Pro-Inflammatory Food, Gut Microbiota, and Cardiovascular and Pancreatic Diseases
Abstract
1. Introduction
2. Methods
3. Cardiovascular Diseases and TMAO
4. Pancreatic Diseases and TMAO
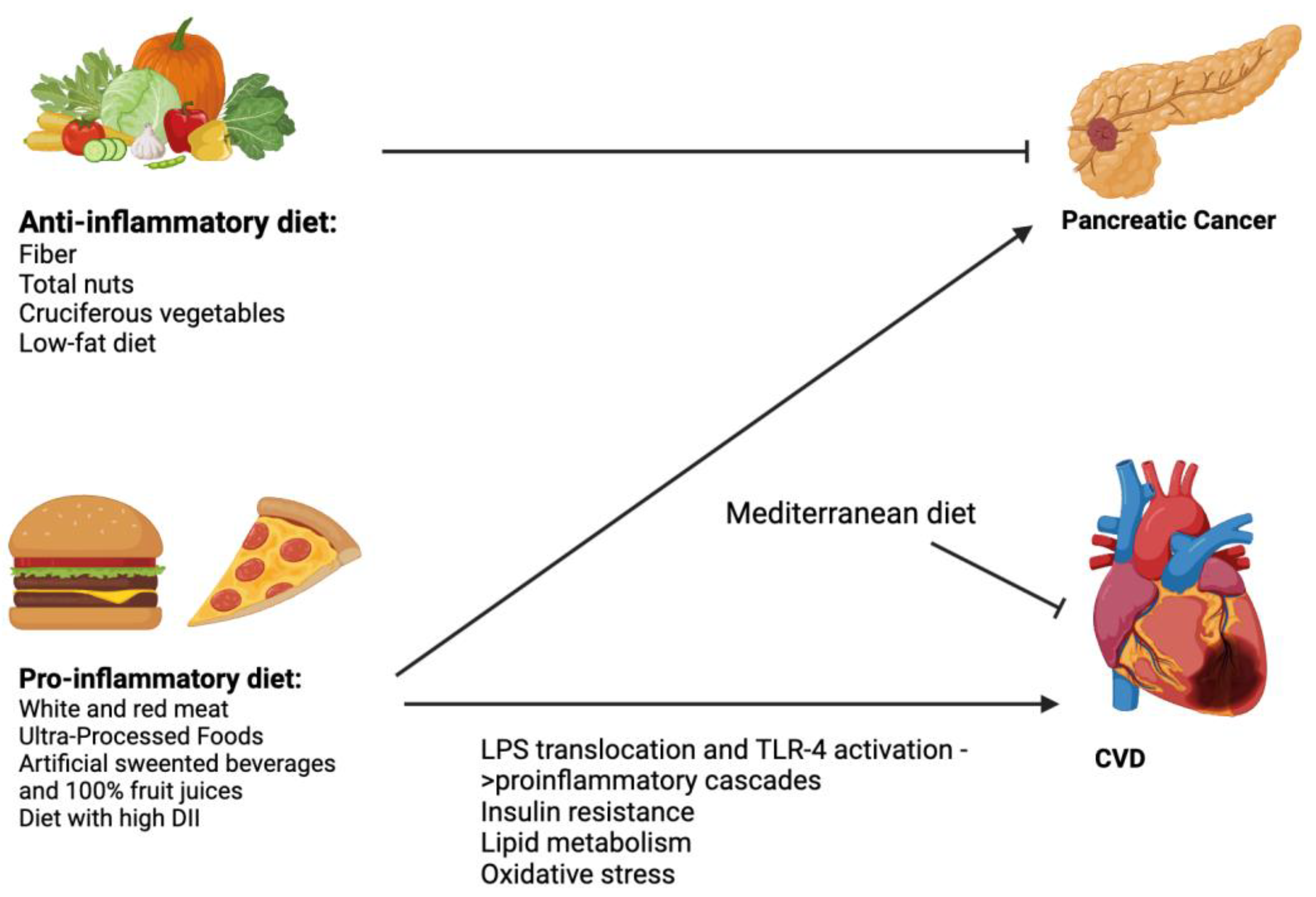
5. Pro-Inflammatory Foods and Pancreatic Diseases
6. Inflammatory Foods and Cardiovascular Diseases
7. Mechanism of Pro-Inflammatory Food
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Szypowska, A.; Zatońska, K.; Szuba, A.; Regulska-Ilow, B. Dietary Inflammatory Index (DII)® and Metabolic Syndrome in the Selected Population of Polish Adults: Results of the PURE Poland Sub-Study. Int. J. Environ. Res. Public Health 2023, 20, 1056. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Anjom-Shoae, J.; Najafi, F.; Ayati, M.H.; Darbandi, M.; Pasdar, Y. Pro-Inflammatory Diet, Cardio-Metabolic Risk Factors and Risk of Type 2 Diabetes: A Cross-Sectional Analysis Using Data from RaNCD Cohort Study. BMC Cardiovasc. Disord. 2023, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Aslani, Z.; Sadeghi, O.; Heidari-Beni, M.; Zahedi, H.; Baygi, F.; Shivappa, N.; Hébert, J.R.; Moradi, S.; Sotoudeh, G.; Asayesh, H.; et al. Association of Dietary Inflammatory Potential with Cardiometabolic Risk Factors and Diseases: A Systematic Review and Dose–Response Meta-Analysis of Observational Studies. Diabetol. Metab. Syndr. 2020, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Hong, Y.; Cheng, Y. Dietary Inflammatory Index and Pancreatic Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Public Health Nutr. 2021, 24, 6427–6435. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Bosetti, C.; Zucchetto, A.; Serraino, D.; La Vecchia, C.; Hébert, J.R. Dietary Inflammatory Index and Risk of Pancreatic Cancer in an Italian Case-Control Study. Br. J. Nutr. 2015, 113, 292–298. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A Small Molecule of Great Expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-Oxide (TMAO) in Human Health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Filippis, F.D.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; Storia, A.L.; Laghi, L.; Serrazanetti, D.I.; Cagno, R.D.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Moyano, J.; Abondano, D.; Echavarria, V. A Review of Acute Pancreatitis. JAMA 2021, 325, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Frossard, M.; Fuchs, I.; Leitner, J.M.; Hsieh, K.; Vlcek, M.; Losert, H.; Domanovits, H.; Schreiber, W.; Laggner, A.N.; Jilma, B. Platelet Function Predicts Myocardial Damage in Patients with Acute Myocardial Infarction. Circulation 2004, 110, 1392–1397. [Google Scholar] [CrossRef]
- Petzold, T.; Thienel, M.; Dannenberg, L.; Mourikis, P.; Helten, C.; Ayhan, A.; M’Pembele, R.; Achilles, A.; Trojovky, K.; Konsek, D.; et al. Rivaroxaban Reduces Arterial Thrombosis by Inhibition of FXa-Driven Platelet Activation via Protease Activated Receptor-1. Circ. Res. 2020, 126, 486–500. [Google Scholar] [CrossRef]
