Role of DNase Activity in Human Sperm DNA Fragmentation
Abstract
:1. Introduction
2. DNases
3. Histone–Protamine Replacement: Endogenous Enzymes
4. ROS Triggering Apoptotic Pathways
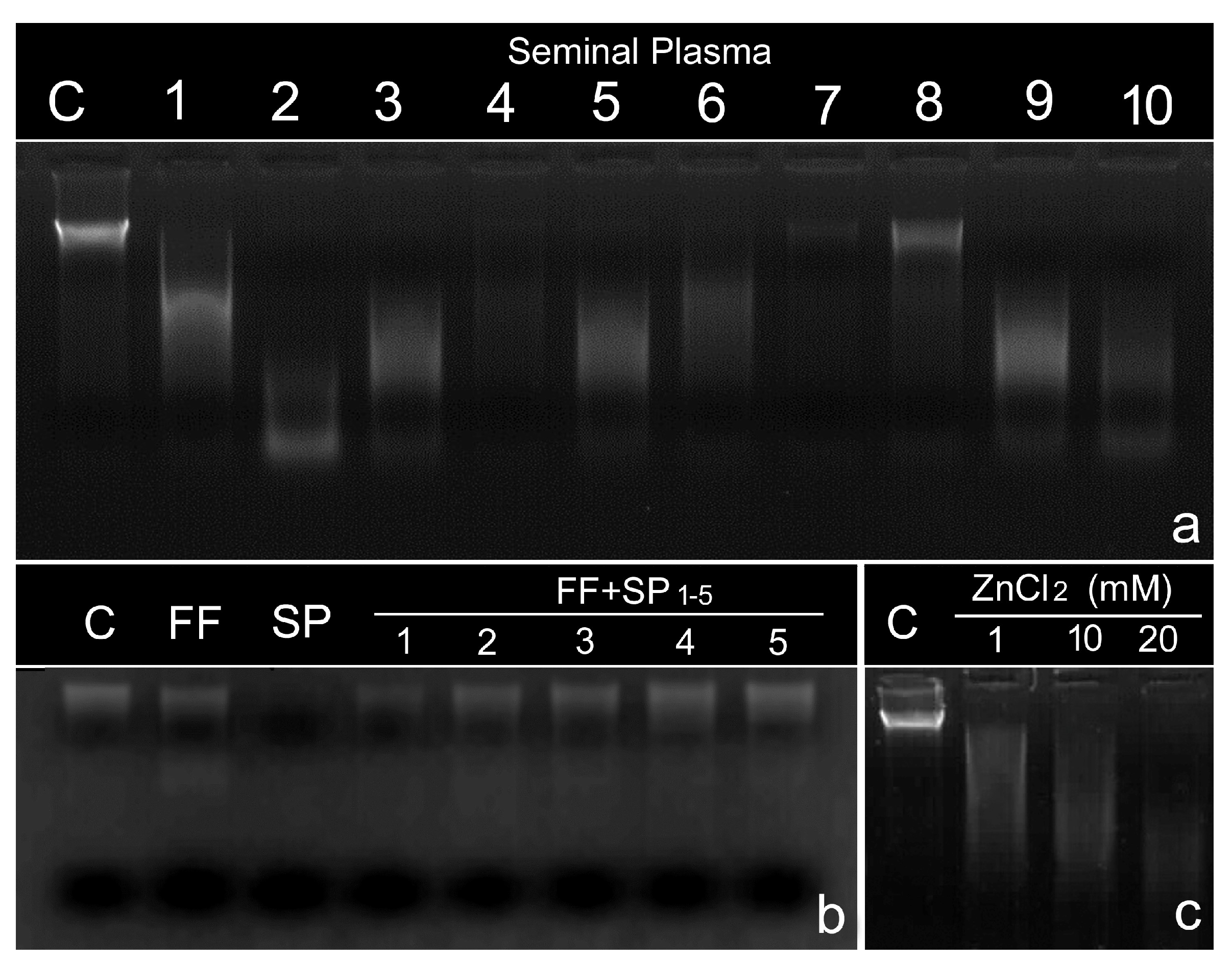
5. Presence of DNase Activity in the Ejaculate
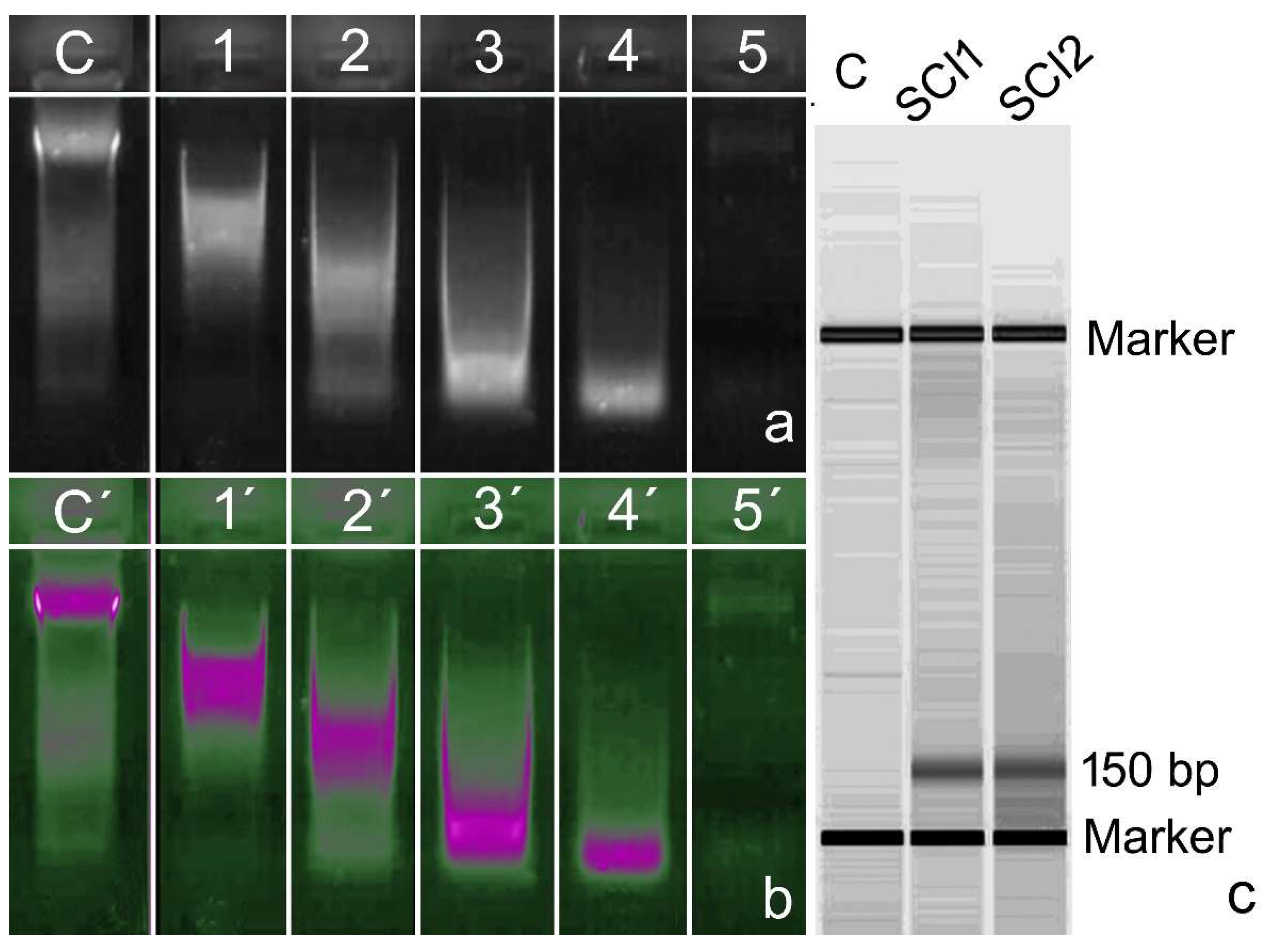
6. The Role of Follicular Fluid in Controlling DNase Activity
7. Antioxidant Therapy and Homeostatic Modifications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chunhui, Z.; Fang, C.; Shengmin, Z.; Hong, S.; Yun, J.; Xidong, W.; Chunxia, Y.; Ya, S.; Naijun, D.; Tongmin, D.X.; et al. Influence of sperm DNA fragmentation on the clinical outcome of in vitro fertilization-embryo transfer (IVF-ET). Front. Endocrinol. 2022, 13, 945242. [Google Scholar] [CrossRef]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Pacey, A.A. Environmental and lifestyle factors associated with sperm DNA damage. Hum. Fertil. 2010, 13, 189–193. [Google Scholar] [CrossRef]
- Gosálvez, J.; López-Fernández, C.; Fernández, J.L.; Gouraud, A.; Holt, W.V. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev. 2011, 78, 951–961. [Google Scholar] [CrossRef]
- López-Fernández, C.; Crespo, F.; Arroyo, F.; Fernández, J.L.; Arana, P.; Johnston, S.D.; Gosalvez, J. Dynamics of sperm DNA fragmentation in domestic animals: II. The stallion. Theriogenology 2008, 68, 1240–1250. [Google Scholar] [CrossRef]
- Marti, T.M.; Fleck, O. DNA repair nucleases. Cell. Mol. Life Sci. 2004, 61, 336–354. [Google Scholar] [CrossRef]
- Shen, B.; Singh, P.; Liu, R.; Qiu, J.; Zheng, L.; Finger, L.D.; Alas, S. Multiple but dissectible functions of FEN-1 nucleases in nucleic acid processing, genome stability and diseases. Bioessays 2005, 27, 717–729. [Google Scholar] [CrossRef]
- Ganai, R.A.; Johansson, E. DNA replication- a matter of fidelity. Mol. Cell 2016, 62, 745–755. [Google Scholar] [CrossRef]
- Pollard, H.; Toumaniantz, G.; Amos, J.L.; Avet-Loiseau, H.; Guihard, G.; Behr, J.P.; Escande, D. Ca2+-sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. J. Gene Med. 2001, 3, 153–164. [Google Scholar] [CrossRef]
- Lyon, C.J.; Aguilera, R.J. Purification and characterization of the immunoglobulin switch sequence-specific endonuclease (Endo-SR) from bovine spleen. Mol. Immunol. 1997, 34, 209–219. [Google Scholar] [CrossRef]
- Evans, C.J.; Aguilera, R.J. DNase II: Genes, enzymes and function. Gene 2003, 322, 1–15. [Google Scholar] [CrossRef]
- Campbell, V.W.; Jackson, D.A. The effect of divalent cations on the mode of action of DNase I. The initial reaction products produced from covalently closed circular DNA. J. Biol. Chem. 1980, 255, 3726–3735. [Google Scholar] [CrossRef]
