LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases
Abstract
1. Introduction
2. Antimicrobial Peptides (AMPs) as Potential Alternatives to Antibiotic Resistance
3. Cathelicidin-Derived AMPs
4. Importance of Structures of AMPs
5. Biological Properties of LL37
5.1. Antimicrobial and Antiviral Activities of LL-37
5.2. Anticancer Activity of LL-37
5.3. Other Functional Properties of LL-37
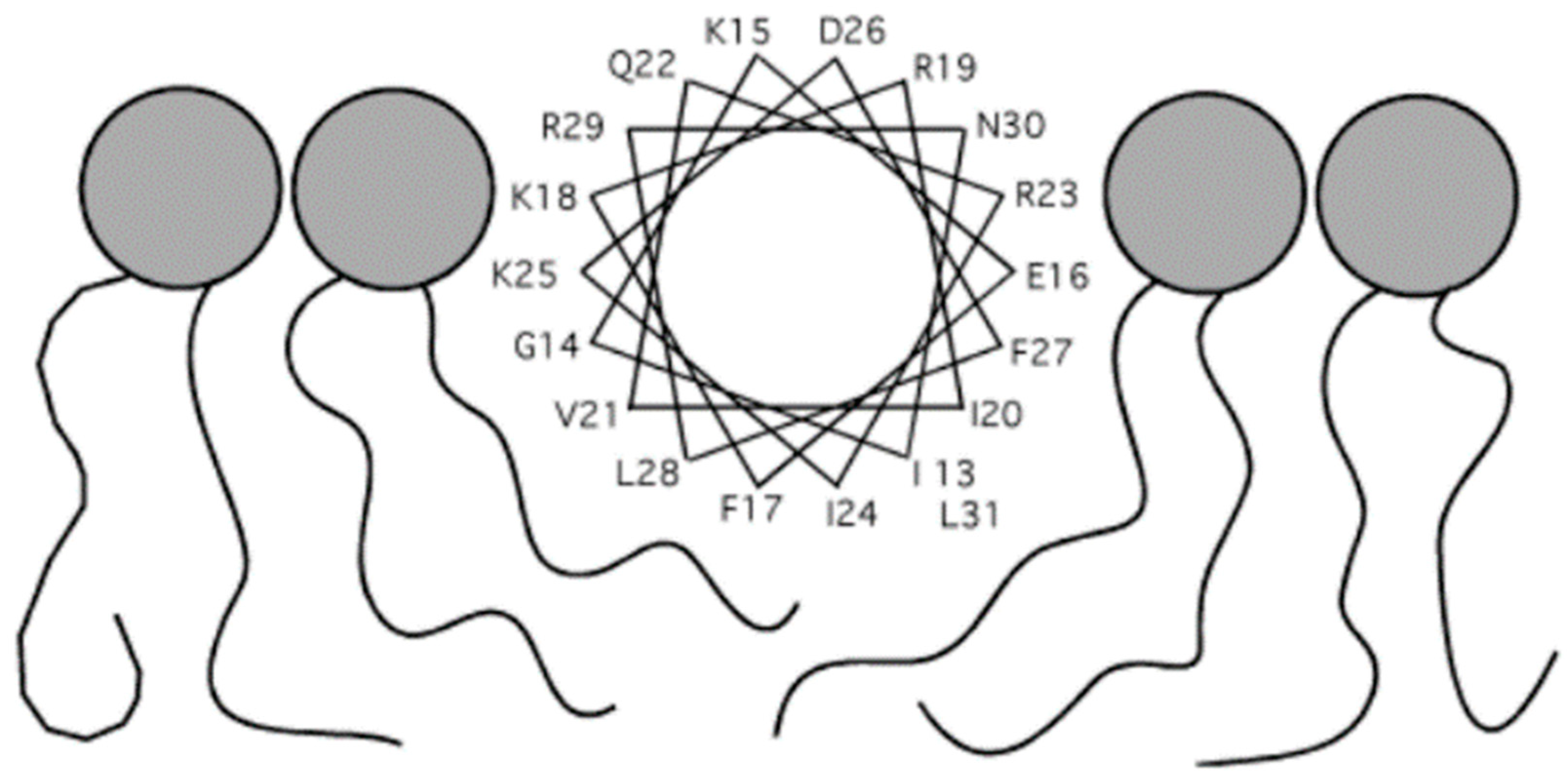
6. Structures of LL-37
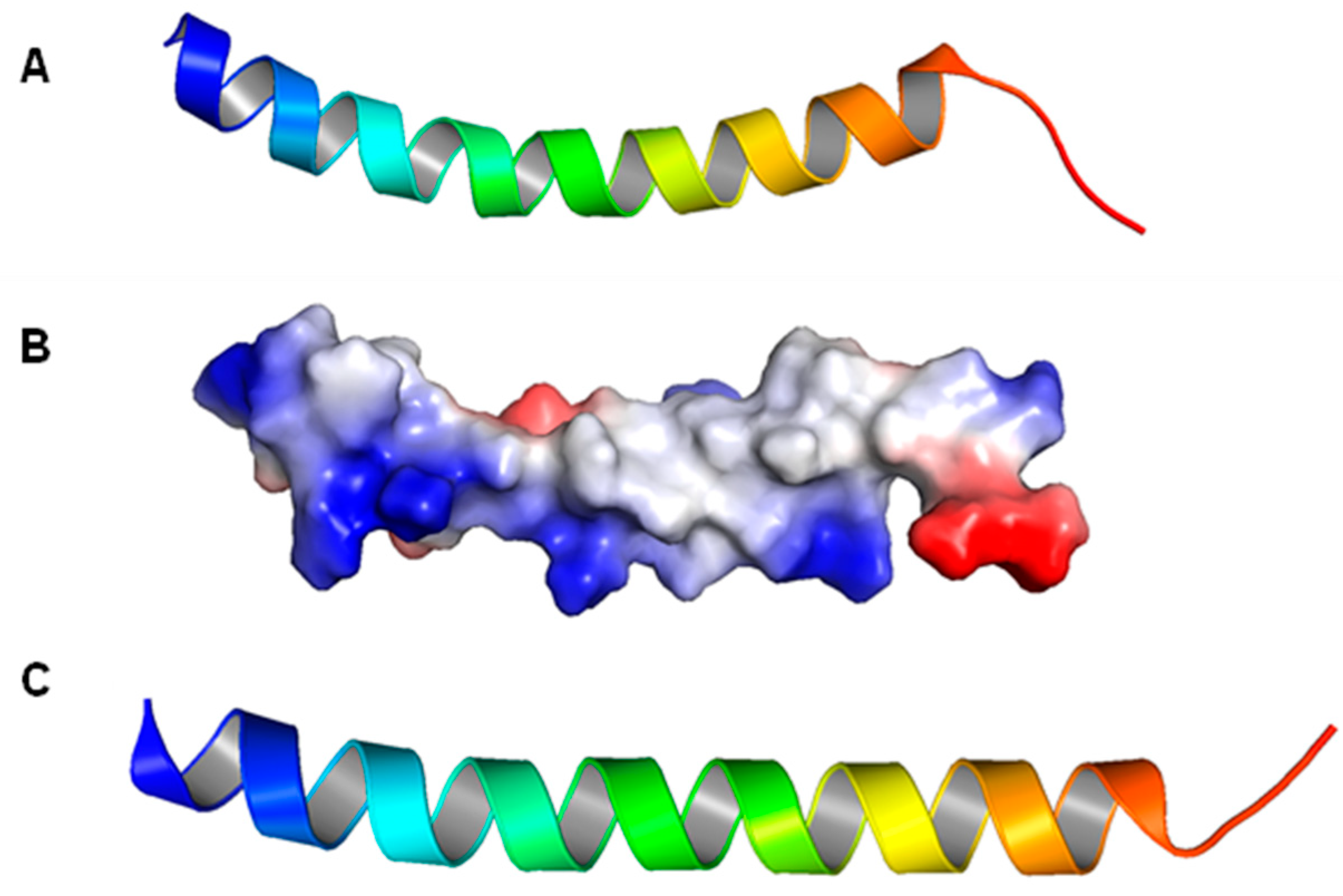
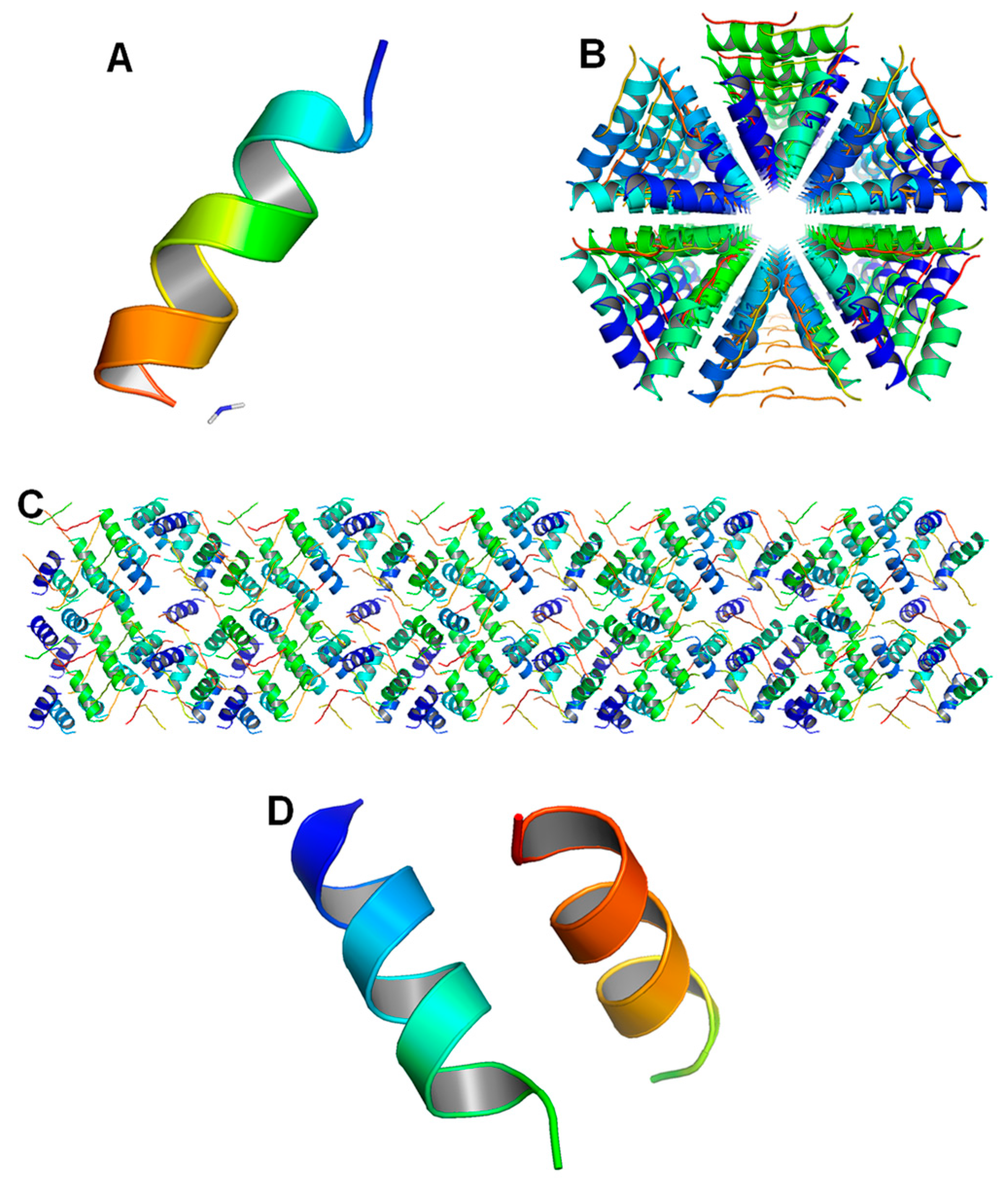
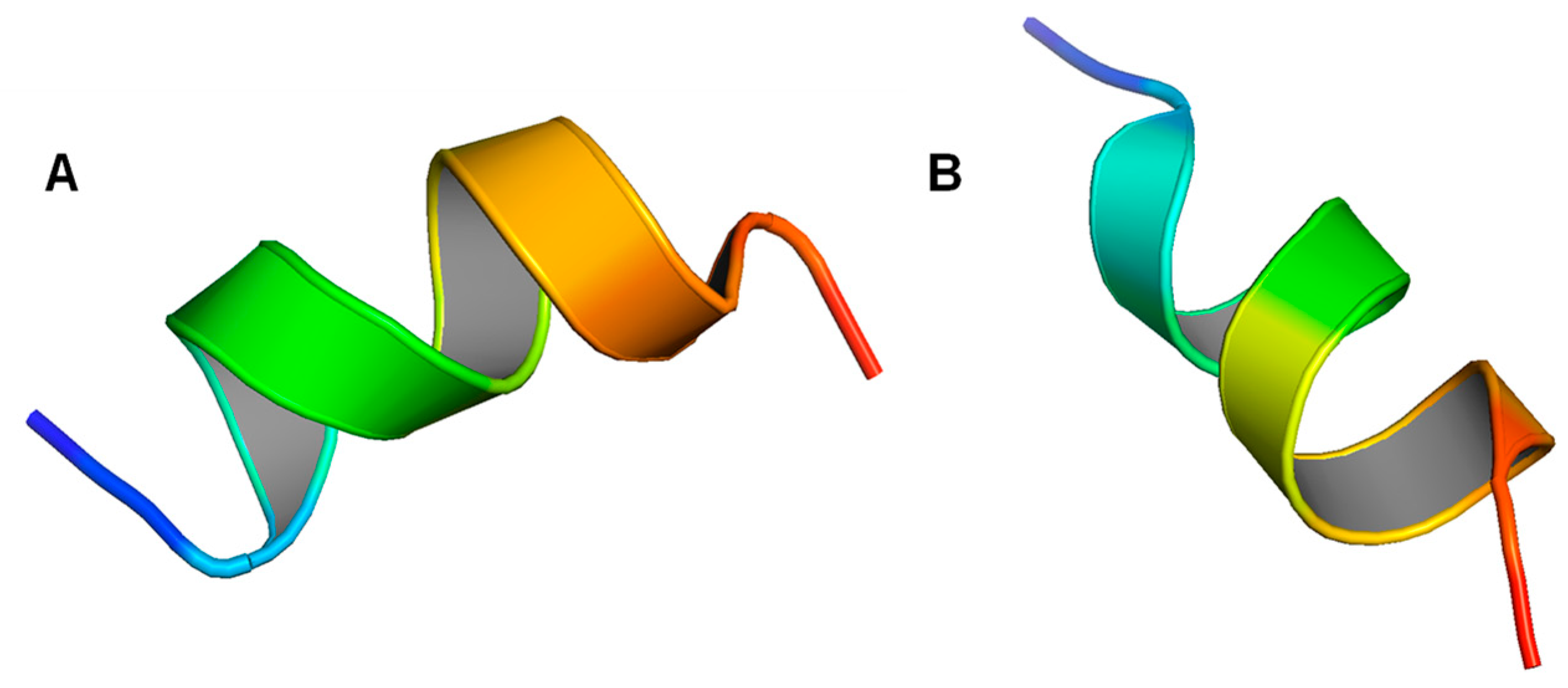
6.1. Monomeric Structures of LL-37
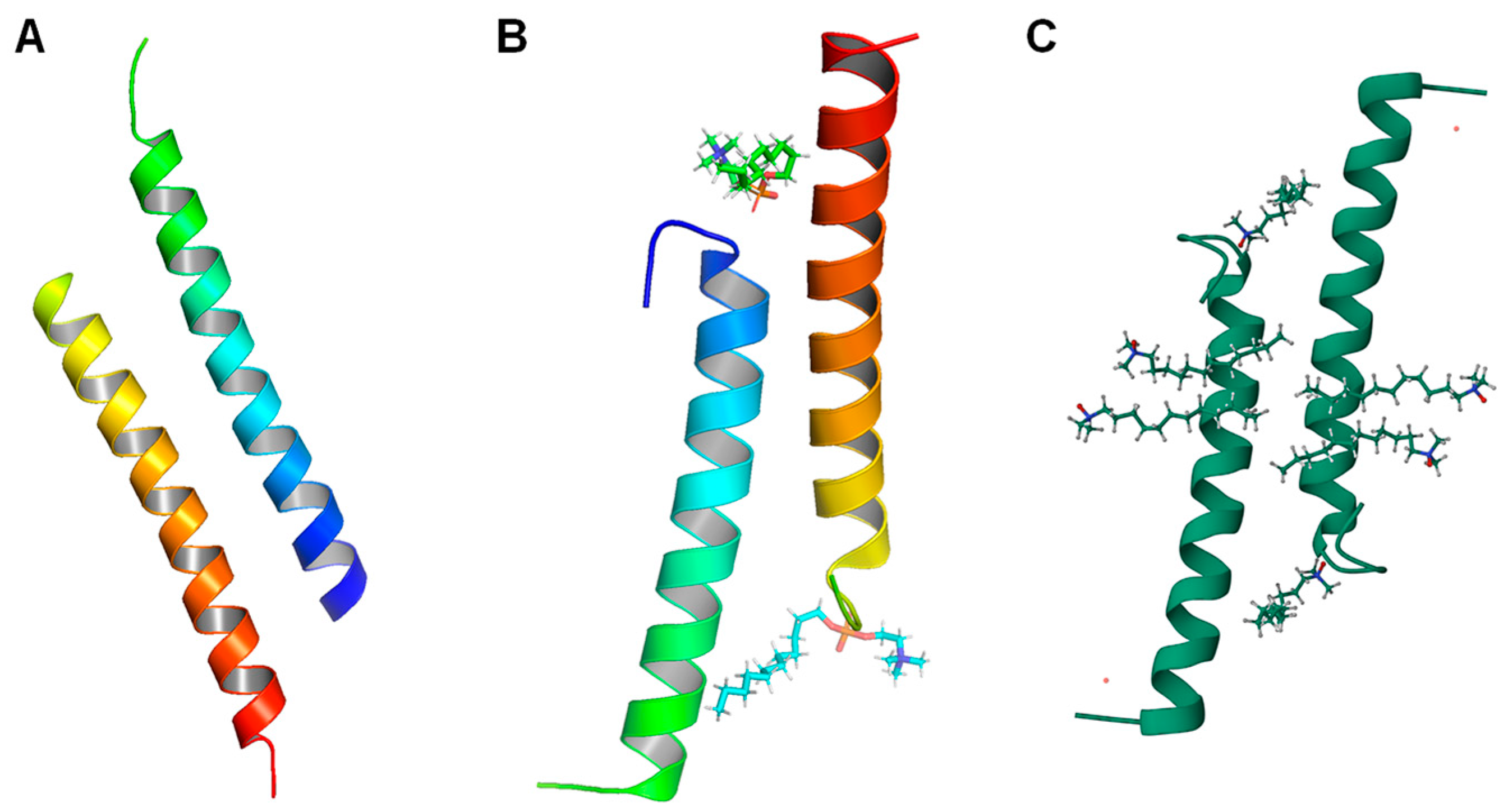
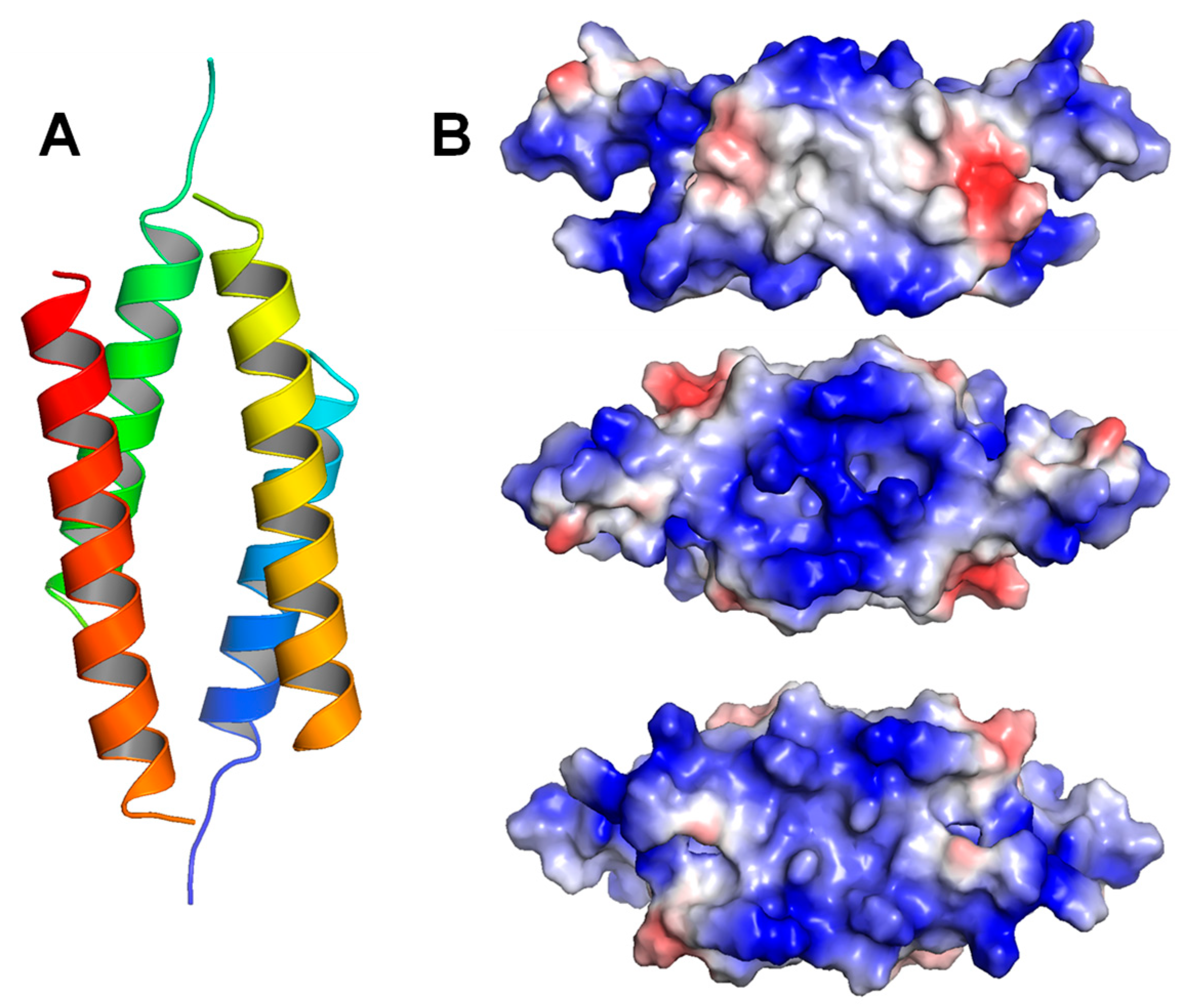