- Skye, S.M.; Zhu, W.; Romano, K.A.; Guo, C.-J.; Wang, Z.; Jia, X.; Kirsop, J.; Haag, B.; Lang, J.M.; DiDonato, J.A.; et al. Microbial Transplantation with Human Gut Commensals Containing CutC Is Sufficient to Transmit Enhanced Platelet Reactivity and Thrombosis Potential. Circ. Res. 2018, 123, 1164–1176. [Google Scholar] [CrossRef]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota-Dependent TMAO Predicts Risk of Cardiovascular Events in Patients with Stroke and Is Related to Proinflammatory Monocytes. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lindskog Jonsson, A.; Caesar, R.; Akrami, R.; Reinhardt, C.; Fåk Hållenius, F.; Borén, J.; Bäckhed, F. Impact of Gut Microbiota and Diet on the Development of Atherosclerosis in Apoe-/- Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, S.; Zhu, J.; Jiang, H.; Jia, D.; Ou, T.; Qi, Z.; Zou, Y.; Qian, J.; Sun, A.; et al. Gut Microbe-Derived Metabolite Trimethylamine N-Oxide Accelerates Fibroblast-Myofibroblast Differentiation and Induces Cardiac Fibrosis. J. Mol. Cell. Cardiol. 2019, 134, 119–130. [Google Scholar] [CrossRef]
- Qiu, L.; Yang, D.; Tao, X.; Yu, J.; Xiong, H.; Wei, H. Enterobacter Aerogenes ZDY01 Attenuates Choline-Induced Trimethylamine N-Oxide Levels by Remodeling Gut Microbiota in Mice. J. Microbiol. Biotechnol. 2017, 27, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Bocchino, B.; Novellino, E. Lactofermented Annurca Apple Puree as a Functional Food Indicated for the Control of Plasma Lipid and Oxidative Amine Levels: Results from a Randomised Clinical Trial. Nutrients 2019, 11, 122. [Google Scholar] [CrossRef]
- Costanza, A.C.; Moscavitch, S.D.; Faria Neto, H.C.C.; Mesquita, E.T. Probiotic Therapy with Saccharomyces Boulardii for Heart Failure Patients: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of Probiotics on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jiménez, R.; Sánchez, M.; Pérez-Vizcaíno, F.; Duarte, J. Antihypertensive Effects of Probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Liu, Y.; Peng, X.; Zhu, S.; Guo, H.; Liu, Y.-L.; Wan, M.; Tang, W. 1HNMR-Based Metabolomic Profile of Rats with Experimental Acute Pancreatitis. BMC Gastroenterol. 2014, 14, 115. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, X. TMAO Promotes Apoptosis and Oxidative Stress of Pancreatic Acinar Cells by Mediating IRE1α-XBP-1 Pathway. Saudi J. Gastroenterol. 2021, 27, 361–369. [Google Scholar] [CrossRef] [PubMed]
- OuYang, D.; Xu, J.; Huang, H.; Chen, Z. Metabolomic Profiling of Serum from Human Pancreatic Cancer Patients Using 1H NMR Spectroscopy and Principal Component Analysis. Appl. Biochem. Biotechnol. 2011, 165, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mirji, G.; Worth, A.; Bhat, S.A.; Sayed, M.E.; Kannan, T.; Goldman, A.R.; Tang, H.-Y.; Liu, Q.; Auslander, N.; Dang, C.V.; et al. The Microbiome-Derived Metabolite TMAO Drives Immune Activation and Boosts Responses to Immune Checkpoint Blockade in Pancreatic Cancer. Sci. Immunol. 2022, 7, eabn0704. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Luu, H.N.; Butler, L.M.; Midttun, Ø.; Ulvik, A.; Wang, R.; Jin, A.; Gao, Y.-T.; Tan, Y.; Ueland, P.M.; et al. A Prospective Evaluation of Serum Methionine-Related Metabolites in Relation to Pancreatic Cancer Risk in Two Prospective Cohort Studies. Int. J. Cancer 2020, 147, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, X. Trimethylamine N-Oxide Promotes Hyperlipidemia Acute Pancreatitis via Inflammatory Response. Can. J. Physiol. Pharmacol. 2022, 100, 61–67. [Google Scholar] [CrossRef]
- Kim, Y. The Association between Red, Processed and White Meat Consumption and Risk of Pancreatic Cancer: A Meta-Analysis of Prospective Cohort Studies. Cancer Causes Control 2023, 34, 569–581. [Google Scholar] [CrossRef]
