Neutrophil Activity and Extracellular Matrix Degradation: Drivers of Lung Tissue Destruction in Fatal COVID-19 Cases and Implications for Long COVID
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection of the Lung Autopsy Samples, Plasma, and Ethical Committee Approvals
2.3. Histopathology, Immunohistochemistry, Morphometry, Western Blotting, Colorimetry, and ELISA
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Tissue Donors
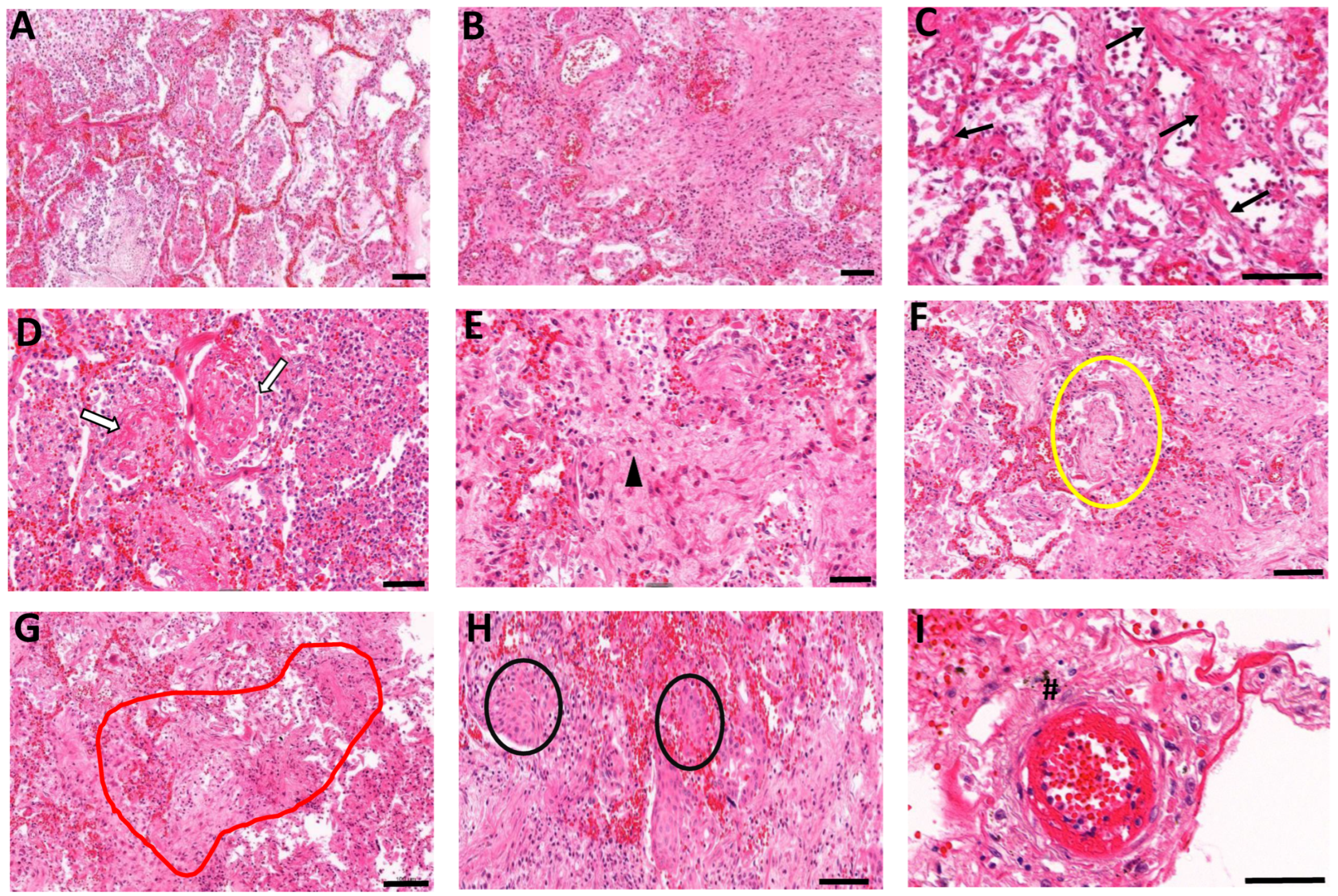
3.2. Histopathological Manifestations of Fibrotic Abnormalities in COVID-19
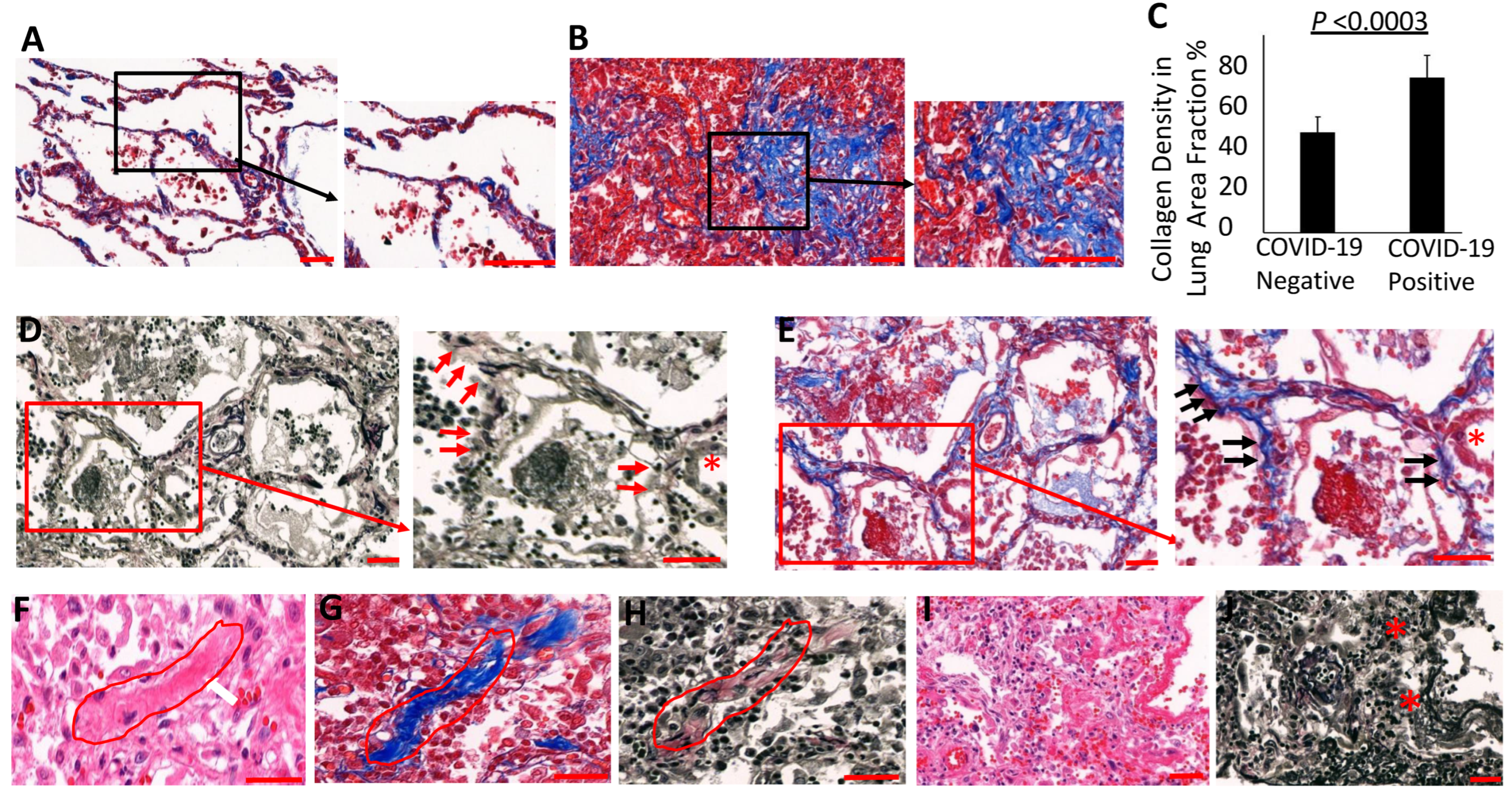
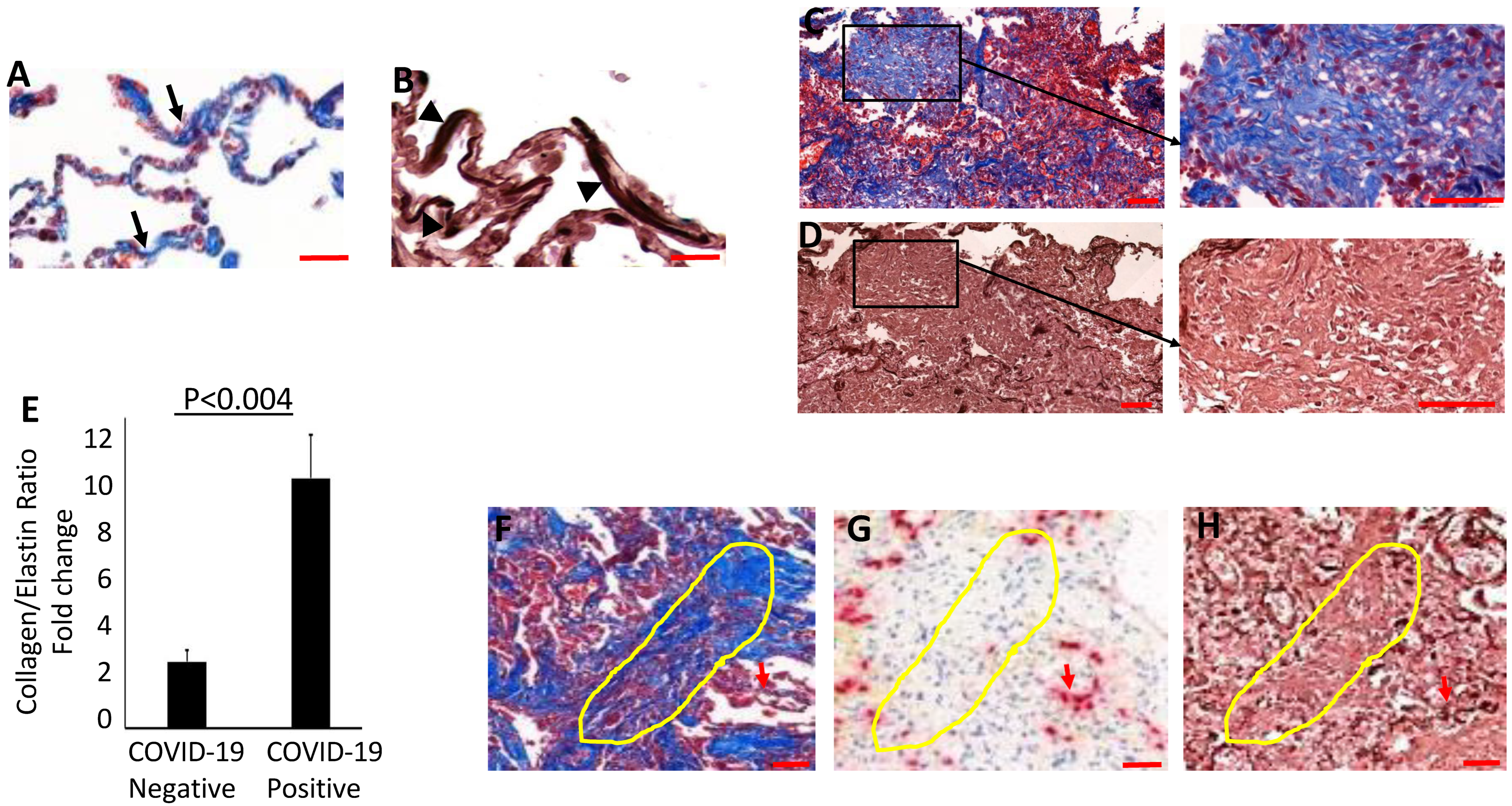
3.3. Evidence for Elastolytic Activity in COVID-19 Patients
3.4. Collagen Accumulates and Replaces Elastin in the Lung Parenchyma of COVID-19 Lungs
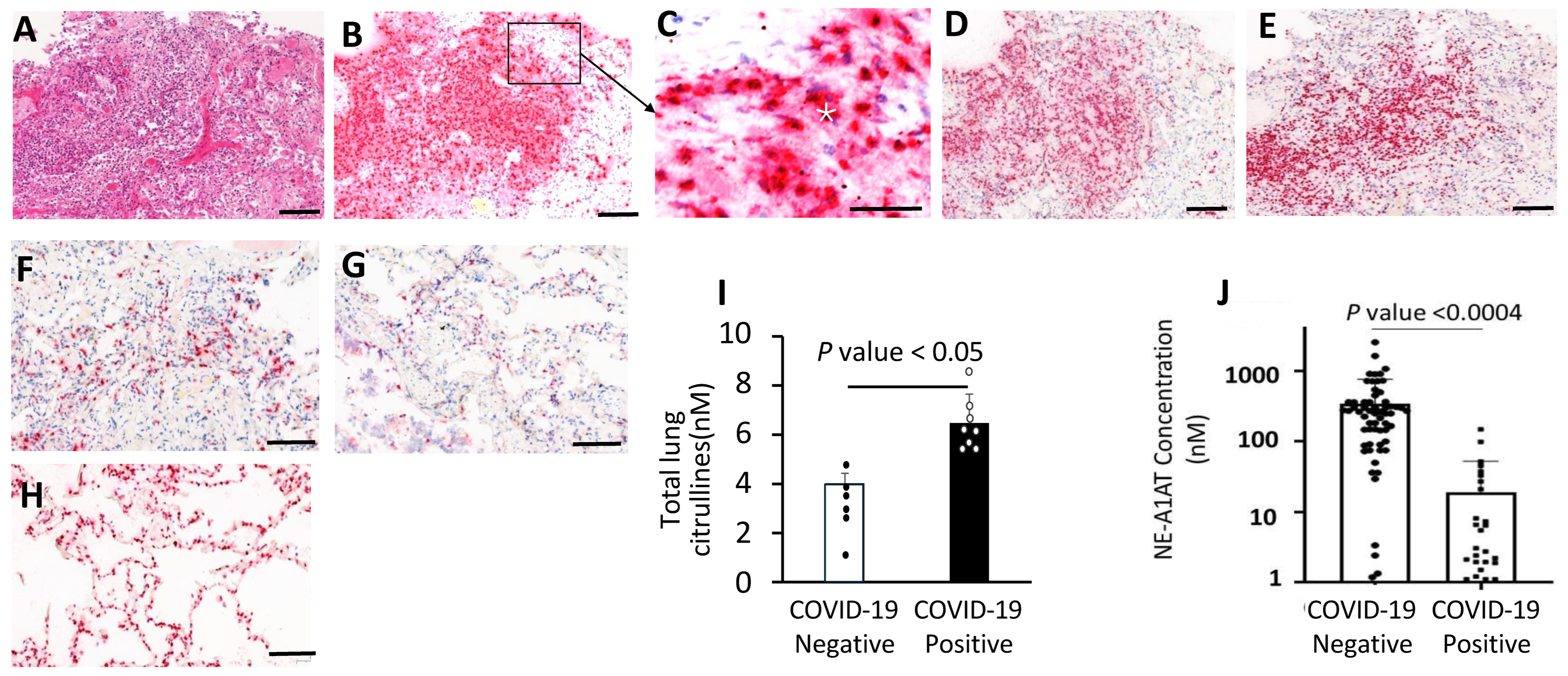
3.5. Neutrophil Aggregates and Active NETosis in the COVID-19 Lungs
3.6. Increased NE-A1AT Complexes in Plasma from COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ravaglia, C.; Doglioni, C.; Chilosi, M.; Piciucchi, S.; Dubini, A.; Rossi, G.; Pedica, F.; Puglisi, S.; Donati, L.; Tomassetti, S.; et al. Clinical, radiological and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection. Eur. Respir. J. 2022, 60, 2102411. [Google Scholar] [CrossRef] [PubMed]
- Mauad, T.; Duarte-Neto, A.N.; da Silva, L.F.F.; de Oliveira, E.P.; de Brito, J.M.; Nascimento, E.C.T.D.; Monteiro, R.A.d.A.; Ferreira, J.C.; de Carvalho, C.R.R.; Saldiva, P.H.D.N.; et al. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir. Res. 2021, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Merdji, H.; Mayeur, S.; Schenck, M.; Oulehri, W.; Clere-Jehl, R.; Cunat, S.; Herbrecht, J.E.; Janssen-Langenstein, R.; Nicolae, A.; Helms, J.; et al. Histopathological features in fatal COVID-19 acute respiratory distress syndrome. Med. Intensiv. 2021, 45, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2020, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, S.; Bessis, S.; Annane, D.; Lorin de la Grandmaison, G.; Cramer-Bordé, E.; Prideaux, B.; Eugenin, E.A.; Bomsel, M. COVID-19 Lung Pathogenesis in SARS-CoV-2 Autopsy Cases. Front. Immunol. 2021, 12, 735922. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.-H.; Luo, T.; Shi, Y.; He, Z.-C.; Tang, R.; Zhang, P.-P.; Cai, J.; Zhou, X.-D.; Jiang, D.-P.; Fei, X.-C.; et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021, 31, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Wang, S.; Li, X.; Zhou, J.; Huang, B.; Luo, D.; Cao, Q.; Chen, Y.; Chen, S.; et al. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology 2021, 78, 542–555. [Google Scholar] [CrossRef]
