Copines, a Family of Calcium Sensor Proteins and Their Role in Brain Function
Abstract
:1. Introduction
2. Structure of Copines
2.1. Conserved Molecular Architecture of Copines
2.2. Tandem C2 Domains and VWA Domain in Copine Proteins
3. Dynamic Distribution of Copines in Cells and Their Molecular Mechanism of Action
4. Role of Copines in Mammalian Brain
4.1. Copines and Calcium Signaling in Brain
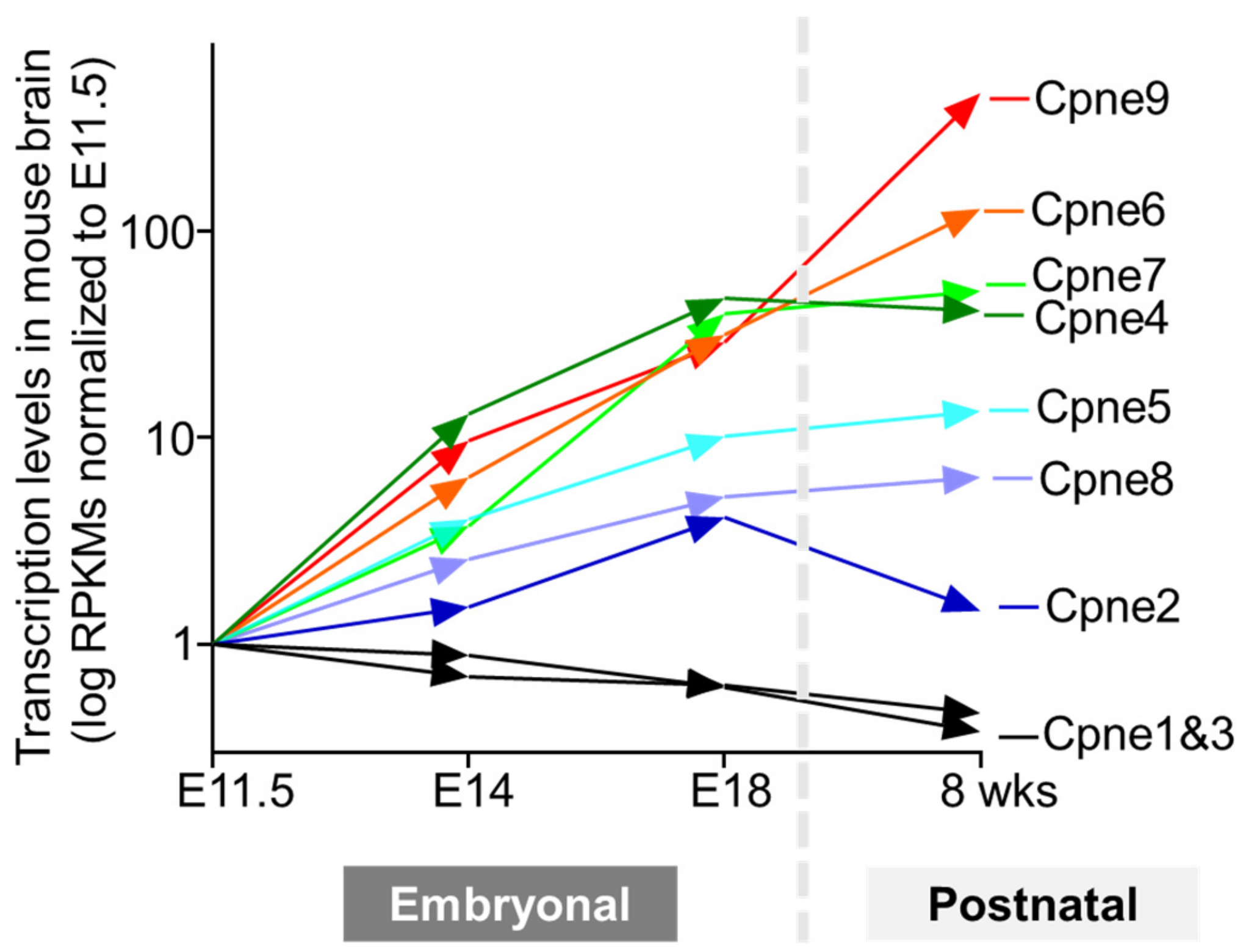
4.2. Spatiotemporal Heterogeneity of Copine Expression in Mammalian Brain
4.3. Neuronal Copine 6
4.3.1. Dynamic Expression of Copine 6 in Brain
4.3.2. Copine 6 Role in Neurotransmission
4.3.3. Copine 6 and Synaptic Plasticity
4.3.4. Copine 6 in Brain Dysfunction
4.4. Ubiquitously Expressed Copines 1 and 3
4.5. Copines with Restricted Tissue Distribution: Copines 4, 5, 7 and 8
4.6. Copines with Unknown Functions in the Brain: Copines 2 and 9
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Creutz, C.E.; Tomsig, J.L.; Snyder, S.L.; Gautier, M.C.; Skouri, F.; Beisson, J.; Cohen, J. The copines, a novel class of C2 domain-containing, calcium-dependent, phospholipid-binding proteins conserved from Paramecium to humans. J. Biol. Chem. 1998, 273, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yaoi, T.; Yasui, M.; Kuwajima, G. N-copine: A novel two C2-domain-containing protein with neuronal activity-regulated expression. FEBS Lett. 1998, 428, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Tomsig, J.L.; Creutz, C.E. Copines: A ubiquitous family of Ca(2+)-dependent phospholipid-binding proteins. Cell. Mol. Life Sci. 2002, 59, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A.; Almedom, R.B.; Schedletzky, T.; Anderson, S.D.; Yates, J.R., 3rd; Schafer, W.R. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J. 2005, 24, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Church, D.L.; Lambie, E.J. The promotion of gonadal cell divisions by the Caenorhabditis elegans TRPM cation channel GON-2 is antagonized by GEM-4 copine. Genetics 2003, 165, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.; Xiong, G.; Qadota, H.; Rogalski, T.; Vogl, A.W.; Moerman, D.G.; Benian, G.M. CPNA-1, a copine domain protein, is located at integrin adhesion sites and is required for myofilament stability in Caenorhabditis elegans. Mol. Biol. Cell 2013, 24, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Damer, C.K.; Bayeva, M.; Hahn, E.S.; Rivera, J.; Socec, C.I. Copine A, a calcium-dependent membrane-binding protein, transiently localizes to the plasma membrane and intracellular vacuoles in Dictyostelium. BMC Cell Biol. 2005, 6, 46. [Google Scholar] [CrossRef]
