Intersections of Fibrodysplasia Ossificans Progressiva and Traumatic Heterotopic Ossification
Abstract
1. Introduction
2. Clinical Picture of tHO and FOP
3. Current Understanding of Mechanisms behind tHO and FOP
3.1. Transforming Growth Factor Beta (TGF-β) Superfamily Signaling
3.1.1. TGF-β Ligands Regulating Traumatic HO
3.1.2. BMP Ligands Regulating Traumatic HO
3.2. Genetic Mutations in ALK2/ACVR1 Causing gHO in Fibrodysplasia Ossificans Progressiva (FOP)
ALK2 Signaling Is Dysregulated in FOP
3.3. Understanding of FOP Mechanism Informs the Future of tHO Studies
3.3.1. Activin A and ALK2
3.3.2. Hypoxia
4. Identification and Diagnosis of HO
4.1. Traumatic HO
4.2. Fibrodysplasia Ossificans Progessiva (FOP)
5. Progenitor Cell Populations in tHO and FOP
5.1. Hematopoietic Stem Cells
5.2. Endothelial Progenitor Cells
5.3. Mesenchymal Stem Cells
5.4. Muscle Stem Cells
5.5. Fibro/Adipogenic Progenitor Cells
5.6. Tendon Stem/Progenitor Cells
6. Inflammatory Control of HO
7. Nervous System Involvement in HO
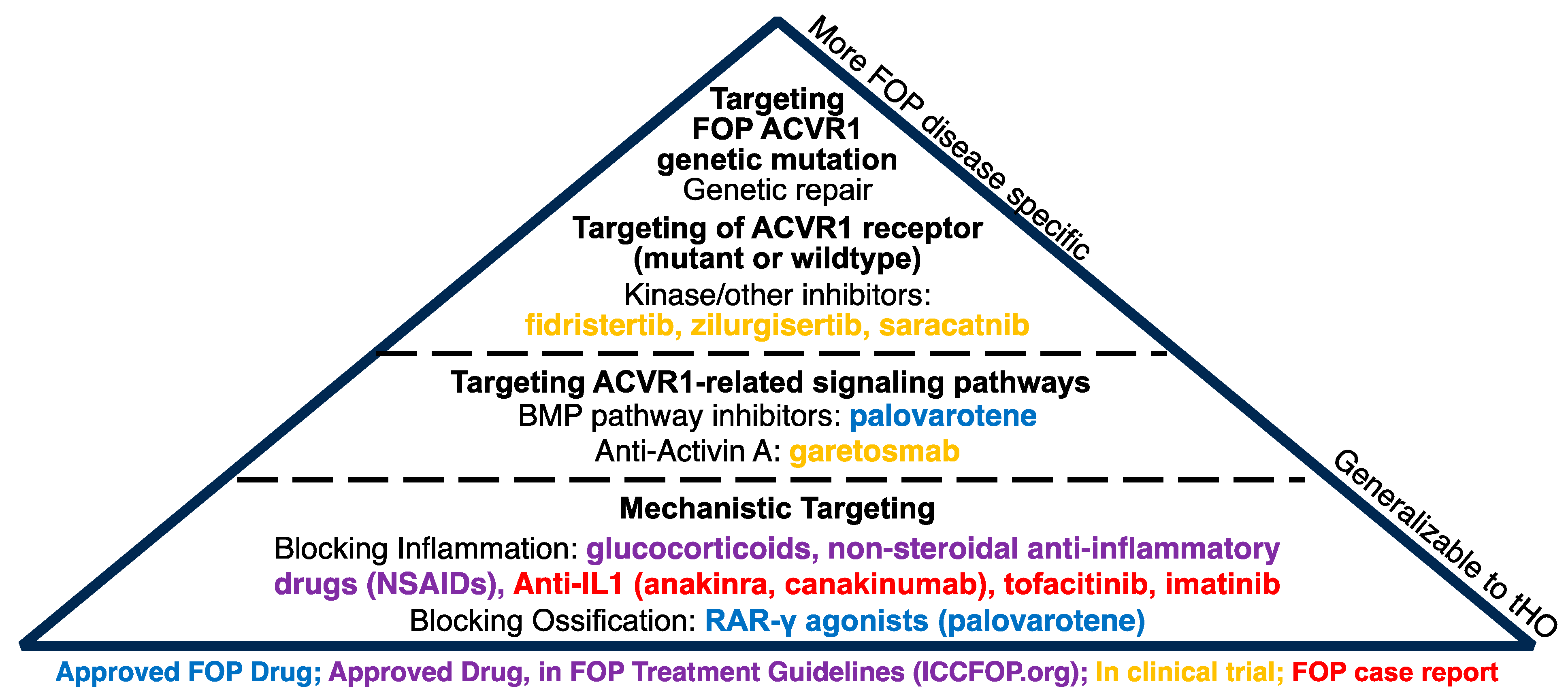
8. Current Therapeutics for HO
8.1. Traumatic HO
8.2. Genetic HO
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ranganathan, K.; Loder, S.; Agarwal, S.; Wong, V.W.; Forsberg, J.; Davis, T.A.; Wang, S.; James, A.W.; Levi, B. Heterotopic Ossification: Basic-Science Principles and Clinical Correlates. J. Bone Jt. Surg. 2015, 97, 1101–1111. [Google Scholar] [CrossRef]
- Eckardt, J.J.; Ivins, J.C.; Perry, H.O.; Unni, K.K. Osteosarcoma arising in heterotopic ossification of dermatomyositis: Case report and review of the literature. Cancer 1981, 48, 1256–1261. [Google Scholar] [CrossRef]
- Cipriano, C.A.; Pill, S.G.; Keenan, M.A. Heterotopic Ossification Following Traumatic Brain Injury and Spinal Cord Injury. J. Am. Acad. Orthop. Surg. 2009, 17, 689–697. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Hsiao, E.C.; Baujat, G.; Lapidus, D.; Sherman, A.; Kaplan, F.S. Prevalence of fibrodysplasia ossificans progressiva (FOP) in the United States: Estimate from three treatment centers and a patient organization. Orphanet J. Rare Dis. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Hwang, C.D.; Pagani, C.A.; Nunez, J.H.; Cherief, M.; Qin, Q.; Gomez-Salazar, M.; Kadaikal, B.; Kang, H.; Chowdary, A.R.; Patel, N.; et al. Contemporary perspectives on heterotopic ossification. J. Clin. Investig. 2022, 7, e158996. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; Torres, S.J.; Mehta, S.; Ahn, J. Heterotopic ossification after central nervous system trauma. Bone Jt. Res. 2013, 2, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, T.; Bouchankouk, A.D.; Özkan, S.; Gilijamse, M.; Bouvy-Berends, E.; Netelenbos, C.; Lobbezoo, F.; Eekhoff, E.M.W.; de Vries, T.J. Limitations of Jaw Movement in Fibrodysplasia Ossificans Progressiva: A Review. Front. Med. 2022, 9, 852678. [Google Scholar] [CrossRef] [PubMed]
- Akesson, L.S.; Savarirayan, R. Fibrodysplasia Ossificans Progressiva. In GeneReviews; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2023. [Google Scholar]
- Furia, J.P.; Pellegrini, V.D. Heterotopic ossification following primary total knee arthroplasty. J. Arthroplast. 1995, 10, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Healy, W.L. Heterotopic Ossification After Hip and Knee Arthroplasty: Risk Factors, Prevention, and Treatment. J. Am. Acad. Orthop. Surg. 2002, 10, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, K.L.; Bigay, K.; Chan, T.V.; Ho, J.P.; Morales, B.M.; Connor, J.; Brooks, E.; Salamat, M.S.; Sanchez, H.C.; Wool, G.; et al. Clinical-pathological correlations in three patients with fibrodysplasia ossificans progressiva. Bone 2018, 109, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.K.; Burns, T.C.; Lacap, A.P.; Granville, R.R.; Gajewski, D. Heterotopic Ossification in the Residual Limbs of Traumatic and Combat-Related Amputees. J. Am. Acad. Orthop. Surg. 2006, 14, S191–S197. [Google Scholar] [CrossRef] [PubMed]
- Appelt, E.A.; Kenkel, J.M.; Ballard, J.R.; Lopez, J.A.; Anthony, T.; Castillo, T. Preoperative Embolization of Heterotopic Ossification for the Treatment of a Recalcitrant Pressure Sore. Plast. Reconstr. Surg. 2005, 116, 50e–53e. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Al Mukaddam, M.; Baujat, G.; Brown, M.; Cali, A.; Cho, T.J.; Crowe, C.; De Cunto, C.; Delai, P.; Diecidue, R.; et al. The Medical Management of Fibrodysplasia Ossificans Progressiva: Current Treatment Considerations. Available online: https://www.iccfop.org/dvlp/wp-content/uploads/2022/05/guidelines-updated-May-2022.pdf (accessed on 7 March 2024).