- Wells, A.U.; Devaraj, A.; Desai, S.R. Interstitial Lung Disease after COVID-19 Infection: A Catalog of Uncertainties. Radiology 2021, 299, E216–E218. [Google Scholar] [CrossRef]
- Berezowska, S.; Schmid, A.; Losmanová, T.; Trippel, M.; Blank, A.; Banz, Y.; Jakob, S.M.; Langer, R. Frequency and Significance of Path-ologic Pulmonary Findings in Postmortem Examinations-A Single Center Experience before COVID-19. Diagnostics 2021, 11, 894. [Google Scholar] [CrossRef]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; Della Casa, G.; Sverzellati, N.; Maher, T.M. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020, 8, 750–752. [Google Scholar] [CrossRef]
- Caruso, D.; Guido, G.; Zerunian, M.; Polidori, T.; Lucertini, E.; Pucciarelli, F.; Polici, M.; Rucci, C.; Bracci, B.; Nicolai, M.; et al. Post-Acute Sequelae of COVID-19 Pneumonia: Six-month Chest CT Follow-up. Radiology 2021, 301, E396–E405. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Tang, X.X. Virus infection induced pulmonary fibrosis. J. Transl. Med. 2021, 19, 496. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, M.; Bi, J.; Wang, X.; Deng, G.; He, G.; Luan, Z.; Lv, N.; Xu, T.; Zhao, L. Pulmonary fibrosis induced by H5N1 viral infection in mice. Respir. Res. 2009, 10, 107. [Google Scholar] [CrossRef]
- Wu, X.; Dong, D.; Ma, D. Thin-Section Computed Tomography Manifestations During Convalescence and Long-Term Follow-Up of Patients with Severe Acute Respiratory Syndrome (SARS). J. Pharmacol. Exp. Ther. 2016, 22, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Balkhy, H.H.; Hayden, F.G.; Bouchama, A.; Luke, T.; Baillie, J.K.; Al-Omari, A.; Hajeer, A.H.; Senga, M.; Denison, M.R.; et al. Middle East Respiratory Syndrome. N. Engl. J. Med. 2017, 376, 584–594. [Google Scholar] [CrossRef]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Aspects Med. 2019, 65, 56–69. [Google Scholar] [CrossRef]
- Sinha, S.; Castillo, V.; Espinoza, C.R.; Tindle, C.; Fonseca, A.G.; Dan, J.M.; Katkar, G.D.; Das, S.; Sahoo, D.; Ghosh, P. COVID-19 lung disease shares driver AT2 cytopathic features with Idiopathic pulmonary fibrosis. EBioMedicine 2022, 82, 104185. [Google Scholar] [CrossRef]