6.2. Oligomeric Structures of LL-37
7. LL-37 Derivatives
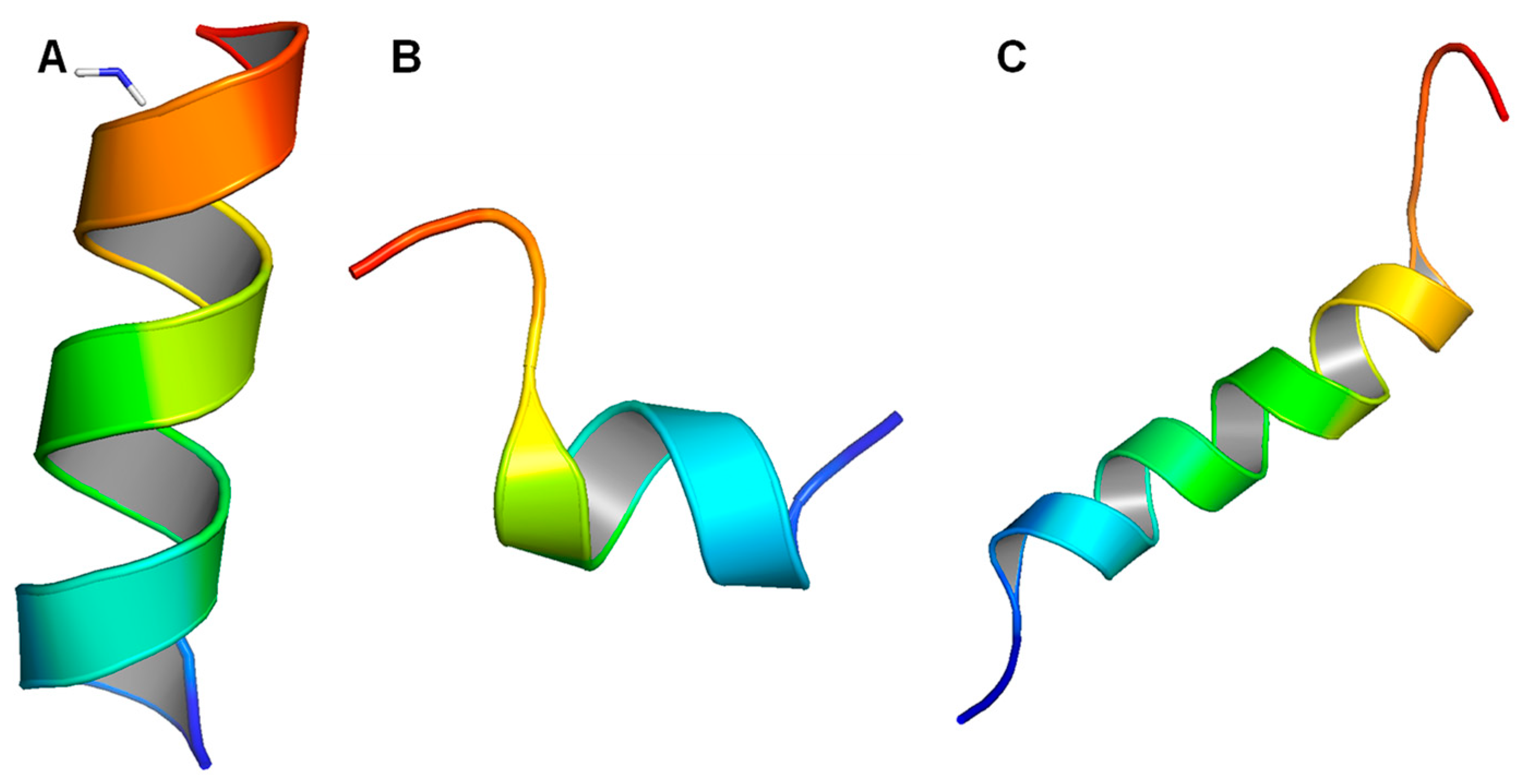
7.1. Core Peptide and Related Fragments
7.2. KR-12 Based Peptides
8. Solid-State NMR Studies on the Mechanism of Membrane Disruption by LL-37
9. Influence of LL-37 on Amyloid Aggregation
10. Summary and Future Directions
Funding
Conflicts of Interest
References
- Taubes, G. The Bacteria Fight Back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, K. Resistance Fighters. Science 2016, 352, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A Naturally Inspired Antibiotic to Target Multidrug-resistant Pathogens. Nature 2022, 601, 606–611. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2019.