- Schmierer, B.; Hill, C.S. TGFβ–SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007, 8, 970–982. [Google Scholar] [CrossRef]
- Wrighton, K.H.; Lin, X.; Feng, X.-H. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009, 19, 8–20. [Google Scholar] [CrossRef]
- Xie, L.; Law, B.K.; Chytil, A.M.; Brown, K.A.; Aakre, M.E.; Moses, H.L. Activation of the Erk Pathway Is Required for TGF-β1-Induced EMT In Vitro. Neoplasia 2004, 6, 603–610. [Google Scholar] [CrossRef]
- Yang, H.; Guo, Y.; Wang, D.; Yang, X.; Ha, C. Effect of TAK1 on osteogenic differentiation of mesenchymal stem cells by regulating BMP-2 via Wnt/β-catenin and MAPK pathway. Organogenesis 2018, 14, 36–45. [Google Scholar] [CrossRef]
- Yu, L.; Hébert, M.C.; Zhang, Y.E. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002, 21, 3749–3759. [Google Scholar] [CrossRef]
- Bakin, A.V.; Tomlinson, A.K.; Bhowmick, N.A.; Moses, H.L.; Arteaga, C.L. Phosphatidylinositol 3-Kinase Function Is Required for Transforming Growth Factor β-mediated Epithelial to Mesenchymal Transition and Cell Migration. J. Biol. Chem. 2000, 275, 36803–36810. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-β1–induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef]
- Chen, Y.; Wurtz, T.; Wang, C.; Kuo, Y.; Yang, K.D.; Huang, H.; Wang, F. Recruitment of mesenchymal stem cells and expression of TGF-β1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J. Orthop. Res. 2004, 22, 526–534. [Google Scholar] [CrossRef]
- Zhou, S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J. Cell. Biochem. 2011, 112, 1651–1660. [Google Scholar] [CrossRef]
- Jian, H.; Shen, X.; Liu, I.; Semenov, M.; He, X.; Wang, X.-F. Smad3-dependent nuclear translocation of β-catenin is required for TGF-β1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006, 20, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, V.; Voncken, J.W.; Shuler, C.; Warburton, D.; Bu, D.; Heisterkamp, N.; Groffen, J. Abnormal lung development and cleft palate in mice lacking TGF–β3 indicates defects of epithelial–mesenchymal interaction. Nat. Genet. 1995, 11, 415–421. [Google Scholar] [CrossRef]
- Sanford, L.P.; Ormsby, I.; Groot, A.C.G.-D.; Sariola, H.; Friedman, R.; Boivin, G.P.; Cardell, E.L.; Doetschman, T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development 1997, 124, 2659–2670. [Google Scholar] [CrossRef]
- Kulkarni, A.B.; Huh, C.G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Serra, R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev. Biol. 2007, 310, 304–316. [Google Scholar] [CrossRef]
- Mohammad, K.S.; Chen, C.G.; Balooch, G.; Stebbins, E.; McKenna, C.R.; Davis, H.; Niewolna, M.; Peng, X.H.; Nguyen, D.H.N.; Ionova-Martin, S.S.; et al. Pharmacologic Inhibition of the TGF-β Type I Receptor Kinase Has Anabolic and Anti-Catabolic Effects on Bone. PLoS ONE 2009, 4, e5275. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.M.; Stempien, S.A.; Thompson, A.Y.; Seyedin, S.M. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J. Cell. Physiol. 1988, 134, 337–346. [Google Scholar] [CrossRef]
- Kang, J.S.; Alliston, T.; Delston, R.; Derynck, R. Repression of Runx2 function by TGF-β through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005, 24, 2543–2555. [Google Scholar] [CrossRef]
- Alliston, T.; Choy, L.; Ducy, P.; Karsenty, G.; Derynck, R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001, 20, 2254–2272. [Google Scholar] [CrossRef]
- Maeda, S.; Hayashi, M.; Komiya, S.; Imamura, T.; Miyazono, K. Endogenous TGF-β signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004, 23, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Grafe, I.; Yang, T.; Alexander, S.; Homan, E.P.; Lietman, C.; Jiang, M.M.; Bertin, T.; Munivez, E.; Chen, Y.; Dawson, B.; et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat. Med. 2014, 20, 670–675. [Google Scholar] [CrossRef]
- Erlebacher, A.; Derynck, R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J. Cell Biol. 1996, 132, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Li, J.; Sun, Z.; Zhang, T.; Liu, H.; Yuan, F.; Fan, C. Macrophage-Derived TGF-β and VEGF Promote the Progression of Trauma-Induced Heterotopic Ossification. Inflammation 2022, 46, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; et al. Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat. Commun. 2018, 9, 551. [Google Scholar] [CrossRef]
- Patel, N.K.; Nunez, J.H.; Sorkin, M.; Marini, S.; Pagani, C.A.; Strong, A.L.; Hwang, C.D.; Li, S.; Padmanabhan, K.R.; Kumar, R.; et al. Macrophage TGF-β signaling is critical for wound healing with heterotopic ossification after trauma. J. Clin. Investig. 2022, 7. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Kang, Q.; Song, W.-X.; Luo, Q.; Tang, N.; Luo, J.; Luo, X.; Chen, J.; Bi, Y.; He, B.-C.; Park, J.K.; et al. A Comprehensive Analysis of the Dual Roles of BMPs in Regulating Adipogenic and Osteogenic Differentiation of Mesenchymal Progenitor Cells. Stem Cells Dev. 2009, 18, 545–558. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Tsuji, K.; Cox, K.; Harfe, B.D.; Rosen, V.; Tabin, C.J. Genetic Analysis of the Roles of BMP2, BMP4, and BMP7 in Limb Patterning and Skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef]
- Ebisawa, T.; Tada, K.; Kitajima, I.; Tojo, K.; Sampath, T.K.; Kawabata, M.; Miyazono, K.; Imamura, T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999, 112, 3519–3527. [Google Scholar] [CrossRef]
- Kemmis, C.M.; Vahdati, A.; Weiss, H.E.; Wagner, D.R. Bone morphogenetic protein 6 drives both osteogenesis and chondrogenesis in murine adipose-derived mesenchymal cells depending on culture conditions. Biochem. Biophys. Res. Commun. 2010, 401, 20–25. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Q.; Lin, X.; Hu, N.; Liao, J.-Y.; Lin, L.-B.; Zhao, C.; Hu, Z.-M.; Liang, X.; Xu, W.; et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016, 366, 101–111. [Google Scholar] [CrossRef]
- Semba, I.; Nonaka, K.; Takahashi, I.; Takahashi, K.; Dashner, R.; Shum, L.; Nuckolls, G.H.; Slavkin, H.C. Positionally-dependent chondrogenesis induced by BMP4 is co-regulated by sox9 and msx2. Dev. Dyn. 2000, 217, 401–414. [Google Scholar] [CrossRef]
- Pan, Q.; Yu, Y.; Chen, Q.; Li, C.; Wu, H.; Wan, Y.; Ma, J.; Sun, F. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J. Cell. Physiol. 2008, 217, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Zehentner, B.K.; Dony, C.; Burtscher, H. The Transcription Factor Sox9 Is Involved in BMP-2 Signaling. J. Bone Miner. Res. 1999, 14, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Song, W.; Luo, J.; Luo, X.; Chen, J.; Sharff, K.A.; Bi, Y.; He, B.; Huang, J.; Zhu, G.; et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/β-catenin signalling. J. Cell. Mol. Med. 2008, 13, 2448–2464. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 Regulates Osterix through Msx2 and Runx2 during Osteoblast Differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef] [PubMed]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bi, D.; Cheng, C.; Ma, S.; Liu, Y.; Cheng, K. Bone morphogenetic protein 7 enhances the osteogenic differentiation of human dermal-derived CD105+ fibroblast cells through the Smad and MAPK pathways. Int. J. Mol. Med. 2018, 43, 37–46. [Google Scholar] [CrossRef]
- Myllylä, R.M.; Haapasaari, K.-M.; Lehenkari, P.; Tuukkanen, J. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2013, 355, 463–470. [Google Scholar] [CrossRef]
- Zhu, F.; Friedman, M.S.; Luo, W.; Woolf, P.; Hankenson, K.D. The transcription factor osterix (SP7) regulates BMP6-induced human osteoblast differentiation. J. Cell. Physiol. 2011, 227, 2677–2685. [Google Scholar] [CrossRef]
