Non-Coding RNAs in Kidney Stones
Abstract
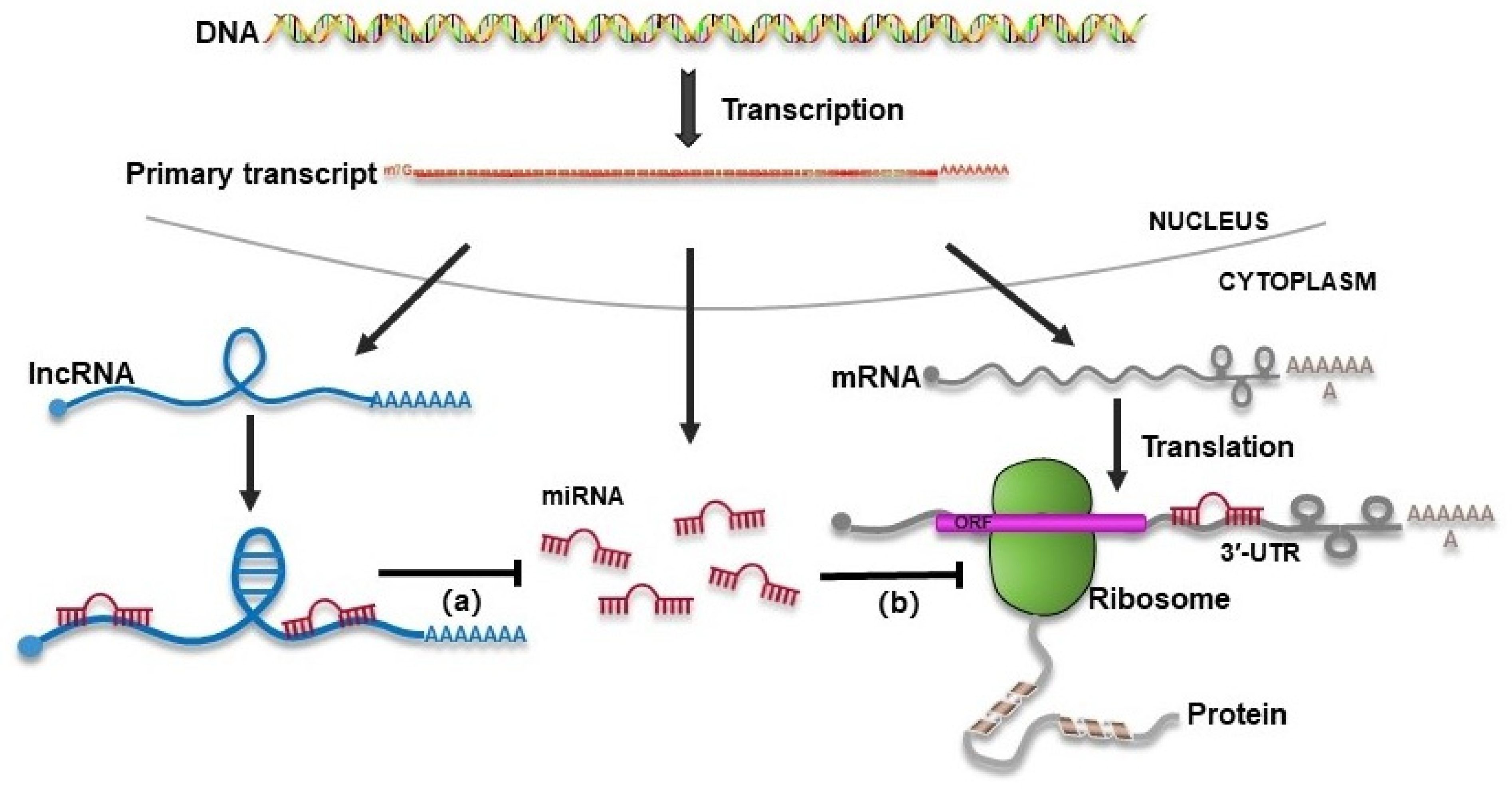
:1. Introduction
2. Differential Expression of Non-Coding RNAs in Kidney Stones
2.1. In Animal Models
2.2. Patients’ Biospecimens
3. The Role and Mechanism of miRNAs in Kidney Stones
3.1. Regulation of Calcium Metabolism
3.2. Metabolism of Oxalate
3.3. Oxidative Stress and Renal Tubular Epithelial Cell (RTEC) Injury
3.4. Cell–Crystal Adhesion
3.5. Macrophage Polarization and Metabolism
3.6. Cellular Autophagy
3.7. Apoptosis
| MiRNA | Expression in Patients | Function | Upstream Effector | Target | Model/Sample | Manipulation | Refs |
|---|---|---|---|---|---|---|---|
| miR-9, miR-374 | NA | Protective | CaSR | claudin-14 | Mouse MKTAL cells, mouse TAL cells, human HEK293 cells, mice |
| [36,37,38,39,40] |
| miRNA-130a-3p, miRNA-148b-3p, miRNA-374b-5p | NA | Protective | Histone H3K9 and H3K27 | Nadc1 and claudin-14 | HK-2 cells, rats |
| [37,42] |
| miR-4660 | Down | Pathogenic | NA | AGXT | Human serum, human liver tissues, HepG2 and L02 cell lines | In vitro: mimic and anti-miR | [49] |
| miRNA-411-3p | NA | Protective | glycine | Slc26a6 and Nadc1 | HK-2 and NRK-52E cells, rats |
| [50] |
| miR-155-5p | Up | Pathogenic | NA | MGP | Human serum and urinary, HK-2 cells, mouse |
| [55,58] |
| miR-21 | Up | Pathogenic | NA | PPARA | Human urine, HK-2 cells, mouse |
| [59] |
| miR-128-3p | NA | Pathogenic | Theaflavin | SIRT1 | HK-2 cells, mouse |
| [60] |
| miR-204 | NA | Protective | NA | mucin 4 | HK-2 cells | In vitro: mimic and anti-miR | [61] |
| miR-141-3p | NA | Protective | NA | NLRP3 | HK-2 cells | In vitro: mimic | [63] |
| miR-93-5p | Down | Protective | PUFA | Pknox1 | Urine samples of patients, biopsy tissue samples from patients, mice, HK-2 cells |
| [64] |
| miR-93-5p | NA | Protective | Nrf2 | TLR4 and IRF1 | BMDMs, TECs, mouse, |
| [77] |
| miR-34a | NA | Protective | NA | CD44 | HK-2 cells, mice |
| [67] |
| miR-103a-3p | NA | Pathogenic | NA | UMOD | NRK-52E cells, rat |
| [68] |
| miR-484 | NA | Protective | NA | VDR | RTECs, rat |
| [69] |
| miR-23 | NA | Protective | PPAR-γ | IRF1/Pknox1 | BMDMs, mice |
| [70] |
| miRNA-185-5p | Up | Pathogenic | AR | CSF-1 | human plasma samples; HK-2 and HKC-8 cells, mice |
| [72,73] |
| miR-20b-3p | Down | Protective | NA | ATG7, TLR4 | Urine of patients, NRK-52E cells, rat |
| [78] |
| miR-30c-5p | NA | Protective | NA | ATG5 | HK-2 cells |
| [80] |
4. The Role and Mechanism of lncRNAs in Kidney Stones
4.1. Function as miRNA Sponges
| LncRNAs | Expression in Patients | Function | Target Pathway | Model | Refs |
|---|---|---|---|---|---|
| LINC00339 | NA | Pathogenic | miR-22-3p/NLRP3 | HK-2 cells | [85] |
| H19 | Up | Pathogenic | miR-216b/HMGB1/TLR4/NF-kB | Randall’s plaques, HK-2 cells, mouse | [86] |
| HOXA11-AS | NA | Pathogenic | miR-124-3p/MCP-1 | HK-2 cell, mouse | [87] |
| XIST | NA | Pathogenic | miR223/NLRP3/Caspase-1/IL-1β | HK-2 cell, mouse | [88] |
| lncRNA-ATB | NA | Pathogenic | miR-200 family | HK-2 cell | [89] |
| LINC01197 | Down | Protective | miR-516b-5p/SIRT3/FOXO1 | Renal tissues of patients, HK-2 cell | [90] |
| CHCHD4P4 | NA | Pathogenic | NA | HK-2 cells, mouse | [92] |
| OLMALINC | NA | Pathogenic | OCT4/BMP2 | hRIFs | [93] |
4.2. Facilitating Fibrosis
5. Non-Coding RNAs as Biomarkers for Kidney Stone
6. Potential Therapeutic Applications
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Chen, Y.; Liao, B.; Luo, D.; Wang, K.; Li, H.; Zeng, G. Epidemiology of Urolithiasis in Asia. Asian J. Urol. 2018, 5, 205–214. [Google Scholar] [CrossRef]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of Stone Disease across the World. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Krambeck, A.E.; Rule, A.D. Determining the True Burden of Kidney Stone Disease. Nat. Rev. Nephrol. 2020, 16, 736–746. [Google Scholar] [CrossRef]