- Shiokawa, D.; Tanuma, S. Characterization of human DNase I family endonucleases and activation of DNase gamma during apoptosis. Biochemistry 2001, 40, 43–52. [Google Scholar] [CrossRef]
- Bernardi, G. Spleen acid deoxyribonuclease. In Hydrolysis; Boyer, P.D., Ed.; Academic Press: New York, NY, USA, 1971; pp. 271–287. [Google Scholar]
- Hevelone, J.; Hartman, P.S. An endonuclease from Caenorhabditis elegans: Partial purification and characterization. Biochem. Genet. 1988, 26, 447–461. [Google Scholar] [CrossRef]
- Kolade, O.O.; Carr, S.B.; Kühlmann, U.C.; Pommer, A.; Kleanthous, C.; Bouchcinsky, C.A.; Hemmings, A.M. Structural aspects of the inhibition of DNase and rRNase colicins by their immunity proteins. Biochimie 2002, 84, 439–446. [Google Scholar] [CrossRef]
- Stoddard, B.L. Homing endonuclease structure and function. Q. Rev. Biophys. 2005, 38, 49–95. [Google Scholar] [CrossRef]
- Widlak, P.; Garrard, W.T. Discovery, regulation, and action of the major apoptotic nucleases DFF40/CAD and endonuclease G. J. Cell. Biochem. 2005, 94, 1078–1087. [Google Scholar] [CrossRef]
- Chowdhury, D.; Beresford, P.J.; Zhu, P.; Zhang, D.; Sung, J.S.; Demple, B.; Perrino, F.W.; Lieberman, J. The exonuclease TREX1 is in the SET complex and acts in concert with NM23-H1 to degrade DNA during granzyme A-mediated cell death. Mol. Cell 2006, 23, 133–412. [Google Scholar] [CrossRef]
- Han, D.S.C.; Lo, Y.M.D. The nexus of cfDNA and nuclease biology. Trends Genet. 2021, 37, 758–770. [Google Scholar] [CrossRef]
- Gromova, I.I.; Nielsenr, O.F.; Sergey, V.; Razin, S.V. Long-range fragmentation of the eukaryotic genome by exogenous and endogenous nucleases proceeds in a specific fashion via preferential DNA cleavage at matrix attachment sites. J. Biol. Chem. 1995, 31, 8685–8690. [Google Scholar] [CrossRef]
- Maione, B.; Pittoggi, C.; Achene, L.; Lorenzini, R.; Spadafora, C. Activation of endogenous nucleases in mature sperm cells upon interaction with exogenous DNA. DNA Cell Biol. 1997, 16, 1087–1097. [Google Scholar] [CrossRef]
- Sotolongo, B.; Lino, E.; Ward, W.S. Ability of hamster spermatozoa to digest their own DNA. Biol. Reprod. 2003, 69, 2029–2035. [Google Scholar] [CrossRef]
- Sotolongo, B.; Huang, T.T.; Isenberger, E.; Ward, W.S. An endogenous nuclease in hamster, mouse, and human spermatozoa cleaves DNA into loop-sized fragments. J. Androl. 2005, 26, 72–80. [Google Scholar] [CrossRef]