- Damer, C.K.; Bayeva, M.; Kim, P.S.; Ho, L.K.; Eberhardt, E.S.; Socec, C.I.; Lee, J.S.; Bruce, E.A.; Goldman-Yassen, A.E.; Naliboff, L.C. Copine A is required for cytokinesis, contractile vacuole function, and development in Dictyostelium. Eukaryot. Cell 2007, 6, 430–442. [Google Scholar] [CrossRef]
- Smith, T.S.; Pineda, J.M.; Donaghy, A.C.; Damer, C.K. Copine A plays a role in the differentiation of stalk cells and the initiation of culmination in Dictyostelium development. BMC Dev. Biol. 2010, 10, 59. [Google Scholar] [CrossRef]
- Wight, E.M.; Ide, A.D.; Damer, C.K. Copine A regulates the size and exocytosis of contractile vacuoles and postlysosomes in Dictyostelium. FEBS Open Bio 2020, 10, 979–994. [Google Scholar] [CrossRef]
- Ide, A.D.; Wight, E.M.; Damer, C.K. Phosphatidylserine exposure promotes increased adhesion in Dictyostelium Copine A mutants. PLoS ONE 2021, 16, e0250710. [Google Scholar] [CrossRef]
- Flegel, K.A.; Pineda, J.M.; Smith, T.S.; Laszczyk, A.M.; Price, J.M.; Karasiewicz, K.M.; Damer, C.K. Copine A is expressed in prestalk cells and regulates slug phototaxis and thermotaxis in developing Dictyostelium. Dev. Growth Differ. 2011, 53, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Grisafi, P.; Cheng, S.H.; Fink, G.R. Plant growth homeostasis is controlled by the Arabidopsis BON1 and BAP1 genes. Genes Dev. 2001, 15, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Jambunathan, N.; McNellis, T.W. Regulation of Arabidopsis COPINE 1 gene expression in response to pathogens and abiotic stimuli. Plant Physiol. 2003, 132, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Jambunathan, N.; Siani, J.M.; McNellis, T.W. A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 2001, 13, 2225–2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Meng, P.; Zhang, X.; Ren, D.; Yang, S. BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J. 2011, 67, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.F.; McNellis, T.W. Evidence that the BONZAI1/COPINE1 protein is a calcium- and pathogen-responsive defense suppressor. Plant Mol. Biol. 2009, 69, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, H.; Grisafi, P.; Sanchatjate, S.; Fink, G.R.; Sun, Q.; Hua, J. The BON/CPN gene family represses cell death and promotes cell growth in Arabidopsis. Plant J. 2006, 45, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Ding, Y.; Liu, H.; Hua, J. Silencing of copine genes confers common wheat enhanced resistance to powdery mildew. Mol. Plant Pathol. 2018, 19, 1343–1352. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Sohma, H.; Creutz, C.E. Calcium-dependent regulation of tumour necrosis factor-alpha receptor signalling by copine. Biochem. J. 2004, 378, 1089–1094. [Google Scholar] [CrossRef]
- Ramsey, C.S.; Yeung, F.; Stoddard, P.B.; Li, D.; Creutz, C.E.; Mayo, M.W. Copine-I represses NF-kappaB transcription by endoproteolysis of p65. Oncogene 2008, 27, 3516–3526. [Google Scholar] [CrossRef] [PubMed]