- Zhong, G.-C.; Zhu, Q.; Cai, D.; Hu, J.-J.; Dai, X.; Gong, J.-P.; Sun, W.-P. Ultra-Processed Food Consumption and the Risk of Pancreatic Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer 2023, 152, 835–844. [Google Scholar] [CrossRef]
- Bae, J.-M.; Shim, S.R. Coffee Consumption and Pancreatic Cancer Risk: A Meta-Epidemiological Study of Population-Based Cohort Studies. Asian Pac. J. Cancer Prev. 2020, 21, 2793–2798. [Google Scholar] [CrossRef]
- Arafa, A.; Eshak, E.S.; Dong, J.-Y.; Shirai, K.; Muraki, I.; Iso, H.; Tamakoshi, A.; The JACC Study Group. Dairy Intake and the Risk of Pancreatic Cancer: The Japan Collaborative Cohort Study (JACC Study) and Meta-Analysis of Prospective Cohort Studies. Br. J. Nutr. 2022, 128, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, S.; Xu, F.; Chang, J.; Guo, Y.; Zhou, Z.; Xu, R.; Wang, T.; Wang, X.; Wang, M.; et al. The Association between Dietary Protein Intake and the Risk of Pancreatic Cancer: Evidence from 14 Publications. Nutr. Cancer 2022, 74, 3172–3178. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Y.; Yu, M.; Li, G.; Chen, Y.; Li, X.; Chen, X.; Xie, Y.; Wang, X. Poultry and Fish Intake and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 55–67. [Google Scholar] [CrossRef]
- Pan, B.; Lai, H.; Ma, N.; Li, D.; Deng, X.; Wang, X.; Zhang, Q.; Yang, Q.; Wang, Q.; Zhu, H.; et al. Association of Soft Drinks and 100% Fruit Juice Consumption with Risk of Cancer: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Chen, L.; White, D.L.; Tinker, L.; Chlebowski, R.T.; Van Horn, L.V.; Richardson, P.; Lane, D.; Sangi-Haghpeykar, H.; El-Serag, H.B. Low-Fat Dietary Pattern and Pancreatic Cancer Risk in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl. Cancer Inst. 2018, 110, 49–56. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Lu, M.; Xu, X.; Lin, H.; Zheng, Z. Cruciferous Vegetable Consumption and the Risk of Pancreatic Cancer: A Meta-Analysis. World J. Surg. Oncol. 2015, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Santangelo, O.E.; Provenzano, S.; Fatigoni, C.; Nardi, M.; Ferrara, P.; Gianfredi, V. Dietary Fiber Intake and Risk of Pancreatic Cancer: Systematic Review and Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 11556. [Google Scholar] [CrossRef]
- Naghshi, S.; Sadeghian, M.; Nasiri, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Association of Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption with Cancer Incidence and Mortality: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zeng, J.; Liu, C.; Gu, Y.; Zou, Y.; Chang, H. Folate Intake and Risk of Pancreatic Cancer: A Systematic Review and Updated Meta-Analysis of Epidemiological Studies. Dig. Dis. Sci. 2021, 66, 2368–2379. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications after Myocardial Infarction: Final Report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association–Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Tit, D.M.; Banica, F.; Bratu, O.G.; Diaconu, C.C.; Nistor-Cseppento, C.D.; Bustea, C.; Aron, R.A.C.; Vesa, C.M. Interactions between Leptin and Insulin Resistance in Patients with Prediabetes, with and without NAFLD. Exp. Ther. Med. 2020, 20, 197. [Google Scholar] [CrossRef]
- Caldart, F.; de Pretis, N.; Luchini, C.; Ciccocioppo, R.; Frulloni, L. Pancreatic Steatosis and Metabolic Pancreatic Disease: A New Entity? Intern. Emerg. Med. 2023, 18, 2199–2208. [Google Scholar] [CrossRef]