- Shaman, J.A.; Prisztoka, R.; Ward, W.S. Topoisomerase IIB and an extracellular nuclease interact to digest sperm DNA in an apoptotic-like manner. Biol. Reprod. 2006, 75, 741–748. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ajduk, A.; Riel, J.M.; Ward, M.A. Ejaculated and epididymal mouse spermatozoa are different in their susceptibility to nuclease-dependent DNA damage and in their nuclease activity. Biol. Reprod. 2007, 77, 636–647. [Google Scholar] [CrossRef]
- Agarwal, A.; Barbăroșie, C.; Ambar, R.; Finelli, R. The impact of single- and double-strand DNA breaks in human spermatozoa on assisted reproduction. Int. J. Mol. Sci. 2020, 29, 3882. [Google Scholar] [CrossRef]
- Ward, W.S. Deoxyribonucleic acid loop domain tertiary structure in mammalian spermatozoa. Biol. Reprod. 1993, 48, 1193–1201. [Google Scholar] [CrossRef]
- Boskovic, A.; Torres-Padilla, M.E. How mammals pack their sperm: A variant matter. Genes Dev. 2013, 27, 1635–1639. [Google Scholar] [CrossRef]
- Cho, C.; Willis, W.D.; Goulding, E.H.; Haesook, J.H.; Choi, Y.C.; Hecht, N.B.; Eddy, E.M. Haploinsufficiency of protamine-1 or-2 causes infertility in mice. Nat. Genet. 2001, 28, 82–86. [Google Scholar] [CrossRef]
- Lewis, S.E.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef]
- Gunes, S.; Al-Sadaan, M.; Agarwal, A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod. Biomed. Online 2015, 31, 309–319. [Google Scholar] [CrossRef]
- Henkel, R.; Leisegang, K. Origins of sperm DNA damage. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants, 2nd ed.; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer: London, UK, 2020; pp. 361–375. [Google Scholar] [CrossRef]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746–754. [Google Scholar] [CrossRef]
- Sakkas, D.; Mariethoz, E.; St. John, J.C. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp. Cell Res. 1999, 251, 350–355. [Google Scholar] [CrossRef]
- Platt, N.; da Silva, R.P.; Gordon, S. Recognizing death: The phagocytosis of apoptotic cells. Trends Cell Biol. 1998, 8, 365–372. [Google Scholar] [CrossRef]
- Badouard, C.; Ménézo, Y.; Panteix, G.; Ravanat, J.L.; Douki, T.; Cadet, J.; Favier, A. Determination of new types of DNA lesions in human sperm. Zygote 2008, 6, 9–13. [Google Scholar] [CrossRef]
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS ONE 2013, 8, e68490. [Google Scholar] [CrossRef]
- Vorilhon, S.; Brugnon, F.; Kocer, A.; Dollet, S.; Bourgne, C.; Berger, M.; Janny, L.; Pereira, B.; Aitken, R.J.; Moazamian, A.; et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum. Reprod. 2018, 33, 553–562. [Google Scholar] [CrossRef]