- Jang, W.-G.; Kim, E.-J.; Kim, D.-K.; Ryoo, H.-M.; Lee, K.-B.; Kim, S.-H.; Choi, H.-S.; Koh, J.-T. BMP2 Protein Regulates Osteocalcin Expression via Runx2-mediated Atf6 Gene Transcription. J. Biol. Chem. 2012, 287, 905–915. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Ishizuyaa, T.; Kintouab, N.; Wadaac, Y.; Katagirid, T.; Wozney, J.M.; Rosene, V.; Yoshikia, S. Effects of BMP-2, BMP-4, and BMP-6 on Osteoblastic Differentiation of Bone Marrow-Derived Stromal Cell Lines, ST2 and MC3T3-G2/PA6. Biochem. Biophys. Res. Commun. 1996, 220, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lee, M.-H.; Wozney, J.M.; Cho, J.-Y.; Ryoo, H.-M. Bone Morphogenetic Protein-2-induced Alkaline Phosphatase Expression Is Stimulated by Dlx5 and Repressed by Msx2. J. Biol. Chem. 2004, 279, 50773–50780. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.; Lin, H.; Shen, H.; Sohn, J.; Alexander, P.G.; Tuan, R.S. Muscle injury promotes heterotopic ossification by stimulating local bone morphogenetic protein-7 production. J. Orthop. Transl. 2019, 18, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Li, Y.; Liu, J.; Zhang, C.; Chen, M.; Lu, P.; Rui, Y. Higher BMP Expression in Tendon Stem/Progenitor Cells Contributes to the Increased Heterotopic Ossification in Achilles Tendon With Aging. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Jane, J.A.; Dunford, B.A.; Kron, A.; Pittman, D.D.; Sasaki, T.; Li, J.Z.; Li, H.; Alden, T.D.; Dayoub, H.; Hankins, G.R.; et al. Ectopic Osteogenesis Using Adenoviral Bone Morphogenetic Protein (BMP)-4 and BMP-6 Gene Transfer. Mol. Ther. 2002, 6, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, E.; Trensz, F.; Haroun, S.; Drouin, G.; Bergeron, É.; Penton, C.M.; Montanaro, F.; Roux, S.; Faucheux, N.; Grenier, G. BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J. Bone Miner. Res. 2010, 26, 1166–1177. [Google Scholar] [CrossRef]
- Prados, B.; del Toro, R.; MacGrogan, D.; Gómez-Apiñániz, P.; Papoutsi, T.; Muñoz-Cánoves, P.; Méndez-Ferrer, S.; de la Pompa, J.L. Heterotopic ossification in mice overexpressing Bmp2 in Tie2+ lineages. Cell Death Dis. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Kan, L.; Hu, M.; Gomes, W.A.; Kessler, J.A. Transgenic Mice Overexpressing BMP4 Develop a Fibrodysplasia Ossificans Progressiva (FOP)-Like Phenotype. Am. J. Pathol. 2004, 165, 1107–1115. [Google Scholar] [CrossRef]
- Agarwal, S.; Loder, S.J.; Breuler, C.; Li, J.; Cholok, D.; Brownley, C.; Peterson, J.; Hsieh, H.H.; Drake, J.; Ranganathan, K.; et al. Strategic Targeting of Multiple BMP Receptors Prevents Trauma-Induced Heterotopic Ossification. Mol. Ther. 2017, 25, 1974–1987. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Spreadborough, P.J.; Dey, D.; Yang, P.; Li, S.; Lee, A.; Haskins, R.M.; Grimm, P.D.; Kumar, R.; Bradley, M.J.; et al. BMP Ligand Trap ALK3-Fc Attenuates Osteogenesis and Heterotopic Ossification in Blast-Related Lower Extremity Trauma. Stem Cells Dev. 2021, 30, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Pagani, C.A.; Das, N.; Marini, S.; Huber, A.K.; Xie, L.; Jimenez, J.; Brydges, S.; Lim, W.K.; Nannuru, K.C.; et al. Activin A does not drive post-traumatic heterotopic ossification. Bone 2020, 138, 115473. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.H.S.; Agarwal, S.; Cholok, D.J.; Loder, S.J.; Kaneko, K.; Huber, A.; Chung, M.T.; Ranganathan, K.; Habbouche, J.; Li, J.; et al. Coordinating Tissue Regeneration Through Transforming Growth Factor-β Activated Kinase 1 Inactivation and Reactivation. Stem Cells 2019, 37, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Spreadborough, P.J.; Pagani, C.A.; Haskins, R.M.; Dey, D.; Grimm, P.D.; Kaneko, K.; Marini, S.; Huber, A.K.; Hwang, C.; et al. Small molecule inhibition of non-canonical (TAK1-mediated) BMP signaling results in reduced chondrogenic ossification and heterotopic ossification in a rat model of blast-associated combat-related lower limb trauma. Bone 2020, 139, 115517. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Greenblatt, M.B.; Xie, M.; Schneider, M.D.; Zou, W.; Zhai, B.; Gygi, S.; Glimcher, L.H. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J. 2009, 28, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad pathways in TGF-β signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Gao, L.; Sheu, T.-J.; Dong, Y.; Hoak, D.M.; Zuscik, M.J.; Schwarz, E.M.; Hilton, M.J.; O’keefe, R.J.; Jonason, J.H. TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J. Cell Sci. 2013, 126, 5704–5713. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Fukuda, K. Transforming Growth Factor β-Activated Kinase 1 Regulates Mesenchymal Stem Cell Proliferation Through Stabilization of Yap1/Taz Proteins. Stem Cells 2019, 37, 1595–1605. [Google Scholar] [CrossRef]
- Cong, Q.; Liu, Y.; Zhou, T.; Zhou, Y.; Xu, R.; Cheng, C.; Chung, H.S.; Yan, M.; Zhou, H.; Liao, Z.; et al. A self-amplifying loop of YAP and SHH drives formation and expansion of heterotopic ossification. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Khan, F.; Yu, X.; Hsiao, E.C. Cardiopulmonary and Neurologic Dysfunctions in FibrodysplasiaOssificans Progressiva. Biomedicines 2021, 9, 155. [Google Scholar] [CrossRef]
- Valer, J.A.; Sánchez-De-Diego, C.; Pimenta-Lopes, C.; Rosa, J.L.; Ventura, F. ACVR1 Function in Health and Disease. Cells 2019, 8, 1366. [Google Scholar] [CrossRef]
- Wang, T.; Donahoe, P.K. The immunophilin FKBP12: A molecular guardian of the TGF-beta family type I receptors. Front. Biosci. 2004, 9, 619–631. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Liu, F.; Massagué, J. Mechanism of TGFβ receptor inhibition by FKBP12. EMBO J. 1997, 16, 3866–3876. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.-Y.; Danielson, P.D.; Shah, P.C.; Rockwell, S.; Lechleider, R.J.; Martin, J.; Manganaro, T.; Donahoe, P.K. The Immunophilin FKBP12 Functions as a Common Inhibitor of the TGFβ Family Type I Receptors. Cell 1996, 86, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Scott, G.; Nagy, A.; Kaartinen, V.; Mishina, Y. BMP type I receptor ALK2 is essential for proper patterning at late gastrulation during mouse embryogenesis. Dev. Dyn. 2006, 236, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ton, A.N.; Niu, Z.; Morales, B.M.; Chen, J.; Braz, J.; Lai, M.H.; Barruet, E.; Liu, H.; Cheung, K.; et al. ACVR1-activating mutation causes neuropathic pain and sensory neuron hyperexcitability in humans. Pain 2022, 164, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Barruet, E.; Morales, B.M.; Cain, C.J.; Ton, A.N.; Wentworth, K.L.; Chan, T.V.; Moody, T.A.; Haks, M.C.; Ottenhoff, T.H.; Hellman, J.; et al. NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification. J. Clin. Investig. 2018, 3. [Google Scholar] [CrossRef]
- Rigueur, D.; Brugger, S.; Anbarchian, T.; Kil Kim, J.; Lee, Y.; Lyons, K.M. The Type I BMP Receptor ACVR1/ALK2 is Required for Chondrogenesis During Development. J. Bone Miner. Res. 2014, 30, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Culbert, A.L.; Chakkalakal, S.A.; Theosmy, E.G.; Brennan, T.A.; Kaplan, F.S.; Shore, E.M. Alk2 Regulates Early Chondrogenic Fate in Fibrodysplasia Ossificans Progressiva Heterotopic Endochondral Ossification. Stem Cells 2014, 32, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tang, M.; Huang, J.; He, B.-C.; Gao, J.-L.; Chen, L.; Zuo, G.-W.; Zhang, W.; Luo, Q.; Shi, Q.; et al. TGFβ/BMP Type I Receptors ALK1 and ALK2 Are Essential for BMP9-induced Osteogenic Signaling in Mesenchymal Stem Cells. J. Biol. Chem. 2010, 285, 29588–29598. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Xu, M.; Seemann, P.; Connor, J.M.; Glaser, D.L.; Carroll, L.; Delai, P.; Fastnacht-Urban, E.; Forman, S.J.; Gillessen-Kaesbach, G.; et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum. Mutat. 2008, 30, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Groppe, J.C.; Wu, J.; Shore, E.M.; Kaplan, F.S. In vitro Analyses of the Dysregulated R206H ALK2 Kinase-FKBP12 Interaction Associated with Heterotopic Ossification in FOP. Cells Tissues Organs 2011, 194, 291–295. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.A.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1 R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015, 7, 303ra137. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Ikeya, M.; Horigome, K.; Matsumoto, Y.; Ebise, H.; Nishio, M.; Sekiguchi, K.; Shibata, M.; Nagata, S.; Matsuda, S.; et al. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc. Natl. Acad. Sci. USA 2015, 112, 15438–15443. [Google Scholar] [CrossRef] [PubMed]