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, Extensively Drug-resistant and Pandrug-resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- May, M. Drug Development: Time for Teamwork. Nature 2014, 509, S4–S5. [Google Scholar] [CrossRef]
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.; Chen, Y.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J. Optimized Arylomycins Are a New Class of Gram-negative Antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, J.D.; Birkelbach, J.; Walesch, S.; Müller, R. Current developments in antibiotic discovery: Global microbial diversity as a source for evolutionary optimized anti-bacterials: Global microbial diversity as a source for evolutionary optimized anti-bacterials. EMBO Rep. 2023, 24, e56184. [Google Scholar] [CrossRef]
- Madden, J.; Outterson, K. Trends in the Global Antibiotics Market. Nat. Rev. Drug Discov. 2023, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.; Farhadi, T.; Ganjparvar, M. Linezolid: A Review of Its Properties, Function, and Use in Critical Care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Adams-Sapper, S.; Nolen, S.; Donzelli, G.F.; Lal, M.; Chen, K.; Justo Da Silva, L.H.; Moreira, B.M.; Riley, L.W. Rapid Induction of High-level Carbapenem Resistance in Heteroresistant Kpc-producing Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 2015, 59, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Balm, M.N.D.; La, M.-V.; Krishnan, P.; Jureen, R.; Lin, R.T.P.; Teo, J.W.P. Emergence of Klebsiella Pneumoniae Co-producing Ndm-type and OXA-181 Carbapenemases. Clin. Microbiol. Infect. 2013, 19, E421–E423. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, F.; Cajal, Y. Recent Advances and Perspectives in the Design and Development of Polymyxins. Nat. Prod. Rep. 2017, 34, 886–908. [Google Scholar] [CrossRef]
- Brown, P.; Dawson, M.J. Development of New Polymyxin Derivatives for Multi-drug Resistant Gram-negative Infections. J. Antibiot. 2017, 70, 386–394. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms: My Perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Straus, S.K. Design, Engineering and Discovery of Novel α-Helical and β-Boomerang Antimicrobial Peptides against Drug Resistant Bacteria. Int. J. Mol. Sci. 2020, 21, 5773. [Google Scholar] [CrossRef]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the Host Defense Peptide Landscape. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Intern. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 43, 3547–3567. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Madera, L.; Hoskin, D.W. Protocols for Studying Antimicrobial Peptides (AMPs) as Anticancer Agents. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 331–343. [Google Scholar]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self defence? Biochem. Soc. Trans. 2001, 29, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U. Global antimicrobial resistance in Gram-negative pathogens and clinical need. Curr. Opin. Microbiol. 2017, 39, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. Antibiotic Resistance Breakers: Can Repurposed Drugs Fill the Antibiotic Discovery Void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The Drug-resistant Bacteria That Pose the Greatest Health Threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; López, C.A.; Gnanakaran, S. Permeability Barrier of Gram-negative Cell Envelopes and Approaches to Bypass It. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef]
- Bhattacharjya, S. NMR Structures and Interactions of Antimicrobial Peptides with Lipopolysaccharide: Connecting Structures to Functions. Curr. Top. Med. Chem. 2015, 16, 4–15. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Mohid, S.A.; Bhunia, A. Atomic-resolution Structures and Mode of Action of Clinically Relevant Antimicrobial Peptides. Lancet Infect. Dis. 2022, 23, 4558. [Google Scholar] [CrossRef]
- Luther, A.; Urfer, M.; Zahn, M.; Müller, M.; Wang, S.-Y.; Mondal, M.; Vitale, A.; Hartmann, J.-B.; Sharpe, T.; Monte, F.L. Chimeric Peptidomimetic Antibiotics Against Gram-negative Bacteria. Nature 2019, 576, 452–458. [Google Scholar] [CrossRef]
- Nicolas, I.; Bordeau, V.; Bondon, A.; Baudy-Floc’H, M.; Felden, B. Novel Antibiotics Effective Against Gram-positive and -negative Multi-resistant Bacteria with Limited Resistance. PLoS Biol. 2019, 17, e3000337. [Google Scholar] [CrossRef]
- Chen, C.H.; Bepler, T.; Pepper, K.; Fu, D.; Lu, T.K. Synthetic molecular evolution of antimicrobial peptides. Curr. Opin. Biotechnol. 2022, 75, 102718. [Google Scholar] [CrossRef]
- Shelburne, C.E.; An, F.Y.; Dhople, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The spectrum of antimicrobial activity of bacteriocin subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Lakshmaiah Narayana, J.; Lushnikova, T.; Wang, X.; Wang, G. Low Cationicity Is Important for Systemic in Vivo Efficacy of Database-derived Peptides Against Drug-resistant Gram-positive Pathogens. Proc. Natl. Acad. Sci. USA 2019, 116, 13517–13522. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Brabet, E.; Oi, K.K.; Desjonquères, N.; Moehle, K.; Le Poupon, K.; Hell, S.; Gable, S.; Rithié, V.; Dillinger, S. Peptidomimetic Antibiotics Disrupt the Lipopolysaccharide Transport Bridge of Drug-resistant Enterobacteriaceae. Sci. Adv. 2023, 9, eadg3683. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Lancet Infect. Dis. 2021, 22, 1522. [Google Scholar] [CrossRef]
- Nyembe, P.L.; Ntombela, T.; Makatini, M.M. Review: Structure-activity Relationship of Antimicrobial Peptoids. Pharmaceutics 2023, 15, 1506. [Google Scholar] [CrossRef]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K. Integrated Evolutionary Analysis Reveals Antimicrobial Peptides with Limited Resistance. Nat. Commun. 2019, 10, 4538. [Google Scholar] [CrossRef]
- Lázár, V.; Martins, A.; Spohn, R.; Daruka, L.; Grézal, G.; Fekete, G.; Számel, M.; Jangir, P.K.; Kintses, B.; Csörgő, B. Antibiotic-resistant Bacteria Show Widespread Collateral Sensitivity to Antimicrobial Peptides. Nat. Microbiol. 2018, 3, 718–731. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Zhong, S.; Tschachler, A.; Mlitz, V.; Karner, S.; Elbe-Bürger, A.; Mildner, M. Fetal Human Keratinocytes Produce Large Amounts of Antimicrobial Peptides: Involvement of Histone-methylation Processes. J. Investig. Dermatol. 2014, 134, 2192–2201. [Google Scholar] [CrossRef]
- Underwood, M.; Bakaletz, L. Innate Immunity and the Role of Defensins in Otitis Media. Curr. Allergy Asthma Rep. 2011, 11, 499–507. [Google Scholar] [CrossRef]
- Jones, D.E.; Bevins, C.L. Defensin-6 Mrna in Human Paneth Cells: Implications for Antimicrobia Peptides in Host Defense of the Human Bowel. FEBS Lett. 1993, 315, 187–192. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Thomas, U.; Pellegrini, A. A Helix-loop-helix Peptide at the Upper Lip of the Active Site Cleft of Lysozyme Confers Potent Antimicrobial Activity with Membrane Permeabilization Action. J. Biol. Chem. 2001, 276, 43767–43774. [Google Scholar] [CrossRef] [PubMed]
- Pane, K.; Sgambati, V.; Zanfardino, A.; Smaldone, G.; Cafaro, V.; Angrisano, T.; Pedone, E.; Di Gaetano, S.; Capasso, D.; Haney, E.F. A New Cryptic Cationic Antimicrobial Peptide from Human Apolipoprotein E with Antibacterial Activity and Immunomodulatory Effects on Human Cells. FEBS J. 2016, 283, 2115–2131. [Google Scholar] [CrossRef]
- Sinha, S.; Harioudh, M.K.; Dewangan, R.P.; Ng, W.J.; Ghosh, J.K.; Bhattacharjya, S. Cell-selective Pore Forming Antimicrobial Peptides of the Prodomain of Human Furin: A Conserved Aromatic/cationic Sequence Mapping, Membrane Disruption, and Atomic-resolution Structure and Dynamics. ACS Omega 2018, 3, 14650–14664. [Google Scholar] [CrossRef]
- Agerberth, B.; Gunne, H.; Odeberg, J.; Kogner, P.; Boman, H.G.; Gudmundsson, G.H. FALL-39, a Putative Human Peptide Antibiotic, Is Cysteine-free and Expressed in Bone Marrow and Testis. Proc. Natl. Acad. Sci. USA 1995, 92, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP18: A Novel Antimicrobial Lipopolysaccharide-binding Protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar] [CrossRef]
- Cowland, J.B.; Johnsen, A.H.; Borregaard, N. Hcap-18, a Cathelin/pro-bactenecin-like Protein of Human Neutrophil Specific Granules. FEBS Lett. 1995, 368, 173–176. [Google Scholar] [CrossRef]
- Selsted, M.E.; Harwig, S.S.; Ganz, T.; Schilling, J.W.; Lehrer, R.I. Primary Structures of Three Human Neutrophil Defensins. J. Clin. Investig. 1985, 76, 1436–1439. [Google Scholar] [CrossRef]
- Wilde, C.G.; Griffith, J.E.; Marra, M.N.; Snable, J.L.; Scott, R.W. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J. Biol. Chem. 1989, 264, 11200–11203. [Google Scholar] [CrossRef]
- Jones, D.E.; Bevins, C.L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 1992, 267, 23216–23225. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [CrossRef]
- Bensch, K.W.; Raida, M.; Mägert, H.-J.; Schulz-Knappe, P.; Forssmann, W.-G. Hbd-1: A Novel Β-defensin from Human Plasma. FEBS Lett. 1995, 368, 331–335. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.-M. A Peptide Antibiotic from Human Skin. Nature 1997, 387, 861. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.-M. Isolation and Characterization of Human Μ-defensin-3, a Novel Human Inducible Peptide Antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- García, J.R.; Krause, A.; Schulz, S.; Rodríguez-Jiménez, F.J.; Klüver, E.; Adermann, K.; Forssmann, U.; Frimpong-Boateng, A.; Bals, R.; Forssmann, W.G. Human beta-defensin 4: A novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001, 15, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F. Dermcidin: A Novel Human Antibiotic Peptide Secreted by Sweat Glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Stenger, S.; Hanson, D.A.; Teitelbaum, R.; Dewan, P.; Niazi, K.R.; Froelich, C.J.; Ganz, T.; Thoma-Uszynski, S.; Melián, A.; Bogdan, C.; et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998, 282, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hieshima, K.; Ohtani, H.; Shibano, M.; Izawa, D.; Nakayama, T.; Kawasaki, Y.; Shiba, F.; Shiota, M.; Katou, F.; Saito, T.; et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 2003, 170, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Krijgsveld, J.; Zaat, S.A.J.; Meeldijk, J.; Van Veelen, P.A.; Fang, G.; Poolman, B.; Brandt, E.; Ehlert, J.E.; Kuijpers, A.J.; Engbers, G.H.M. Thrombocidins, Microbicidal Proteins from Human Blood Platelets, Are C-terminal Deletion Products of CXC Chemokines. J. Biol. Chem. 2000, 275, 20374–20381. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Neitz, S.; Mägert, H.-J.; Schulz, A.; Forssmann, W.-G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a Novel Highly Disulfide-bonded Human Peptide, Exhibits Antimicrobial Activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, M.; Cristiani, S.; Lipton, J.M.; Catania, A. Antimicrobial Effects of A-msh Peptides. J. Leukoc. Biol. 2000, 67, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Chan, L.C.; Wang, H.; Lieng, J.; Hung, M.; Srinivasan, Y.; Wang, J.; Waschek, J.A.; Ferguson, A.L.; Lee, K.-F. PACAP Is a Pathogen-inducible Resident Antimicrobial Neuropeptide Affording Rapid and Contextual Molecular Host Defense of the Brain. Proc. Natl. Acad. Sci. USA 2021, 118, e1917623117. [Google Scholar] [CrossRef]