- Barroso Oquendo, M.; Siegel-Axel, D.; Gerst, F.; Lorza-Gil, E.; Moller, A.; Wagner, R.; Machann, J.; Fend, F.; Königsrainer, A.; Heni, M.; et al. Pancreatic Fat Cells of Humans with Type 2 Diabetes Display Reduced Adipogenic and Lipolytic Activity. Am. J. Physiol. Cell Physiol. 2021, 320, C1000–C1012. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hsiao, M. Leptin and Cancer: Updated Functional Roles in Carcinogenesis, Therapeutic Niches, and Developments. Int. J. Mol. Sci. 2021, 22, 2870. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, J.-L.; Li, M.-L.; Zhou, J.; Sun, X.-L. The Association between Pancreas Steatosis and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Diabetes/Metab. Res. Rev. 2019, 35, e3142. [Google Scholar] [CrossRef]
- Ocké, M.C. Evaluation of Methodologies for Assessing the Overall Diet: Dietary Quality Scores and Dietary Pattern Analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the Effect of Ketogenic Diet as a Preventive Measure to Obesity and Diabetes Mellitus. Life Sci. 2021, 264, 118661. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Saidoung, P.; Pham, T.; Phisalprapa, P.; Lee, Y.Y.; Varady, K.A.; Veettil, S.K.; Chaiyakunapruk, N. Effects of Ketogenic Diet on Health Outcomes: An Umbrella Review of Meta-Analyses of Randomized Clinical Trials. BMC Med. 2023, 21, 196. [Google Scholar] [CrossRef]
- de Freitas Junior, L.M.; de Almeida, E.B. Medicinal Plants for the Treatment of Obesity: Ethnopharmacological Approach and Chemical and Biological Studies. Am. J. Transl. Res. 2017, 9, 2050–2064. [Google Scholar] [PubMed]
- Rani, N.; Sharma, S.K.; Vasudeva, N. Assessment of Antiobesity Potential of Achyranthes aspera Linn. Seed. Evid. Based Complement. Altern. Med. 2012, 2012, 715912. [Google Scholar] [CrossRef] [PubMed]
- Bais, S.; Singh, G.S.; Sharma, R. Antiobesity and Hypolipidemic Activity of Moringa oleifera Leaves against High Fat Diet-Induced Obesity in Rats. Adv. Biol. 2014, 2014, e162914. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid Intake and Cardiovascular Disease Mortality in a Prospective Cohort of US Adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function12. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Haș, I.M.; Tit, D.M.; Bungau, S.G.; Pavel, F.M.; Teleky, B.-E.; Vodnar, D.C.; Vesa, C.M. Cardiometabolic Risk: Characteristics of the Intestinal Microbiome and the Role of Polyphenols. Int. J. Mol. Sci. 2023, 24, 13757. [Google Scholar] [CrossRef]
- Li, R.-L.; Wang, L.-Y.; Liu, S.; Duan, H.-X.; Zhang, Q.; Zhang, T.; Peng, W.; Huang, Y.; Wu, C. Natural Flavonoids Derived From Fruits Are Potential Agents Against Atherosclerosis. Front. Nutr. 2022, 9, 862277. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Ceriello, A.; Taboga, C.; Tonutti, L.; Quagliaro, L.; Piconi, L.; Bais, B.; Da Ros, R.; Motz, E. Evidence for an Independent and Cumulative Effect of Postprandial Hypertriglyceridemia and Hyperglycemia on Endothelial Dysfunction and Oxidative Stress Generation: Effects of Short- and Long-Term Simvastatin Treatment. Circulation 2002, 106, 1211–1218. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic Syndrome: A Comprehensive Perspective Based on Interactions between Obesity, Diabetes, and Inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Sun, T.; Guo, F.; Huang, S.; Chandalia, M.; Abate, N.; Fan, D.; Xin, H.-B.; Chen, Y.E.; et al. Dietary Obesity-Induced Egr-1 in Adipocytes Facilitates Energy Storage via Suppression of FOXC2. Sci. Rep. 2013, 3, 1476. [Google Scholar] [CrossRef]