- Karampoor, S.; Hesamizadeh, K.; Maleki, F.; Farahmand, M.; Zahednasab, H.; Mirzaei, R.; Banoun, H.; Zamani, F.; Hajibaba, M.; Tabibzadeh, A.; et al. A possible pathogenic correlation between neutrophil elastase (NE) enzyme and inflammation in the pathogenesis of coronavirus disease 2019 (COVID-19). Int. Immunopharmacol. 2021, 100, 108137. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Lofaro, F.D.; Cossarizza, A.; Quaglino, D. The “Elastic Perspective” of SARS-CoV-2 Infection and the Role of Intrinsic and Extrinsic Factors. Int. J. Mol. Sci. 2022, 23, 1559. [Google Scholar] [CrossRef]
- Mecham, R.P. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef]
- Davidson, J. Biochemistry and turnover of lung interstitium. Eur. Respir. J. 1990, 3, 1048–1068. [Google Scholar] [CrossRef]
- Mäki, J.M.; Sormunen, R.; Lippo, S.; Kaarteenaho-Wiik, R.; Soininen, R.; Myllyharju, J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am. J. Pathol. 2005, 167, 927–936. [Google Scholar] [CrossRef]
- Kozel, B.A.; Mecham, R.P.; Rosenbloom, J. Elastin. In Biology of Extracellular Matrix; Mecham, R.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 267–301. [Google Scholar]
- Deslee, G.; Woods, J.C.; Moore, C.M.; Liu, L.; Conradi, S.H.; Milne, M.; Gierada, D.S.; Pierce, J.; Patterson, A.; Lewit, R.A.; et al. Elastin expression in very severe human COPD. Eur. Respir. J. 2009, 34, 324–331. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Endicott, S.K.; AProvince, M.; APierce, J.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, S.; Turino, G.; Cantor, J. The pattern of elastic fiber breakdown in bleomycin-induced pulmonary fibrosis may reflect microarchitectural changes. Lung 2016, 195, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.T.; Plosa, E.J.; Sucre, J.M.; van der Meer, R.; Dave, S.; Gutor, S.S.; Nichols, D.S.; Gulleman, P.M.; Jetter, C.S.; Han, W.; et al. Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD. J. Clin. Investig. 2021, 131, e139481. [Google Scholar] [CrossRef] [PubMed]
- Starcher, B.; Peterson, B. The kinetics of elastolysis: Elastin catabolism during experimentally induced fibrosis. Exp. Lung Res. 1999, 25, 407–424. [Google Scholar] [PubMed]
- Ackermann, M.; Anders, H.-J.; Bilyy, R.; Bowlin, G.L.; Daniel, C.; De Lorenzo, R.; Egeblad, M.; Henneck, T.; Hidalgo, A.; Hoffmann, M.; et al. Patients with COVID-19: In the dark-NETs of neutrophils. Cell Death Differ. 2021, 28, 3125–3139. [Google Scholar] [CrossRef]
- Ouwendijk, W.J.D.; Raadsen, M.P.; van Kampen, J.J.A.; Verdijk, R.M.; von der Thusen, J.H.; Guo, L.; Hoek, R.A.S.; Akker, J.P.C.V.D.; Endeman, H.; Langerak, T.; et al. High Levels of Neutrophil Extracellular Traps Persist in the Lower Respiratory Tract of Critically Ill Patients with Coronavirus Disease 2019. J. Infect. Dis. 2021, 223, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schughart, K.; Pelaia, T.M.; Chew, T.; Kim, K.; Karvunidis, T.; Knippenberg, B.; Teoh, S.; Phu, A.L.; Short, K.R.; et al. Blood transcriptome responses in patients correlate with severity of COVID-19 disease. Front. Immunol. 