- Shimono, K.; Tung, W.-E.; Macolino, C.; Chi, A.H.-T.; Didizian, J.H.; Mundy, C.; A Chandraratna, R.; Mishina, Y.; Enomoto-Iwamoto, M.; Pacifici, M.; et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat. Med. 2011, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Bedford-Gay, C.; Liljesthröm, M.; Durbin-Johnson, B.P.; Shore, E.M.; Rocke, D.M.; Kaplan, F.S. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J. Bone Miner. Res. 2015, 31, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Chavez, R.D.; Barruet, E.; Hsiao, E.C. Inflammation in Fibrodysplasia Ossificans Progressiva and Other Forms of Heterotopic Ossification. Curr. Osteoporos. Rep. 2019, 17, 387–394. [Google Scholar] [CrossRef]
- Convente, M.R.; A Chakkalakal, S.; Yang, E.; Caron, R.J.; Zhang, D.; Kambayashi, T.; Kaplan, F.S.; Shore, E.M. Depletion of Mast Cells and Macrophages Impairs Heterotopic Ossification in an Acvr1R206H Mouse Model of Fibrodysplasia Ossificans Progressiva. J. Bone Miner. Res. 2017, 33, 269–282. [Google Scholar] [CrossRef]
- Matsuo, K.; Matsuo, K.; Lepinski, A.; Lepinski, A.; Chavez, R.D.; Chavez, R.D.; Barruet, E.; Barruet, E.; Pereira, A.; Pereira, A.; et al. ACVR1R206H extends inflammatory responses in human induced pluripotent stem cell-derived macrophages. Bone 2021, 153, 116129. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, S.; Shan, C.; Zhu, Q.; Xue, Y.; Zhang, K. The serum levels of activin A and bone morphogenetic protein-4 and -6 in patients with fibrodysplasia ossificans progressiva. Orphanet J. Rare Dis. 2023, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lees-Shepard, J.B.; Stoessel, S.J.; Chandler, J.T.; Bouchard, K.; Bento, P.; Apuzzo, L.N.; Devarakonda, P.M.; Hunter, J.W.; Goldhamer, D.J. An anti-ACVR1 antibody exacerbates heterotopic ossification by fibro-adipogenic progenitors in fibrodysplasia ossificans progressiva mice. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Stoessel, S.J.; Yamamoto, S.; Goldhamer, D.J. Overexpression of Wild-Type ACVR1 in Fibrodysplasia Ossificans Progressiva Mice Rescues Perinatal Lethality and Inhibits Heterotopic Ossification. J. Bone Miner. Res. 2022, 37, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Bagarova, J.; Kerr, G.; Xia, D.-D.; Place, E.S.; Dey, D.; Shen, Y.; Bocobo, G.A.; Mohedas, A.H.; Huang, X.; et al. Saracatinib is an efficacious clinical candidate for fibrodysplasia ossificans progressiva. J. Clin. Investig. 2021, 6, e95042. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Shore, E.M. Common mutations in ALK2/ACVR1, a multi-faceted receptor, have roles in distinct pediatric musculoskeletal and neural orphan disorders. Cytokine Growth Factor Rev. 2015, 27, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Ciarmela, P.; Cruz, C.D.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in Mammalian Physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.W.; Li, L.; Houston-Hawkins, D.E.; Matzuk, M.M. Activins Are Critical Modulators of Growth and Survival. Mol. Endocrinol. 2003, 17, 2404–2417. [Google Scholar] [CrossRef]
- Morianos, I.; Papadopoulou, G.; Semitekolou, M.; Xanthou, G. Activin-A in the regulation of immunity in health and disease. J. Autoimmun. 2019, 104, 102314. [Google Scholar] [CrossRef]
- Mundy, C.; Yao, L.; Sinha, S.; Chung, J.; Rux, D.; Catheline, S.E.; Koyama, E.; Qin, L.; Pacifici, M. Activin A promotes the development of acquired heterotopic ossification and is an effective target for disease attenuation in mice. Sci. Signal. 2021, 14. [Google Scholar] [CrossRef]
- Wang, H.; Lindborg, C.; Lounev, V.; Kim, J.-H.; McCarrick-Walmsley, R.; Xu, M.; Mangiavini, L.; Groppe, J.C.; Shore, E.M.; Schipani, E.; et al. Cellular Hypoxia Promotes Heterotopic Ossification by Amplifying BMP Signaling. J. Bone Miner. Res. 2016, 31, 1652–1665. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; Bunn, H.F. Activation of Hypoxia-inducible Transcription Factor Depends Primarily upon Redox-sensitive Stabilization of Its α Subunit. J. Biol. Chem. 1996, 271, 32253–32259. [Google Scholar] [CrossRef] [PubMed]
- Kallio, P.J.; Pongratz, I.; Gradin, K.; McGuire, J.; Poellinger, L. Activation of hypoxia-inducible factor 1α: Posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc. Natl. Acad. Sci. USA 1997, 94, 5667–5672. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Loder, S.; Brownley, C.; Cholok, D.; Mangiavini, L.; Li, J.; Breuler, C.; Sung, H.H.; Li, S.; Ranganathan, K.; et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proc. Natl. Acad. Sci. USA 2016, 113, E338–E347. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Horigome, K.; Nishio, M.; Komura, S.; Nagata, S.; Zhao, C.; Jin, Y.; Kawakami, K.; Yamada, Y.; Ohta, A.; et al. Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J. Clin. Investig. 2017, 127, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.C.; Liu, M.; Chiang, G.G.; Otterness, D.M.; Loomis, D.C.; Kaper, F.; Giaccia, A.J.; Abraham, R.T. Regulation of Hypoxia-Inducible Factor 1α Expression and Function by the Mammalian Target of Rapamycin. Mol. Cell. Biol. 2002, 22, 7004–7014. [Google Scholar] [CrossRef] [PubMed]
- Land, S.C.; Tee, A.R. Hypoxia-inducible Factor 1α Is Regulated by the Mammalian Target of Rapamycin (mTOR) via an mTOR Signaling Motif. J. Biol. Chem. 2007, 282, 20534–20543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Yin, N.; Zhang, J. The Expression Regulation and Biological Function of Autotaxin. Cells 2021, 10, 939. [Google Scholar] [CrossRef]
- Qureshi, A.T.; Dey, D.; Sanders, E.M.; Seavey, J.G.; Tomasino, A.M.; Moss, K.; Wheatley, B.; Cholok, D.; Loder, S.; Li, J.; et al. Inhibition of Mammalian Target of Rapamycin Signaling with Rapamycin Prevents Trauma-Induced Heterotopic Ossification. Am. J. Pathol. 2017, 187, 2536–2545. [Google Scholar] [CrossRef]
- Lin, L.; Shen, Q.; Leng, H.; Duan, X.; Fu, X.; Yu, C. Synergistic Inhibition of Endochondral Bone Formation by Silencing Hif1α and Runx2 in Trauma-induced Heterotopic Ossification. Mol. Ther. 2011, 19, 1426–1432. [Google Scholar] [CrossRef]
- Hwang, C.; Marini, S.; Huber, A.K.; Stepien, D.M.; Sorkin, M.; Loder, S.; Pagani, C.A.; Li, J.; Visser, N.D.; Vasquez, K.; et al. Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification. Bone Res. 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; He, P.; Li, Y.; Wu, Y.; Lei, W.; Liu, N.; de Bruijn, J.D.; Zhang, H.; Zhang, H.; et al. Hypoxia Drives Material-Induced Heterotopic Bone Formation by Enhancing Osteoclastogenesis via M2/Lipid-Loaded Macrophage Axis. Adv. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Wyndaele, J.J. Heterotopic ossification following spinal cord injury. Spinal Cord 2010, 48, 511. [Google Scholar] [CrossRef][Green Version]
- Franz, S.; Rust, L.; Heutehaus, L.; Rupp, R.; Schuld, C.; Weidner, N. Impact of Heterotopic Ossification on Functional Recovery in Acute Spinal Cord Injury. Front. Cell. Neurosci. 2022, 16, 842090. [Google Scholar] [CrossRef]