- Ceriello, A.; Quagliaro, L.; Catone, B.; Pascon, R.; Piazzola, M.; Bais, B.; Marra, G.; Tonutti, L.; Taboga, C.; Motz, E. Role of Hyperglycemia in Nitrotyrosine Postprandial Generation. Diabetes Care 2002, 25, 1439–1443. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Giugliano, F.; Di Palo, C.; Ciotola, M.; Barbieri, M.; Paolisso, G.; Giugliano, D. Meal Modulation of Circulating Interleukin 18 and Adiponectin Concentrations in Healthy Subjects and in Patients with Type 2 Diabetes Mellitus. Am. J. Clin. Nutr. 2003, 78, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans: Role of Oxidative Stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2018, 8, 1091–1115. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T.; et al. Olfactory Receptor Responding to Gut Microbiota-Derived Signals Plays a Role in Renin Secretion and Blood Pressure Regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Li, F.; Gonzalez, F.J. FXR Signaling in the Enterohepatic System. Mol. Cell Endocrinol. 2013, 368, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Moschetta, A.; Lee, Y.-K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of Antibacterial Defense in the Small Intestine by the Nuclear Bile Acid Receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative Stress—Complex Pathological Issues Concerning the Hallmark of Cardiovascular and Metabolic Disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The Role of Reactive Oxygen Species in the Pathophysiology of Cardiovascular Diseases and the Clinical Significance of Myocardial Redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef] [PubMed]

| First Author | Type of Study | Patients, n | Outcomes |
|---|---|---|---|
| Skye et al. [19] | Animal and human study | N/A | TMA/TMAO-enhanced platelet reactivity and thrombosis potential. |
| Haghikia et al. [20] | Cohort study | 593 | High TMAO is associated with increased risk of adverse cardiovascular events in patients with recent first-ever stroke with an adjusted HR of 3.3 (95% CI: 1.2–10.9) during 1-year follow-up. |
| Chen et al. [23] | Cell and animal study | N/A | TMAO promoted vascular inflammation by activating the NLRP3 pathway. |
| Yang et al. [24] | Animal study | N/A | TMAO accelerated cardiac fibrosis by activating the TGF-βRI/Smad2 pathway. |
| Koeth et al. [13] | Animal and human cohort study | 2595 | Chronic dietary L-carnitine supplementation in mice enhanced the synthesis of TMAO and increased atherosclerosis. In humans, plasma L-carnitine levels predicted risks for prevalent CVD and incident major adverse cardiovascular events only in subjects with high TMAO levels. |
| Li et al. [30] | Animal study | N/A | TMAO level was decreased in rats with experimental acute pancreatitis. |
| Yang et al. [31] | Animal study | N/A | TMAO increased the incidence of acute pancreatitis by activating the inositol-requiring enzyme 1α (IRE1α)/X-box binding protein 1 pathway. |
| OuYang et al. [32] | Human study | 17 with pancreatic cancer vs. 24 controls | Pancreatic cancer patients had lower plasma levels of TMAO. |