- Oliva, R. Protamines and male infertility. Hum. Reprod. Update 2006, 12, 417–435. [Google Scholar] [CrossRef]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signalling pathways in apoptosis. Int. J. Mol. Sci. 2020, 28, 2346. [Google Scholar] [CrossRef]
- Ranawat, P.; Bansal, M.P. Apoptosis induced by modulation in selenium status involves p38 MAPK and ROS: Implications in spermatogenesis. Mol. Cell. Biochem. 2009, 330, 83–95. [Google Scholar] [CrossRef]
- Sato, F.; Soh, T.; Hattori, M.A.; Fujihara, N. Evaluation of deoxyribonuclease activity in seminal plasma of ejaculated chicken semen. Asian J. Androl. 2003, 5, 213–216. [Google Scholar]
- Dorado-Silva, M.; Bartolomé-Nebreda, J.; Sánchez-Martín, P.; Johnston, S.; Gosálvez, J. Co-incubation of spermatozoa with human follicular fluid reduces sperm DNA fragmentation by mitigating DNase activity in the seminal plasma. J. Assist. Reprod. Genet. 2020, 37, 63–69. [Google Scholar] [CrossRef]
- Bahmanpour, S.; Namavar, M.R.; Talaei-Khozani, T.; Mazaheri, Z. The effect of the follicular fluid on sperm chromatin quality in comparison with conventional media. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1840–1846. [Google Scholar]
- Cortés-Gutiérrez, E.I.; García De La Vega, C.; Bartolomé-Nebreda, J.; Gosálvez, J. Characterization of DNA cleavage produced by seminal plasma using leukocytes as a cell target. Syst. Biol. Reprod. Med. 2019, 65, 420–429. [Google Scholar] [CrossRef]
- Singer, R.; Sagiv, M.; Allalouf, D.; Levinsky, H.; Servadio, C. Deoxyribonuclease activity in human seminal fluid. Arch. Androl. 1983, 10, 169–172. [Google Scholar] [CrossRef]
- Vargas-Baquero, E.; Johnston, S.D.; Sánchez-Ramos, A.; Arévalo-Martín, A.; Wilson, R.; Gosálvez, J. The incidence and etiology of sperm DNA fragmentation in the ejaculates of males with spinal cord injuries. Spinal Cord 2020, 58, 803–810. [Google Scholar] [CrossRef]
- Bartolomé-Nebreda, J.; Vargas-Baquero, E.; López-Fernández, C.; Fernández, J.L.; Johnston, S.D.; Gosálvez, J. Free circulating DNA and DNase activity in the ejaculates of men with spinal cord injury. Spinal Cord 2021, 59, 167–174. [Google Scholar] [CrossRef]
- Bartolomé-Nebreda, J.; Romeo, S.C.; Dorado-Silva, M.; García de la Vega, C.; López, C.; Sánchez-Martín, P.; Johnston, S.; Gosálvez, J. DNase activity in human seminal plasma and follicular fluid and its inhibition by follicular fluid chelating agents. Reprod. Biomed. Online 2021, 43, 1079–1086. [Google Scholar] [CrossRef]