- El-Huneidi, W.; Anjum, S.; Mohammed, A.K.; Unnikannan, H.; Saeed, R.; Bajbouj, K.; Abu-Gharbieh, E.; Taneera, J. Copine 3 “CPNE3” is a novel regulator for insulin secretion and glucose uptake in pancreatic β-cells. Sci. Rep. 2021, 11, 20692. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, K.S.; Yoon, G.J.; Lee, H.J.; Lee, J.Y.; Park, Y.S.; Park, J.C.; Lee, G.; Chung, C.P.; Park, Y.J. Identification of cell-penetrating osteogenic peptide from copine-7 protein and its delivery system for enhanced bone formation. J. Biomed. Mater. Res. A 2019, 107, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lee, Y.S.; Park, J.S.; Kim, S.H.; Bae, H.S.; Park, J.C. Expression of CPNE7 during mouse dentinogenesis. J. Mol. Histol. 2019, 50, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Son, C.; Seo, Y.M.; Lee, Y.S.; Har, A.; Park, J.C. CPNE7-Induced Autophagy Restores the Physiological Function of Mature Odontoblasts. Front. Cell. Dev. Biol. 2021, 9, 655498. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.M.; Park, S.J.; Lee, H.K.; Park, J.C. Copine-7 binds to the cell surface receptor, nucleolin, and regulates ciliogenesis and Dspp expression during odontoblast differentiation. Sci. Rep. 2017, 7, 11283. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.E.; Flenniken, A.M.; Ji, X.; Teboul, L.; Wong, M.D.; White, J.K.; Meehan, T.F.; Weninger, W.J.; Westerberg, H.; Adissu, H.; et al. High-throughput discovery of novel developmental phenotypes. Nature 2016, 537, 508–514. [Google Scholar] [CrossRef]
- Tang, H.; Pang, P.; Qin, Z.; Zhao, Z.; Wu, Q.; Song, S.; Li, F. The CPNE Family and Their Role in Cancers. Front. Genet. 2021, 12, 689097. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, M.; Isupov, M.N.; Chen, Y.; Littlechild, J.A.; Sun, L.; Wu, X.; Wang, Q.; Yang, W.; Chen, L.; et al. The crystal structure of Arabidopsis BON1 provides insights into the copine protein family. Plant J. 2020, 103, 1215–1232. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Tan, J.Z.A.; Jang, S.E.; Batallas-Borja, A.; Bhembre, N.; Chandra, M.; Zhang, L.; Guo, H.; Ringuet, M.T.; Widagdo, J.; Collins, B.M.; et al. Copine-6 is a Ca(2+) sensor for activity-induced AMPA receptor exocytosis. Cell. Rep. 2023, 42, 113460. [Google Scholar] [CrossRef]
- Perestenko, P.; Watanabe, M.; Beusnard-Bee, T.; Guna, P.; McIlhinney, J. The second C2-domain of copine-2, copine-6 and copine-7 is responsible for their calcium-dependent membrane association. FEBS J. 2015, 282, 3722–3736. [Google Scholar] [CrossRef]
