioia, L.; Bottazzai, B.; Peri, G.; Breviario, F.; Salmona, M.; De Gregorio, L.; et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood 1996, 87, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Alberti, L.; Gilardini, L.; Zulian, A.; Micheletto, G.; Peri, G.; Doni, A.; Mantovani, A.; Invitti, C. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis 2009, 202, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Nebuloni, M.; Pasqualini, F.; Zerbi, P.; Lauri, E.; Mantovani, A.; Vago, L.; Garlanda, C. PTX3 expression in the heart tissues of patients with myocardial infarction and infectious myocarditis. Cardiovasc. Pathol. 2011, 20, e27–e35. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, J.; Sugiyama, S.; Nozaki, T.; Akiyama, E.; Matsuzawa, Y.; Kurokawa, H.; Maeda, H.; Fujisue, K.; Sugamura, K.; Yamamoto, E.; et al. Incremental prognostic significance of the elevated levels of pentraxin 3 in patients with heart failure with normal left ventricular ejection fraction. J. Am. Heart Assoc. 2014, 3, e000928. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Takeishi, Y.; Niizeki, T.; Koyama, Y.; Kitahara, T.; Sasaki, T.; Sagara, M.; Kubota, I. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am. Heart J. 2008, 155, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Latini, R.; Gullestad, L.; Masson, S.; Nymo, S.H.; Ueland, T.; Cuccovillo, I.; Vårdal, M.; Bottazzi, B.; Mantovani, A.; Lucci, D.; et al. Pentraxin-3 in chronic heart failure: The CORONA and GISSI-HF trials. Eur. J. Heart Fail. 2012, 14, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Klimcakova, E.; Lolmède, K.; Berlan, M.; Lafontan, M.; Stich, V.; Bouloumié, A.; Galitzky, J.; Arner, P.; Langin, D. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia 2007, 50, 1038–1047. [Google Scholar] [CrossRef]
- Hendricks, S.; Dykun, I.; Balcer, B.; Totzeck, M.; Rassaf, T.; Mahabadi, A.A. Higher BNP/NT-pro BNP levels stratify prognosis equally well in patients with and without heart failure: A meta-analysis. ESC Heart Fail. 2022, 9, 3198–3209. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Prabhu, S.D. The cardiosplenic axis is essential for the pathogenesis of ischemic heart failure. Trans. Am. Clin. Climatol. Assoc. 2018, 129, 202–214. [Google Scholar]
- Hedayat, M.; Mahmoudi, M.J.; Taghvaei, M.; Nematipour, E.; Farhadi, E.; Esfahanian, N.; Mahmoudi, M.; Sadr, M.; Nourijelyani, K.; Amirzargar, A.A.; et al. Tumor necrosis factor-alpha and interleukin-6 gene polymorphisms in iranian patients with ischemic heart failure. Avicenna J. Med. Biotechnol. 2018, 10, 105–109. [Google Scholar]
- Zheng, G.-H.; Chen, H.-Y.; Xiong, S.-Q. Polymorphisms of −174G>C and −572G>C in the interleukin 6 (IL-6) gene and coronary heart disease risk: A meta-analysis of 27 research studies. PLoS ONE 2012, 7, e34839. [Google Scholar] [CrossRef]
- Lim, G.B. New mouse model reveals nitrosative stress as a novel driver of HFpEF. Nat. Rev. Cardiol. 2019, 16, 383–384. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Q.; Chen, F.; Pang, J.; Pan, C.; Xu, F.; Chen, Y. Fundamental mechanisms of the cell death caused by nitrosative stress. Front. Cell Dev. Biol. 2021, 9, 742483. [Google Scholar] [CrossRef] [PubMed]
- van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.; Laarman, G.J.; Somsen, A.; et al. Low Myocardial Protein Kinase G Activity in Heart Failure with Preserved Ejection Fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef]
- Dimitrow, P.P.; Undas, A.; Bober, M.; Tracz, W.; Dubiel, J.S. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol. Rep. 2007, 59, 715–720. [Google Scholar]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef]
- Liu, X.; Hou, L.; Xu, D.; Chen, A.; Yang, L.; Zhuang, Y.; Xu, Y.; Fassett, J.T.; Chen, Y. Effect of asymmetric dimethylarginine (ADMA) on heart failure development. Nitric Oxide 2016, 54, 73–81. [Google Scholar] [CrossRef]
- Du, Z.; Jiang, W.; Yu, C.; Lu, X.; Xia, W. Asymmetric dimethylarginine is associated with the phenomenon of coronary slow flow in patients with nonvalvular atrial fibrillation. Cardiology, 2024; ahead of print. [Google Scholar] [CrossRef]
- Debette, S.; Bauters, C.; Leys, D.; Lamblin, N.; Pasquier, F.; de Groote, P. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest. Heart Fail. 2007, 13, 205–208. [Google Scholar] [CrossRef]
- Dridi, H.; Liu, X.; Yuan, Q.; Reiken, S.; Yehia, M.; Sittenfeld, L.; Apostolou, P.; Buron, J.; Sicard, P.; Matecki, S.; et al. Role of defective calcium regulation in cardiorespiratory dysfunction in Huntington’s disease. J. Clin. Investig. 2020, 5, e140614. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Schumacker, P.T.; Guzman, J.D.; Ilijic, E.; Yang, B.; Zampese, E. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 1013–1019. [Google Scholar] [CrossRef]
- Tran, D.H.; Wang, Z.V. Glucose metabolism in cardiac hypertrophy and heart failure. J. Am. Heart Assoc. 2019, 8, e012673. [Google Scholar] [CrossRef]
- Doehner, W.; Ural, D.; Haeusler, K.G.; Čelutkienė, J.; Bestetti, R.; Cavusoglu, Y.; Peña-Duque, M.A.; Glavas, D.; Iacoviello, M.; Laufs, U.; et al. Heart and brain interaction in patients with heart failure: Overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur. J. Heart Fail. 2018, 20, 199–215. [Google Scholar] [CrossRef]
| Biomarker | Study | Aim | Sample (N) | Type of Study/Procedure | Results |
|---|---|---|---|---|---|