- D’Costa, M.R.; Haley, W.E.; Mara, K.C.; Enders, F.T.; Vrtiska, T.J.; Pais, V.M.; Jacobsen, S.J.; McCollough, C.H.; Lieske, J.C.; Rule, A.D. Symptomatic and Radiographic Manifestations of Kidney Stone Recurrence and Their Prediction by Risk Factors: A Prospective Cohort Study. J. Am. Soc. Nephrol. 2019, 30, 1251–1260. [Google Scholar] [CrossRef]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney Stones. Nat. Rev. Dis. Prim. 2016, 2, 16008. [Google Scholar] [CrossRef]
- Ushimoto, C.; Sugiki, S.; Kunii, K.; Inoue, S.; Kuroda, E.; Akai, R.; Iwawaki, T.; Miyazawa, K. Dynamic Change and Preventive Role of Stress Response via Keap1-Nrf2 during Renal Crystal Formation. Free Radic. Biol. Med. 2023, 207, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Canela, V.H.; Bowen, W.S.; Ferreira, R.M.; Syed, F.; Lingeman, J.E.; Sabo, A.R.; Barwinska, D.; Winfree, S.; Lake, B.B.; Cheng, Y.-H.; et al. A Spatially Anchored Transcriptomic Atlas of the Human Kidney Papilla Identifies Significant Immune Injury in Patients with Stone Disease. Nat. Commun. 2023, 14, 4140. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. Kidney Stone Prevention. Adv. Nutr. 2023, 14, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.S.C.; Pearle, M.S. Medical Management of Renal Stones. BMJ 2016, 352, i52. [Google Scholar] [CrossRef] [PubMed]
- di Iulio, J.; Bartha, I.; Wong, E.H.M.; Yu, H.-C.; Lavrenko, V.; Yang, D.; Jung, I.; Hicks, M.A.; Shah, N.; Kirkness, E.F.; et al. The Human Noncoding Genome Defined by Genetic Diversity. Nat. Genet. 2018, 50, 333–337. [Google Scholar] [CrossRef]
- Popławski, P.; Bogusławska, J.; Hanusek, K.; Piekiełko-Witkowska, A. Nucleolar Proteins and Non-Coding RNAs: Roles in Renal Cancer. Int. J. Mol. Sci. 2021, 22, 13126. [Google Scholar] [CrossRef]
- Kato, M. Noncoding RNAs as Therapeutic Targets in Early Stage Diabetic Kidney Disease. Kidney Res. Clin. Pract. 2018, 37, 197–209. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Shu, S.; Cai, J.; Tang, C.; Dong, Z. Non-Coding RNAs in Kidney Injury and Repair. Am. J. Physiol. Cell Physiol. 2019, 317, C177–C188. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Kaneko, S.; Yanai, K.; Aomatsu, A.; Hirai, K.; Ookawara, S.; Morishita, Y. MicroRNA Expression Profiling in Diabetic Kidney Disease. Transl. Res. 2021, 237, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, Q. Non-Coding RNA and Diabetic Kidney Disease. DNA Cell Biol. 2021, 40, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Aomatsu, A.; Kaneko, S.; Yanai, K.; Ishii, H.; Ito, K.; Hirai, K.; Ookawara, S.; Kobayashi, Y.; Sanui, M.; Morishita, Y. MicroRNA Expression Profiling in Acute Kidney Injury. Transl. Res. 2022, 244, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Li, X. Non-Coding RNAs in Hereditary Kidney Disorders. Int. J. Mol. Sci. 2021, 22, 3014. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, B.; Liu, J.; Yao, W.; Xia, D.; Li, L.; Chen, Z.; Ye, Z.; Yu, X. Analysis of Altered microRNA Expression Profiles in Proximal Renal Tubular Cells in Response to Calcium Oxalate Monohydrate Crystal Adhesion: Implications for Kidney Stone Disease. PLoS ONE 2014, 9, e101306. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Chen, D.; Liang, X.; Huang, J.; Zeng, T.; Duan, X.; Chen, K.; Lai, Y.; Yang, D.; Li, S.; et al. Integrative Analysis of miRNA and mRNA Expression Profiles in Calcium Oxalate Nephrolithiasis Rat Model. BioMed Res. Int. 2017, 2017, 8306736. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, H.; Yang, J.; Wang, T.; Ding, Y.; Liu, J.; Wang, S.; Ye, Z. Analysis of Altered microRNA Expression Profiles in the Kidney Tissues of Ethylene Glycol-Induced Hyperoxaluric Rats. Mol. Med. Rep. 2016, 14, 4650–4658. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, B.; Hu, H.; Zhang, J.; Wang, Y.; Wang, Q.; Wang, S. Integrative microRNA-Gene Expression Network Analysis in Genetic Hypercalciuric Stone-Forming Rat Kidney. PeerJ 2016, 4, e1884. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, X.; Yang, Y.; Ye, Z.; Wang, E.; Dong, Z. Changing Expression Profiles of Long Non-Coding RNAs, mRNAs and Circular RNAs in Ethylene Glycol-Induced Kidney Calculi Rats. BMC Genom. 2018, 19, 660. [Google Scholar] [CrossRef]