- Tam, C.; Mun, J.J.; Evans, D.J.; Fleiszig, S.M.J. Cytokeratins Mediate Epithelial Innate Defense Through Their Antimicrobial Properties. J. Clin. Investig. 2012, 122, 3665–3677. [Google Scholar] [CrossRef]
- Tollner, T.L.; Yudin, A.I.; Tarantal, A.F.; Treece, C.A.; Overstreet, J.W.; Cherr, G.N. Beta-defensin 126 on the Surface of Macaque Sperm Mediates Attachment of Sperm to Oviductal Epithelia1. Biol. Reprod. 2008, 78, 400–412. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Lenarčič, B.; Ritonja, A.; Dolenc, I.; Stoka, V.; Berbič, S.; Pungerčar, J.; Štrukelj, B.; Turk, V. Pig Leukocyte Cysteine Proteinase Inhibitor (PLCPI), a New Member of the Stefin Family. FEBS Lett. 1993, 336, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ritonja, A.; Kopitar, M.; Jerala, R.; Turk, V. Primary Structure of a New Cysteine Proteinase Inhibitor from Pig Leucocytes. FEBS Lett. 1989, 255, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Storici, P.; Tossi, A.; Lenarčič, B.; Romeo, D. Purification and Structural Characterization of Bovine Cathelicidins, Precursors of Antimicrobial Peptides. Eur. J. Biochem. 1996, 238, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Wang, S.; Zanetti, M. Structural Organization of the Bovine Cathelicidin Gene Family and Identification of a Novel Member1. FEBS Lett. 1997, 417, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.E.; Berndt, K.D.; Agerberth, B. Conformation-dependent Antibacterial Activity of the Naturally Occurring Human Peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Brock, R.; Luh, F.; Chou, P.-J.; Larrick, J.W.; Huang, R.-F.; Huang, T.-H. The Solution Structure of the Active Domain of CAP18—A Lipopolysaccharide Binding Protein from Rabbit Leukocytes. FEBS Lett. 1995, 370, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Cady, S.D.; Tang, M.; Waring, A.J.; Lehrer, R.I.; Hong, M. Membrane-dependent Oligomeric Structure and Pore Formation of a Β-hairpin Antimicrobial Peptide in Lipid Bilayers from Solid-state NMR. Proc. Natl. Acad. Sci. USA 2006, 103, 16242–16247. [Google Scholar] [CrossRef] [PubMed]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, B.; Davis, E.G.; Ross, C.R.; Blecha, F. Cathelicidins: Microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002, 4, 361–372. [Google Scholar] [CrossRef]
- Xiao, Y.; Cai, Y.; Bommineni, Y.R.; Fernando, S.C.; Prakash, O.; Gilliland, S.E.; Zhang, G. Identification and Functional Characterization of Three Chicken Cathelicidins with Potent Antimicrobial Activity. J. Biol. Chem. 2006, 281, 2858–2867. [Google Scholar] [CrossRef]
- Bhunia, A.; Mohanram, H.; Bhattacharjya, S. Lipopolysaccharide bound structures of the active fragments of fowlicidin-1, a cathelicidin family of antimicrobial and antiendotoxic peptide from chicken, determined by transferred nuclear Overhauser effect spectroscopy. Biopolymers 2009, 92, 9–22. [Google Scholar] [CrossRef]
- Bals, R.; Lang, C.; Weiner, D.J.; Vogelmeier, C.; Welsch, U.; Wilson, J.M. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin. Diagn. Lab. Immunol. 2001, 9, 370–375. [Google Scholar] [CrossRef]
- Gallo, R.L.; Kim, K.J.; Bernfield, M.; Kozak, C.A.; Zanetti, M.; Merluzzi, L.; Gennaro, R. Identification of CRAMP, a Cathelin-related Antimicrobial Peptide Expressed in the Embryonic and Adult Mouse. J. Biol. Chem. 1997, 272, 13088–13093. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tsutsumi-Ishii, Y.; Yomogida, S.; Yamashita, T. Isolation of Cdna Encoding Guinea Pig Neutrophil Cationic Antibacterial Polypeptide of 11 Kda (CAP11) and Evaluation of CAP11 Mrna Expression During Neutrophil Maturation. J. Biol. Chem. 1997, 272, 22742–22750. [Google Scholar] [CrossRef][Green Version]
- Lawyer, C.; Pai, S.; Watabe, M.; Borgia, P.; Mashimo, T.; Eagleton, L.; Watabe, K. Antimicrobial Activity of a 13 Amino Acid Tryptophan-rich Peptide Derived from a Putative Porcine Precursor Protein of a Novel Family of Antibacterial Peptides. FEBS Lett. 1996, 390, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Gidalevitz, D.; Ishitsuka, Y.; Muresan, A.S.; Konovalov, O.; Waring, A.J.; Lehrer, R.I.; Lee, K.Y.C. Interaction of Antimicrobial Peptide Protegrin with Biomembranes. Proc. Natl. Acad. Sci. USA 2003, 100, 6302–6307. [Google Scholar] [CrossRef]
- Tossi, A.; Scocchi, M.; Zanetti, M.; Storici, P.; Gennaro, R. PMAP-37, a Novel Antibacterial Peptide from Pig Myeloid Cells. Cdna Cloning, Chemical Synthesis and Activity. Eur. J. Biochem. 1995, 228, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Lee, J.; Bergman, T.; Carlquist, M.; Boman, H.G.; Mutt, V.; Jörnvall, H. Amino Acid Sequence of PR-39. Eur. J. Biochem. 1991, 202, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.; Skerlavaj, B.; Bolognesi, M.; Gennaro, R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J. Biol. Chem. 1988, 263, 9573–9575. [Google Scholar] [CrossRef] [PubMed]
- Bagella, L.; Scocchi, M.; Zanetti, M. Cdna Sequences of Three Sheep Myeloid Cathelicidins. FEBS Lett. 1995, 376, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Skerlavaj, B.; Gennaro, R.; Bagella, L.; Merluzzi, L.; Risso, A.; Zanetti, M. Biological Characterization of Two Novel Cathelicidin-derived Peptides and Identification of Structural Requirements for Their Antimicrobial and Cell Lytic Activities. J. Biol. Chem. 1996, 271, 28375–28381. [Google Scholar] [CrossRef]
- Thennarasu, S.; Tan, A.; Penumatchu, R.; Shelburne, C.E.; Heyl, D.L.; Ramamoorthy, A. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL-37. Biophys. J. 2010, 98, 248–257. [Google Scholar] [CrossRef]
- Travis, S.M.; Anderson, N.N.; Forsyth, W.R.; Espiritu, C.; Conway, B.D.; Greenberg, E.P.; Mccray, P.B.; Lehrer, R.I.; Welsh, M.J.; Tack, B.F. Bactericidal Activity of Mammalian Cathelicidin-derived Peptides. Infect. Immun. 2000, 68, 2748–2755. [Google Scholar] [CrossRef]
- Schlusselhuber, M.; Torelli, R.; Martini, C.; Leippe, M.; Cattoir, V.; Leclercq, R.; Laugier, C.; Grötzinger, J.; Sanguinetti, M.; Cauchard, J. The Equine Antimicrobial Peptide Ecath1 Is Effective Against the Facultative Intracellular Pathogen Rhodococcus Equi in Mice. Antimicrob. Agents Chemother. 2013, 57, 4615–4621. [Google Scholar] [CrossRef]
- Uzzell, T.; Stolzenberg, E.D.; Shinnar, A.E.; Zasloff, M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides 2003, 24, 1655–1667. [Google Scholar] [CrossRef]
- Santana, F.L.; Estrada, K.; Alford, M.A.; Wu, B.C.; Dostert, M.; Pedraz, L.; Akhoundsadegh, N.; Kalsi, P.; Haney, E.F.; Straus, S.K. Novel Alligator Cathelicidin As-cath8 Demonstrates Anti-infective Activity Against Clinically Relevant and Crocodylian Bacterial Pathogens. Antibiotics 2022, 11, 1603. [Google Scholar] [CrossRef]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, F.; Verardi, R.; Shi, L.; Henzler-Wildman, K.A.; Ramamoorthy, A.; Veglia, G. NMR Structure of the Cathelicidin-derived Human Antimicrobial Peptide LL-37 in Dodecylphosphocholine Micelles. Biochemistry 2008, 47, 5565–5572. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Soblosky, L.; Nguyen, K.; Geng, J.; Yu, X.; Ramamoorthy, A.; Chen, Z. Physiologically-relevant Modes of Membrane Interactions by the Human Antimicrobial Peptide, LL-37, Revealed by SFG Experiments. Sci. Rep. 2013, 3, srep01854. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Structures of Human Host Defense Cathelicidin LL-37 and Its Smallest Antimicrobial Peptide KR-12 in Lipid Micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [PubMed]
- Valore, E.V.; Park, C.H.; Quayle, A.J.; Wiles, K.R.; Mccray, P.B.; Ganz, T. Human Beta-defensin-1: An Antimicrobial Peptide of Urogenital Tissues. J. Clin. Investig. 1998, 101, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta 2006, 1758, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Lichtenstein, A.K.; Ganz, T. Defensins: Antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 1993, 11, 105–128. [Google Scholar] [CrossRef]
- Gudmundsson, G.H.; Agerberth, B.; Odeberg, J.; Bergman, T.; Olsson, B.; Salcedo, R. The Human Gene FALL39 and Processing of the Cathelin Precursor to the Antibacterial Peptide LL-37 in Granulocytes. Eur. J. Biochem. 1996, 238, 325–332. [Google Scholar] [CrossRef]
- Sinha, S.; Zheng, L.; Mu, Y.; Ng, W.J.; Bhattacharjya, S. Structure and Interactions of A Host Defense Antimicrobial Peptide Thanatin in Lipopolysaccharide Micelles Reveal Mechanism of Bacterial Cell Agglutination. Sci. Rep. 2017, 7, 17795. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.N.; Bhunia, A.; Ramamoorthy, A.; Bhattacharjya, S. Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: Role of the helical hairpin conformation in outer-membrane permeabilization. J. Am. Chem. Soc. 2010, 132, 18417–18428. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Mohanram, H.; Domadia, P.N.; Torres, J.; Bhattacharjya, S. Designed Β-boomerang Antiendotoxic and Antimicrobial Peptides. J. Biol. Chem. 2009, 284, 21991–22004. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, H.; Kim, J.; Lee, D.; Malmsten, M.; Bhunia, A. Structural Insights into the Combinatorial Effects of Antimicrobial Peptides Reveal a Role of Aromatic–aromatic Interactions in Antibacterial Synergism. J. Biol. Chem. 2019, 294, 14615–14633. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Jaiswal, N.; Ilyas, H.; Debnath, S.; Biswas, K.; Kumar, D.; Bhunia, A. Glycine-Mediated Short Analogue of a Designed Peptide in Lipopolysaccharide Micelles: Correlation Between Compact Structure and Anti-Endotoxin Activity. Biochemistry 2017, 56, 1348–1362. [Google Scholar] [CrossRef]
- Jakubec, M.; Rylandsholm, F.G.; Rainsford, P.; Silk, M.; Bril’Kov, M.; Kristoffersen, T.; Juskewitz, E.; Ericson, J.U.; Svendsen, J.S.M. Goldilocks Dilemma: LPS Works Both as the Initial Target and a Barrier for the Antimicrobial Action of Cationic Amps on E. Coli. Biomolecules 2023, 13, 1155. [Google Scholar] [CrossRef]
