Sulforaphane-Enriched Extracts from Broccoli Exhibit Antimicrobial Activity against Plant Pathogens, Promising a Natural Antimicrobial Agent for Crop Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Microorganism Strains
2.2. DNA Preparation and Genetic Diversity Analysis by SSR Method
2.3. Determination of Total GSLs
2.4. SFN Extraction and HPLC Assay
2.5. RNA Isolation and Quantitative Real-Time RT-PCR (qRT-PCR) Analysis
2.6. Detection of Antimicrobial Activity of SFN-ee In Vitro
2.7. Determination of the Minimum Inhibitory Concentration (MIC)
2.8. Membrane Integrity Assay
2.9. Measurement of ROS Accumulation in Broccoli Leaves
2.10. Statistical Analysis
3. Results
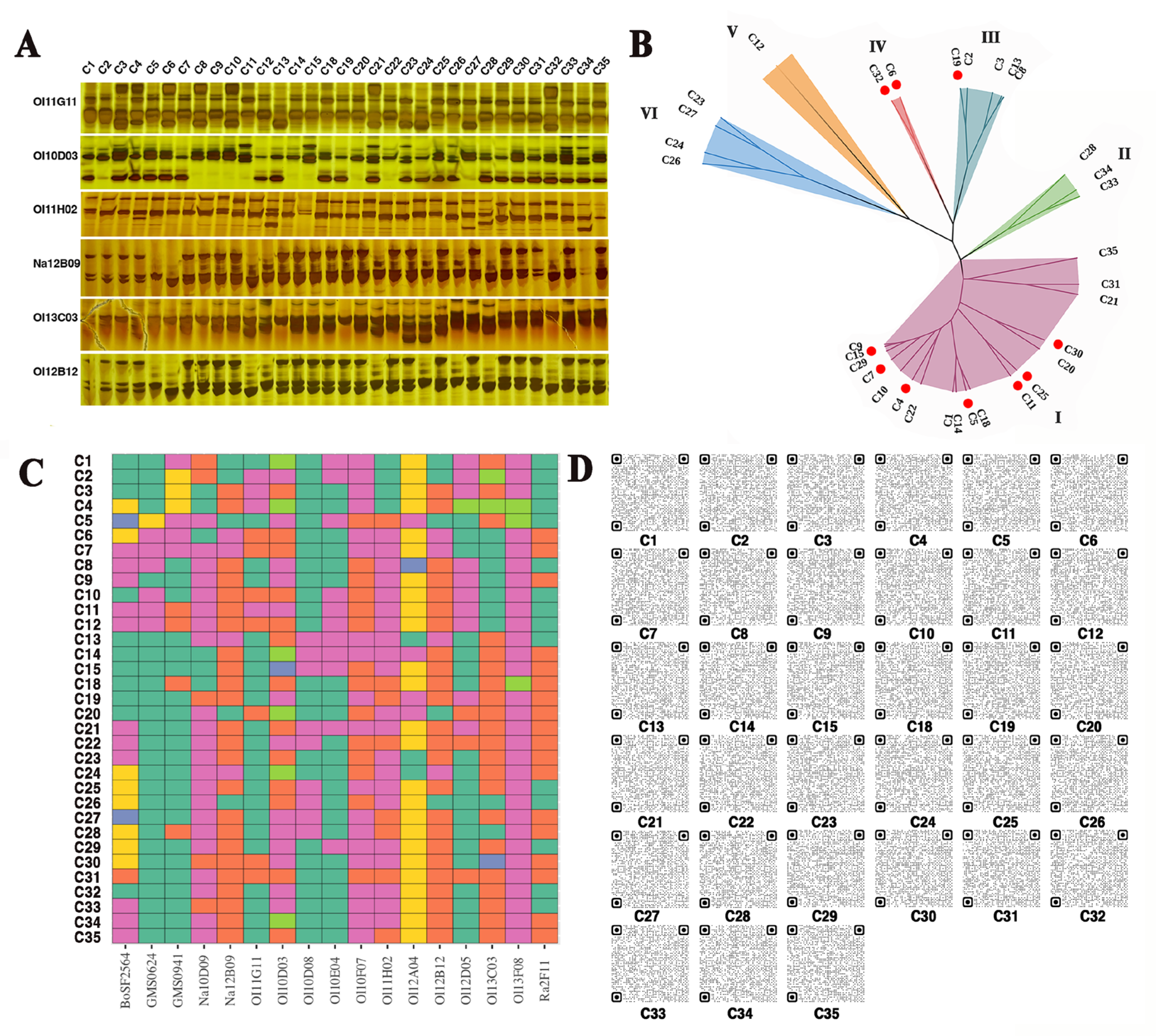
3.1. Molecular IDs of 33 B. oleracea Varieties Were Identified
3.2. Genetic Background of Different B. oleracea Varieties Influences GSL Content
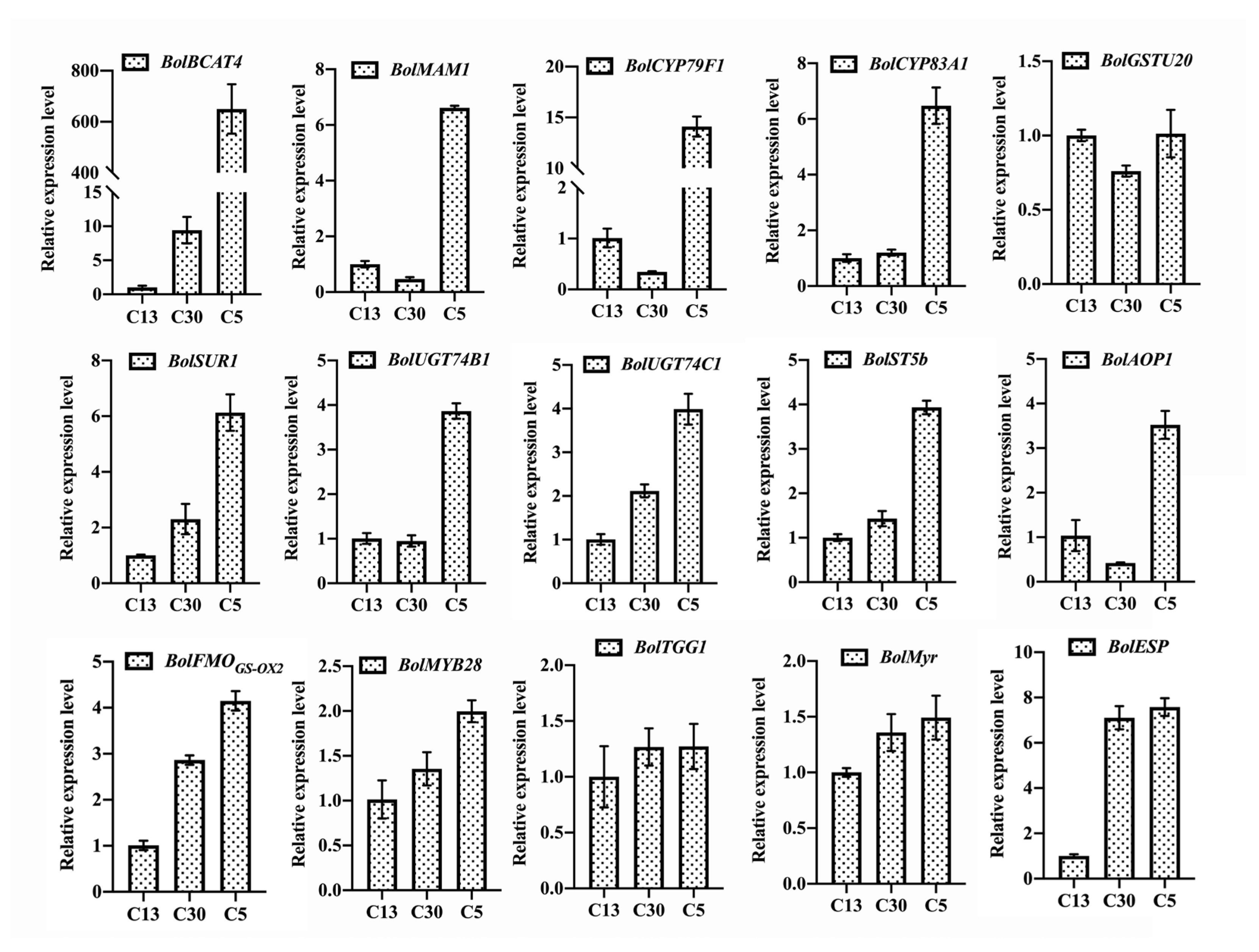