2023, 13, 1043219. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Bonham, K.S.; Anam, F.A.; Walker, T.A.; Faliti, C.E.; Ishii, Y.; Kaminski, C.Y.; Ruunstrom, M.C.; Cooper, K.R.; Truong, A.D.; et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat. Commun. 2023, 14, 4201. [Google Scholar] [CrossRef]
- Cakir, S.N.; Brás, L.E.d.C. Injury-specific inflammation leads to organ-specific fibrosis. Am. J. Physiol. Circ. Physiol. 2020, 319, H610–H612. [Google Scholar] [CrossRef]
- de Brouwer, B.; Drent, M.; Ouweland, J.M.W.v.D.; Wijnen, P.A.; van Moorsel, C.H.M.; Bekers, O.; Grutters, J.C.; White, E.S.; Janssen, R. Increased circulating desmosine and age-dependent elastinolysis in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 45. [Google Scholar] [CrossRef]
- D’souza, R.F.; Masson, S.W.C.; Woodhead, J.S.T.; James, S.L.; MacRae, C.; Hedges, C.P.; Merry, T.L. α1-Antitrypsin A treatment attenuates neutrophil elastase accumulation and enhances insulin sensitivity in adipose tissue of mice fed a high-fat diet. Am. J. Physiol. Metab. 2021, 321, E560–E570. [Google Scholar] [CrossRef]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef]
- Bar, K.J.; Shaw, P.A.; Choi, G.H.; Aqui, N.; Fesnak, A.; Yang, J.B.; Soto-Calderon, H.; Grajales, L.; Starr, J.; Andronov, M.; et al. A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. J. Clin. Investig. 2021, 131, e155114. [Google Scholar] [CrossRef]
- Ochs, M.; Timm, S.; Elezkurtaj, S.; Horst, D.; Meinhardt, J.; Heppner, F.L.; Weber-Carstens, S.; Hocke, A.C.; Witzenrath, M. Collapse in-duration of alveoli is an ultrastructural finding in a COVID-19 patient. Eur. Respir. J. 2021, 57, 2004165. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; McEvoy, N.L.; Boland, F.; McElvaney, O.F.; Hogan, G.; Donnelly, K.; Friel, O.; Browne, E.; Fraughen, D.D.; Murphy, M.P.; et al. A randomized, double-blind, place-bo-controlled trial of intravenous alpha-1 antitrypsin for ARDS secondary to COVID-19. Med 2022, 3, 233–248.e6. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Seida, I.; Esirgün, S.N.; Bragazzi, N.L. The COVID-19 pandemic—How many times were we warned before? Eur. J. Intern. Med. 2022, 105, 8–14. [Google Scholar] [CrossRef]