- Liang, X.; Lai, Y.; Wu, W.; Chen, D.; Zhong, F.; Huang, J.; Zeng, T.; Duan, X.; Huang, Y.; Zhang, S.; et al. LncRNA-miRNA-mRNA Expression Variation Profile in the Urine of Calcium Oxalate Stone Patients. BMC Med. Genom. 2019, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Q.; Xun, Y.; Li, C.; Wang, S. The Preliminary Exploration of What Role miRNAs Derived From Urinary Exosomes Play in Kidney Stone Formation. Urology 2022, 166, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, X.; Ye, Z.; Yu, W.; Ning, J.; Ruan, Y.; Yuan, R.; Lin, F.; Ye, P.; Zheng, D.; et al. Construction and Analysis of Immune Infiltration-Related ceRNA Network for Kidney Stones. Front. Genet. 2021, 12, 774155. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Shin, S.; Jung, Y.; Uhm, H.; Song, M.; Son, S.; Goo, J.; Jeong, C.; Song, J.-J.; Kim, V.N.; Hohng, S. Quantification of Purified Endogenous miRNAs with High Sensitivity and Specificity. Nat. Commun. 2020, 11, 6033. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, X.; Xiong, J.; Han, W.; Xiao, T.; Xu, X.; Yang, K.; Liu, C.; Jiang, W.; He, T.; et al. MicroRNA-34a Promotes Renal Fibrosis by Downregulation of Klotho in Tubular Epithelial Cells. Mol. Ther. 2019, 27, 1051–1065. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, D.; Wang, F.; Liu, J.; Huang, B.; Baker, M.A.; Yin, J.; Wu, R.; Liu, X.; Regner, K.R.; et al. Endogenous miR-204 Protects the Kidney against Chronic Injury in Hypertension and Diabetes. J. Am. Soc. Nephrol. 2020, 31, 1539–1554. [Google Scholar] [CrossRef]
- Gebeshuber, C.A.; Kornauth, C.; Dong, L.; Sierig, R.; Seibler, J.; Reiss, M.; Tauber, S.; Bilban, M.; Wang, S.; Kain, R.; et al. Focal Segmental Glomerulosclerosis Is Induced by microRNA-193a and Its Downregulation of WT1. Nat. Med. 2013, 19, 481–487. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Karolina, D.S.; Sepramaniam, S.; Armugam, A.; Wintour, E.M.; Bertram, J.F.; Jeyaseelan, K. Role of microRNAs in Kidney Homeostasis and Disease. Kidney Int. 2012, 81, 617–627. [Google Scholar] [CrossRef]
- Singh, P.; Enders, F.T.; Vaughan, L.E.; Bergstralh, E.J.; Knoedler, J.J.; Krambeck, A.E.; Lieske, J.C.; Rule, A.D. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo Clin. Proc. 2015, 90, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Flocks, R.H. Calcium and Phosphorus Excretion in the Urine: Of Patients with Renal or Ureteral Calculi. J. Am. Med. Assoc. 1939, 113, 1466–1471. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Feng, D.; Fu, Y.; Wu, H.; Lu, J.; Bao, J. Calcium-Sensing Receptor Promotes Calcium Oxalate Crystal Adhesion and Renal Injury in Wistar Rats by Promoting ROS Production and Subsequent Regulation of PS Ectropion, OPN, KIM-1, and ERK Expression. Ren. Fail. 2021, 43, 465–476. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Shi, W.; Su, Y.; Fu, X.; Yang, Y.; Lu, J.; Yue, Z. Calcium Oxalate Induces Renal Injury through Calcium-Sensing Receptor. Oxid. Med. Cell. Longev. 2016, 2016, 5203801. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Renigunta, V.; Himmerkus, N.; Zhang, J.; Renigunta, A.; Bleich, M.; Hou, J. Claudin-14 Regulates Renal Ca++ Transport in Response to CaSR Signalling via a Novel microRNA Pathway. EMBO J. 2012, 31, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Himmerkus, N.; Plain, A.; Bleich, M.; Hou, J. Epigenetic Regulation of microRNAs Controlling CLDN14 Expression as a Mechanism for Renal Calcium Handling. J. Am. Soc. Nephrol. 2015, 26, 663–676. [Google Scholar] [CrossRef]