- Mujtaba, B.; Taher, A.; Fiala, M.J.; Nassar, S.; Madewell, J.E.; Hanafy, A.K.; Aslam, R. Heterotopic ossification: Radiological and pathological review. Radiol. Oncol. 2019, 53, 275–284. [Google Scholar] [CrossRef]
- Meyers, C.; Lisiecki, J.; Miller, S.; Levin, A.; Fayad, L.; Ding, C.; Sono, T.; McCarthy, E.; Levi, B.; James, A.W. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019, 3, e10172. [Google Scholar] [CrossRef]
- Edsberg, L.E.; Crowgey, E.L.; Osborn, P.M.; Wyffels, J.T. A survey of proteomic biomarkers for heterotopic ossification in blood serum. J. Orthop. Surg. Res. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Garland, D.E.; A Hanscom, D.; A Keenan, M.; Smith, C.; Moore, T. Resection of heterotopic ossification in the adult with head trauma. J. Bone Jt. Surg. 1985, 67, 1261–1269. [Google Scholar] [CrossRef]
- Charter, R.A.; Chai, C.J.; Kim, S.K.; Kim, E.S. Serum alkaline phosphatase and inorganic phosphorus values in spinal cord injury patients with heterotopic ossification. Spinal Cord 1990, 28, 441–447. [Google Scholar] [CrossRef]
- Hammond, S.P. Yes, You Can! A Guide to Self-Care for Persons with Spinal Cord Injury; Paralyzed Veterans of America: Oklahoma City, OK, USA, 2009. [Google Scholar]
- Zakel, J.C.; Harrington, A.L. Heterotopic Ossification After Spinal Cord Injury: Current Clinical Approaches. Curr. Phys. Med. Rehabilitation Rep. 2020, 8, 172–178. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, P.; Li, P.; Song, X.; Hu, H.; Li, X.; Chen, W.; Wang, X. Ultrasonography Monitoring of Trauma-Induced Heterotopic Ossification: Guidance for Rehabilitation Procedures. Front. Neurol. 2018, 9, 771. [Google Scholar] [CrossRef]
- McKean, D.; Ather, S.; Gandhi, A.; Hubble, T.; Belci, M.; Tiberti, S.; Papanikitas, J.; Yanny, S.; King, D.; Hughes, R.; et al. Pelvic MRI in spinal cord injury patients: Incidence of muscle signal change and early heterotopic ossification. Spinal Cord 2020, 59, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.M.; Xu, M.; Feldman, G.J.; A Fenstermacher, D.; Cho, T.-J.; Choi, I.H.; Connor, J.M.; Delai, P.; Glaser, D.L.; LeMerrer, M.; et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Little, S.C.; Xu, M.; Haupt, J.; Ast, C.; Katagiri, T.; Mundlos, S.; Seemann, P.; Kaplan, F.S.; Mullins, M.C.; et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J. Clin. Investig. 2009, 119, 3462–3472. [Google Scholar] [CrossRef]
- Kaplan, F.S.; A Tabas, J.; Gannon, F.H.; Finkel, G.; Hahn, G.V.; A Zasloff, M. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J. Bone Jt. Surg. 1993, 75, 220–230. [Google Scholar] [CrossRef] [PubMed]
- A Chakkalakal, S.; Zhang, D.; Culbert, A.L.; Convente, M.R.; Caron, R.J.; Wright, A.C.; DA Maidment, A.; Kaplan, F.S.; Shore, E.M. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J. Bone Miner. Res. 2012, 27, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Gannon, F.H.; Valentine, B.A.; Shore, E.M.; Zasloff, M.A.; Kaplan, F.S. Acute Lymphocytic Infiltration in an Extremely Early Lesion of Fibrodysplasia Ossificans Progressiva. Clin. Orthop. Relat. Res. 1998, 346, 19–25. [Google Scholar] [CrossRef]
- Davis, T.A.; Lazdun, Y.; Potter, B.K.; Forsberg, J.A. Ectopic bone formation in severely combat-injured orthopedic patients — A hematopoietic niche. Bone 2013, 56, 119–126. [Google Scholar] [CrossRef]
- Lounev, V.Y.; Ramachandran, R.; Wosczyna, M.N.; Yamamoto, M.; DA Maidment, A.; Shore, E.M.; Glaser, D.L.; Goldhamer, D.J.; Kaplan, F.S. Identification of Progenitor Cells That Contribute to Heterotopic Skeletogenesis. J. Bone Jt. Surg. 2009, 91, 652–663. [Google Scholar] [CrossRef]
- Medici, D.; Shore, E.M.; Lounev, V.Y.; Kaplan, F.S.; Kalluri, R.; Olsen, B.R. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010, 16, 1400–1406. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Zhou, W.; Dai, C.; Chen, X.; Xie, Y.; Han, S.; Liu, H.; Hu, Y.; Tang, C.; et al. Single cell analysis reveals inhibition of angiogenesis attenuates the progression of heterotopic ossification in Mkx−/− mice. Bone Res. 2022, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Loder, S.J.; Brownley, C.; Eboda, O.; Peterson, J.R.; Hayano, S.; Wu, B.; Zhao, B.; Kaartinen, V.; Wong, V.C.; et al. BMP signaling mediated by constitutively active Activin type 1 receptor (ACVR1) results in ectopic bone formation localized to distal extremity joints. Dev. Biol. 2015, 400, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Barruet, E.; Garcia, S.M.; Wu, J.; Morales, B.M.; Tamaki, S.; Moody, T.; Pomerantz, J.H.; Hsiao, E.C. Modeling the ACVR1R206H mutation in human skeletal muscle stem cells. eLife 2021, 10, e66107. [Google Scholar] [CrossRef] [PubMed]
- Lees-Shepard, J.B.; Yamamoto, M.; Biswas, A.A.; Stoessel, S.J.; Nicholas, S.-A.E.; Cogswell, C.A.; Devarakonda, P.M.; Schneider, M.J.; Cummins, S.M.; Legendre, N.P.; et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat. Commun. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Bagarova, J.; Hatsell, S.J.; Armstrong, K.A.; Huang, L.; Ermann, J.; Vonner, A.J.; Shen, Y.; Mohedas, A.H.; Lee, A.; et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci. Transl. Med. 2016, 8, 366ra163. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Loder, S.J.; Cholok, D.; Peterson, J.; Li, J.; Breuler, C.; Brownley, R.C.; Sung, H.H.; Chung, M.T.; Kamiya, N.; et al. Scleraxis-Lineage Cells Contribute to Ectopic Bone Formation in Muscle and Tendon. Stem Cells 2016, 35, 705–710. [Google Scholar] [CrossRef]
- Yea, J.-H.; Gomez-Salazar, M.; Onggo, S.; Li, Z.; Thottappillil, N.; Cherief, M.; Negri, S.; Xing, X.; Qin, Q.; Tower, R.J.; et al. Tppp3+ synovial/tendon sheath progenitor cells contribute to heterotopic bone after trauma. Bone Res. 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Pietras, E.M.; Warr, M.R.; Passegué, E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 2011, 195, 709–720. [Google Scholar] [CrossRef]
- Martinbianco, E.M.; Lilley, C.M.; Grech, J.; Mirza, K.M.; Chen, X. Heterotopic Mesenteric Ossification With Trilineage Hematopoiesis. Cureus 2022, 14, e24620. [Google Scholar] [CrossRef]
- Christofi, T.; A Raptis, D.; Kallis, A.; Ambasakoor, F. True trilineage haematopoiesis in excised heterotopic ossification from a laparotomy scar: Report of a case and literature review. Ind. Mark. Manag. 2008, 90, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Borgia, A.; Manara, S.; Balzarotti, M.; Vinciguerra, P.; Di Maria, A. Small lymphocytic lymphoma in true trilineage hematopoietic tissue within heterotopic ossification in an enucleated blind painful eye: A case report. J. Med Case Rep. 2020, 14, 1–4. [Google Scholar] [CrossRef]
- Wang, D.; Shurafa, M.S.; Acharya, D.R.; Strand, V.F.; Linden, M.D. Chronic Abdominal Pain Caused by Heterotopic Ossification With Functioning Bone Marrow: A Case Report and Review of the Literature. Arch. Pathol. Lab. Med. 2004, 128, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Song, J.; Shiozawa, Y.; Wang, J.; Wang, Z.; Williams, B.; Havens, A.; Schneider, A.; Ge, C.; Franceschi, R.T.; et al. Hematopoietic Stem Cells Regulate Mesenchymal Stromal Cell Induction into Osteoblasts Thereby Participating in the Formation of the Stem Cell Niche. Stem Cells 2008, 26, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006, 6, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.S.; Bab, I.A.; Lian, J.B.; Stein, G.S.; Jazrawi, L.; Majeska, R.J.; Attar-Namdar, M.; Einhorn, T.A. Stimulation of Systemic Bone Formation Induced by Experimental Blood Loss. Clin. Orthop. Relat. Res. 1997, 340, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Otsuru, S.; Overholt, K.M.; Olson, T.S.; Hofmann, T.J.; Guess, A.J.; Velazquez, V.M.; Kaito, T.; Dominici, M.; Horwitz, E.M. Hematopoietic derived cells do not contribute to osteogenesis as osteoblasts. Bone 2017, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, S.-H. Hematopoietic Stem Cells and Their Roles in Tissue Regeneration. Int. J. Stem Cells 2020, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Minato, N. Myeloid cells. Int. J. Biochem. Cell Biol. 2004, 36, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Crinier, A.; Vély, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef]