- Zinkova, A.; Brynychova, I.; Svacina, A.; Jirkovska, M.; Korabecna, M. Cell-free DNA from human plasma and serum differs in content of telomeric sequences and its ability to promote immune response. Sci. Rep. 2017, 7, 2591. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. The role of nitric oxide on male and female reproduction. Malays. J. Med. Sci. 2022, 29, 8–30. [Google Scholar] [CrossRef]
- Collares, T.; Campos, V.F.; de Leon, P.M.M.; Marques De Leon, P.; Cavalcanti, P.V.; Amaral, M.G.; Dellagostin, O.A.; Deschamps, J.C.; Seixas, F.K. Transgene transmission in chickens by sperm-mediated gene transfer after seminal plasma removal and exogenous DNA treated with dimethylsulfoxide or N,N-dimethylacetamide. J. Biosci. 2011, 36, 613–620. [Google Scholar] [CrossRef]
- Singh, N.P.; Danner, D.B.; Tice, R.R.; McCoy, M.T.; Collins, G.D.; Schneider, E.L. Abundant alkali labile sites in DNA of human and mouse sperm. Exp. Cell Res. 1989, 184, 461–470. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; Fernández, J.L.; Gosálvez, J.; Johnston, S.D.; López-Fernández, C. Mapping alkali-labile sites in mammalian spermatozoa. In Animal Reproduction: New Research Developments; Dahnof, L.T., Ed.; Nova Publishers: New York, NY, USA, 2007; pp. 219–231. [Google Scholar]
- Gosálvez, J.; López-Fernández, C.; García de la Vega, C.; Mezzanotte, R.; Fernández, J.L.; Goyanes, V. Selective digestion of mouse chromosomes with restriction endonucleases. Oligonucleotide priming of single-stranded DNA produced with exonuclease III. Genome 1993, 36, 230–234. [Google Scholar] [CrossRef]
- Brackett, B.G.; Baranska, W.; Sawicki, W.; Koprowski, H. Uptake of heterologous genome by mammalian spermatozoa and its transfer to ova through fertilization. Proc. Natl. Acad. Sci. USA 1971, 68, 353–357. [Google Scholar] [CrossRef]
- Celebi, C.; Guillaudeux, T.; Auvray, P.; Vallet-Erdtmann, V.; Jégou, B. The making of transgenic spermatozoa. Biol. Reprod. 2003, 68, 1477–1483. [Google Scholar] [CrossRef]
- Gallegos, G.; Ramos, B.; Santiso, R.; Goyanes, V.; Gosálvez, J.; Fernández, J.L. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil. Steril. 2008, 90, 328–334. [Google Scholar] [CrossRef]
- Pagliuca, C.; Cariati, F.; Bagnulo, F.; Scaglione, E.; Carotenuto, C.; Farina, F.; D’Argenio, V.; Carraturo, F.; D’Aprile, P.; Vitiello, M.; et al. Microbiological evaluation and sperm DNA fragmentation in semen samples of patients undergoing fertility investigation. Genes 2021, 27, 654. [Google Scholar] [CrossRef]
- Eini, F.; Kutenaei, M.A.; Zareei, F.; Dastjerdi, Z.S.; Shirzeyli, M.H.; Salehi, E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with leukocytospermia. BMC Mol. Cell Biol. 2021, 22, 42. [Google Scholar] [CrossRef]