- Gong, Y.; Hou, J. Claudin-14 Underlies Ca++-Sensing Receptor-Mediated Ca++ Metabolism via NFAT-microRNA-Based Mechanisms. J. Am. Soc. Nephrol. 2014, 25, 745–760. [Google Scholar] [CrossRef]
- Hou, J. Lecture: New Light on the Role of Claudins in the Kidney. Organogenesis 2012, 8, 1–9. [Google Scholar] [CrossRef]
- Hou, J. Claudins and Mineral Metabolism. Curr. Opin. Nephrol. Hypertens. 2016, 25, 308–313. [Google Scholar] [CrossRef]
- Zeng, G.; Mai, Z.; Xia, S.; Wang, Z.; Zhang, K.; Wang, L.; Long, Y.; Ma, J.; Li, Y.; Wan, S.P.; et al. Prevalence of Kidney Stones in China: An Ultrasonography Based Cross-Sectional Study. BJU Int. 2017, 120, 109–116. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Lan, Y.; Li, X.; Luo, L.; Duan, X.; Lei, M.; Liu, G.; Yang, Z.; Mai, X.; et al. Dietary Vinegar Prevents Kidney Stone Recurrence via Epigenetic Regulations. EBioMedicine 2019, 45, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A. Aspects of Calcium Oxalate Crystallization: Theory, in Vitro Studies, and in Vivo Implementation. J. Am. Soc. Nephrol. 1999, 10 (Suppl. S14), S351–S354. [Google Scholar]
- Pak, C.Y.C.; Adams-Huet, B.; Poindexter, J.R.; Pearle, M.S.; Peterson, R.D.; Moe, O.W. Relative Effect of Urinary Calcium and Oxalate on Saturation of Calcium Oxalate Rapid Communication. Kidney Int. 2004, 66, 2032–2037. [Google Scholar] [CrossRef]
- Siener, R.; Ebert, D.; Nicolay, C.; Hesse, A. Dietary Risk Factors for Hyperoxaluria in Calcium Oxalate Stone Formers. Kidney Int. 2003, 63, 1037–1043. [Google Scholar] [CrossRef]
- Knight, J.; Jiang, J.; Assimos, D.G.; Holmes, R.P. Hydroxyproline Ingestion and Urinary Oxalate and Glycolate Excretion. Kidney Int. 2006, 70, 1929–1934. [Google Scholar] [CrossRef]
- M’Dimegh, S.; Aquaviva-Bourdain, C.; Omezzine, A.; M’Barek, I.; Souche, G.; Zellama, D.; Abidi, K.; Achour, A.; Gargah, T.; Abroug, S.; et al. A Novel Mutation in the AGXT Gene Causing Primary Hyperoxaluria Type I: Genotype-Phenotype Correlation. J. Genet. 2016, 95, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B. Evidence of True Genotype-Phenotype Correlation in Primary Hyperoxaluria Type 1. Kidney Int. 2010, 77, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Zhao, Y.; Li, Q.; Yu, X.; Yang, Y.; Shi, S.; Ding, Z.; Miao, Y.; Zou, Z.; Wang, X.; et al. Human MiR-4660 Regulates the Expression of Alanine-Glyoxylate Aminotransferase and May Be a Biomarker for Idiopathic Oxalosis. Clin. Exp. Nephrol. 2019, 23, 890–897. [Google Scholar] [CrossRef]
- Lan, Y.; Zhu, W.; Duan, X.; Deng, T.; Li, S.; Liu, Y.; Yang, Z.; Wen, Y.; Luo, L.; Zhao, S.; et al. Glycine Suppresses Kidney Calcium Oxalate Crystal Depositions via Regulating Urinary Excretions of Oxalate and Citrate. J. Cell. Physiol. 2021, 236, 6824–6835. [Google Scholar] [CrossRef]
- Xi, J.; Jing, J.; Zhang, Y.; Liang, C.; Hao, Z.; Zhang, L.; Chen, Y. SIRT3 Inhibited the Formation of Calcium Oxalate-induced Kidney Stones through Regulating NRF2/HO-1 Signaling Pathway. J. Cell. Biochem. 2019, 120, 8259–8271. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.L.; Liu, Y.L.; Tao, Z.W.; Wang, X. The role of cell-crystal reaction mediated inflammation in the formation of intrarenal calcium oxalate crystals. Zhonghua Wai Ke Za Zhi Chin. J. Surg. 2018, 56, 733–736. [Google Scholar]
- Ming, S.; Tian, J.; Ma, K.; Pei, C.; Li, L.; Wang, Z.; Fang, Z.; Liu, M.; Dong, H.; Li, W.; et al. Oxalate-Induced Apoptosis through ERS-ROS-NF-κB Signalling Pathway in Renal Tubular Epithelial Cell. Mol. Med. 2022, 28, 88. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive Oxygen Species, Inflammation and Calcium Oxalate Nephrolithiasis. Transl. Androl. Urol. 2014, 3, 256–276. [Google Scholar] [PubMed]