| β-OHB | Voorrips S.N. et al., 2023 [17] | To test if ketogenesis is increased in patients with acute decompensated HF. | 79 by the EMPA-RESPONSE-AHF study | Post hoc analysis of patients with acute heart failure: three ketone bodies were measured in nonfasting plasma samples, which were drawn at six timepoints during the treatment phase | Circulating ketone body concentrations were significantly higher during an episode of acute decompensated HF compared with after stabilization. |
| Flores-Guerrero J.L. et al., 2021 [18] | To assess whether β-OHB was prospectively associated with HF in the general population. | 6134 by the PREVEND study | A prospective population-based cohort study with screening examinations at three different times over a period of about 8 years | High plasma concentrations of β-OHB are associated with an increased risk of HFrEF, particularly in women. | |
| PTX3 | Yamamoto M. et al., 2021 [19] | The secondary endpoint was to evaluate the prognostic value of some markers, including PTX-3, in comparison with BNP. | 616 by the Ibaraki Cardiac Assessment Study, Heart Failure Registry | A prospective 3-year study of patients with acute decompensated heart failure | This study did not show the additional clinical value of PTX-3 for risk stratification in addition to BNP. |
| IL-6 | Fish-Trotter H. et al., 2020 [20] | Examining correlations between inflammation and NP levels, including associations between circulating levels of IL6 and NT-proBNP. | 5481 by the MESA study | A prospective observational study | Increased IL6 was associated with increased NT-proBNP at both baseline and follow-up. |
| Chia Y.C. et al., 2021 [21] | To investigate whether a higher plasma level of IL-6 in the general population is associated with an increased risk of developing new-onset HF. | 961 by the PREVEND study | Cohort study | IL-6 levels were significantly associated with the development of HFpEF, while the association with HFrEF was not significant. | |
| Perez A.L. et al., 2021 [22] | Analyze associations between IL-6 and acute decompensation, readmission, and mortality. | 883 by the ASCEND-HF study | Cohort substudy | IL-6 at follow-up 48/72 h was independently associated with 30-day mortality but not with 180-day mortality. | |
| Alogna A. et al., 2023 [23] | To determine the association of IL-6 with changes in cardiac function, congestion, body composition, and exercise tolerance in HFpEF. | 374 | Cohort substudy | IL-6 levels are commonly found to be elevated in HFpEF, and they are associated with greater symptom severity. | |
| Markousis-Mavrogenis et al., 2021 [24] | To investigate the prognostic value of serial IL-6 measurements in a cohort of patients with acute HF. | 1256 by the PROTECT study | Retrospective cohort analysis | The temporal evolution patterns of IL-6 in patients with acute HF have an additive prognostic value independent of changes in BNP. | |
| Nitric oxide | Momot K. et al., 2023 [25] | To evaluate the circulating levels of markers of inflammation and oxidative/nitrosative stress. | 90 (27 HFpEF, 22 HFrEF, and 41 controls) | Observational cohort study | HFpEF is associated with a significantly increased plasma concentration of 3-NT compared to other groups, suggesting that the pathogenesis of HFpEF is associated with oxidative/nitrosative stress. |
| Lewsey S.C. et al., 2022 [26] | To test the hypothesis that NO-mediated CEF is impaired in HFpEF patients compared with control subjects by coronary MRI. | 32 (10 HFpEF and 22 controls) | Prospective study | ~60% of stable HFpEF patients exhibit abnormal CEF. | |
| Cognitive Biomarkers | Dridi H. et al., 2023 [27] | To identify a mechanism for cognitive dysfunction after heart failure in which hyper-adrenergic signaling and transforming growth factor beta activation induce Ca2+ leak by RyR2 channels in hippocampal neurons. | 13 (9 with HF and 4 control subjects) | Hippocampal biopsy samples were obtained from the Brain Bank | An increased inflammatory response in HFrEF caused intracellular Ca2+ leakage mediated by neuronal RyR2 that subsequently affected cognition and memory. |
| Lyra V. et al., 2020 [28] | To investigate whole-brain and regional brain glucose metabolism in HF patients and its association with depression comorbidity. | 59 (29 subjects with HF and 30 controls) | Cohort study | Heart failure patients with more severe disease showed whole-brain and regional brain hypometabolism in 18F-FDG PET/CT. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, M.; Corallo, F.; D’Aleo, P.; Duca, A.; Bramanti, P.; Bramanti, A.; Cappadona, I. A Set of Possible Markers for Monitoring Heart Failure and Cognitive Impairment Associated: A Review of Literature from the Past 5 Years. Biomolecules 2024, 14, 185. https://doi.org/10.3390/biom14020185
Pagano M, Corallo F, D’Aleo P, Duca A, Bramanti P, Bramanti A, Cappadona I. A Set of Possible Markers for Monitoring Heart Failure and Cognitive Impairment Associated: A Review of Literature from the Past 5 Years. Biomolecules. 2024; 14(2):185. https://doi.org/10.3390/biom14020185
Chicago/Turabian StylePagano, Maria, Francesco Corallo, Piercataldo D’Aleo, Antonio Duca, Placido Bramanti, Alessia Bramanti, and Irene Cappadona. 2024. "A Set of Possible Markers for Monitoring Heart Failure and Cognitive Impairment Associated: A Review of Literature from the Past 5 Years" Biomolecules 14, no. 2: 185. https://doi.org/10.3390/biom14020185