- Mares, J.; Kumaran, S.; Gobbo, M.; Zerbe, O. Interactions of Lipopolysaccharide and Polymyxin Studied by NMR Spectroscopy. J. Biol. Chem. 2009, 284, 11498–11506. [Google Scholar] [CrossRef]
- Swarbrick, J.D.; Karas, J.A.; Li, J.; Velkov, T. Structure of Micelle Bound Cationic Peptides by NMR Spectroscopy Using a Lanthanide Shift Reagent. Chem. Commun. 2020, 56, 2897–2900. [Google Scholar] [CrossRef]
- Yu, K.; Park, K.; Kang, S.W.; Shin, S.Y.; Hahm, K.S.; Kim, Y. Solution structure of a cathelicidin-derived antimicrobial peptide, CRAMP as determined by NMR spectroscopy. J. Pept. Res. 2002, 60, 1–9. [Google Scholar] [CrossRef]
- Park, K.; Oh, D.; Shin, S.Y.; Hahm, K.S.; Kim, Y. Structural studies of porcine myeloid antibacterial peptide PMAP-23 and its analogues in DPC micelles by NMR spectroscopy. Biochem. Biophys. Res. Commun. 2002, 290, 204–212. [Google Scholar] [CrossRef]
- Tack, B.F.; Sawai, M.V.; Kearney, W.R.; Robertson, A.D.; Sherman, M.A.; Wang, W.; Hong, T.; Boo, L.M.; Wu, H.; Waring, A.J. SMAP-29 Has Two Lps-binding Sites and a Central Hinge. Eur. J. Biochem. 2002, 269, 1181–1189. [Google Scholar] [CrossRef]
- Yang, S.; Lee, C.W.; Kim, H.J.; Jung, H.H.; Kim, J.I.; Shin, S.Y.; Shin, S.H. Structural analysis and mode of action of BMAP-27, a cathelicidin-derived antimicrobial peptide. Peptides 2019, 118, 170106. [Google Scholar] [CrossRef]
- Xiao, Y.; Dai, H.; Bommineni, Y.R.; Soulages, J.L.; Gong, Y.; Prakash, O.; Zhang, G. Structure–activity Relationships of Fowlicidin-1, a Cathelicidin Antimicrobial Peptide in Chicken. FEBS J. 2006, 273, 2581–2593. [Google Scholar] [CrossRef]
- Bommineni, Y.R.; Dai, H.; Gong, Y.X.; Soulages, J.L.; Fernando, S.C.; Desilva, U.; Prakash, O.; Zhang, G. Fowlicidin-3 is an alpha-helical cationic host defense peptide with potent antibacterial and lipopolysaccharide-neutralizing activities. FEBS J. 2007, 274, 418–428. [Google Scholar] [CrossRef]
- Saravanan, R.; Bhattacharjya, S. Oligomeric structure of a cathelicidin antimicrobial peptide in dodecylphosphocholine micelle determined by NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 2011, 1808, 369–381. [Google Scholar] [CrossRef]
- Chen, J.; Falla, T.J.; Liu, H.; Hurst, M.A.; Fujii, C.A.; Mosca, D.A.; Embree, J.R.; Loury, D.J.; Radel, P.A.; Cheng Chang, C.; et al. Development of protegrins for the treatment and prevention of oral mucositis: Structure-activity relationships of synthetic protegrin analogues. Biopolymers 2000, 55, 88–98. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, N.; Yang, J.; Meng, X.; Yang, R.; Li, J.; Sun, T. Antimicrobial Peptide LL-37 and IDR-1 Ameliorate MRSA Pneumonia in Vivo. Cell. Physiol. Biochem. 2013, 32, 614–623. [Google Scholar] [CrossRef]
- Aronen, M.; Viikari, L.; Langen, H.; Kohonen, I.; Wuorela, M.; Vuorinen, T.; Söderlund-Venermo, M.; Viitanen, M.; Camargo, C.A.; Vahlberg, T. The Long-term Prognostic Value of Serum 25(OH)D, Albumin, and LL-37 Levels in Acute Respiratory Diseases Among Older Adults. BMC Geriatr. 2022, 22, 146. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, Y.; Zhu, J.; Liu, Y.; Sun, M. Proteína 3 Contendo Um Domínio NACHT, Porção C-terminal Rica Em Repetições De Leucina E De Domínio Pirina E LL-37: Valor Prognóstico De Novos Biomarcadores Em Pneumonia Adquirida Na Comunidade. J. Bras. Pneumol. 2019, 45, e20190001. [Google Scholar] [CrossRef]
- Majewski, K.; Żelechowska, P.; Brzezińska-Błaszczyk, E. Circulating Cathelicidin LL-37 in Adult Patients with Pulmonary Infectious Diseases. Clin. Investig. Med. 2017, 40, 34. [Google Scholar] [CrossRef]
- Kozłowska, E.; Wysokiński, A.; Majewski, K.; Agier, J.; Margulska, A.; Brzezińska-Błaszczyk, E. Human Cathelicidin LL-37—Does It Influence the Homeostatic Imbalance in Mental Disorders? J. Biosci. 2018, 43, 321–327. [Google Scholar] [CrossRef]
- Majewski, K.; Kozłowska, E.; Żelechowska, P.; Brzezińska-Błaszczyk, E. Serum Concentrations of Antimicrobial Peptide Cathelicidin LL-37 in Patients with Bacterial Lung Infections. Cent. Eur. J. Immunol. 2018, 43, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Mücke, P.-A.; Maaß, S.; Kohler, T.P.; Hammerschmidt, S.; Becher, D. Proteomic Adaptation of Streptococcus Pneumoniae to the Human Antimicrobial Peptide LL-37. Microorganisms 2020, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.D.; Hesse, L.; Wu, X.; Allam, V.S.R.R.; Van Oldeniel, D.; Bhiekharie, L.J.; Phipps, S.; Oliver, B.G.; Gosens, R.; Sukkar, M.B. LL-37 and HMGB1 Induce Alveolar Damage and Reduce Lung Tissue Regeneration via RAGE. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2021, 321, L641–L652. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, M.; Kan-o, K.; Ishii, Y.; Yamamoto, N.; Ogawa, T.; Fukuyama, S.; Ogawa, A.; Fujita, A.; Nakanishi, Y.; Matsumoto, K. Effects of Cigarette Smoke on Barrier Function and Tight Junction Proteins in the Bronchial Epithelium: Protective Role of Cathelicidin LL-37. Respir. Res. 2019, 20, 251. [Google Scholar] [CrossRef] [PubMed]
- Uysal, P.; Simsek, G.; Durmus, S.; Sozer, V.; Aksan, H.; Yurt, S.; Cuhadaroglu, C.; Kosar, F.; Gelisgen, R.; Uzun, H. evaluation of Plasma Antimicrobial Peptide LL-37 and Nuclear Factor-kappab Levels in Stable Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Y.; Xiao, W.; Zhu, M.-X.; Yang, Z.-H.; Pan, X.-J.; Zhang, Y.; Sun, C.-C.; Xing, Y. The Effect of Human Antibacterial Peptide LL-37 in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Respir. Med. 2012, 106, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhu, M.; Yang, Z.; Pan, X.; Zhang, Y.; Wang, Q.; Xiao, W. LL-37 Secreted by Epithelium Promotes Fibroblast Collagen Production: A Potential Mechanism of Small Airway Remodeling in Chronic Obstructive Pulmonary Disease. Lab. Investig. 2014, 94, 991–1002. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Li, L.; Wu, J.; Yi, W.; Lai, Y.; Qin, L. Ll-37-coupled Porous Composite Scaffold for the Treatment of Infected Segmental Bone Defect. Pharmaceutics 2022, 15, 88. [Google Scholar] [CrossRef]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral Activity and Increased Host Defense Against Influenza Infection Elicited by the Human Cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef]
- Tripathi, S.; Tecle, T.; Verma, A.; Crouch, E.; White, M.; Hartshorn, K.L. The Human Cathelicidin LL-37 Inhibits Influenza A Viruses Through a Mechanism Distinct from That of Surfactant Protein D or Defensins. J. Gen. Virol. 2013, 94, 40–49. [Google Scholar] [CrossRef] [PubMed]
- White, M.R.; Tripathi, S.; Verma, A.; Kingma, P.; Takahashi, K.; Jensenius, J.; Thiel, S.; Wang, G.; Crouch, E.C.; Hartshorn, K.L. Collectins, H-ficolin and LL-37 Reduce Influence Viral Replication in Human Monocytes and Modulate Virus-induced Cytokine Production. Innate Immun. 2017, 23, 77–88. [Google Scholar] [CrossRef]
- Lee, I.H.; Jung, Y.-J.; Cho, Y.G.; Nou, I.S.; Huq, M.A.; Nogoy, F.M.; Kang, K.-K. SP-LL-37, Human Antimicrobial Peptide, Enhances Disease Resistance in Transgenic Rice. PLoS ONE 2017, 12, e0172936. [Google Scholar] [CrossRef] [PubMed]
- Palusinska-Szysz, M.; Jurak, M.; Gisch, N.; Waldow, F.; Zehethofer, N.; Nehls, C.; Schwudke, D.; Koper, P.; Mazur, A. The human LL-37 peptide exerts antimicrobial activity against Legionella micdadei interacting with membrane phospholipids. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2022, 1867, 159138. [Google Scholar] [CrossRef]
- Tripathi, S.; Wang, G.; White, M.; Rynkiewicz, M.; Seaton, B.; Hartshorn, K. Identifying the Critical Domain of LL-37 Involved in Mediating Neutrophil Activation in the Presence of Influenza Virus: Functional and Structural Analysis. PLoS ONE 2015, 10, e0133454. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.I.; Stohl, E.A.; Seifert, H.S. The Neisseria Gonorrhoeae Type IV Pilus Promotes Resistance to Hydrogen Peroxide- and Ll-37-mediated Killing by Modulating the Availability of Intracellular, Labile Iron. PLoS Pathog. 2022, 18, e1010561. [Google Scholar] [CrossRef] [PubMed]
- Kiattiburut, W.; Zhi, R.; Lee, S.G.; Foo, A.C.; Hickling, D.R.; Keillor, J.W.; Goto, N.K.; Li, W.; Conlan, W.; Angel, J.B. Antimicrobial Peptide LL-37 and Its Truncated Forms, GI-20 and GF-17, Exert Spermicidal Effects and Microbicidal Activity Against Neisseria Gonorrhoeae. Hum. Reprod. 2018, 33, 2175–2183. [Google Scholar] [CrossRef]
- Pashapour, A.; Sardari, S.; Ehsani, P. In Silicodesign and in Vitro Evaluation of Some Novel Amps Derived from Human LL-37 as Potential Antimicrobial Agents for Keratitis. Iran. J. Pharm. Res. 2022, 21, e124017. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N.; Mishra, P.; Joseph, J.; Mishra, D.K.; Garg, P.; Roy, S. Differential Expression of Antimicrobial Peptides in Streptococcus Pneumoniae Keratitis and Stat3-dependent Expression of LL-37 by Streptococcus Pneumoniae in Human Corneal Epithelial Cells. Pathogens 2019, 8, 31. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Courrol, D.S.; Chura-Chambi, R.M.; Morganti, L.; Souza, G.O.; Franzolin, M.R.; Wunder, E.A., Jr.; Heinemann, M.B.; Barbosa, A.S. Inactivation of the antimicrobial peptide LL-37 by pathogenic leptospira. Microb. Pathog. 2021, 150, 104704. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Angarita, A.; Aragón, C.C.; Tobón, G.J. Cathelicidin ll-37: A new important molecule in the pathophysiology of systemic lupus erythematosus. J. Transl. Autoimmun. 2020, 3, 100029. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Kaplan, M.J. Little Peptide, Big Effects: The Role of LL-37 in Inflammation and Autoimmune Disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-dna–peptide Complexes in Systemic Lupus Erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory Role of the Antimicrobial LL-37 Peptide in Autoimmune Diseases and Viral Infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Geörg, M.; Maudsdotter, L.; Jonsson, A.-B. Endotoxin, Capsule, and Bacterial Attachment Contribute to Neisseria Meningitidis Resistance to the Human Antimicrobial Peptide LL-37. J. Bacteriol. 2009, 191, 3861–3868. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Svoboda, P.; Pohl, J.; Stephens, D.S.; Shafer, W.M. The Human Host Defense Peptide LL-37 Interacts with Neisseria Meningitidis Capsular Polysaccharides and Inhibits Inflammatory Mediators Release. PLoS ONE 2010, 5, e13627. [Google Scholar] [CrossRef]
- Seib, K.L.; Serruto, D.; Oriente, F.; Delany, I.; Adu-Bobie, J.; Veggi, D.; Aricò, B.; Rappuoli, R.; Pizza, M. Factor H-binding Protein Is Important for Meningococcal Survival in Human Whole Blood and Serum and in the Presence of the Antimicrobial Peptide LL-37. Infect. Immun. 2009, 77, 292–299. [Google Scholar] [CrossRef]