- Hu, Y.-Y.; Dong, W.-D.; Xu, Y.-F.; Yao, X.-D.; Peng, B.; Liu, M.; Zheng, J.-H. Elevated Levels of miR-155 in Blood and Urine from Patients with Nephrolithiasis. BioMed Res. Int. 2014, 2014, 295651. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.F.; Kapffer, S.; Paust, H.-J.; Schmidt, T.; Bennstein, S.B.; Peters, A.; Stege, G.; Brix, S.R.; Meyer-Schwesinger, C.; Müller, R.-U.; et al. MicroRNA-155 Drives TH17 Immune Response and Tissue Injury in Experimental Crescentic GN. J. Am. Soc. Nephrol. 2013, 24, 1955–1965. [Google Scholar] [CrossRef]
- Yin, Q.; Zhao, Y.-J.; Ni, W.-J.; Tang, T.-T.; Wang, Y.; Cao, J.-Y.; Yin, D.; Wen, Y.; Li, Z.-L.; Zhang, Y.-L.; et al. MiR-155 Deficiency Protects Renal Tubular Epithelial Cells from Telomeric and Genomic DNA Damage in Cisplatin-Induced Acute Kidney Injury. Theranostics 2022, 12, 4753–4766. [Google Scholar] [CrossRef]
- Assimos, D.G. Re: miR-155-5p Promotes Oxalate- and Calcium-Induced Kidney Oxidative Stress Injury by Suppressing MGP Expression. J. Urol. 2020, 204, 381. [Google Scholar] [CrossRef]
- Su, B.; Han, H.; Ji, C.; Hu, W.; Yao, J.; Yang, J.; Fan, Y.; Li, J. MiR-21 Promotes Calcium Oxalate-Induced Renal Tubular Cell Injury by Targeting PPARA. Am. J. Physiol. Ren. Physiol. 2020, 319, F202–F214. [Google Scholar] [CrossRef]
- Ye, T.; Yang, X.; Liu, H.; Lv, P.; Lu, H.; Jiang, K.; Peng, E.; Ye, Z.; Chen, Z.; Tang, K. Theaflavin Protects against Oxalate Calcium-Induced Kidney Oxidative Stress Injury via Upregulation of SIRT1. Int. J. Biol. Sci. 2021, 17, 1050–1060. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, J.; Chen, Z. MicroRNA-204 Attenuates Oxidative Stress Damage of Renal Tubular Epithelial Cells in Calcium Oxalate Kidney-Stone Formation via MUC4-Mediated ERK Signaling Pathway. Urolithiasis 2022, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pino, V.; Ramsauer, V.P.; Salas, P.; Carothers Carraway, C.A.; Carraway, K.L. Membrane Mucin Muc4 Induces Density-Dependent Changes in ERK Activation in Mammary Epithelial and Tumor Cells: Role in Reversal of Contact Inhibition. J. Biol. Chem. 2006, 281, 29411–29420. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.-G.; Wang, Z.-H.; Xu, H.-T. Mechanism of miRNA-141-3p in Calcium Oxalate-Induced Renal Tubular Epithelial Cell Injury via NLRP3-Mediated Pyroptosis. Kidney Blood Press. Res. 2022, 47, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, J.; Chen, Z.; Wei, L.; Chen, J.; Xie, Z. Polyunsaturated Fatty Acids Ameliorate Renal Stone-Induced Renal Tubular Damage via miR-93-5p/Pknox1 Axis. Nutrition 2023, 105, 111863. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, A.; Asselman, M.; Persy, V.P.; Schepers, M.S.J.; Helbert, M.F.; Verkoelen, C.F.; De Broe, M.E. Crystal Retention Capacity of Cells in the Human Nephron: Involvement of CD44 and Its Ligands Hyaluronic Acid and Osteopontin in the Transition of a Crystal Binding- into a Nonadherent Epithelium. J. Am. Soc. Nephrol. 2003, 14, 107–115. [Google Scholar] [CrossRef]
- Asselman, M.; Verhulst, A.; De Broe, M.E.; Verkoelen, C.F. Calcium Oxalate Crystal Adherence to Hyaluronan-, Osteopontin-, and CD44-Expressing Injured/Regenerating Tubular Epithelial Cells in Rat Kidneys. J. Am. Soc. Nephrol. 2003, 14, 3155–3166. [Google Scholar] [CrossRef]
- Wang, B.; He, G.; Xu, G.; Wen, J.; Yu, X. miRNA-34a Inhibits Cell Adhesion by Targeting CD44 in Human Renal Epithelial Cells: Implications for Renal Stone Disease. Urolithiasis 2020, 48, 109–116. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Y.; Liu, G.; Jiang, Y. miR-103a-3p Silencing Ameliorates Calcium Oxalate Deposition in Rat Kidney by Activating the UMOD/TRPV5 Axis. Dis. Mark. 2022, 2022, 2602717. [Google Scholar] [CrossRef]