- Reinhard, J.R.; Kriz, A.; Galic, M.; Angliker, N.; Rajalu, M.; Vogt, K.E.; Ruegg, M.A. The calcium sensor Copine-6 regulates spine structural plasticity and learning and memory. Nat. Commun. 2016, 7, 11613. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Südhof, T.C. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 1998, 273, 15879–15882. [Google Scholar] [CrossRef] [PubMed]
- Burk, K.; Ramachandran, B.; Ahmed, S.; Hurtado-Zavala, J.I.; Awasthi, A.; Benito, E.; Faram, R.; Ahmad, H.; Swaminathan, A.; McIlhinney, J.; et al. Regulation of Dendritic Spine Morphology in Hippocampal Neurons by Copine-6. Cereb. Cortex 2018, 28, 1087–1104. [Google Scholar] [CrossRef]
- Perestenko, P.V.; Pooler, A.M.; Noorbakhshnia, M.; Gray, A.; Bauccio, C.; Jeffrey McIlhinney, R.A. Copines-1, -2, -3, -6 and -7 show different calcium-dependent intracellular membrane translocation and targeting. FEBS J. 2010, 277, 5174–5189. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Li, T.; Badea, T.C. Differential expression and subcellular localization of Copines in mouse retina. J. Comp. Neurol. 2019, 527, 2245–2262. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, A.N.; Price, J.E.; Graham, B.N.; Buccilli, M.J.; McKellar, D.R.; Damer, C.K. Cyclic AMP signaling in Dictyostelium promotes the translocation of the copine family of calcium-binding proteins to the plasma membrane. BMC Cell. Biol. 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yaoi, T.; Kuwajima, G.; Yoshie, O.; Sakata, T. Ca2(+)-dependent interaction of N-copine, a member of the two C2 domain protein family, with OS-9, the product of a gene frequently amplified in osteosarcoma. FEBS Lett. 1999, 453, 77–80. [Google Scholar] [CrossRef]
- Yoo, J.C.; Park, N.; Choi, H.Y.; Park, J.Y.; Yi, G.S. JAB1 regulates CPNE1-related differentiation via direct binding to CPNE1 in HiB5 hippocampal progenitor cells. Biochem. Biophys. Res. Commun. 2018, 497, 424–429. [Google Scholar] [CrossRef]
- Liu, P.; Khvotchev, M.; Li, Y.C.; Chanaday, N.L.; Kavalali, E.T. Copine-6 Binds to SNAREs and Selectively Suppresses Spontaneous Neurotransmission. J. Neurosci. 2018, 38, 5888–5899. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Snyder, S.L.; Creutz, C.E. Identification of targets for calcium signaling through the copine family of proteins. Characterization of a coiled-coil copine-binding motif. J. Biol. Chem. 2003, 278, 10048–10054. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.A.; Hynes, R.O. Distribution and evolution of von Willebrand/integrin A domains: Widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell. 2002, 13, 3369–3387. [Google Scholar] [CrossRef] [PubMed]
- Tomsig, J.L.; Creutz, C.E. Biochemical characterization of copine: A ubiquitous Ca2+-dependent, phospholipid-binding protein. Biochemistry 2000, 39, 16163–16175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gou, M.; Sun, Q.; Hua, J. Requirement of calcium binding, myristoylation, and protein-protein interaction for the Copine BON1 function in Arabidopsis. J. Biol. Chem. 2010, 285, 29884–29891. [Google Scholar] [CrossRef] [PubMed]