3.3. Myrosinase and ESP Genes Play Important Roles in Affecting the SFN Content besides Genetic Background
3.4. SFN Is Efficiently Extracted from Broccoli Curds
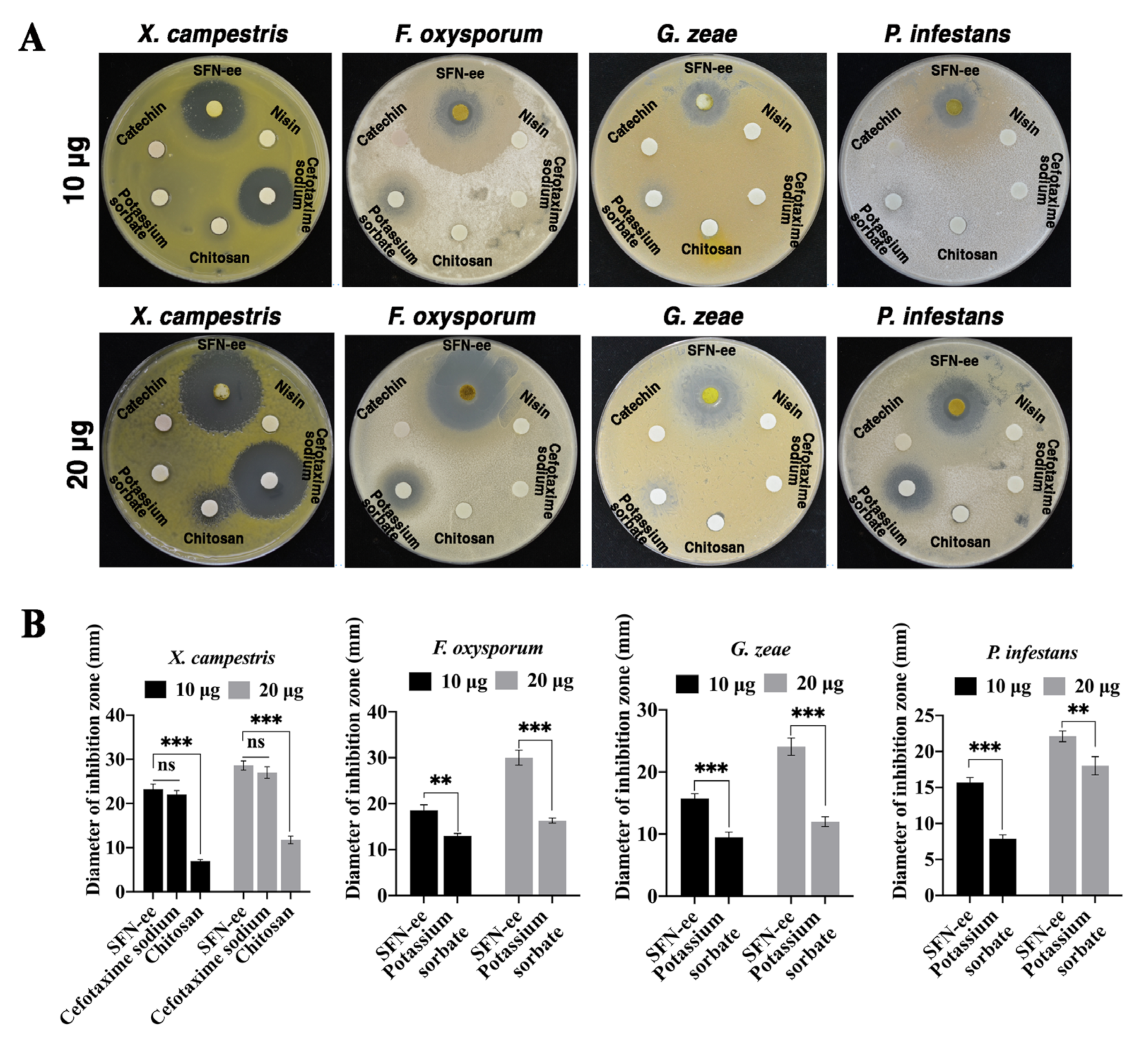
3.5. SFN-ee Display Strong and Broad-Spectrum Antimicrobial Activity In Vitro
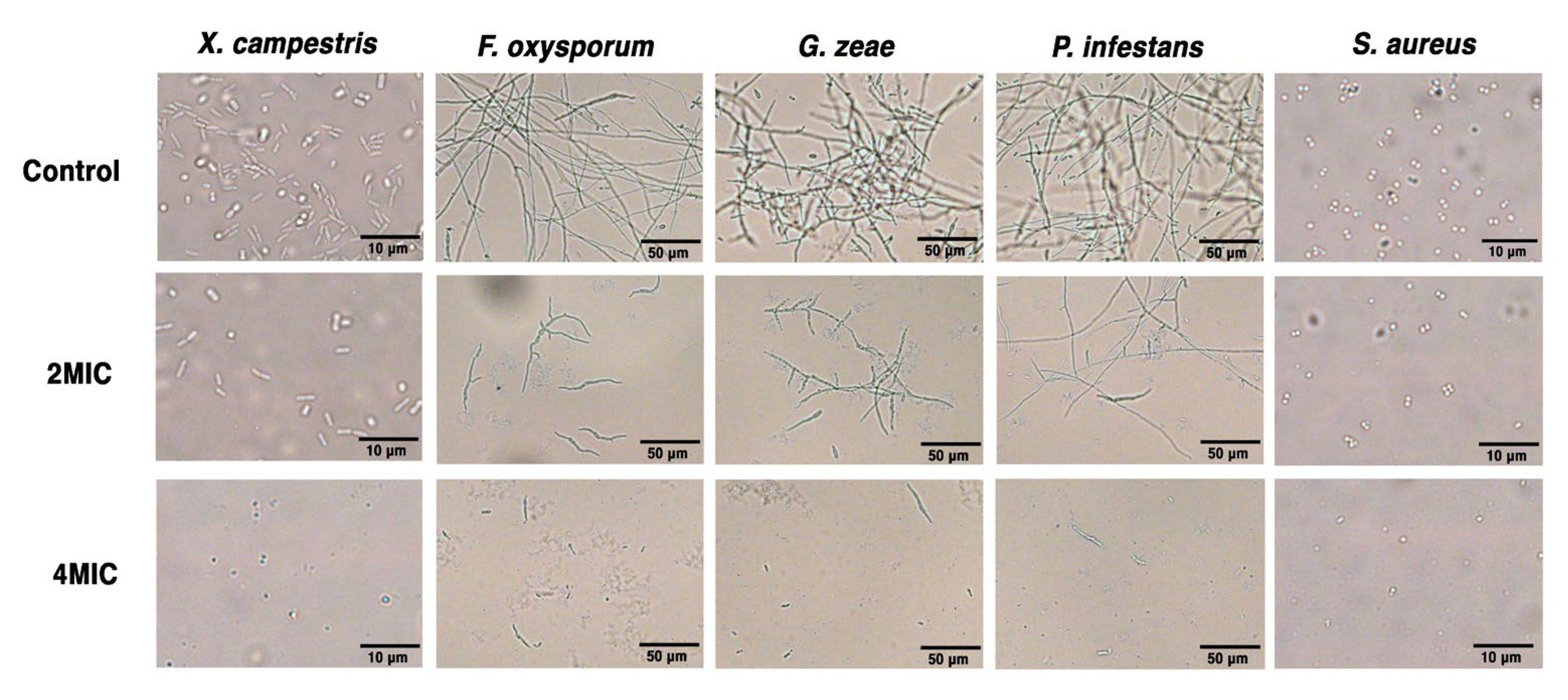
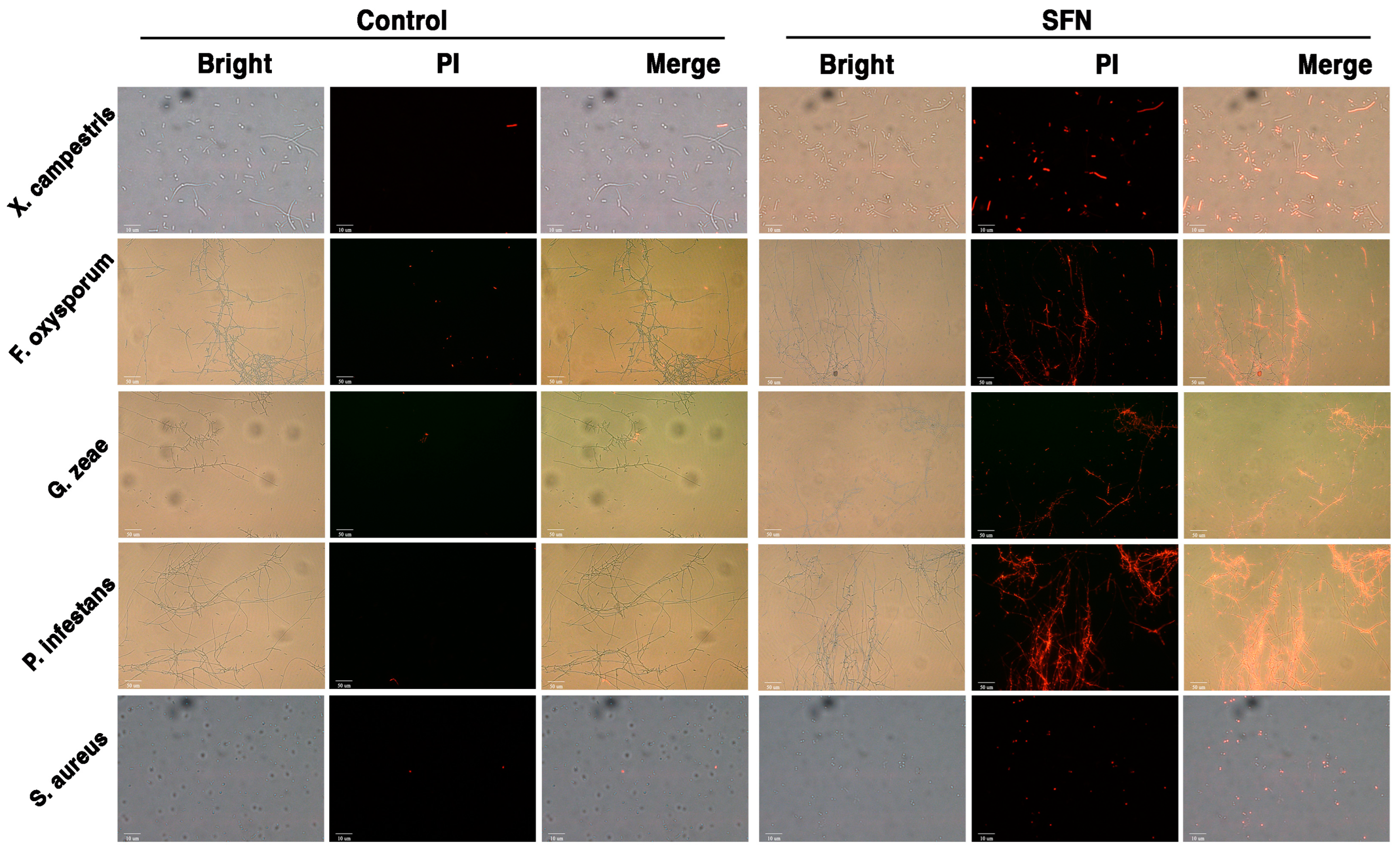
3.6. SFN-ee Inhibit the Proliferation and Destroy the Membrane Integrity of Pathogens
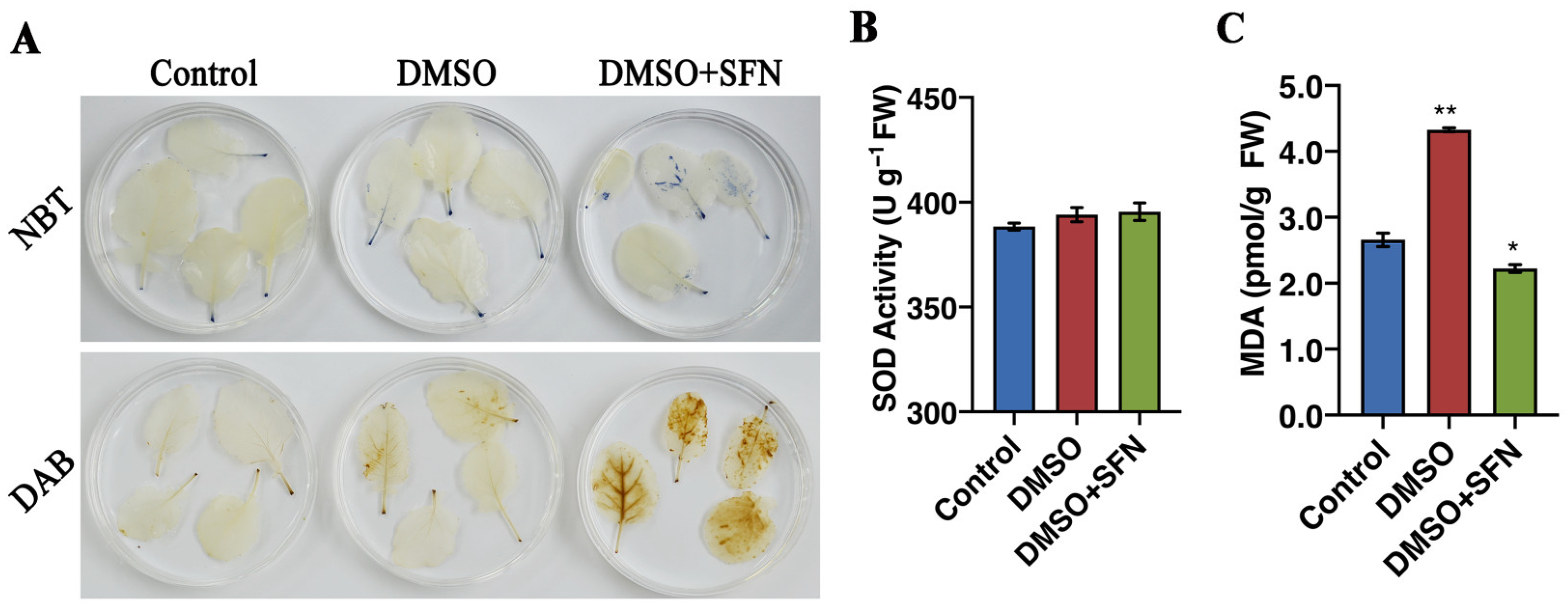
3.7. SFN-ee Induce Endogenous ROS Accumulation in Broccoli Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Singh Sidhu, G.P.; Handa, N.; Kaur Kohli, S.; Yadav, P.; Shreeya Bali, A.; Daman Parihar, R.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Li Destri Nicosia, M.G.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Su, Y.; Li, Y.; Zuo, D.; Liu, H.; Liu, Y.; Mei, X.; Huang, H.; Yang, M.; et al. Allyl