- Marchiani, S.; Baccani, I.; Tamburrino, L.; Mattiuz, G.; Nicolò, S.; Bonaiuto, C.; Panico, C.; Vignozzi, L.; Antonelli, A.; Rossolini, G.M.; et al. Effects of common Gram-negative pathogens causing male genitourinary-tract infections on human sperm functions. Sci. Rep. 2021, 11, 19177. [Google Scholar] [CrossRef]
- González-Marín, C.; Roy, R.; López-Fernández, C.; Diez, B.; Carabaño, M.J.; Fernández, J.L.; Kjelland, M.E.; Moreno, J.F.; Gosálvez, J. Bacteria in bovine semen can increase sperm DNA fragmentation rates: A kinetic experimental approach. Anim. Reprod. Sci. 2011, 123, 139–148. [Google Scholar] [CrossRef]
- Sumby, P.; Barbian, K.D.; Gardner, D.J.; Whitney, A.R.; Welty, D.M.; Long, R.D.; Bailey, J.R.; Parnell, M.J.; Hoe, N.P.; Adams, G.G.; et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. USA 2005, 102, 1679–1684. [Google Scholar] [CrossRef]
- Palmer, L.J.; Chapple, I.L.; Wright, H.J.; Roberts, A.; Cooper, P.R. Extracellular deoxyribonuclease production by periodontal bacteria. J. Periodontal Res. 2012, 47, 439–445. [Google Scholar] [CrossRef]
- Takeshita, H.; Yasuda, T.; Nadano, D.; Tenjo, E.; Sawazaki, K.; Iida, R.; Kishi, K. Detection of deoxyribonucleases I and II (DNases I and II) activities in reproductive organs of male rabbits. Int. J. Biochem. 1994, 26, 1025–1031. [Google Scholar] [CrossRef]
- Carballada, R.; Esponda, P. Regulation of foreign DNA uptake by mouse spermatozoa. Exp. Cell Res. 2001, 262, 104–113. [Google Scholar] [CrossRef]
- Lanes, C.F.; Sampaio, L.A.; Marins, L.F. Evaluation of DNase activity in seminal plasma and uptake of exogenous DNA by spermatozoa of the Brazilian flounder Paralichthys orbignyanus. Theriogenology 2009, 71, 525–533. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Funnell, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, D.N. Comparative studies on bull and stallion seminal DNase activity and interaction with semen extender and spermatozoa. Anim. Reprod. Sci. 2010, 121, 249–258. [Google Scholar] [CrossRef]
- Dalin, A.M.; Kaeoket, K.; Persson, E. Immune cell infiltration of normal and impaired sow endometrium. Anim. Reprod. Sci. 2004, 83, 401–413. [Google Scholar] [CrossRef]
- Wicherek, L. The role of the endometrium in the regulation of immune cell activity. Front. Biosci. 2008, 13, 1018–1035. [Google Scholar] [CrossRef]