- Gutner, M.; Chaushu, S.; Balter, D.; Bachrach, G. Saliva Enables the Antimicrobial Activity of LL-37 in the Presence of Proteases of Porphyromonas Gingivalis. Infect. Immun. 2009, 77, 5558–5563. [Google Scholar] [CrossRef]
- Chinipardaz, Z.; Zhong, J.M.; Yang, S. Regulation of LL-37 in Bone and Periodontium Regeneration. Life 2022, 12, 1533. [Google Scholar] [CrossRef]
- Tada, H.; Shimizu, T.; Matsushita, K.; Takada, H. Porphyromonas Gingivalisinduced IL-33 Down-regulates Hcap-18/ll-37 Production in Human Gingival Epithelial cells. Biomed. Res. 2017, 38, 167–173. [Google Scholar] [CrossRef]
- Bedran, T.B.L.; Mayer, M.P.A.; Spolidorio, D.P.; Grenier, D. Synergistic Anti-inflammatory Activity of the Antimicrobial Peptides Human Beta-defensin-3 (hbd-3) and Cathelicidin (LL-37) in a Three-dimensional Co-culture Model of Gingival Epithelial Cells and Fibroblasts. PLoS ONE 2014, 9, e106766. [Google Scholar] [CrossRef] [PubMed]
- Puklo, M.; Guentsch, A.; Hiemstra, P.S.; Eick, S.; Potempa, J. Analysis of Neutrophil-derived Antimicrobial Peptides in Gingival Crevicular Fluid Suggests Importance of Cathelicidin LL-37 in the Innate Immune Response Against Periodontogenic Bacteria. Oral Microbiol. Immunol. 2008, 23, 328–335. [Google Scholar] [CrossRef]
- Lao, J.; Xie, Z.; Qin, Q.; Qin, R.; Li, S.; Yuan, Y. Serum LL-37 and Inflammatory Cytokines Levels in Psoriasis. Immun. Inflamm. Dis. 2023, 11, e802. [Google Scholar] [CrossRef]
- Dombrowski, Y.; Schauber, J. Cathelicidin LL-37: A Defense Molecule with a Potential Role in Psoriasis Pathogenesis. Exp. Dermatol. 2012, 21, 327–330. [Google Scholar] [CrossRef]
- Morizane, S.; Yamasaki, K.; Mühleisen, B.; Kotol, P.F.; Murakami, M.; Aoyama, Y.; Iwatsuki, K.; Hata, T.; Gallo, R.L. Cathelicidin Antimicrobial Peptide LL-37 in Psoriasis Enables Keratinocyte Reactivity Against TLR9 Ligands. J. Investig. Dermatol. 2012, 132, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.H.; Bruns, H.; Bäckdahl, L.; Neregård, P.; Niederreiter, B.; Herrmann, M.; Catrina, A.I.; Agerberth, B.; Holmdahl, R. The Cathelicidins LL-37 and Rcramp Are Associated with Pathogenic Events of Arthritis in Humans and Rats. Ann. Rheum. Dis. 2013, 72, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.W.; Al-Maleki, A.R.; Vaithilingam, R.D.; Vadivelu, J.; Sockalingam, S.; Baharuddin, N.A.; Bartold, P.M. Associations Between Inflammation-related LL-37 with Subgingival Microbial Dysbiosis in Rheumatoid Arthritis Patients. Clin. Oral Investig. 2022, 26, 4161–4172. [Google Scholar] [CrossRef]
- Chow, L.N.Y.; Choi, K.-Y.; Piyadasa, H.; Bossert, M.; Uzonna, J.; Klonisch, T.; Mookherjee, N. Human Cathelicidin Ll-37-derived Peptide IG-19 Confers Protection in a Murine Model of Collagen-induced Arthritis. Mol. Immunol. 2014, 57, 86–92. [Google Scholar] [CrossRef]
- Kuensaen, C.; Chomdej, S.; Kongdang, P.; Sirikaew, N.; Jaitham, R.; Thonghoi, S.; Ongchai, S. LL-37 Alone and in Combination with IL17A Enhances Proinflammatory Cytokine Expression in Parallel with Hyaluronan Metabolism in Human Synovial Sarcoma Cell Line SW982—A Step Toward Understanding the Development of Inflammatory Arthritis. PLoS ONE 2019, 14, e0218736. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.; Bryzek, D.; Sroka, A.; Maresz, K.; Glowczyk, I.; Bielecka, E.; Kantyka, T.; Pyrć, K.; Svoboda, P.; Pohl, J.; et al. Citrullination Alters Immunomodulatory Function of LL-37 Essential for Prevention of Endotoxin-Induced Sepsis. J. Immunol. 2014, 192, 5363–5372. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.; Duque, H.M.; Rodrigues, G.R.; da Cunha, N.B.; Franco, O.L. The LL-37 domain: A clue to cathelicidin immunomodulatory response? Peptide 2023, 165, 171011. [Google Scholar] [CrossRef]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial Cathelicidin Peptide LL-37 Inhibits the Lps/atp-induced Pyroptosis of Macrophages by Dual Mechanism. PLoS ONE 2014, 9, e85765. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Lancet Infect. Dis. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Hernandez-Pando, R.; Carranza, C.; Juarez, E.; Contreras, J.L.; Aguilar-Leon, D.; Torres, M.; Sada, E. Expression of Cathelicidin LL-37 Duringmycobacterium Tuberculosisinfection in Human Alveolar Macrophages, Monocytes, Neutrophils, and Epithelial Cells. Infect. Immun. 2008, 76, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.S.; Rao Muvva, S.J.; Wan, M.; Raqib, R.; Bergman, P.; Brighenti, S.; Gudmundsson, G.H.; Agerberth, B. Phenylbutyrate Induces Ll-37-dependent Autophagy and Intracellular Killing of Mycobacterium Tuberculosis in Human Macrophages. Autophagy 2015, 11, 1688–1699. [Google Scholar] [CrossRef]
- Torres-Juarez, F.; Cardenas-Vargas, A.; Montoya-Rosales, A.; González-Curiel, I.; Garcia-Hernandez, M.H.; Enciso-Moreno, J.A.; Hancock, R.E.W.; Rivas-Santiago, B. LL-37 Immunomodulatory Activity During Mycobacterium Tuberculosis Infection in Macrophages. Infect. Immun. 2015, 83, 4495–4503. [Google Scholar] [CrossRef]
- Dhiman, A.; Talukdar, S.; Chaubey, G.K.; Dilawari, R.; Modanwal, R.; Chaudhary, S.; Patidar, A.; Boradia, V.M.; Kumbhar, P.; Raje, C.I. Regulation of Macrophage Cell Surface GAPDH Alters LL-37 Internalization and Downstream Effects in the Cell. J. Innate Immun. 2023, 15, 581–598. [Google Scholar] [CrossRef]
- Duan, Z.; Fang, Y.; Sun, Y.; Luan, N.; Chen, X.; Chen, M.; Han, Y.; Yin, Y.; Mwangi, J.; Niu, J.; et al. Antimicrobial peptide LL-37 forms complex with bacterial DNA to facilitate blood translocation of bacterial DNA and aggravate ulcerative colitis. Sci. Bull. 2018, 63, 1364–1375. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M. Antibiofilm Properties of Cathelicidin LL-37: An In-depth Review. World J. Microbiol. Biotechnol. 2023, 39, 99. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef]
- Schmidtchen, A.; Frick, I.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of Common Pathogenic Bacteria Degrade and Inactivate the Antibacterial Peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar] [CrossRef]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wójcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J. Degradation of Human Antimicrobial Peptide LL-37 by Staphylococcus Aureus -derived Proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.; Karim, A.Y.; Przybyszewska, K.; Ksiazek, M.; Rapala-Kozik, M.; Nguyen, K.-A.; Potempa, J. Proteolytic Inactivation of LL-37 by Karilysin, a Novel Virulence Mechanism of Tannerella forsythia. J. Innate Immun. 2010, 2, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Thomassin, J.-L.; Brannon, J.R.; Gibbs, B.F.; Gruenheid, S.; Le Moual, H. Ompt Outer Membrane Proteases of Enterohemorrhagic and Enteropathogenic Escherichia Coli Contribute Differently to the Degradation of Human LL-37. Infect. Immun. 2012, 80, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Brannon, J.R.; Thomassin, J.-L.; Desloges, I.; Gruenheid, S.; Le Moual, H. Role of Uropathogenicescherichia Coliompt in the Resistance Against Human Cathelicidin LL-37. FEMS Microbiol. Lett. 2013, 345, 64–71. [Google Scholar] [CrossRef]
- Papo, N.; Shahar, M.; Eisenbach, L.; Shai, Y. A Novel Lytic Peptide Composed of Dl-amino Acids Selectively Kills Cancer Cells in Culture and in Mice. J. Biol. Chem. 2003, 278, 21018–21023. [Google Scholar] [CrossRef] [PubMed]
- Makovitzki, A.; Fink, A.; Shai, Y. Suppression of Human Solid Tumor Growth in Mice by Intratumor and Systemic Inoculation of Histidine-rich and Ph-dependent Host Defense–like Lytic Peptides. Cancer Res. 2009, 69, 3458–3463. [Google Scholar] [CrossRef]
- Kamarajan, P.; Hayami, T.; Matte, B.; Liu, Y.; Danciu, T.; Ramamoorthy, A.; Worden, F.; Kapila, S.; Kapila, Y. Nisin ZP, a Bacteriocin and Food Preservative, Inhibits Head and Neck Cancer Tumorigenesis and Prolongs Survival. PLoS ONE 2015, 10, e0131008. [Google Scholar] [CrossRef]
- Wang, G.; Vaisman, I.I.; Van Hoek, M.L. Machine Learning Prediction of Antimicrobial Peptides. In Single Cell Analysis; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–37. [Google Scholar]
- Heilborn, J.D.; Nilsson, M.F.; Jimenez, C.I.C.; Sandstedt, B.; Borregaard, N.; Tham, E.; Sørensen, O.E.; Weber, G.; Ståhle, M. Antimicrobial Protein Hcap18/ll-37 Is Highly Expressed in Breast Cancer and Is a Putative Growth Factor for Epithelial Cells. Int. J. Cancer 2005, 114, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, X.; Qi, G.; Tang, Y.; Guo, Y.; Si, J.; Liang, L. Roles and Mechanisms of Human Cathelicidin LL-37 in Cancer. Cell. Physiol. Biochem. 2018, 47, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Niemirowicz, K.; Wnorowska, U.; Wątek, M.; Wollny, T.; Głuszek, K.; Góźdź, S.; Levental, I.; Bucki, R. The Role of Cathelicidin LL-37 in Cancer Development. Arch. Immunol. Ther. Exp. 2016, 64, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K.; Wang, G.; Coffelt, S.B.; Betancourt, A.M.; Lee, C.W.; Fan, D.; Wu, K.; Yu, J.; Sung, J.J.Y.; Cho, C.H. Emerging Roles of the Host Defense Peptide LL-37 in Human Cancer and Its Potential Therapeutic Applications. Int. J. Cancer 2010, 127, 1741–1747. [Google Scholar] [CrossRef]
- Verjans, E.T.; Zels, S.; Luyten, W.; Landuyt, B.; Schoofs, L. Molecular mechanisms of LL-37-induced receptor activation: An overview. Peptides 2016, 85, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; White, M.R.; Tecle, T.; Hartshorn, K.L. Human Defensins and LL-37 in Mucosal Immunity. J. Leukoc. Biol. 2009, 87, 79–92. [Google Scholar] [CrossRef]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 215–240. [Google Scholar]
- Yang, B.; Good, D.; Mosaiab, T.; Liu, W.; Ni, G.; Kaur, J.; Liu, X.; Jessop, C.; Yang, L.; Fadhil, R. Significance of LL-37 on Immunomodulation and Disease Outcome. BioMed Res. Int. 2020, 2020, 8349712. [Google Scholar] [CrossRef]
- Bucki, R.; Leszczyńska, K.; Namiot, A.; Sokołowski, W. Cathelicidin LL-37: A Multitask Antimicrobial Peptide. Arch. Immunol. Ther. Exp. 2010, 58, 15–25. [Google Scholar] [CrossRef]
- De Lorenzi, E.; Chiari, M.; Colombo, R.; Cretich, M.; Sola, L.; Vanna, R.; Gagni, P.; Bisceglia, F.; Morasso, C.; Lin, J.S. Evidence That the Human Innate Immune Peptide LL-37 May Be a Binding Partner of Amyloid-β and Inhibitor of Fibril Assembly. J. Alzheimer’s Dis. 2017, 59, 1213–1226. [Google Scholar] [CrossRef]