- Voskarides, K. SARS-CoV-2: Tracing the origin, tracking the evolution. BMC Med. Genomics 2022, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.A. The Future of the COVID-19 Pandemic: How Good (or Bad) Can the SARS-CoV2 Spike Protein Get? Cells 2022, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A. Pathogenesis of pulmonary fibrosis. Hum. Pathol. 1986, 17, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H. Pathogenesis of interstitial fibrosis of the lung. Progr Respir. Res. 1975, 8, 34–44. [Google Scholar]
- Froese, A.R.; Shimbori, C.; Bellaye, P.S.; Inman, M.; Obex, S.; Fatima, S.; Jenkins, G.; Gauldie, J.; Ask, K.; Kolb, M. Stretch-induced Activation of Transforming Growth Factor-β1 in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 84–96. [Google Scholar] [CrossRef]
- D’Agnillo, F.; Walters, K.A.; Xiao, Y.; Sheng, Z.M.; Scherler, K.; Park, J.; Gygli, S.; Rosas, L.A.; Sadtler, K.; Kalish, H.; et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021, 13, eabj7790. [Google Scholar] [CrossRef]
- Nagel, D.; Clough, R.; Sime, P.J.; Kottamann, R.M. Elastin degradative products induce myofibroblast differentiation. Am. J. Resp. Crit. Care Med. 2019, 199, A5333. [Google Scholar]
- Neeli, I.; Moarefian, M.; Kuseladass, J.; Dwivedi, N.; Jones, C.; Radic, M. Neutrophil attachment via Mac-1 (αMβ2; CD11b/CD18; CR3) integrins induces PAD4 deimination of profilin and histone H3. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220247. [Google Scholar] [CrossRef]
- Shafqat, A.; Omer, M.H.; Albalkhi, I.; Razzak, G.A.; Abdulkader, H.; Rab, S.A.; Sabbah, B.N.; Alkattan, K.; Yaqinuddin, A. Neutrophil extracellular traps and long COVID. Front. Immunol. 2023, 14, 1254310. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Shang, Y.; Wang, J.; Zhang, X.; Su, D.; Zhao, S.; Wang, Q.; Liu, L.; Li, Y.; et al. Ratios of neutrophil-to-lymphocyte and platelet-to-lymphocyte predict all-cause mortality in inpatients with coronavirus disease 2019 (COVID-19): A retrospective cohort study in a single medical centre. Epidemiol. Infect. 2020, 148, e211. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2021, 51, 446–453. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-Y.; Chen, C.-Y.; Lin, Y.-L.; Lin, C.-M.; Tsai, W.-C.; Tsai, Y.-L.; Lin, G.-J.; Chen, Y.-G.; Wang, S.-Y.; Sun, R.-N.; et al. RNF128 regulates neutrophil infiltration and myeloperoxidase functions to prevent acute lung injury. Cell Death Dis. 2023, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Zweers, M.C.; van Vlijmen-Willems, I.M.; van Kuppevelt, T.H.; Mecham, R.P.; Steijlen, P.M.; Bristow, J.; Schalkwijk, J. Deficiency of tenascin-X causes abnormalities in dermal elastic fiber morphology. J. Invest. Dermatol. 