- Buschow, S.I.; van Balkom, B.W.; Aalberts, M.; Heck, A.J.; Wauben, M.; Stoorvogel, W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell. Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Principe, S.; Jones, E.E.; Kim, Y.; Sinha, A.; Nyalwidhe, J.O.; Brooks, J.; Semmes, O.J.; Troyer, D.A.; Lance, R.S.; Kislinger, T.; et al. In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine. Proteomics 2013, 13, 1667–1671. [Google Scholar] [CrossRef]
- Sun, B.; Li, Y.; Zhou, Y.; Ng, T.K.; Zhao, C.; Gan, Q.; Gu, X.; Xiang, J. Circulating exosomal CPNE3 as a diagnostic and prognostic biomarker for colorectal cancer. J. Cell. Physiol. 2019, 234, 1416–1425. [Google Scholar] [CrossRef]
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef]
- Südhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef]
- Kaeser, P.S.; Regehr, W.G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014, 76, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Kavalali, E.T.; Chung, C.; Khvotchev, M.; Leitz, J.; Nosyreva, E.; Raingo, J.; Ramirez, D.M. Spontaneous neurotransmission: An independent pathway for neuronal signaling? Physiology 2011, 26, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.J. Structural LTP: Signal transduction, actin cytoskeleton reorganization, and membrane remodeling of dendritic spines. Curr. Opin. Neurobiol. 2022, 74, 102534. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Duszkiewicz, A.J.; Morris, R.G. The synaptic plasticity and memory hypothesis: Encoding, storage and persistence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130288. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 1469–1658. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Sheng, M.; Hanson, J.E. Targeting synapse function and loss for treatment of neurodegenerative diseases. Nat. Rev. Drug Discov. 2024, 23, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Uzay, B.; Kavalali, E.T. Genetic disorders of neurotransmitter release machinery. Front. Synaptic Neurosci. 2023, 15, 1148957. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef]
- Yu, Y.; Fuscoe, J.C.; Zhao, C.; Guo, C.; Jia, M.; Qing, T.; Bannon, D.I.; Lancashire, L.; Bao, W.; Du, T.; et al. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat. Commun. 2014, 5, 3230. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yaoi, T.; Kuwajima, G. Localization and subcellular distribution of N-copine in mouse brain. J. Neurochem. 1999, 72, 373–379. [Google Scholar] [CrossRef]