- Chen, X.; Deng, S.; Wang, W.; Castiglione, S.; Duan, Z.; Luo, L.; Cianci, F.; Zhang, X.; Xu, J.; Li, H. Human Antimicrobial Peptide LL-37 Contributes to Alzheimer’s Disease Progression. Mol. Psychiatry 2022, 27, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Armiento, V.; Hille, K.; Naltsas, D.; Lin, J.S.; Barron, A.E.; Kapurniotu, A. The Human Host-defense Peptide Cathelicidin LL-37 Is a Nanomolar Inhibitor of Amyloid Self-assembly of Islet Amyloid Polypeptide (IAPP). Angew. Chem. Int. Ed. 2020, 59, 12837–12841. [Google Scholar] [CrossRef]
- Sancho-Vaello, E.; François, P.; Bonetti, E.-J.; Lilie, H.; Finger, S.; Gil-Ortiz, F.; Gil-Carton, D.; Zeth, K. Structural Remodeling and Oligomerization of Human Cathelicidin on Membranes Suggest Fibril-like Structures as Active Species. Sci. Rep. 2017, 7, 15371. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Vaello, E.; Gil-Carton, D.; François, P.; Bonetti, E.-J.; Kreir, M.; Pothula, K.R.; Kleinekathöfer, U.; Zeth, K. The Structure of the Antimicrobial Human Cathelicidin LL-37 Shows Oligomerization and Channel Formation in the Presence of Membrane Mimics. Sci. Rep. 2020, 10, 17356. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution Structures of Human LL-37 Fragments and NMR-Based Identification of a Minimal Membrane-Targeting Antimicrobial and Anticancer Region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Backbone Cyclization and Dimerization of Ll-37-derived Peptides Enhance Antimicrobial Activity and Proteolytic Stability. Front. Microbiol. 2020, 11, 168. [Google Scholar] [CrossRef]
- Henzler Wildman, K.A.; Lee, D.K.; Ramamoorthy, A. Mechanism of bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Alford, M.A.; Yung, D.B.Y.; Molchanova, N.; Fortkort, J.A.; Lin, J.S.; Diamond, G.; Hancock, R.E.W.; Jenssen, H.; Pletzer, D. Self-assembly of Antimicrobial Peptoids Impacts Their Biological Effects on ESKAPE Bacterial Pathogens. ACS Infect. Dis. 2022, 8, 533–545. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, C.; Qiu, J.; Ma, D.; Xu, C.; Hu, S.; Han, W.; Yuan, B.; Lu, Y. Nanomolar LL-37 Induces Permeability of a Biomimetic Mitochondrial Membrane. Nanoscale 2022, 14, 17654–17660. [Google Scholar] [CrossRef]
- Mitra, A.; Paul, S. Pathways of hLL-3717-29 Aggregation Give Insight into the Mechanism of α-Amyloid Formation. J. Phys. Chem. B 2023, 127, 8162–8175. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Peterkosfsky, A.; Wang, G. NMR studies of aurein 1.2 analogs. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1203–1214. [Google Scholar] [CrossRef]
- Engelberg, Y.; Landau, M. The Human LL-37(17-29) Antimicrobial Peptide Reveals a Functional Supramolecular Structure. Nat. Commun. 2020, 11, 3894. [Google Scholar] [CrossRef]
- Engelberg, Y.; Ragonis-Bachar, P.; Landau, M. Rare by Natural Selection: Disulfide-bonded Supramolecular Antimicrobial Peptides. Biomacromolecules 2022, 23, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Min, H.J.; Lee, C.W. NMR Structure and Bactericidal Activity of KR-12 Analog Derived from Human LL-37 as a Potential Cosmetic Preservative. J. Anal. Sci. Technol. 2020, 11, 14. [Google Scholar] [CrossRef]
- Shcherbakov, A.A.; Spreacker, P.J.; Dregni, A.J.; Henzler-Wildman, K.A.; Hong, M. High-ph Structure of Emre Reveals the Mechanism of Proton-coupled Substrate Transport. Nat. Commun. 2022, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Hou, G.; Agarwal, V.; Su, Y.; Ramamoorthy, A. Ultrafast Magic Angle Spinning Solid-State NMR Spectroscopy: Advances in Methodology and Applications. Chem. Rev. 2023, 123, 918–988. [Google Scholar] [CrossRef]
- Hellmich, U.A.; Lyubenova, S.; Kaltenborn, E.; Doshi, R.; van Veen, H.W.; Prisner, T.F.; Glaubitz, C. Probing the ATP Hydrolysis Cycle of the ABC Multidrug Transporter LmrA by Pulsed EPR Spectroscopy. J. Am. Chem. Soc. 2013, 134, 5857–5862. [Google Scholar] [CrossRef]
- Rogawski, R.; MecDermott, A.E. New NMR tools for protein structure and function: Spin tags for dynamic nuclear polarization solid state NMR. Arch. Biochem. Biophys. 2017, 628, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Reif, B.; Ashbrook, S.E.; Emsley, L.; Hong, M. Solid-state NMR Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 2. [Google Scholar] [CrossRef]
- Gopinath, T.; Weber, D.; Wang, S.; Larsen, E.; Veglia, G. Solid-State NMR of Membrane Proteins in Lipid Bilayers: To Spin or Not to Spin? Acc. Chem. Res. 2021, 54, 1430–1439. [Google Scholar] [CrossRef]
- Roversi, D.; Troiano, C.; Salnikov, E.; Giordano, L.; Riccitelli, F.; De Zotti, M.; Casciaro, B.; Loffredo, M.R.; Park, Y.; Formaggio, F.; et al. Effects of antimicrobial peptides on membrane dynamics: A comparison of fluorescence and NMR experiments. Biophys. Chem. 2023, 300, 107060. [Google Scholar] [CrossRef]
- Salnikov, E.; Aisenbrey, C.; Bechinger, B. Lipid saturation and head group composition have a pronounced influence on the membrane insertion equilibrium of amphipathic helical polypeptides. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183844. [Google Scholar] [CrossRef] [PubMed]
- Schweigardt, F.; Strandberg, E.; Wadhwani, P.; Reichert, J.; Bürck, J.; Cravo, H.L.P.; Burger, L.; Ulrich, A.S. Membranolytic Mechanism of Amphiphilic Antimicrobial Β-stranded [kl]n Peptides. Biomedicines 2022, 10, 2071. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, A. Beyond NMR Spectra of Antimicrobial Peptides: Dynamical Images at Atomic Resolution and Functional Insights. Solid State Nucl. Magn. Reson. 2009, 35, 201–207. [Google Scholar] [CrossRef]
- Mihailescu, M.; Sorci, M.; Seckute, J.; Silin, V.I.; Hammer, J.; Perrin, B.S.; Hernandez, J.I.; Smajic, N.; Shrestha, A.; Bogardus, K.A. Structure and Function in Antimicrobial Piscidins: Histidine Position, Directionality of Membrane Insertion, and Ph-dependent Permeabilization. J. Am. Chem. Soc. 2019, 141, 9837–9853. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Morgera, F.; Zinth, U.; Rizzo, R.; Pacor, S.; Tossi, A. New Aspects of the Structure and Mode of Action of the Human Cathelicidin LL-37 Revealed by the Intrinsic Probe P-cyanophenylalanine. Biochem. J. 2015, 465, 443–457. [Google Scholar] [CrossRef]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and Organization of the Human Antimicrobial Peptide LL-37 in Phospholipid Membranes: Relevance to the Molecular Basis for Its Non-cell-selective Activity. Biochem. J. 1999, 341, 501–513. [Google Scholar] [CrossRef]
- Sood, R.; Domanov, Y.; Pietiäinen, M.; Kontinen, V.P.; Kinnunen, P.K. Binding of LL-37 to model biomembranes: Insight into target vs host cell recognition. Biochim. Biophys. Acta 2008, 1778, 983–996. [Google Scholar] [CrossRef]
- Liu, C.; Henning-Knechtel, A.; Österlund, N.; Wu, J.; Wang, G.; Gräslund, R.A.O.; Kirmizialtin, S.; Luo, J. Oligomer Dynamics of LL-37 Truncated Fragments Probed by A-hemolysin Pore and Molecular Simulations. Small 2023, 19, e2206232. [Google Scholar] [CrossRef] [PubMed]
- Zeth, K.; Sancho-Vaello, E. The Human Antimicrobial Peptides Dermcidin and LL-37 Show Novel Distinct Pathways in Membrane Interactions. Front. Chem. 2017, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- Henzler-Wildman, K.A.; Martinez, G.V.; Brown, M.F.; Ramamoorthy, A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry 2004, 43, 8459–8469. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Michele; Samuel; Jeffrey; Chen, J.; Lee, D.-K.; Ramamoorthy, A. Two-step Mechanism of Membrane Disruption by Aβ Through Membrane Fragmentation and Pore Formation. Biophys. J. 2012, 103, 702–710. [Google Scholar] [CrossRef]
- Kotler, S.A.; Brender, J.R.; Vivekanandan, S.; Suzuki, Y.; Yamamoto, K.; Monette, M.; Krishnamoorthy, J.; Walsh, P.; Cauble, M.; Holl, M.M.B. High-resolution NMR Characterization of Low Abundance Oligomers of Amyloid-β Without Purification. Sci. Rep. 2015, 5, 11811. [Google Scholar] [CrossRef]
- Colombo, L.; Gamba, A.; Cantù, L.; Salmona, M.; Tagliavini, F.; Rondelli, V.; Del Favero, E.; Brocca, P. Pathogenic Aβ A2V Versus Protective Aβ A2T Mutation: Early Stage Aggregation and Membrane Interaction. Biophys. Chem. 2017, 229, 11–18. [Google Scholar] [CrossRef]
- Ma, L.; Li, X.; Peterson, R.B.; Peng, A.; Huang, K. Probing the interactions between amyloidogenic proteins and bio-membranes. Biophys. Chem. 2023, 296, 106984. [Google Scholar] [CrossRef] [PubMed]
- Fatafta, H.; Kav, B.; Bundschuh, B.F.; Loschwitz, J.; Strodel, B. Disorder-to-order transition of the amyloid-β peptide upon lipid binding. Biophys. Chem. 2022, 280, 106700. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, P.; Jemiola-Rzeminska, M.; Muñoz-Torrero, D.; Suwalsky, M.; Strzalka, K. A rhein-huprine hybrid protects erythrocyte membrane integrity against Alzheimer’s disease related Aβ(1-42) peptide. Biophys. Chem. 2023, 300, 107061. [Google Scholar] [CrossRef]
- Nicastro, M.C.; Spigolon, D.; Librizzi, F.; Moran, O.; Ortore, M.G.; Bulone, D.; Biagio, P.L.; Carrotta, R. Amyloid β-peptide insertion in liposomes containing GM1-cholesterol domains. Biophys. Chem. 2016, 208, 9–16. [Google Scholar] [CrossRef]
- Saha, J.; Ford, B.J.; Wang, X.; Boyd, S.; Morgan, S.E.; Rangachari, V. Sugar distributions on gangliosides guide the formation and stability of amyloid-β oligomers. Biophys. Chem. 2023, 300, 197073. [Google Scholar] [CrossRef]
- Kenyaga, J.M.; Oteino, S.A.; Sun, Y.; Qiang, W. In-cell 31P solid-state NMR measurements of the lipid dynamics and influence of exogeneous β-amyloid peptides on live neuroblastoma neuro-2a cells. Biophys. Chem. 2023, 297, 107008. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Vestergaard, M.; Hamada, T.; Takagi, M. Real-time observation of model membrane dynamics induced by Alzheimer’s amyloid beta. Biophys. Chem. 2009, 147, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ivanova, M.I.; Ramamoorthy, A. Non-micellar ganglioside GM1 induces an instantaneous conformational change in Aβ42 leading to the modulation of the peptide amyloid-fibril pathway. Biophys. Chem. 2023, 301, 107091. [Google Scholar] [CrossRef]
- Kumar, M.; I Ivanova, M.; Ramamoorthy, A. Ganglioside GM1 Produces Stable, Short, and Cytotoxic Aβ40 Protofibrils. Chem. Commun. 2023, 59, 7040–7043. [Google Scholar] [CrossRef]
- Lee, M.; Shi, X.; Barron, A.E.; McGeer, E.; McGeer, P.L. Human antimicrobial peptide LL-37 induces glial-mediated neuroinflammation. Biochem. Pharmacol. 2015, 94, 130–141. [Google Scholar] [CrossRef]
- Fülöp, T.; Itzhaki, R.F.; Balin, B.J.; Miklossy, J.; Barron, A.E. Role of Microbes in the Development of Alzheimer’s Disease: State of the Art—An International Symposium Presented at the 2017 IAGG Congress in San Francisco. Front. Genet. 2018, 9, 362. [Google Scholar] [CrossRef]