2004, 122, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kottmann, R.M.; Sharp, J.; Owens, K.; Salzman, P.; Xiao, G.Q.; Phipps, R.P.; Sime, P.J.; Brown, E.B.; Perry, S.W. Second harmonic generation microscopy reveals altered collagen microstructure in usual interstitial pneumonia versus healthy lung. Respir. Res. 2015, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Hedberg, A.; Zheng, Y.Y.; Neeli, I.; Satoh, M.; Morel, L.; Rekvig, O.P.; Radic, M. B Cell Tolerance to Deiminated Histones in BALB/c, C57BL/6, and Autoimmune-Prone Mouse Strains. Front. Immunol. 2017, 8, 362. [Google Scholar] [CrossRef]
- Neumann, S.; Hennrich, N.; Gunzer, G.; Lang, H. Enzyme-linked immunoassay for human granulocyte elastase in complex with alpha 1-proteinase inhibitor. Adv. Exp. Med. Biol. 1984, 167, 379–390. [Google Scholar]
- Engelmaier, A.; Weber, A. Sensitive and specific measurement of alpha1-antitrypsin activity with an elastase complex formation immunosorbent assay (ECFISA). J. Pharm. Biomed. Anal. 2022, 209, 114476. [Google Scholar] [CrossRef]

| No. | Sex | Age | Upon Hospitalization | Days in the Hospital | Complications | Disease Duration | SARS-CoV-2 PCR Positive, at Day | SpO2 | ESR | Hb | WBC | Lymphocytes | Neutrophils | Platelets, ×103/uL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| For all—cause of death is Acute hemorrhagic tracheobronchitis, respirators distress syndrome (according to local classification). | upon hospitalization | last record | upon hospitalization | last record | g/L | Hct % | last record | upon hospitalization | upon hospitalization | last record | last record | upon hospitalization | upon hospitalization | last record | last record | upon hospitalization | last record | |||||||||
| with O2 | air | with O2 | air | ×109/L | ×109/L | % | ×109/L | % | ×109/L | % | ×109/L | % | ||||||||||||||
| 204 | M | 61 | bilateral pneumonia COVID-19 | 10 | other viral and bacterial pneumonia, acute respiratory syndrome, hypertensive heart disease, chronic lymphocytic leukemia, arterial hypertension, gastric ulcer | 16 | 6 | n/a | 90 | 80 | n/a | 55 | 46 | 99 | 29.6 | 25.2 | 18.3 | 72.5 | 26.6 | 61.2 | 4.6 | 18.3 | 12.5 | 24 | 210 | 280 |
| 205 | F | 64 | bilateral pneumonia COVID-19 | 21 | tongue carcinoma, hypertension, hypothyroidism | 27 | 6 | n/a | 96 | 97 | n/a | 72 | 49 | 96 | 23.4 | 4.7 | 0.2 | 14.8 | 0.4 | 8.7 | 1 | 80.8 | 4.2 | 87.4 | 202 | 361 |