- Gannon, F.H.; Glaser, D.; Caron, R.; Thompson, L.D.; Shore, E.M.; Kaplan, F.S. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum. Pathol. 2001, 32, 842–848. [Google Scholar] [CrossRef]
- Glaser, D.L.; Economides, A.N.; Wang, L.; Liu, X.; Kimble, R.D.; Fandl, J.P.; Wilson, J.M.; Stahl, N.; Kaplan, F.S.; Shore, E.M. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. JBJS 2003, 85, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Shore, E.M.; Gupta, R.; Billings, P.C.; Glaser, D.L.; Pignolo, R.J.; Graf, D.; Kamoun, M. Immunological Features of Fibrodysplasia Ossificans Progressiva and the Dysregulated BMP4 Pathway. Clin. Rev. Bone Miner. Metab. 2005, 3, 189–194. [Google Scholar] [CrossRef]
- Kan, L.; Liu, Y.; McGuire, T.L.; Berger, D.M.P.; Awatramani, R.B.; Dymecki, S.M.; Kessler, J.A. Dysregulation of Local Stem/Progenitor Cells as a Common Cellular Mechanism for Heterotopic Ossification. Stem Cells 2009, 27, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Wosczyna, M.N.; A Biswas, A.; A Cogswell, C.; Goldhamer, D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner. Res. 2012, 27, 1004–1017. [Google Scholar] [CrossRef]
- Tirone, M.; Giovenzana, A.; Vallone, A.; Zordan, P.; Sormani, M.; Nicolosi, P.A.; Meneveri, R.; Gigliotti, C.R.; Spinelli, A.E.; Bocciardi, R.; et al. Severe Heterotopic Ossification in the Skeletal Muscle and Endothelial Cells Recruitment to Chondrogenesis Are Enhanced by Monocyte/Macrophage Depletion. Front. Immunol. 2019, 10, 1640. [Google Scholar] [CrossRef]
- El-Labban, N.G.; Hopper, C.; Barber, P. Ultrastructural finding of vascular degeneration in fibrodysplasia ossificans progressiva (FOP). J. Oral Pathol. Med. 1995, 24, 125–190. [Google Scholar] [CrossRef]
- Qin, Y.; Guan, J.; Zhang, C. Mesenchymal stem cells: Mechanisms and role in bone regeneration. Postgrad. Med. J. 2014, 90, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Billings, P.C.; Fiori, J.L.; Bentwood, J.L.; O’Connell, M.P.; Jiao, X.; Nussbaum, B.; Caron, R.J.; Shore, E.M.; Kaplan, F.S. Dysregulated BMP Signaling and Enhanced Osteogenic Differentiation of Connective Tissue Progenitor Cells From Patients With Fibrodysplasia Ossificans Progressiva (FOP). J. Bone Miner. Res. 2008, 23, 305–313. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Katagiri, T.; Ikeda, T.; Wozney, J.M.; Rosen, V.; A Wang, E.; Kahn, A.J.; Suda, T.; Yoshiki, S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J. Cell Biol. 1991, 113, 681–687. [Google Scholar] [CrossRef]
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2012, 13, 127–133. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J.; Mula, J.; Miyazaki, M.; Erfani, R.; Garrison, K.; Farooqui, A.B.; Srikuea, R.; Lawson, B.A.; Grimes, B.; Keller, C.; et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 2011, 138, 3657–3666. [Google Scholar] [CrossRef]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage [published erratum appears in J Cell Biol 1995 Feb;128(4):following 713]. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Kiyono, T.; Wada, M.R.; Umeda, R.; Goto, Y.-I.; Nonaka, I.; Shimizu, S.; Yasumoto, S.; Inagawa-Ogashiwa, M. Osteogenic properties of human myogenic progenitor cells. Mech. Dev. 2008, 125, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Kaplan, F.S.; Pignolo, R.J. Clearance of Senescent Cells From Injured Muscle Abrogates Heterotopic Ossification in Mouse Models of Fibrodysplasia Ossificans Progressiva. J. Bone Miner. Res. 2021, 37, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Wosczyna, M.N.; Konishi, C.T.; Carbajal, E.E.P.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Wagner, M.W.; Rando, T.A. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019, 27, 2029–2035.e5. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.-I.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Stanley, A.; Tichy, E.D.; Kocan, J.; Roberts, D.W.; Shore, E.M.; Mourkioti, F. Dynamics of skeletal muscle-resident stem cells during myogenesis in fibrodysplasia ossificans progressiva. npj Regen. Med. 2022, 7, 1–15. [Google Scholar] [CrossRef]
- Levesque, J.-P.; A Sims, N.; Pettit, A.R.; A Alexander, K.; Tseng, H.-W.; Torossian, F.; Genêt, F.; Lataillade, J.-J.; Le Bousse-Kerdilès, M.-C. Macrophages Driving Heterotopic Ossification: Convergence of Genetically-Driven and Trauma-Driven Mechanisms. J. Bone Miner. Res. 2017, 33, 365–366. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; A Inkson, C.; Embree, M.C.; Sonoyama, W.; Li, L.; I Leet, A.; Seo, B.-M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Udagawa, N. The mechanism of osteoclast differentiation from macrophages: Possible roles of T lymphocytes in osteoclastogenesis. J. Bone Miner. Metab. 2003, 21, 337–343. [Google Scholar] [CrossRef]
- Vi, L.; Baht, G.S.; Whetstone, H.; Ng, A.; Wei, Q.; Poon, R.; Mylvaganam, S.; Grynpas, M.; A Alman, B. Macrophages Promote Osteoblastic Differentiation In Vivo: Implications in Fracture Repair and Bone Homeostasis. J. Bone Miner. Res. 2014, 30, 1090–1102. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Wullschleger, M.E.; Alexander, K.A.; Wu, A.C.K.; Millard, S.M.; Kaur, S.; Maugham, M.L.; Gregory, L.S.; Steck, R.; Pettit, A.R. Fracture Healing via Periosteal Callus Formation Requires Macrophages for Both Initiation and Progression of Early Endochondral Ossification. Am. J. Pathol. 2014, 184, 3192–3204. [Google Scholar] [CrossRef]
- Wolken, D.M.A.; Idone, V.; Hatsell, S.J.; Yu, P.B.; Economides, A.N. The obligatory role of Activin A in the formation of heterotopic bone in Fibrodysplasia Ossificans Progressiva. Bone 2017, 109, 210–217. [Google Scholar] [CrossRef]
- Shafritz, A.B.; Shore, E.M.; Gannon, F.H.; Zasloff, M.A.; Taub, R.; Muenke, M.; Kaplan, F.S. Overexpression of an Osteogenic Morphogen in Fibrodysplasia Ossificans Progressiva. N. Engl. J. Med. 1996, 335, 555–561. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Glaser, D.L.; Shore, E.M.; Pignolo, R.J.; Xu, M.; Zhang, Y.; Senitzer, D.; Forman, S.J.; Emerson, S.G. Hematopoietic Stem-Cell Contribution to Ectopic Skeletogenesis. J. Bone Jt. Surg. 2007, 89, 347–357. [Google Scholar] [CrossRef]
- Champagne, C.M.; Takebe, J.; Offenbacher, S.; Cooper, L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002, 30, 26–31. [Google Scholar] [CrossRef]
- Del Zotto, G.; Antonini, F.; Azzari, I.; Ortolani, C.; Tripodi, G.; Giacopelli, F.; Cappato, S.; Moretta, L.; Ravazzolo, R.; Bocciardi, R. Peripheral Blood Mononuclear Cell Immunophenotyping in Fibrodysplasia Ossificans Progressiva Patients: Evidence for Monocyte DNAM1 Up-regulation. Cytom. Part B Clin. Cytom. 2017, 94, 613–622. [Google Scholar] [CrossRef]
- Maekawa, H.; Jin, Y.; Nishio, M.; Kawai, S.; Nagata, S.; Kamakura, T.; Yoshitomi, H.; Niwa, A.; Saito, M.K.; Matsuda, S.; et al. Recapitulation of pro-inflammatory signature of monocytes with ACVR1A mutation using FOP patient-derived iPSCs. Orphanet J. Rare Dis. 2022, 17, 1–15. [Google Scholar] [CrossRef]
- Evans, K.N.; Forsberg, J.A.; Potter, B.K.; Hawksworth, J.S.; Brown, T.S.; Andersen, R.; Dunne, J.R.; Tadaki, D.; Elster, E.A. Inflammatory Cytokine and Chemokine Expression is Associated With Heterotopic Ossification in High-Energy Penetrating War Injuries. J. Orthop. Trauma 2012, 26, e204–e213. [Google Scholar] [CrossRef]