- Zhu, B.; Zha, J.; Long, Y.; Hu, X.; Chen, G.; Wang, X. Increased expression of copine VI in patients with refractory epilepsy and a rat model. J. Neurol. Sci. 2016, 360, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yamatani, H.; Kawasaki, T.; Mita, S.; Inagaki, N.; Hirata, T. Proteomics analysis of the temporal changes in axonal proteins during maturation. Dev. Neurobiol. 2010, 70, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Chen, Y.; Hu, D.; Hanashima, S.; Yamamoto, K.; Yamaguchi, Y. Structural basis for oligosaccharide recognition of misfolded glycoproteins by OS-9 in ER-associated degradation. Mol. Cell. 2010, 40, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Bacaj, T.; Morishita, W.; Goswami, D.; Arendt, K.L.; Xu, W.; Chen, L.; Malenka, R.C.; Südhof, T.C. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 2017, 544, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Hu, X.; Chen, G.; Wang, X.; Zhu, B. Down-regulation of adenylate kinase 5 in temporal lobe epilepsy patients and rat model. J. Neurol. Sci. 2016, 366, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, Y.Y.; Wu, R.; Han, Y.X.; Yu, Y.; Ge, J.F.; Chen, F.H. Impaired learning and memory in rats induced by a high-fat diet: Involvement with the imbalance of nesfatin-1 abundance and copine 6 expression. J. Neuroendocrinol. 2017, 29, 1–12. [Google Scholar] [CrossRef]
- Greene, N.D.; Leung, K.Y.; Wait, R.; Begum, S.; Dunn, M.J.; Copp, A.J. Differential protein expression at the stage of neural tube closure in the mouse embryo. J. Biol. Chem. 2002, 277, 41645–41651. [Google Scholar] [CrossRef]
- Park, N.; Yoo, J.C.; Ryu, J.; Hong, S.G.; Hwang, E.M.; Park, J.Y. Copine1 enhances neuronal differentiation of the hippocampal progenitor HiB5 cells. Mol. Cells 2012, 34, 549–554. [Google Scholar] [CrossRef]
- Kim, T.H.; Sung, S.E.; Cheal Yoo, J.; Park, J.Y.; Yi, G.S.; Heo, J.Y.; Lee, J.R.; Kim, N.S.; Lee, D.Y. Copine1 regulates neural stem cell functions during brain development. Biochem. Biophys. Res. Commun. 2018, 495, 168–173. [Google Scholar] [CrossRef]
- Park, N.; Yoo, J.C.; Lee, Y.S.; Choi, H.Y.; Hong, S.G.; Hwang, E.M.; Park, J.Y. Copine1 C2 domains have a critical calcium-independent role in the neuronal differentiation of hippocampal progenitor HiB5 cells. Biochem. Biophys. Res. Commun. 2014, 454, 228–233. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, N.; Lee, B.; Choe, Y.I.; Woo, D.K.; Park, J.Y.; Yoo, J.C. CPNE1-mediated neuronal differentiation can be inhibited by HAX1 expression in HiB5 cells. Biochem. Biophys. Res. Commun. 2020, 533, 319–324. [Google Scholar] [CrossRef]