| 208 | M | 59 | bilateral pneumonia COVID-19, DM | 5 | hypertension, diabetes mellitus, varicose disease | 8 | 2 | n/a | 86 | 85 | n/a | 45 | 42 | 135 | 39.1 | 6.5 | 0.76 | 13 | 0.5 | 8.1 | 4.63 | 79 | 58 | 89 | 155 | 155 |
| 210 | F | 80 | bilateral pneumonia COVID-19 | 19 | hypertension, kidney disease, COPD | 26 | 7 | 98 | n/a | 73 | 55 | 47 | 24 | 147 | 44.2 | 14.3 | 0.2 | n/a | 0.9 | 6.1 | 11.4 | 84.1 | 13 | 91 | 179 | 179 |
| 211 | F | 61 | bilateral pneumonia COVID-19 | 19 | hypertension, diabetes mellitus, COPD | 24 | 5 | 78 | 65 | 98 | n/a | 42 | 17 | 141 | 40.8 | 10.5 | 1.1 | 10.6 | 1 | 14.9 | 8.6 | 81.9 | 5.5 | 80.30 | 143 | 389 |
| 212 | M | 30 | pneumonia (unilateral, R) COVID-19 | 17 | pneumothorax (R) | 26 | 9 | 97 | 90 | 78 | 62 | 53 | 42 | 100 | 30.1 | 18.6 | 0.6 | 9 | 0.5 | 2.5 | 11.6 | 95 | 17.4 | 93.8 | 238 | 324 |
| 214 | M | 71 | pneumonia (unilateral, R) COVID-20 | 16 | bacterial pneumonia | 24 | 8 | 97 | 81 | 80 | n/a | 37 | 9 | 162 | 46 | 10.2 | 0.7 | 6.9 | 1.1 | 10.8 | 9.6 | 89.2 | 5.26 | 85.5 | 225 | 226 |
| 216 | F | 56 | bilateral pneumonia COVID-19 | 13 | hypertension, diabetes mellitus, obesity, multiple organ failure | 27 | 14 | 90 | n/a | 70 | n/a | 49 | 29 | 120 | 36.4 | 6.5 | 0.4 | 7.2 | 0.4 | 6.5 | 5 | 89.1 | 5.7 | 87.7 | 287 | 421 |
| mean | 60.3 | 15.0 | 22.3 | 7.1 | 92.0 | 84.7 | 82.6 | 58.5 | 50.0 | 32.3 | 125.0 | 36.2 | 12.1 | 2.8 | 19.1 | 3.9 | 14.9 | 7.1 | 77.2 | 15.2 | 79.8 | 204.9 | 291.9 | |||
| 809 | F | 81 | cecum cancer pT3N0M0G2 | 4 | emphysema, anemia, atherosclerosis, thromboemboly of lung artery | 0 | 98 | 98 | 28 | 78 | 4.6 | 4.6 | 32 | 3.5 | 58 | 242 | ||||||||||
| 825 | M | 85 | atherosclerotic aneurism of infrarenal aorta with rupture | 1 | hypertension, obesity, chronic bronchitis | 1 | 0 | 95 | 9 | 124 | 45 | 6.8 | 6.2 | 37 | 6.2 | 62 | 280 | |||||||||
| 835 | F | 66 | hypertensive microangiopathy of brain, kidney, and pancreas | 21 | fracture L2-L4 7 years ago | 0 | 96 | 96 | 14 | 12 | 132 | 42 | 5.4 | 5.4 | 39 | 5.5 | 52 | 245 | ||||||||
| mean | 77.3 | 8.7 | 1.0 | - | 96.3 | 97.0 | 17.0 | 12.0 | 111.3 | 43.5 | 5.6 | 5.4 | 36.0 | 5.1 | 57.3 | 255.7 | ||||||||||
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 |
|---|---|---|---|---|---|---|---|---|
| Alveolar changes | ||||||||
| Hemorrhage | 1 | 1 | 1 | 3 | 0 | 1 | 1 | 2 |
| Edema | 1 | 2 | 0 | 0 | 1 | 3 | 0 | 3 |
| Fibrin deposition | 1 | 0 | 0 | 3 | 1 | 3 | 1 | 1 |
| Hyaline membrane | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Exfoliation | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 |
| Necrosis of epithelium | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Type 2 Pneumocyte hyperplasia | 3 | 3 | 1 | 3 | 1 | 3 | 3 | 3 |