- Fan, L.; Li, H.; Huo, W. Inhibitory Role of microRNA-484 in Kidney Stone Formation by Repressing Calcium Oxalate Crystallization via a VDR/FoxO1 Regulator Axis. Urolithiasis 2022, 50, 665–678. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, P.; Sun, X.; Tang, K.; Liu, H.; Han, S.; Ye, T.; Liu, X.; Yang, X.; Zeng, J.; et al. Pioglitazone Decreased Renal Calcium Oxalate Crystal Formation by Suppressing M1 Macrophage Polarization via the PPAR-γ-miR-23 Axis. Am. J. Physiol. Ren. Physiol. 2019, 317, F137–F151. [Google Scholar] [CrossRef]
- Lu, H.; Sun, X.; Jia, M.; Sun, F.; Zhu, J.; Chen, X.; Chen, K.; Jiang, K. Rosiglitazone Suppresses Renal Crystal Deposition by Ameliorating Tubular Injury Resulted from Oxidative Stress and Inflammatory Response via Promoting the Nrf2/HO-1 Pathway and Shifting Macrophage Polarization. Oxid. Med. Cell. Longev. 2021, 2021, 5527137. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, Z.; Chou, F.; Zuo, L.; Liu, T.; Yeh, S.; Bushinsky, D.; Zeng, G.; Chang, C. Loss of the Androgen Receptor Suppresses Intrarenal Calcium Oxalate Crystals Deposition via Altering Macrophage Recruitment/M2 Polarization with Change of the miR-185-5p/CSF-1 Signals. Cell Death Dis. 2019, 10, 275. [Google Scholar] [CrossRef]
- Elshal, A.M.; Shamshoun, H.; Awadalla, A.; Elbaz, R.; Ahmed, A.E.; El-Khawaga, O.Y.; Shokeir, A.A. Hormonal and Molecular Characterization of Calcium Oxalate Stone Formers Predicting Occurrence and Recurrence. Urolithiasis 2023, 51, 76. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Li, C.; Lu, Y.; Hu, H.; Qin, B.; Xun, Y.; Zhu, Y.; Wu, Y.; Zhang, J.; et al. Inhibiting Inflammation and Modulating Oxidative Stress in Oxalate-Induced Nephrolithiasis with the Nrf2 Activator Dimethyl Fumarate. Free Radic. Biol. Med. 2019, 134, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.-J.; Huang, J.-H.; Lv, B.-D.; Huang, X.-J.; Hu, Q.; Fu, J.; Huang, W.-J.; Tao, T.-T. Protective Effects of Total Flavonoids from Lysimachia Christinae on Calcium Oxalate-Induced Oxidative Stress in a Renal Cell Line and Renal Tissue. Evid. Based Complement. Altern. Med. eCAM 2021, 2021, 6667902. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, Y.; Yin, S.; Cui, J.; Zhang, Y.; Wang, X.; Zhang, F.; Li, H.; Tang, Y.; Wang, J. Circadian Clock Gene BMAL1 Reduces Urinary Calcium Oxalate Stones Formation by Regulating NRF2/HO-1 Pathway. Life Sci. 2021, 265, 118853. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Tang, K.; Ye, T.; Duan, C.; Lv, P.; Yan, L.; Wu, X.; Chen, Z.; Liu, J.; et al. Sulforaphane Elicts Dual Therapeutic Effects on Renal Inflammatory Injury and Crystal Deposition in Calcium Oxalate Nephrocalcinosis. Theranostics 2020, 10, 7319–7334. [Google Scholar] [CrossRef] [PubMed]
- Assimos, D.G. Re: Exosomes from miR-20b-3p-Overexpressing Stromal Cells Ameliorate Calcium Oxalate Deposition in Rat Kidney. J. Urol. 2020, 203, 246. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Dai, X.-M.; Li, S.; Qi, G.-L.; Cao, G.-X.; Zhong, Y.; Yin, P.; Yang, X.-S. MiR-30c Regulates Cisplatin-Induced Apoptosis of Renal Tubular Epithelial Cells by Targeting Bnip3L and Hspa5. Cell Death Dis. 2017, 8, e2987. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Han, S.; Chen, H.; Chen, C.; Ji, L.; Gao, B. Overexpression of miR-30c-5p Reduces Cellular Cytotoxicity and Inhibits the Formation of Kidney Stones through ATG5. Int. J. Mol. Med. 2020, 45, 375–384. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Valentin, M.D.; Canalle, R.; Queiroz, R.D.P.; Tone, L.G. Frequency of Polymorphisms and Protein Expression of Cyclin-Dependent Kinase Inhibitor 1A (CDKN1A) in Central Nervous System Tumors. Sao Paulo Med. J. 2009, 127, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Uszczynska-Ratajczak, B.; Lagarde, J.; Frankish, A.; Guigó, R.; Johnson, R. Towards a Complete Map of the Human Long Non-Coding RNA Transcriptome. Nat. Rev. Genet. 