- Cohen, O.S.; McCoy, S.Y.; Middleton, F.A.; Bialosuknia, S.; Zhang-James, Y.; Liu, L.; Tsuang, M.T.; Faraone, S.V.; Glatt, S.J. Transcriptomic analysis of postmortem brain identifies dysregulated splicing events in novel candidate genes for schizophrenia. Schizophr. Res. 2012, 142, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Z.; Chen, C.; Xue, G.; Lu, S.; Liu, H.; Dong, Q.; Zhang, M. CPNE3 moderates the association between anxiety and working memory. Sci. Rep. 2021, 11, 6891. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, S.; Janning, P.; Seidel, R.; Matschke, J.; Gonsberg, A.; Jung, S.; Glatzel, M.; Engelhard, M.; Winklhofer, K.F.; Tatzelt, J. Alterations in the brain interactome of the intrinsically disordered N-terminal domain of the cellular prion protein (PrPC) in Alzheimer’s disease. PLoS ONE 2018, 13, e0197659. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.I.; Dasen, J.S.; Jessell, T.M. Divergent Hox Coding and Evasion of Retinoid Signaling Specifies Motor Neurons Innervating Digit Muscles. Neuron 2017, 93, 792–805.e794. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Aponte, A.M.; Wistow, G.; Badea, T.C. Molecular studies into cell biological role of Copine-4 in Retinal Ganglion Cells. PLoS ONE 2021, 16, e0255860. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, K.; Lal, D.; Li, Y.; Doody, R.S.; Wilhelmsen, K.; Yan, L.; Liu, S.; Ma, C.; Texas Alzheimer, R.; Care, C. Genome-wide scan for copy number variation association with age at onset of Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 517–523. [Google Scholar] [CrossRef]
- Hanley, O.; Zewdu, R.; Cohen, L.J.; Jung, H.; Lacombe, J.; Philippidou, P.; Lee, D.H.; Selleri, L.; Dasen, J.S. Parallel Pbx-Dependent Pathways Govern the Coalescence and Fate of Motor Columns. Neuron 2016, 91, 1005–1020. [Google Scholar] [CrossRef]
- Ding, X.; Jin, Y.; Wu, Y.; Wu, Y.; Wu, H.; Xiong, L.; Song, X.; Liu, S.; Fan, W.; Fan, M. Localization and cellular distribution of CPNE5 in embryonic mouse brain. Brain Res. 2008, 1224, 20–28. [Google Scholar] [CrossRef]
- Ding, X.F.; Wang, H.J.; Qian, L.; Hu, Z.Y.; Feng, S.F.; Wu, Y.; Yang, H.H.; Wu, H.T.; Fan, W.H.; Fan, M. Cpne5 is Involved in Regulating Rodent Anxiety Level. CNS Neurosci. Ther. 2017, 23, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Zuo, L.; Pan, Y.; Xie, C.; Luo, X. Genetic variants in the CPNE5 gene are associated with alcohol dependence and obesity in Caucasian populations. J. Psychiatr. Res. 2015, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Tsai, C.J.; Yasugaki, S.; Nagata, N.; Morita, M.; Isotani, A.; Yanagisawa, M.; Hayashi, Y. Copine-7 is required for REM sleep regulation following cage change or water immersion and restraint stress in mice. Neurosci. Res. 2021, 165, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.E.; Maytham, E.G.; Grizenkova, J.; Hummerich, H.; Collinge, J. A Copine family member, Cpne8, is a candidate quantitative trait gene for prion disease incubation time in mouse. Neurogenetics 2010, 11, 185–191. [Google Scholar] [CrossRef] [PubMed]