| Organization (fibrosis) | 0 | 2 | 3 Bacterial colonies BOOP Masson bodies | 3 | 3 | 1 | 2 | 0 |
| Inflammation | 0 | 2 | 3 | 2 | 3 | 3 | 0 | 1 |
| Multinucleate giant cells | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Others | Atypia of type II pneumocytes | Atypia of type II pneumocytes | Mitosis Reed Sternberg like cells squamous modules | Atypia of type II pneumocytes | Acute eosinophilic pneumonia | Corpora amylacea | - | - |
| Interstitial changes | ||||||||
| Hemorrhage | 2 | 2 | 3 | 3 | 0 | 1 | 1 | 0 |
| Expansion | 2 | 2 | 3 | 3 | 2 | 1 | 2 | 1 |
| Inflammation | 2 | 2 | 3 | 1 | 1 | 1 | 1 | 1 |
| Fibrosis | 1 | 2 | 2 | 3 | 0 | 0 | 3 | 1 |
| Fibrin deposition | 1 | 1 | 1 | 3 | 0 | 1 | 1 | 1 |
| Vasculitis | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Thrombi | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 0 |
| Pleura | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bronchiolitis | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narasaraju, T.; Neeli, I.; Criswell, S.L.; Krishnappa, A.; Meng, W.; Silva, V.; Bila, G.; Vovk, V.; Serhiy, Z.; Bowlin, G.L.; et al. Neutrophil Activity and Extracellular Matrix Degradation: Drivers of Lung Tissue Destruction in Fatal COVID-19 Cases and Implications for Long COVID. Biomolecules 2024, 14, 236. https://doi.org/10.3390/biom14020236
Narasaraju T, Neeli I, Criswell SL, Krishnappa A, Meng W, Silva V, Bila G, Vovk V, Serhiy Z, Bowlin GL, et al. Neutrophil Activity and Extracellular Matrix Degradation: Drivers of Lung Tissue Destruction in Fatal COVID-19 Cases and Implications for Long COVID. Biomolecules. 2024; 14(2):236. https://doi.org/10.3390/biom14020236
Chicago/Turabian StyleNarasaraju, Teluguakula, Indira Neeli, Sheila L. Criswell, Amita Krishnappa, Wenzhao Meng, Vasuki Silva, Galyna Bila, Volodymyr Vovk, Zolotukhin Serhiy, Gary L. Bowlin, and et al. 2024. "Neutrophil Activity and Extracellular Matrix Degradation: Drivers of Lung Tissue Destruction in Fatal COVID-19 Cases and Implications for Long COVID" Biomolecules 14, no. 2: 236. https://doi.org/10.3390/biom14020236
APA StyleNarasaraju, T., Neeli, I., Criswell, S. L., Krishnappa, A., Meng, W., Silva, V., Bila, G., Vovk, V., Serhiy, Z., Bowlin, G. L., Meyer, N., Luning Prak, E. T., Radic, M., & Bilyy, R. (2024). Neutrophil Activity and Extracellular Matrix Degradation: Drivers of Lung Tissue Destruction in Fatal COVID-19 Cases and Implications for Long COVID. Biomolecules, 14(2), 236. https://doi.org/10.3390/biom14020236