2018, 19, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Dong, G.; Liang, X.; Dong, Z. Epigenetic Regulation in AKI and Kidney Repair: Mechanisms and Therapeutic Implications. Nat. Rev. Nephrol. 2019, 15, 220–239. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, Y.; Gong, B.; Xu, H.; Hao, Z.; Liang, C. Long Noncoding RNA LINC00339 Promotes Renal Tubular Epithelial Pyroptosis by Regulating the miR-22-3p/NLRP3 Axis in Calcium Oxalate-Induced Kidney Stone. J. Cell. Biochem. 2019, 120, 10452–10462. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Yang, X.; Liu, J.; Jiang, K.; Lu, H.; Xia, D.; Peng, E.; Chen, Z.; Sun, F.; et al. H19 Promote Calcium Oxalate Nephrocalcinosis-Induced Renal Tubular Epithelial Cell Injury via a ceRNA Pathway. EBioMedicine 2019, 50, 366–378. [Google Scholar] [CrossRef]
- Li, Y.; Yan, G.; Zhang, J.; Chen, W.; Ding, T.; Yin, Y.; Li, M.; Zhu, Y.; Sun, S.; Yuan, J.H.; et al. LncRNA HOXA11-AS Regulates Calcium Oxalate Crystal-Induced Renal Inflammation via miR-124-3p/MCP-1. J. Cell. Mol. Med. 2020, 24, 238–249. [Google Scholar] [CrossRef]
- Lv, P.; Liu, H.; Ye, T.; Yang, X.; Duan, C.; Yao, X.; Li, B.; Tang, K.; Chen, Z.; Liu, J.; et al. XIST Inhibition Attenuates Calcium Oxalate Nephrocalcinosis-Induced Renal Inflammation and Oxidative Injury via the miR-223/NLRP3 Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 1676152. [Google Scholar] [CrossRef]
- Li, Y.; Ding, T.; Hu, H.; Zhao, T.; Zhu, C.; Ding, J.; Yuan, J.; Guo, Z. LncRNA-ATB Participates in the Regulation of Calcium Oxalate Crystal-Induced Renal Injury by Sponging the miR-200 Family. Mol. Med. 2021, 27, 143. [Google Scholar] [CrossRef]
- Xi, J.; Chen, Y.; Jing, J.; Qi, W.; Zhang, Y. LncRNA LINC01197 Inhibited the Formation of Calcium Oxalate-Induced Kidney Stones by Regulating miR-516b-5p/SIRT3/FOXO1 Signaling Pathway. Cell Tissue Res. 2023, 392, 553–563. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA Sponges: Evidence and Controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, J.; Hu, H.; Chen, W.; Liu, M.; Zhang, J.; Sun, S.; Guo, Z. Long Non-Coding RNA CHCHD4P4 Promotes Epithelial-Mesenchymal Transition and Inhibits Cell Proliferation in Calcium Oxalate-Induced Kidney Damage. Braz. J. Med. Biol. Res. 2017, 51, e6536. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, F.; Jiang, Y.; Ruan, S.; Liu, M.; Zhang, Y.; Li, Y.; Chen, J.; Cui, Y.; Chen, Z.; et al. OLMALINC/OCT4/BMP2 Axis Enhances Osteogenic-like Phenotype of Renal Interstitial Fibroblasts to Participate in Randall’s Plaque Formation. Mol. Med. 2022, 28, 162. [Google Scholar] [CrossRef]
- Tawfick, A.; Matboli, M.; Shamloul, S.; Agwa, S.H.A.; Saad, M.; Shaker, H.; Selim, M.M.Y.; Salim, M.S.; Radwan, A.; Shorbagy, A.A.; et al. Predictive Urinary RNA Biomarkers of Kidney Injury after Extracorporeal Shock Wave Lithotripsy. World J. Urol. 2022, 40, 1561–1567. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Goyal, B.; Yadav, S.R.M.; Awasthee, N.; Gupta, S.; Kunnumakkara, A.B.; Gupta, S.C. Diagnostic, Prognostic, and Therapeutic Significance of Long Non-Coding RNA MALAT1 in Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188502. [Google Scholar] [CrossRef]
- Sharma, U.; Barwal, T.S.; Malhotra, A.; Pant, N.; Vivek; Dey, D.; Gautam, A.; Tuli, H.S.; Vasquez, K.M.; Jain, A. Long Non-Coding RNA TINCR as Potential Biomarker and Therapeutic Target for Cancer. Life Sci. 2020, 257, 118035. [Google Scholar] [CrossRef]