- Forsberg, J.A.; Potter, B.K.; Polfer, E.M.; Safford, S.D.; Elster, E.A. Do Inflammatory Markers Portend Heterotopic Ossification and Wound Failure in Combat Wounds? Clin. Orthop. Relat. Res. 2014, 472, 2845–2854. [Google Scholar] [CrossRef]
- Sorkin, M.; Huber, A.K.; Hwang, C.; Carson, W.F.; Menon, R.; Li, J.; Vasquez, K.; Pagani, C.; Patel, N.; Li, S.; et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020, 11, 722. [Google Scholar] [CrossRef]
- Nunez, J.H.; Juan, C.B.; Sun, Y.; Hong, J.B.; Bancroft, A.C.B.; Hwang, C.; Medrano, J.M.; Huber, A.K.; Tower, R.J.; Levi, B. Neutrophil and NETosis Modulation in Traumatic Heterotopic Ossification. Ann. Surg. 2023, 278, e1289–e1298. [Google Scholar] [CrossRef]
- Huang, J.; Wu, J.; Lin, J.; Li, C.; Tang, B.; Xiao, H. Palovarotene inhibits the NF-κB signalling pathway to prevent heterotopic ossification. Clin. Exp. Pharmacol. Physiol. 2022, 49, 881–892. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Le Merrer, M.; Glaser, D.L.; Pignolo, R.J.; Goldsby, R.E.; Kitterman, J.A.; Groppe, J.; Shore, E.M. Fibrodysplasia ossificans progressiva. Best Pr. Res. Clin. Rheumatol. 2008, 22, 191–205. [Google Scholar] [CrossRef]
- Peng, K.; Cheung, K.; Lee, A.; Sieberg, C.; Borsook, D.; Upadhyay, J. Longitudinal Evaluation of Pain, Flare-Up, and Emotional Health in Fibrodysplasia Ossificans Progressiva: Analyses of the International FOP Registry. JBMR Plus 2019, 3, e10181. [Google Scholar] [CrossRef]
- Agarwal, S.; Loder, S.; Levi, B. Heterotopic Ossification Following Upper Extremity Injury. Hand Clin. 2017, 33, 363–373. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, C.; Marini, S.; Tower, R.J.; Qin, Q.; Negri, S.; Pagani, C.A.; Sun, Y.; Stepien, D.M.; Sorkin, M.; et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat. Commun. 2021, 12, 4939. [Google Scholar] [CrossRef]
- Qin, Q.; Gomez-Salazar, M.; Cherief, M.; Pagani, C.A.; Lee, S.; Hwang, C.; Tower, R.J.; Onggo, S.; Sun, Y.; Piplani, A.; et al. Neuron-to-vessel signaling is a required feature of aberrant stem cell commitment after soft tissue trauma. Bone Res. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Li, Z.; Zhang, Q.; Goh, B.C.; Li, Z.; Thorek, D.L.; Rajbhandari, L.; Brushart, T.M.; Minichiello, L.; Zhou, F.; et al. NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone. Cell Rep. 2016, 16, 2723–2735. [Google Scholar] [CrossRef]
- Salisbury, E.; Rodenberg, E.; Sonnet, C.; Hipp, J.; Gannon, F.H.; Vadakkan, T.J.; Dickinson, M.E.; Olmsted-Davis, E.A.; Davis, A.R. Sensory nerve induced inflammation contributes to heterotopic ossification. J. Cell. Biochem. 2011, 112, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Şenlikci, H.B.; Yemişci, O. Post-stroke bilateral heterotopic ossification: An acute problem with long-lasting consequences. Jt. Dis. Relat. Surg. 2020, 31, 386–389. [Google Scholar] [CrossRef]
- Pek, C.; Lim, M.; Yong, R.; Wong, H. Neurogenic heterotopic ossification after a stroke: Diagnostic and radiological challenges. Singap. Med. J. 2014, 55, e119–e122. [Google Scholar] [CrossRef]
- Huang, H.; Cheng, W.-X.; Hu, Y.-P.; Chen, J.-H.; Zheng, Z.-T.; Zhang, P. Relationship between heterotopic ossification and traumatic brain injury. J. Orthop. Transl. 2017, 12, 16–25. [Google Scholar] [CrossRef]
- Anthonissen, J.; Steffen, C.T.; Hofmann, A.; Victor, J. The pathogenesis of heterotopic ossification after traumatic brain injury. A review of current literature. Acta Orthop. Belg. 2020, 86, 369–377. [Google Scholar] [PubMed]
- Hofman, M.; Koopmans, G.; Kobbe, P.; Poeze, M.; Andruszkow, H.; Brink, P.R.G.; Pape, H.-C. Improved Fracture Healing in Patients with Concomitant Traumatic Brain Injury: Proven or Not? Mediat. Inflamm. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Liu, W.; Chen, W.; Xie, M.; Chen, C.; Shao, Z.; Zhang, Y.; Zhao, H.; Song, Q.; Hu, H.; Xing, X.; et al. Traumatic brain injury stimulates sympathetic tone-mediated bone marrow myelopoiesis to favor fracture healing. Signal Transduct. Target. Ther. 2023, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chalidis, B.; Stengel, D.; Giannoudis, P.V. Early Excision and Late Excision of Heterotopic Ossification after Traumatic Brain Injury Are Equivalent: A Systematic Review of the Literature. J. Neurotrauma 2007, 24, 1675–1686. [Google Scholar] [CrossRef]
- Genêt, F.; Ruet, A.; Almangour, W.; Gatin, L.; Denormandie, P.; Schnitzler, A. Beliefs relating to recurrence of heterotopic ossification following excision in patients with spinal cord injury: A review. Spinal Cord 2015, 53, 340–344. [Google Scholar] [CrossRef]
- Mertens, M.G.; Meert, L.; Struyf, F.; Schwank, A.; Meeus, M. Exercise Therapy Is Effective for Improvement in Range of Motion, Function, and Pain in Patients With Frozen Shoulder: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 103, 998–1012.e14. [Google Scholar] [CrossRef]
- Casavant, A.M.; Hastings, H. Heterotopic Ossification about the Elbow: A Therapist’s Guide to Evaluation and Management. J. Hand Ther. 2006, 19, 255–267. [Google Scholar] [CrossRef]
- Fijn, R.; Koorevaar, R.; Brouwers, J. Prevention of heterotopic ossification after total hip replacement with NSAIDs. Pharm. Weekbl. 2003, 25, 138–145. [Google Scholar] [CrossRef]
- Griffin, S.M.; Sims, S.H.; Karunakar, M.A.; Seymour, R.; Haines, N. Heterotopic Ossification Rates After Acetabular Fracture Surgery Are Unchanged Without Indomethacin Prophylaxis. Clin. Orthop. Relat. Res. 2013, 471, 2776–2782. [Google Scholar] [CrossRef]
- Anthony, P.; Keys, H.; Evarts, C.M.; Rubin, P.; Lush, C. Prevention of heterotopic bone formation with early post operative irradiation in high risk patients undergoing total HIP arthroplasty: Comparison of 10.00 Gy VS 20.00 Gy schedules. Endocrine 1987, 13, 365–369. [Google Scholar] [CrossRef]
- I Vasileiadis, G.; I Sakellariou, V.; Kelekis, A.; Galanos, A.; Soucacos, P.N.; Papagelopoulos, P.J.; Babis, G.C. Prevention of heterotopic ossification in cases of hypertrophic osteoarthritis submitted to total hip arthroplasty. Etidronate or Indomethacin? J. Musculoskelet. Neuronal Interact. 2010, 10, 159–165. [Google Scholar]
- Brennan, T.A.; Lindborg, C.M.; Bergbauer, C.R.; Wang, H.; Kaplan, F.S.; Pignolo, R.J. Mast cell inhibition as a therapeutic approach in fibrodysplasia ossificans progressiva (FOP). Bone 2017, 109, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Shore, E.M.; Pignolo, R.J.e.; Hsiao, E.C. The Medical Management of Fibrodysplasia Ossificans Progressiva: Current Treatment Considerations. Clinc. Proce. Intl. Clin. Consort. FOP 2011, 4, 1–100. [Google Scholar]
- Wu, X.-B.; Yang, M.-H.; Zhu, S.-W.; Cao, Q.-Y.; Wu, H.-H.; Wang, M.-Y.; Cuellar, D.O.; Mauffrey, C. Surgical resection of severe heterotopic ossification after open reduction and internal fixation of acetabular fractures: A case series of 18 patients. Injury 2014, 45, 1604–1610. [Google Scholar] [CrossRef]
- Kornhaber, R.; Foster, N.; Edgar, D.; Visentin, D.; Ofir, E.; Haik, J.; Harats, M. The development and impact of heterotopic ossification in burns: A review of four decades of research. Scars Burn. Heal. 2017, 3. [Google Scholar] [CrossRef]
- Eekhoff, E.M.W.; Netelenbos, J.C.; de Graaf, P.; Hoebink, M.; Bravenboer, N.; Micha, D.; Pals, G.; de Vries, T.J.; A Lammertsma, A.; Raijmakers, P.G.; et al. Flare-Up After Maxillofacial Surgery in a Patient With Fibrodysplasia Ossificans Progressiva: An [18F]-NaF PET/CT Study and a Systematic Review. JBMR Plus 2017, 2, 55–58. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Hsiao, E.C.; Al Mukaddam, M.; Baujat, G.; Berglund, S.K.; Brown, M.A.; Cheung, A.M.; De Cunto, C.; Delai, P.; Haga, N.; et al. Reduction of New Heterotopic Ossification (HO) in the Open-Label, Phase 3 MOVE Trial of Palovarotene for Fibrodysplasia Ossificans Progressiva (FOP). J. Bone Miner. Res. 2023, 38, 381–394. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Baujat, G.; Hsiao, E.C.; Keen, R.; Wilson, A.; Packman, J.; Strahs, A.L.; Grogan, D.R.; Kaplan, F.S. Palovarotene for Fibrodysplasia Ossificans Progressiva (FOP): Results of a Randomized, Placebo-Controlled, Double-Blind Phase 2 Trial. J. Bone Miner. Res. 2020, 37, 1891–1902. [Google Scholar] [CrossRef]