| Mirji et al. [33] | Animal and human study | N/A | The abundance of TMAO-generating bacteria correlated with improved survival and response to anti-programmed cell death protein 1 in a mouse model of pancreatic cancer. |
| Huang et al. [34] | Human prospective cohort study | 129 cases and 258 controls in the Shanghai Cohort, 58 cases and 104 controls in Singapore Chinese Health Study | TMAO is associated with an OR of 2.81 (95% CI: 1.37–5.76) for pancreatic cancer in the Shanghai cohort, while the association was not significant in the Singapore Chinese Study. |
| Filippis et al. [12] | Human study | 153 | Vegetarian and vegan diet was associated with lower levels of TMAO. |
| Qiu et al. [25] | Animal study | N/A | The use of probiotics reduced the serum TMAO levels. |
| Tenore et al. [26] | Randomized controlled trial | 90 | Lactofermented Annurca apple puree intake reduced plasma TMAO levels. |
| Costanza et al. [27] | Randomized controlled trial | N/A | Among heart failure patients, treatment with probiotics was associated with a reduction in total cholesterol levels, uric acid levels, and left atrial diameter, and an improvement in left ventricular ejection fraction. |
| Khalesi et al. [28] | Meta-analysis | 543 | Probiotic therapy led to a significant reduction of systolic blood pressure by 3.56 mmHg and diastolic blood pressure by 2.38 mmHg. |
| First Author | Types of Study | Patients, n | Outcomes |
|---|---|---|---|
| Guo et al. [6] | Meta-analysis with 2 prospective cohort studies and 4 case-control studies | 634,705 | Highest DII category was associated with 45% increased risk of pancreatic cancer (PC) with an RR of 1.45 (95% CI: 1.11–1.90). Every 1-unit increase in the DII score increased PC risk by 8% with an RR of 1.08 (95% CI: 1.002–1.166). |
| Kim et al. [36] | Meta-analysis | 3,934,909 | The pooled RR for pancreatic cancer in highest vs. lowest intakes of red meat and white meat was 1.09 (95% CI: 0.97–1.21) and 1.14 (95% CI: 1.03–1.27), respectively. |
| Zhong et al. [37] | Prospective cohort study | 98,265 | High intake of ultra-processed foods was associated with PC with an HR of 1.49 (95% CI:1.07–2.07). |
| Bae et al. [38] | Meta-analysis (12 cohort studies) | 3,230,053 with 10,587 pancreatic cancer incidents | Summary RR of PC risk for the highest vs. the lowest level of coffee consumption was 0.98 (95% CI: 0.88–1.10). |
| Arafa et al. [39] | Meta-analysis with 5 prospective studies | N/A | Milk, cheese, and yogurt were not associated with reduced risk of PC with an HR of 0.95 (95% CI: 0.82–1.11), 1.16 (95% CI: 0.87–1.55), 0.91 (95% CI: 0.79–1.05), respectively. |
| Zhang et al. [40] | Meta-analysis (12 case-control and 2 cohort studies) | 77,156 | Total protein intake had no significant association with the risk of pancreatic cancer with an RR = 1.02 (95% CI: 0.85–1.22). Although not statistically significant, the opposite association was found in animal protein intake (RR:1.37, 95% CI: 0.93–2.01) and vegetable protein intake (RR:0.78, 95% CI: 0.54–1.14). |
| Gao et al. [41] | Meta-analysis of 25 studies | 1,258,913 | No appreciable link between fish intake and PC risk (RR: 1.00, 95% CI: 0.93–1.07). |
| Pan et al. [42] | Systematic review and 8 prospective cohort studies | 1,594,301 | There was a linear dose-response association between artificially sweetened beverages and 100% fruit juices and the risk of pancreatic cancer. |