- Betsholtz, C.; Johnson, K.H.; Westermark, P. ‘amylin’ Hormone. Nature 1989, 338, 211. [Google Scholar] [CrossRef] [PubMed]
- Westermark, G.T.; Westermark, P. Islet amyloid polypeptide and diabetes. Curr. Protein Pept. Sci. 2013, 14, 330–337. [Google Scholar] [CrossRef]
- Westermark, P.; Andersson, A.; Westermark, G.T. Is Aggregated IAPP a Cause of Beta-cell Failure in Transplanted Human Pancreatic Islets? Curr. Diabetes Rep. 2005, 5, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Gazit, E.; Radford, S.E.; Xu, Y.; Gallardo, R.U.; Caflisch, A.; Westermark, G.T.; Westermark, P.; Rosa, C.L.; Ramamoorthy, A. Proteostasis of Islet Amyloid Polypeptide: A Molecular Perspective of Risk Factors and Protective Strategies for Type II Diabetes. Chem. Rev. 2021, 121, 1845–1893. [Google Scholar] [CrossRef]
- Pithadia, A.; Brender, J.R.; Fierke, C.A.; Ramamoorthy, A. Inhibition of IAPP Aggregation and Toxicity by Natural Products and Derivatives. J. Diabetes Res. 2016, 2016, 2046327. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Chillemi, R.; Sciuto, S.; Greco, V.; Messineo, C.; Kotler, S.A.; Lee, D.; Brender, J.R.; Ramamoorthy, A.; Rosa, C.L.; et al. A blend of two resveratrol derivatives abolishes hIAPP amyloid growth and membrane damage. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1793–1802. [Google Scholar] [CrossRef]
- Cox, S.J.; Rodriguez Camargo, D.C.; Lee, Y.-H.; Dubini, R.C.A.; Rovó, P.; Ivanova, M.I.; Padmini, V.; Reif, B.; Ramamoorthy, A. Small Molecule Induced Toxic Human-iapp Species Characterized by NMR. Chem. Commun. 2020, 56, 13129–13132. [Google Scholar] [CrossRef]
- Tsai, H.; Huang, C.; Tu, L. TPE conjugated islet amyloid polypeptide probe for detection of peptide oligomers. Biophys. Chem. 2024, 304, 107129. [Google Scholar] [CrossRef]
- Yu, F.; Teng, Y.; Yang, S.; He, Y.; Zhang, Z.; Yang, H.; Ding, C.; Zhou, P. The thermodynamic and kinetic mechanisms of a Ganoderma lucidum proteoglycan inhibiting hIAPP amyloidosis. Biophys. Chem. 2022, 280, 106702. [Google Scholar] [CrossRef]
- Nireeksha; Hegde, M.N.; Kumari, N.S. Potential Role of Salivary Vitamin D Antimicrobial Peptide LL-37 and Interleukins in Severity of Dental Caries: An Exvivo Study. BMC Oral Health 2024, 24, 79. [Google Scholar] [CrossRef]
- Juszczak, M.; Zawrotniak, M.; Rapala-Kozik, M. Complexation of Fungal Extracellular Nucleic Acids by Host LL-37 Peptide Shapes Neutrophil Response to Candida Albicans Biofilm. Front. Immunol. 2024, 15, 1295168. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, S.; Zhao, N.; Nong, C.; He, Y.; Bao, R. Pseudomonas Aeruginosa Two-component System Cprrs Regulates Higba Expression and Bacterial Cytotoxicity in Response to LL-37 Stress. PLoS Pathog. 2024, 20, e1011946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bharathi, V.; Dokoshi, T.; De Anda, J.; Ursery, L.T.; Kulkarni, N.N.; Nakamura, Y.; Chen, J.; Luo, E.W.C.; Wang, L. Viral Afterlife: SARS-CoV-2 as a Reservoir of Immunomimetic Peptides That Reassemble into Proinflammatory Supramolecular Complexes. Proc. Natl. Acad. Sci. USA 2024, 121, e2300644120. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Yang, C.; Sun, Y.; Li, D.; Hao, L.; Li, Y.; Wu, S.; Li, H.; Lan, C.; Fang, X. Turning Cationic Antimicrobial Peptide KR-12 into Self-assembled Nanobiotics with Potent Bacterial Killing and LPS Neutralizing Activities. Nanoscale 2024, 16, 887–902. [Google Scholar] [CrossRef] [PubMed]



| Name | Sequence | Net Change | Activity@ | Ref. |
|---|---|---|---|---|
| LL37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | +6 (pI 10.6) | G+/G− | [60,61,62] |
| α-Defensin HNP-1 | ACYCRIPACIAGERRYGTCIYQGRLWAFCC | +3 (pI 8.68) | G+/G− | [63] |
| α-Defensin HNP-2 | CYCRIPACIAGERRYGTCIYQGRLWAFCC | +3 (pI 8.67) | G+/G− | [63] |
| α-Defensin HNP-3 | DCYCRIPACIAGERRYGTCIYQGRLWAFCC | +2 (pI 8.33) | G+/G− | [63] |
| α-Defensin HNP-4 | VCSCRLVFCRRTELRVGNCLIGGVSFTYCCTRV | +4 (pI 8.98) | G+/G− | [64] |
| α-Defensin HD-5 | ATCYCRTGRCATRESLSGVCEISGRLYRLCCR | +4 (pI 8.96) | G+/G− | [65] |
| Histatin 3 | DSHAKRHHGYKRKFHEKHHSHRGYRSNYLYDN | +5 (pI 9.9) | G+/G− | [66] |
| β-Defensin HBD-1 | DHYNCVSSGGQCLYSACPIF TKIQGTCYRGKAKCCK | +4 (pI 8.87) | G+/G− | [67] |
| β-Defensin HBD-2 | GIGDPVTCLKSGAICHPVFCP RRYKQIGTCGLPGTKCCKKP | +6 (pI 9.3) | G+/G− | [68] |
| β-Defensin HBD-3 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQ IGKCSTRGRKCCRRKK | +11 (pI 10) | G+/G− | [69] |
| β-Defensin HBD-4 | FELDRICGYGTARCRKKCRSQEYRIGRCPNTYACCLRKWDESLLNRTKP | +7 (pI 9.45) | G+/G− | [70] |
| Dermcidin | SSLLEKGLDGAKKAVGGLGKLGKDAVEDLESVGKGAVHDVKDVLDSV | −2 (pI 5.07) | G+/G− | [71] |
| Granulysin | GRDYRTCLTIVQKLKKMVDKPTQRSVSNAATRVCRTGRSRWRDVCRNFMRRYQSRVTQGLVAGETAQQICEDLR | +11 (pI10.83) | G+/G− | [72] |
| Ubiquicidin | KVHGSLARAGKVRGQTPKVAKQEKKKKKTGRAKRRMQYNRRFVNVVPTFGKKKGPNANS | +19 (pI12.15) | G+/G− | [73] |
| Thrombocidin-1 | AELRCMCIKTTSGIHPKNIQSLEVIGKGTHCNQVEVIATLKDGRKICLDPDAPRIKKIVQKKLAGDES | +4 (pI 9.05) | G+/G− | [74] |
| Hepcidin 25 (LEAP-1) | DTHFPICIFCCGCCHRSKCGMCCKT | +2 (pI 8.22) | G+/G− | [75] |
| Neuropeptide α-MSH | SYSMEHFRWGKPV | +1 (pI 8.33) | G+ | [76] |
| PACAP Neuropeptide | HSDGIFTDSYSRYRKQMAVKKYLAAVLGKRYKQRVKNK | +9 (pI 10.41) | G+/G− | [77] |
| KDAMP | RAIGGGLSSVGGGSSTIKY | +2 (pI 9.99) | G− | [78] |
| DEFB114 | DRCTKRYGRCKRDCLESEKQIDICSLPRKICCTEKLYEEDDMF | 0 (pI 6.37) | G+/G− | [79] |
| Source | Name | Sequence | Net Charge | Sec. Structure | Antibacterial Activity@ | Toxicity | References |
|---|---|---|---|---|---|---|---|
| Human | LL37 | 1LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES37 | +6 | Helix (NMR) | G+/G– | Hemolytic | [86] |
| Rhesus Monkey | RL37 | 1RLGNFFRKVKEKIGGGLKKVGQKIKDFLGNLVPRTAS37 | +8 | Helix (CD) | G+/G− | Hemolytic | [93] |
| Rabbit | CAP18 | 1GLRKRLRKFRNKIKEKLKKIGQKIQGFVPKLAPRTDY37 | +12 | Helix (CD) | G+/G− | Non- Hemolytic | [87] |
| Mice | CRAMP | 1GLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPEQ34 | +6 | Helix (NMR) | G+/G− | Hemolytic | [94] |
| Guinea Pig | CAP11 | 1GLRKKFRKTRKRIQKLGRKIGKTGRKVWKAWREYGQIPYPCRI43-dimer -disulfide-linked | +16 | ND | G+/G− | Hemolytic | [95] |
| Pig | Tritrpticin | 1VRRFPWWWPFLRR13 | +4 | b-strand (NMR) | G+/G− | Hemolytic | [96] |
| Pig | Protegrin-1 | 1RGGRLCYCRRRFCVCVGR18 | +7 | b-sheet (NMR) | G+/G− | Hemolytic, cytotoxic | [97] |
| Pig | PMAP37 | 1GLLSRLRDFLSDRGRRLGEKIERIGQKIKDLSEFFQS37 | +4 | Helix (CD) | G+/G− | Hemolytic | [98] |
| Pig | PR39 | 1RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP39 | +11 | ND | G+/G− | ND | [99] |
| Bovine | Bactenecin | 1RLCRIVVIRVCR12 | +4 | b-turn2 | G+/G− | Non- Hemolytic | [100] |
| Cattle | Indolicidin | 1ILPWKWPWWPWRR13 | +4 | b-strand (NMR) | G+/G− | Non- Hemolytic | [89] |
| Sheep | SMAP29 | 1RGLRRLGRKIAHGVKKYGPTVLRIIRIAG29 | +10 | Helix (NMR) | G+/G | Hemolytic | [101] |
| Bovine | BMAP27 | 1GRFKRFRKKFKKLFKKLSPVIPLLHLG27 | +10 | Helix (NMR) | G+/G− | Non Hemolytic | [102] |
| Bovine | BMAP28 | 1GGLRSLGRKILRAWKKYGPIIVPIIRIG28 | +7 | Helix (NMR) | G+/G− | Hemolytic | [102] |
| Bovine | BMAP34 | 1GLFRRLRDSIRRGQQKILEKARRIGERIKDIFRG34 | +8 | Helix (CD) | G+/G− | Non Hemolytic | [103] |
| Pig | PMAP23 | 1RIIDLLWRVRRPQKPKFVTVWVR23 | +6 | Helix (NMR) | G+/G− | Non Hemolytic | [98] |
| Pig | PMAP36 | 1VGRFRRLRKKTRKRLKKIGKVLKWIPPIVGSIPLGCG37 | +13 | ND | G+/G− | Hemolytic | [98] |
| Sheep | SMAP34 | 1GLFGRLRDSLQRGGQKILEKAERIWCKIKDIFR33 | +5 | ND | G+/G− | Hemolytic | [104] |
| Equine | e-CATH1 | 1KRFGRLAKSFLRMRILLPRRKILLAS26 | +9 | Helix (CD) | G+/G− | Non Hemolytic | [105] |
| Chicken | Fowlicidin-1 | 1RVKRVWPLVIRTVIAGYNLYRAIKKK26 | +8 | Helix (NMR) | G+/G− | Hemolytic | [91] |
| Chicken | Fowlicidin-2 | 1RFGRFLRKIRRFRPKVTITIQGSARFG27 | +9 | Helix (NMR) | G+/G− | Hemolytic | [91] |
| Chicken | Fowlicidin-3 | 1RVKRFWPLVPVAINTVAAGINLYKAIRRK29 | +7 | Helix (NMR) | G+/G− | Hemolytic | [91] |
| Hagfish | HFIAP-1 | 1GFFKKAWRKVKHAGRRVLDTAKGVGRHYVNNWLNRYR37 | +10 | ND | G+/G− | ND | [106] |
| Hagfish | HFIAP-3 | 1GWFKKAWRKVKNAGRRVLKGVGIHYGVGLI30 | +8 | ND | G+/G− | ND | [106] |
| Crocodile | As-CATH7 | 1KRVNWRKVGRNTALGASYVLSFLG24 | +6 | Helix (CD) | G+/G− | ND | [107] |
| Crocodile | As-CATH8 | 1KRVNWAKVGRTALKLLPYIFG21 | +6 | Helix (CD) | G+/G− | ND | [107] |
| Crocodile | Gg-CATH5 | 1TRRKWWKKVLNGAIKIAPYILD22 | +6 | Helix (CD) | G+/G− | ND | [107] |
| Crocodile | Gg-CATH7 | 1KRVNWRKVGLGASYVMSWLG20 | +5 | Helix (CD) | G+/G− | ND | [107] |
| Disease Studied | General Conclusion | Ref. |
|---|---|---|
| Bacterial pneumonia | Possible candidate for treatment | [132,133,134,135,136,137,138,139] |
| COPD | Candidate for treatment, though it may also play a role in the pathogenesis process | [140,141,142,143,144] |
| Infected segmental bone defects | Possible candidate for treatment | [145] |
| Influenza A | Possible candidate for treatment | [146,147,148,149,150,151] |
| Gonorrhea | Possible candidate for treatment | [152,153] |
| Keratitis | Possible candidate for treatment | [154,155] |
| Leptospirosis | Bacteria inhibits LL-37 | [156] |
| Lupus | Possible candidate for treatment | [157,158,159,160] |
| Meningitis | Candidate for treatment, though resistance to LL-37 has been reported | [161,162,163] |
| Periodontitis | Possible candidate for treatment | [164,165,166,167,168] |
| Psoriasis | LL-37 plays a role in the pathogenesis process but may still be used for therapeutic purposes. | [158,160,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189] |
| Rheumatoid arthritis | LL-37 plays a role in the pathogenesis process but may still be used for therapeutic purposes. | [158,172,173,174,175] |
| Sepsis | Candidate for treatment, though significant possible side effects have been noted | [176,177,178,179] |
| Tuberculosis | Possible candidate for treatment | [134,180,181,182,183] |
| Ulcerative colitis | Possible candidate for treatment | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharjya, S.; Zhang, Z.; Ramamoorthy, A. LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases. Biomolecules 2024, 14, 320. https://doi.org/10.3390/biom14030320
Bhattacharjya S, Zhang Z, Ramamoorthy A. LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases. Biomolecules. 2024; 14(3):320. https://doi.org/10.3390/biom14030320
Chicago/Turabian StyleBhattacharjya, Surajit, Zhizhuo Zhang, and Ayyalusamy Ramamoorthy. 2024. "LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases" Biomolecules 14, no. 3: 320. https://doi.org/10.3390/biom14030320
APA StyleBhattacharjya, S., Zhang, Z., & Ramamoorthy, A. (2024). LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases. Biomolecules, 14(3), 320. https://doi.org/10.3390/biom14030320