- Vanhoutte, F.; Liang, S.; Ruddy, M.; Zhao, A.; Drewery, T.; Wang, Y.; DelGizzi, R.; Forleo-Neto, E.; Rajadhyaksha, M.; Herman, G.; et al. Pharmacokinetics and Pharmacodynamics of Garetosmab (Anti-Activin A): Results From a First-in-Human Phase 1 Study. J. Clin. Pharmacol. 2020, 60, 1424–1431. [Google Scholar] [CrossRef]
- Rocco, M.D.; Forleo-Neto, E.; Pignolo, R.; Keen, R.; Orcel, P.; Funck-Brentano, T.; Roux, C.; Kolta, S.; Madeo, A.; Bubbear, J.S.; et al. Garetosmab, an inhibitor of activin A, reduces heterotopic ossification and flare-ups in adults with fibrodysplasia ossificans progressiva: A randomized, double-blind, placebo-controlled phase 2 trial. medRxiv 2023, preprint. [Google Scholar]
- Maekawa, H.; Kawai, S.; Nishio, M.; Nagata, S.; Jin, Y.; Yoshitomi, H.; Matsuda, S.; Toguchida, J. Prophylactic treatment of rapamycin ameliorates naturally developing and episode -induced heterotopic ossification in mice expressing human mutant ACVR1. Orphanet J. Rare Dis. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Haviv, R.; Moshe, V.; De Benedetti, F.; Prencipe, G.; Rabinowicz, N.; Uziel, Y. Is fibrodysplasia ossificans progressiva an interleukin-1 driven auto-inflammatory syndrome? Pediatr. Rheumatol. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Nikishina, I.P.; Arsenyeva, S.V.; Matkava, V.G.; Arefieva, A.N.; Kaleda, M.I.; Smirnov, A.V.; Blank, L.M.; Kostik, M.M. Successful experience of tofacitinib treatment in patients with Fibrodysplasia Ossificans Progressiva. Pediatr. Rheumatol. 2023, 21, 1–9. [Google Scholar] [CrossRef]
- Tseng, H.-W.; Kulina, I.; Girard, D.; Gueguen, J.; Vaquette, C.; Salga, M.; Fleming, W.; Jose, B.; Millard, S.M.; Pettit, A.R.; et al. Interleukin-1 Is Overexpressed in Injured Muscles Following Spinal Cord Injury and Promotes Neurogenic Heterotopic Ossification. J. Bone Miner. Res. 2020, 37, 531–546. [Google Scholar] [CrossRef]
- Migliorini, F.; Trivellas, A.; Eschweiler, J.; Driessen, A.; Tingart, M.; Maffulli, N. NSAIDs for Prophylaxis for Heterotopic Ossification After Total Hip Arthroplasty: A Bayesian Network Meta-analysis. Calcif. Tissue Int. 2020, 108, 196–206. [Google Scholar] [CrossRef]
- Schneider, J.; Maffulli, N.; Eschweiler, J.; Bell, A.; Hildebrand, F.; Migliorini, F. Efficacy of ibuprofen and indomethacin as prophylaxis of heterotopic ossification: A comparative study. Sci. Rep. 2023, 13, 20210. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.; Rakow, A.; Gaber, T.; Hahne, M.; Sentürk, U.; Strehl, C.; Fangradt, M.; Schmidt-Bleek, K.; Huscher, D.; Winkler, T.; et al. Preoperative irradiation for the prevention of heterotopic ossification induces local inflammation in humans. Bone 2013, 55, 93–101. [Google Scholar] [CrossRef] [PubMed]


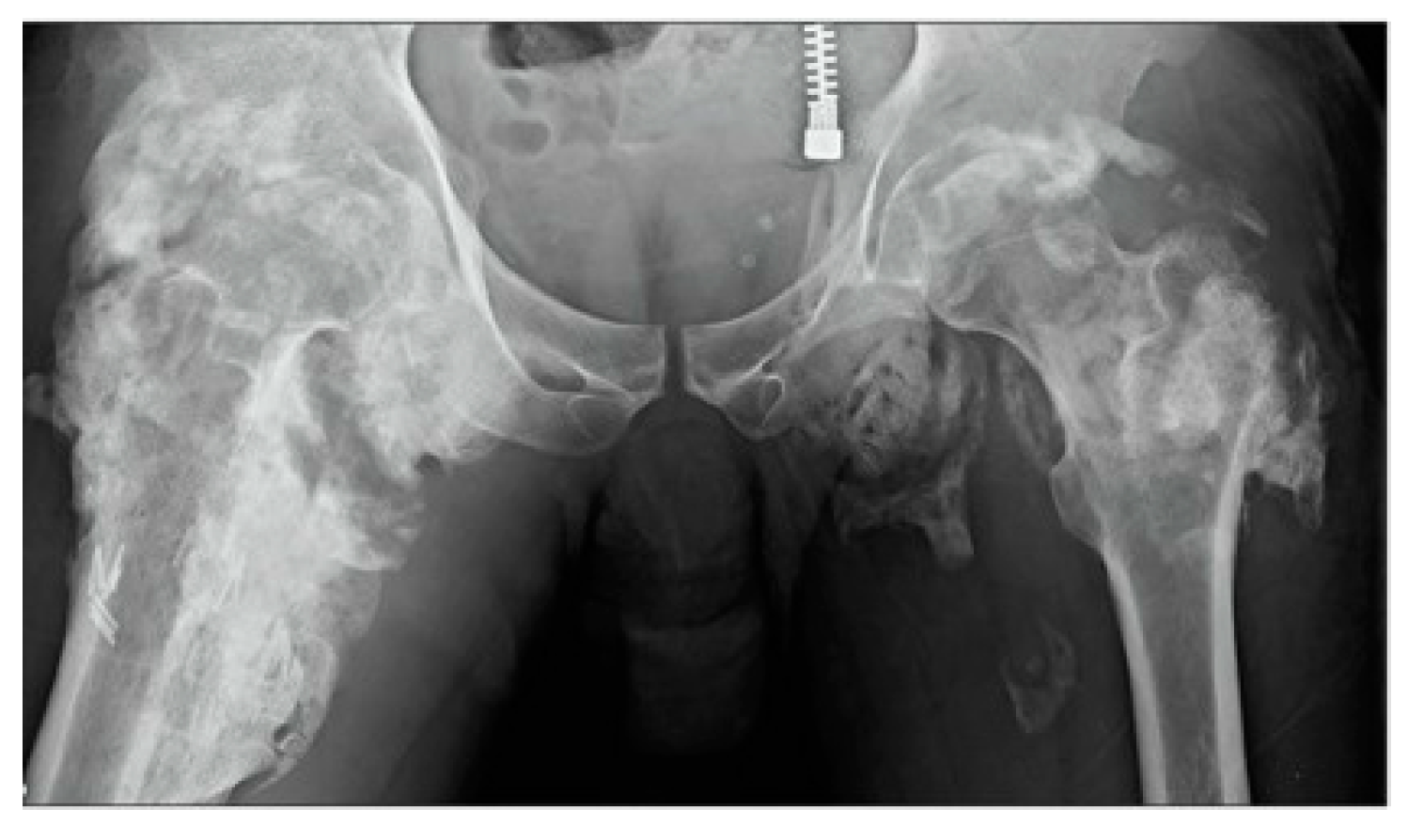
| Parameter | Postinjury Time |
|---|---|
| Transient ↓ in serum Ca2+ | 1 week |
| ↑ CPK and CRP | 1 week |
| ↑ Urinary PGE2 | 1 week |
| ↑ serum ALP level | 2 weeks |
| +ve triple phase bone scan | 3 weeks |
| +ve radiograph | 4–6 weeks |
| Lab Tests | tHO Group | Non-tHO Group |
|---|---|---|
| Alkaline phosphatase (ALP) | >130 U/L | 20–130 U/L |
| C-reactive protein (CRP) | 10–100 mg/L | <1.0 mg/L |
| Creatine phosphokinase (CPK) | 1–10 mg/L | <1 mg/L |
| Erythrocyte sedimentation rate (ESR) | 16–100 mm/h | <15 mm/h |
| X-ray | CT Scan | Triple Phase Bone Scan | MRI | Diagnostic Ultrasound | |
|---|---|---|---|---|---|
| Advantages | Cost-effective, reliable, and sensitive for HO diagnosis | Comprehensive, reliable, and sensitive for HO diagnosis | Early detection before calcification | Comprehensive and reliable to indicate HO formation | Portable, sensitive, and cost-effective to indicate HO formation |
| Risks | Light ionization radiation exposure | Moderate ionization radiation exposure | Contrast agent required and moderate radiation exposure | Not applicable to those with implant, pacemaker, and intracranial aneurysm clips | No measurable risks but may introduce non-measurable bias |
| Limitations | Qualitative and unable to detect pre-HO soft tissue mineralization | Qualitative Limited access in some regions. | Limited access in regions with less resourceful healthcare. | Qualitative, expensive, and not commonly used for HO early detection. Limited access in some regions. | Subjective and qualitative measures |
| Timing of HO diagnosis after SCI | 4–6 weeks | 4–6 weeks | 2–3 weeks | 2–4 weeks | 1–2 weeks |
| Progenitor Cell Type | Disease | Findings | Model (Lineage Tracing Marker) | Study |
|---|---|---|---|---|
| Hematopoietic Stem Cells (HSCs) | FOP/ tHO | HSCs give rise to cells that contribute to early inflammatory and fibroproliferative stage of HO Hematopoiesis evidence found in patient excised tHO | Human | Gannon et al. (1998) [130] Davis et al. (2013) [131] |
| Endothelial Progenitor Cells (EPCs) | FOP/tHO | Tie2+ EPCs contribute to every stage of HO formation Chondrocytes and osteoblasts express endothelial markers, suggesting endothelial-to-mesenchymal transition (EndMT) in FOP-HO lesionsAngiogenesis drives HO formation in FOP; inhibition of angiogenesis attenuates HO progression in tHO | Mouse (Tie2-Cre) | Lounev et al. (2009) [132] Medici et al. (2010) [133] Lin et al. (2022) [134] |
| Mesenchymal Stem Cells (MSCs) | FOP | MSCs increase osteochondrogenesis in FOP Nfatc1+ cells induce spontaneous HO lesions with increased osteogenic potential | Human Mouse (Nfatc1-Cre) | Hino et al. (2015) [87] Agarwal et al. (2015) [135] |
| Muscle Stem Cells | FOP | Muscle stem cells exhibit enhanced osteogenic and chondrogenic fate following muscular injury in FOP | Human | Barruet et al. (2021) [136] |
| Fibro/Adipogenic Progenitor Cells (FAP) | FOP | Activin A drives osteogenesis in FAPs, leading to spontaneous gHO formation | Mouse (MyoD-iCre/Tie2-Cre) | Lees-Shepard et al.(2018) [137] |
| Tendon Stem/Progenitor Cells | FOP/tHO | Scx+ cells induce spontaneous HO formation and are capable of chondrogenic and osteogenic differentiation involved in both gHO and tHO Tppp3+ cells contribute to chondrogenesis and osteogenesis after trauma | Mouse (Scx-Cre/Tppp3+) | Dey et al. (2016) [138] Agarwal et al. (2017) [139] Yea et al. (2023) [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan, C.; Bancroft, A.C.; Choi, J.H.; Nunez, J.H.; Pagani, C.A.; Lin, Y.-S.; Hsiao, E.C.; Levi, B. Intersections of Fibrodysplasia Ossificans Progressiva and Traumatic Heterotopic Ossification. Biomolecules 2024, 14, 349. https://doi.org/10.3390/biom14030349
Juan C, Bancroft AC, Choi JH, Nunez JH, Pagani CA, Lin Y-S, Hsiao EC, Levi B. Intersections of Fibrodysplasia Ossificans Progressiva and Traumatic Heterotopic Ossification. Biomolecules. 2024; 14(3):349. https://doi.org/10.3390/biom14030349
Chicago/Turabian StyleJuan, Conan, Alec C. Bancroft, Ji Hae Choi, Johanna H. Nunez, Chase A. Pagani, Yen-Sheng Lin, Edward C. Hsiao, and Benjamin Levi. 2024. "Intersections of Fibrodysplasia Ossificans Progressiva and Traumatic Heterotopic Ossification" Biomolecules 14, no. 3: 349. https://doi.org/10.3390/biom14030349
APA StyleJuan, C., Bancroft, A. C., Choi, J. H., Nunez, J. H., Pagani, C. A., Lin, Y.-S., Hsiao, E. C., & Levi, B. (2024). Intersections of Fibrodysplasia Ossificans Progressiva and Traumatic Heterotopic Ossification. Biomolecules, 14(3), 349. https://doi.org/10.3390/biom14030349