| Jiao et al. [43] | Randomized controlled trial | 48,835 | Low-fat dietary intervention was associated with a reduced risk of pancreatic cancer in patients with body mass index ≥ 25 kg/m2 with an HR of 0.71 (95% CI: 0.53–0.96). |
| Li et al. [44] | Meta-analysis with five case-control studies | 3207 | High intake of cruciferous vegetables is associated with significantly decreased risk of pancreatic cancer (OR: 0.78, 95% CI: 0.64–0.91) |
| Nucci et al. [45] | Meta-analysis | 343,120 | Higher dietary fiber intake is associated with a significantly lower risk of pancreatic cancer with a pooled effect size of 0.63 (95% CI: 0.53–0.76). |
| Naghshi et al. [46] | Meta-analysis with 51 articles | 1,739,414 | A 5-g/d increase in total nut intake was associated with a 6% lower risk of pancreatic cancer |
| Fu et al. [47] | Meta-analysis of 16 studies | 1,009,374 | There was a significant association between folate intake and decreased risk of PC, with a pooled odds ratio of 0.82 (95% CI: 0.69–0.97). However, the association was observed only in case-control studies (OR: 0.78, 95% CI: 0.65–0.93), but not in cohort studies (RR: 0.85, 95% CI: 0.66–1.09). |
| Lorgeril et al. [48] | Randomized controlled trial | 605 | The Mediterranean diet was associated with reduced all-cause and cardiovascular (p = 0.01) mortality and the combination of recurrent myocardial infarction and cardiac death (p < 0.0001). |
| Estruch et al. [49] | Multicenter randomized controlled trial | 7447 | The hazard ratio for major cardiovascular events was 0.69% (95% CI: 0.53–0.91) for the Mediterranean diet with extra-virgin olive oil and 0.72 (95% CI: 0.54–0.95) for the Mediterranean diet with nuts, compared to the control diet. |
| Namazi et al. [4] | Cross-sectional analysis | 9039 | Results showed that higher DII scores were associated with a 61% (95% CI: 1.27–2.05) increased risk of T2DM after adjusting for confounding factors. |
| Aslani et al. [5] | Systematic review | 291,968 | Individuals with the highest DII score category had a 29% increased risk (HR: 1.29, 95% CI: 1.18–1.41) of cardiovascular and metabolic disease mortality compared to those with the lowest DII category. |
| Shah et al. [50] | Prospective cohort study | 100 | A vegan diet led to 32% lower high-sensitivity C-reactive protein (p = 0.02) compared to the American Heart Association-recommended diet in patients with coronary artery disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Patel, S.; Bao, L.; Nadeem, D.; Krittanawong, C. Pro-Inflammatory Food, Gut Microbiota, and Cardiovascular and Pancreatic Diseases. Biomolecules 2024, 14, 210. https://doi.org/10.3390/biom14020210
Chen B, Patel S, Bao L, Nadeem D, Krittanawong C. Pro-Inflammatory Food, Gut Microbiota, and Cardiovascular and Pancreatic Diseases. Biomolecules. 2024; 14(2):210. https://doi.org/10.3390/biom14020210
Chicago/Turabian StyleChen, Bing, Shriraj Patel, Lingyu Bao, Danial Nadeem, and Chayakrit Krittanawong. 2024. "Pro-Inflammatory Food, Gut Microbiota, and Cardiovascular and Pancreatic Diseases" Biomolecules 14, no. 2: 210. https://doi.org/10.3390/biom14020210
APA StyleChen, B., Patel, S., Bao, L., Nadeem, D., & Krittanawong, C. (2024). Pro-Inflammatory Food, Gut Microbiota, and Cardiovascular and Pancreatic Diseases. Biomolecules, 14(2), 210. https://doi.org/10.3390/biom14020210