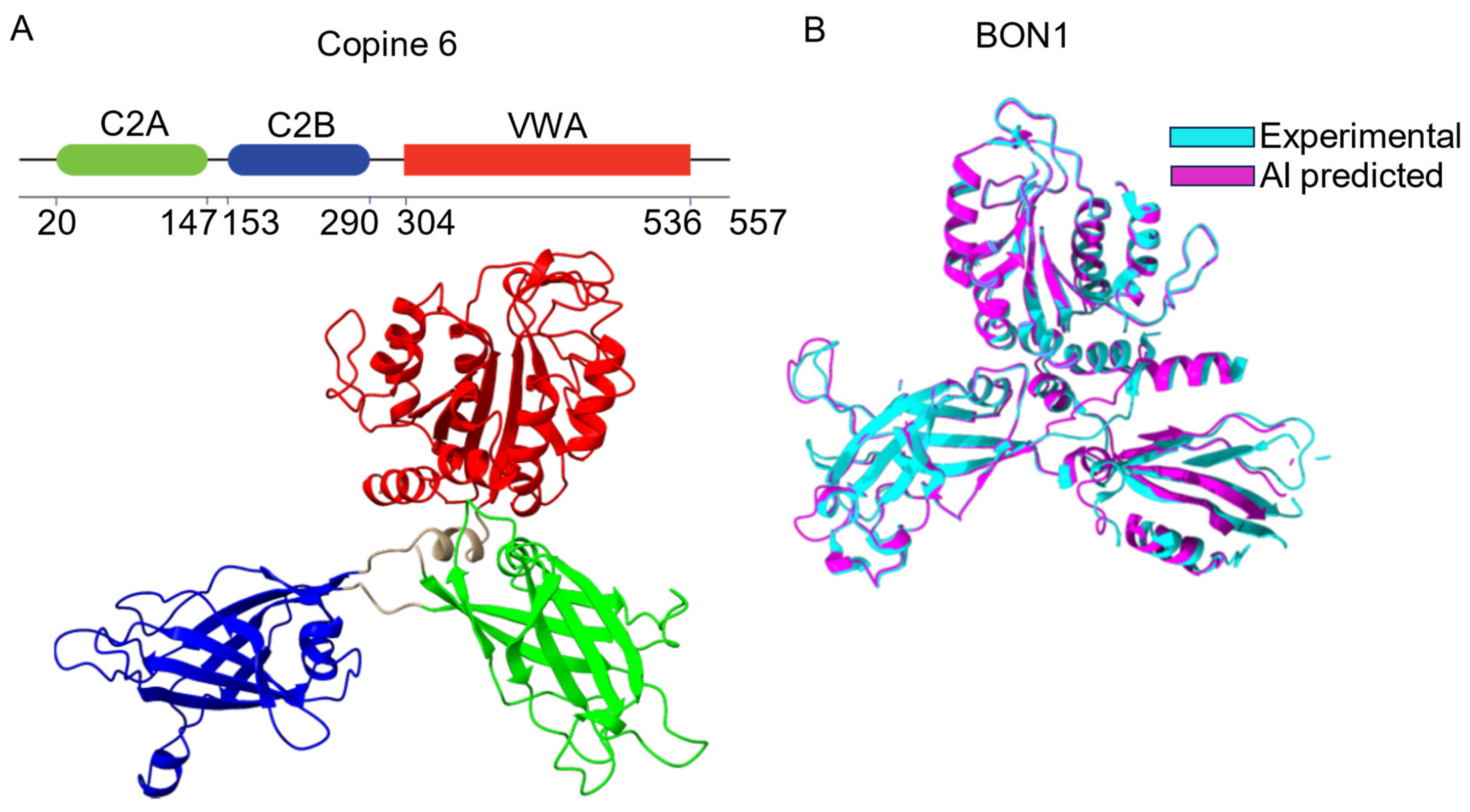
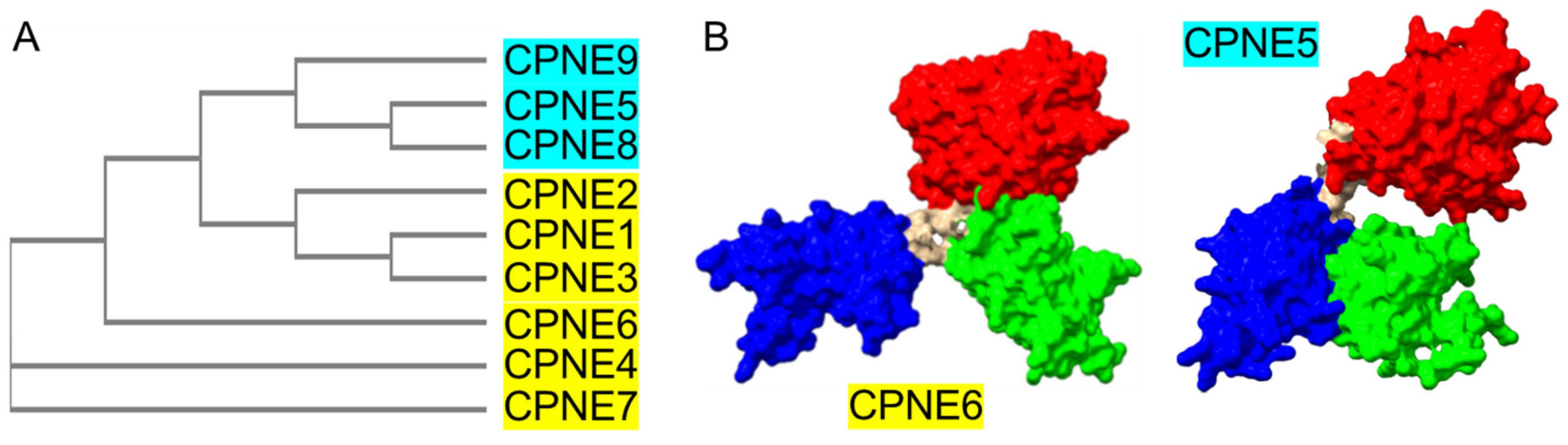
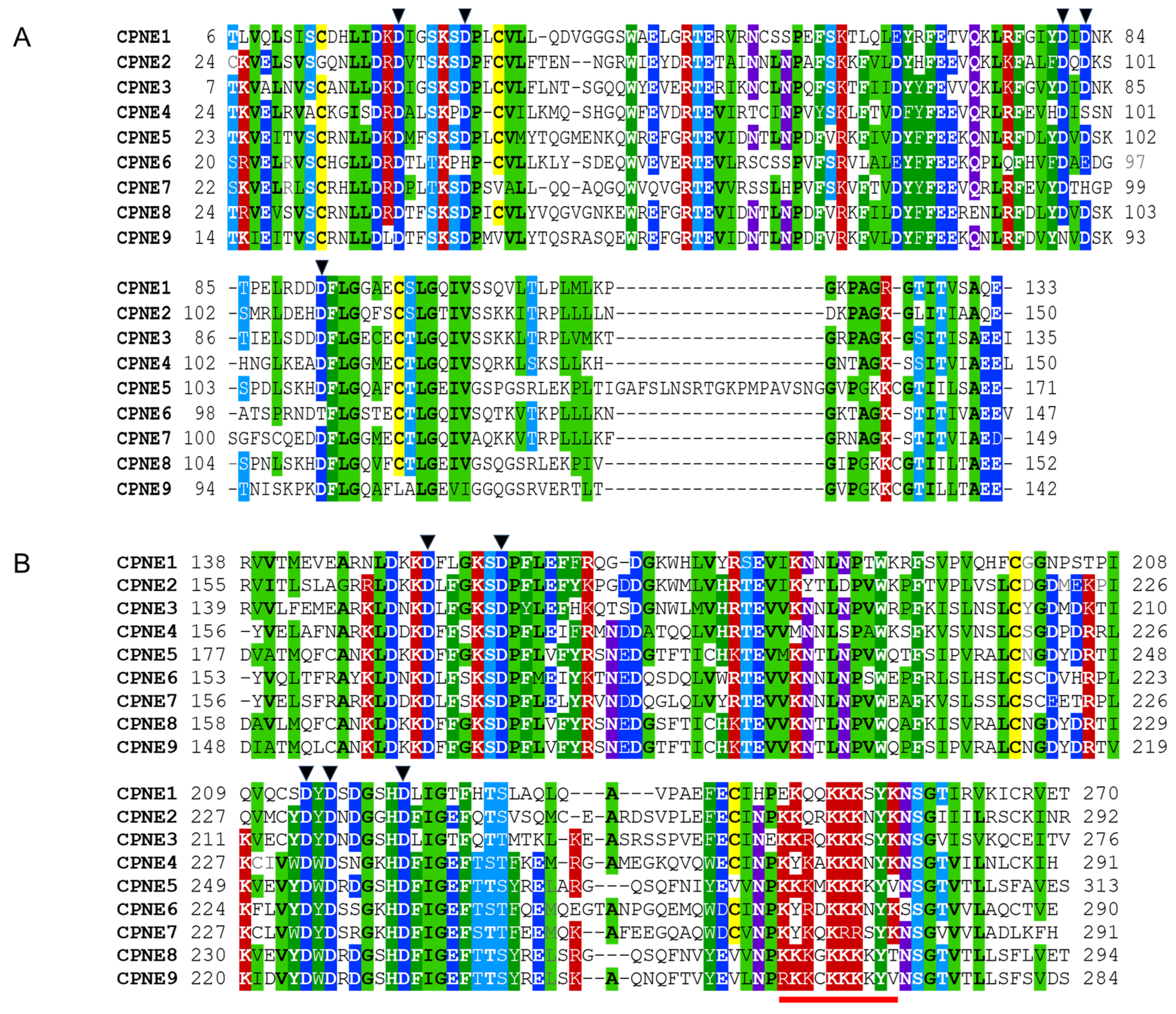
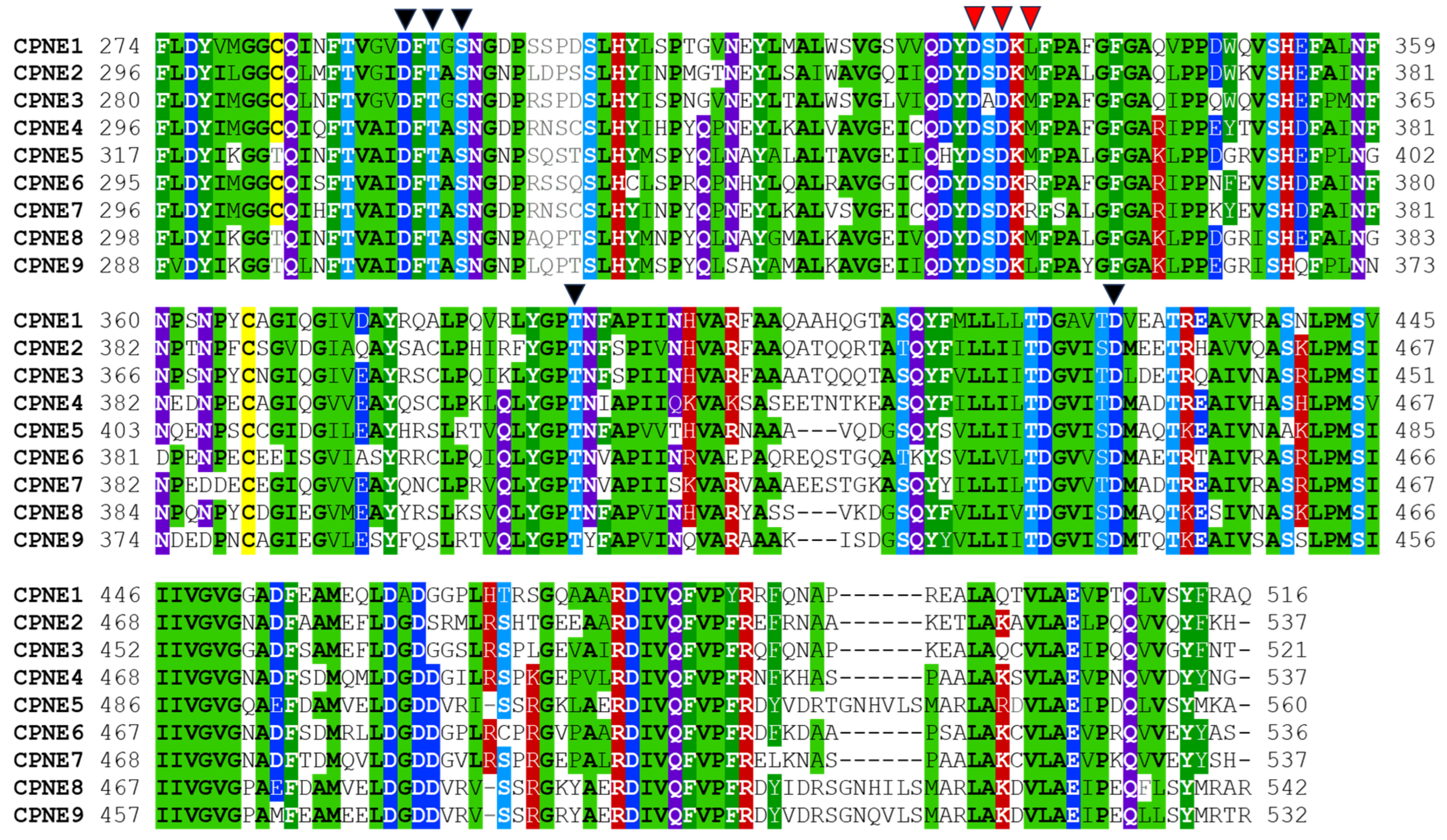

| Copine Proteins (aa) | Molecular Weight 1 (with PTMs), Da | Copine Sequences ID (Name) | Domains and Domain Boundaries 2 | ||
|---|---|---|---|---|---|
| C2A | C2B | vWA | |||
| Human Copines: | |||||
| Copine 1 (1-537) | 59,058 (59,378) | Q99829 (CPNE1) | T6-E133 | R138-T270 | Q283-Q516 |
| Copine 2 (1-548) | 61,190 (61,190) | Q96FN4 (CPNE2) | C24-E150 | R155-R292 | Q305-H537 |
| Copine 3 (1-537) | 60,131 (60,850) | O75131 (CPNE3) | T7-I135 | R139-V276 | Q289-T521 |
| Copine 4 (1-557) | 62,395 (62,395) | Q96A23 (CPNE4) | T24-L150 | Y156-H291 | Q305-G537 |
| Copine 5 (1-593) | 65,734 (66,373) | Q9HCH3 (CPNE5) | T23-E171 | D177-S313 | Q326-A560 |
| Copine 6 (1-557) | 61,991 (62,071) | O95741 (CPNE6) | S20-V147 | Y153-E290 | Q304-S536 |
| Copine 7 (1-633) | 70,294 (70,454) | Q9UBL6 (CPNE7) | S22-D149 | Y156-H291 | Q305-H537 |
| Copine 8 (1-564) | 63,108 (63,348) | Q86YQ8 (CPNE8) | T24-E152 | D158-T294 | Q307-R542 |
| Copine 9 (1-553) | 61,864 (62,067) | Q8IYJ1 (CPNE9) | T14-E142 | D148-S284 | Q297-R532 |
| Plant Copine: | |||||
| BONZAI 1 (1-578) | 63,119 (63,199) | Q941L3 (BON1) | Q49-E188 | I194-V326 | E339-I570 |
| S48-E188 3 | I194-V326 3 | E339-N572 3 | |||
| Identity 1 | Copine 1 | Copine 2 | Copine 3 | Copine 4 | Copine 5 | Copine 6 | Copine 7 | Copine 8 | Copine 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Similarity 1 | ||||||||||
| Copine 1 | 59% | 66% | 52% | 49% | 51% | 54% | 52% | 48% | ||
| Copine 2 | 75% | 65% | 54% | 52% | 51% | 57% | 52% | 49% | ||
| Copine 3 | 80% | 80% | 57% | 53% | 55% | 58% | 53% | 50% | ||
| Copine 4 | 69% | 71% | 73% | 50% | 63% | 74% | 53% | 49% | ||
| Copine 5 | 66% | 68% | 67% | 65% | 48% | 45% | 80% | 77% | ||
| Copine 6 | 67% | 67% | 70% | 79% | 62% | 71% | 50% | 47% | ||
| Copine 7 | 70% | 75% | 76% | 88% | 59% | 86% | 53% | 51% | ||
| Copine 8 | 69% | 69% | 71% | 68% | 89% | 67% | 67% | 79% | ||
| Copine 9 | 68% | 69% | 68% | 66% | 87% | 63% | 66% | 90% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khvotchev, M.; Soloviev, M. Copines, a Family of Calcium Sensor Proteins and Their Role in Brain Function. Biomolecules 2024, 14, 255. https://doi.org/10.3390/biom14030255
Khvotchev M, Soloviev M. Copines, a Family of Calcium Sensor Proteins and Their Role in Brain Function. Biomolecules. 2024; 14(3):255. https://doi.org/10.3390/biom14030255
Chicago/Turabian StyleKhvotchev, Mikhail, and Mikhail Soloviev. 2024. "Copines, a Family of Calcium Sensor Proteins and Their Role in Brain Function" Biomolecules 14, no. 3: 255. https://doi.org/10.3390/biom14030255