- Kaeoket, K.; Persson, E.; Dalin, A.M. Influence of pre-ovulatory insemination and early pregnancy on the infiltration by cells of the immune system in the sow endometrium. Anim. Reprod. Sci. 2003, 75, 55–71. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil extracellular traps in the second decade. J. Innate Immun. 2018, 10, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.S.; Foster, D.N. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol. Reprod. 2005, 73, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Encinas, A.; García-Peiró, A.; Ribas-Maynou, J.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Characterization of nuclease activity in human seminal plasma and its relationship to semen parameters, sperm DNA fragmentation and male Infertility. J. Urol. 2016, 195, 213–219. [Google Scholar] [CrossRef]
- Surai, P. Antioxidant action of carnitine: Molecular mechanisms and practical applications. EC Vet. Sci. 2015, 2, 66–84. [Google Scholar]
- Volek, J.S.; Judelson, D.A.; Silvestre, R.; Yamamoto, L.M.; Spiering, B.A.; Hatfield, D.L.; Vingren, J.L.; Quann, E.E.; Anderson, J.M.; Maresh, C.M.; et al. Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. Am. J. Cardiol. 2008, 102, 1413–1417. [Google Scholar] [CrossRef]
- Tsampoukas, G.; Khan, M.F.; Katsouri, A.; Akhter, W.; Moussa, M.; Deliveliotis, K.; Papatsoris, A.; Buchholz, N. L-carnitine as primary or adjuvant treatment in infertile patients with varicocele. A systematic review. Arch. Ital. Urol. Androl. 2020, 2, 92. [Google Scholar] [CrossRef]
- Torriglia, E.; Chaudun, Y.; Courtois, M.F. On the use of Zn2+ to discriminate endonucleases activated during apoptosis. Biochimie 1997, 79, 435–438. [Google Scholar] [CrossRef]
- García-Contreras, A.; De Loera, Y.; García-Artiga, C.; Palomo, A.; Guevara, J.A.; Herrera-Haro, J.; López-Fernández, C.; Johnston, S.; Gosálvez, J. Elevated dietary intake of Zn-methionate is associated with increased sperm DNA fragmentation in the boar. Reprod. Toxicol. 2011, 31, 570–573. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosálvez, J.; Fernández, C.L.; Johnston, S.D.; Bartolomé-Nebreda, J. Role of DNase Activity in Human Sperm DNA Fragmentation. Biomolecules 2024, 14, 304. https://doi.org/10.3390/biom14030304
Gosálvez J, Fernández CL, Johnston SD, Bartolomé-Nebreda J. Role of DNase Activity in Human Sperm DNA Fragmentation. Biomolecules. 2024; 14(3):304. https://doi.org/10.3390/biom14030304
Chicago/Turabian StyleGosálvez, Jaime, Carmen López Fernández, Stephen D. Johnston, and Javier Bartolomé-Nebreda. 2024. "Role of DNase Activity in Human Sperm DNA Fragmentation" Biomolecules 14, no. 3: 304. https://doi.org/10.3390/biom14030304
APA StyleGosálvez, J., Fernández, C. L., Johnston, S. D., & Bartolomé-Nebreda, J. (2024). Role of DNase Activity in Human Sperm DNA Fragmentation. Biomolecules, 14(3), 304. https://doi.org/10.3390/biom14030304