- Zhu, M.; Li, X.; Zhu, S.; Li, P.; Min, L.; Zhang, S. Long Non-Coding RNA BLACAT1, a Novel Promising Biomarker and Regulator of Human Cancers. Biomed. Pharm. 2020, 132, 110808. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, H.; Tang, J.; Wang, R. Long Non-Coding RNA FAM230B Is a Novel Prognostic and Diagnostic Biomarker for Lung Adenocarcinoma. Bioengineered 2022, 13, 7919–7925. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Yuan, C.; Zhao, C. Long Intragenic Non-Coding RNA P53-Induced Transcript (LINC-PINT) as a Novel Prognosis Indicator and Therapeutic Target in Cancer. Biomed. Pharm. 2021, 143, 112127. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, B.; Ge, H.; Xu, Y.; Li, G.; Wu, J. Long Non-Coding RNA CRNDE as a Potential Prognostic Biomarker in Solid Tumors: A Meta-Analysis. Clin. Chim. Acta 2018, 481, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Koutalianos, D.; Koutsoulidou, A.; Mytidou, C.; Kakouri, A.C.; Oulas, A.; Tomazou, M.; Kyriakides, T.C.; Prokopi, M.; Kapnisis, K.; Nikolenko, N.; et al. miR-223-3p and miR-24-3p as Novel Serum-Based Biomarkers for Myotonic Dystrophy Type 1. Mol. Ther. Methods Clin. Dev. 2021, 23, 169–183. [Google Scholar] [CrossRef]
- Singh, S.; de Ronde, M.W.J.; Kok, M.G.M.; Beijk, M.A.; De Winter, R.J.; van der Wal, A.C.; Sondermeijer, B.M.; Meijers, J.C.M.; Creemers, E.E.; Pinto-Sietsma, S.-J. MiR-223-3p and miR-122-5p as Circulating Biomarkers for Plaque Instability. Open Heart 2020, 7, e001223. [Google Scholar] [CrossRef] [PubMed]
- Mellis, D.; Caporali, A. MicroRNA-Based Therapeutics in Cardiovascular Disease: Screening and Delivery to the Target. Biochem. Soc. Trans. 2018, 46, 11–21. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.D.; Holmes, R.P.; Erbe, D.; Liebow, A.; Fargue, S.; Knight, J. Reduction in Urinary Oxalate Excretion in Mouse Models of Primary Hyperoxaluria by RNA Interference Inhibition of Liver Lactate Dehydrogenase Activity. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2203–2209. [Google Scholar] [CrossRef]
- Cochat, P.; Rumsby, G. Primary Hyperoxaluria. N. Engl. J. Med. 2013, 369, 649–658. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Approves First Drug to Treat Rare Metabolic Disorder. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-rare-metabolic-disorder (accessed on 25 October 2023).
- Liebow, A.; Li, X.; Racie, T.; Hettinger, J.; Bettencourt, B.R.; Najafian, N.; Haslett, P.; Fitzgerald, K.; Holmes, R.P.; Erbe, D.; et al. An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria. JASN 2017, 28, 494–503. [Google Scholar] [CrossRef]
- Debacker, A.J.; Voutila, J.; Catley, M.; Blakey, D.; Habib, N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 2020, 28, 1759–1771. [Google Scholar] [CrossRef]
- Garrelfs, S.F.; Frishberg, Y.; Hulton, S.A.; Koren, M.J.; O’Riordan, W.D.; Cochat, P.; Deschênes, G.; Shasha-Lavsky, H.; Saland, J.M.; Van’t Hoff, W.G.; et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021, 384, 1216–1226. [Google Scholar] [CrossRef]
- Palmer, T.C.; Hunter, R.W. Using RNA-Based Therapies to Target the Kidney in Cardiovascular Disease. Front. Cardiovasc. Med. 2023, 10, 1250073. [Google Scholar] [CrossRef] [PubMed]
- Boada, C.; Sukhovershin, R.; Pettigrew, R.; Cooke, J.P. RNA Therapeutics for Cardiovascular Disease. Curr. Opin. Cardiol. 2021, 36, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Overcoming Cellular Barriers for RNA Therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Mi, J.; Bai, J.; He, Q.; Li, X.; Wang, Z. Non-Coding RNAs in Kidney Stones. Biomolecules 2024, 14, 213. https://doi.org/10.3390/biom14020213
Wang G, Mi J, Bai J, He Q, Li X, Wang Z. Non-Coding RNAs in Kidney Stones. Biomolecules. 2024; 14(2):213. https://doi.org/10.3390/biom14020213
Chicago/Turabian StyleWang, Guilin, Jun Mi, Jiangtao Bai, Qiqi He, Xiaoran Li, and Zhiping Wang. 2024. "Non-Coding RNAs in Kidney Stones" Biomolecules 14, no. 2: 213. https://doi.org/10.3390/biom14020213