isothiocyanate in the volatiles of brassica juncea inhibits the growth of root rot pathogens of panax notoginseng by inducing the accumulation of ROS. J. Agric. Food Chem. 2021, 69, 13713–13723. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar Singh, V.; Kumar Dwivedy, A.; Kumar Chaudhari, A.; Deepika, K.; Dubey, N. Nanostructured Pimpinella anisum essential oil as novel green food preservative against fungal infestation, aflatoxin B1 contamination and deterioration of nutritional qualities. Food Chem. 2021, 344, 128574. [Google Scholar] [CrossRef] [PubMed]
- Otoo, R.A.; Allen, A.R. Sulforaphane’s multifaceted potential: From neuroprotection to anticancer action. Molecules 2023, 28, 6902. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, H.; Qiu, Y.; Dong, Y.; Hamouda, H.I.; Balah, M.A.; Mao, X. Biochemical characterization of a novel myrosinase Rmyr from Rahnella inusitata for high-level preparation of sulforaphene and sulforaphane. J. Agric. Food Chem. 2022, 70, 2303–2311. [Google Scholar] [CrossRef]
- Lambrix, V.; Reichelt, M.; Mitchell-Olds, T.; Kliebenstein, D.J.; Gershenzon, J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 2001, 13, 2793–2807. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.A.; Khamis, G. Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.; Krause, K.; Karczewska, M.; Szalewska-Pałasz, A. Evaluation of the anti-shigellosis activity of dietary isothiocyanates in Galleria mellonella larvae. Nutrients 2021, 13, 3967. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef]
- Krause, K.; Pyrczak-Felczykowska, A.; Karczewska, M.; Narajczyk, M.; Herman-Antosiewicz, A.; Szalewska-Pałasz, A.; Nowicki, D. Dietary isothiocyanates, sulforaphane and 2-phenethyl isothiocyanate, effectively impair vibrio cholerae virulence. Int. J. Mol. Sci. 2021, 22, 10187. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Zhang, J.; Liu, Y.X.; Tian, C.; Qu, B.; Gao, C.; Xin, P.; Cheng, S.; Zhang, W.; et al. An Arabidopsis secondary metabolite directly targets expression of the bacterial type III secretion system to inhibit bacterial virulence. Cell Host Microbe 2020, 27, 601–613. [Google Scholar] [CrossRef]
- Schillheim, B.; Jansen, I.; Baum, S.; Beesley, A.; Bolm, C.; Conrath, U. Sulforaphane modifies histone H3, unpacks chromatin, and primes defense. Plant Physiol. 2018, 176, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yao, W.; Song, Y.; Liu, W.; Wang, Z. Molecular characterization and expression of three galactinol synthase genes that confer stress tolerance in Salvia miltiorrhiza. J. Plant Physiol. 2012, 169, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Huang, S.M.; Liu, Y.M.; Fang, Z.Y.; Yang, L.M.; Hua, W.; Yuan, S.X.; Liu, S.Y.; Sun, J.F.; Zhuang, M.; et al. Construction and analysis of a high-density genetic linkage map in cabbage (Brassica oleracea L. var. capitata). BMC Genom. 2012, 13, 523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, X.; Liu, Q.; Luo, T.; Tang, Z.; Zhou, Y. The genetic diversity and relationships of cauliflower (Brassica oleracea var. botrytis) inbred lines assessed by using SSR markers. PLoS ONE 2018, 13, e0208551. [Google Scholar]
- Lin, M.G.; Wen, H.P.; Xiao, J.Z.; Zheng, Q. Construction of DNA fingerprint for 48 broccoli cultivars. Acta Agric. Zhejiangensis 2021, 33, 2304–2312. (In Chinese) [Google Scholar]
- Ding, Y.; Mu, W.F.; Yang, L.; Zhang, P.; Guan, J.J. SSR core primer screening for purity identification of cauliflower hybrids. China Seed Ind. 2021, 11, 79–84. (In Chinese) [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, M.; Wiciarz, M.; Jajić, I.; Kozieradzka-Kiszkurno, M.; Dobrev, P.; Vanková, R.; Niewiadomska, E. A different pattern of production and scavenging of reactive oxygen species in halophytic Eutrema salsugineum (Thellungiella salsuginea) plants in comparison to Arabidopsis thaliana and its relation to salt stress signaling. Front. Plant Sci. 2016, 7, 1179. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Wallig, M.A.; Juvik, J.A.; Klein, B.P.; Kushad, M.M.; Jeffery, E.H. Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea. J. Agric. Food Chem. 2001, 49, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Boyen, F.; Vangroenweghe, F.; Butaye, P.; De Graef, E.; Castryck, F.; Heylen, P.; Vanrobaeys, M.; Haesebrouck, F. Disk prediffusion is a reliable method for testing colistin susceptibility in porcine E. coli strains. Vet Microbiol. 2010, 144, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Bernardes-Engemann, A.R.; Tomki, G.F.; Rabello, V.B.S.; Almeida-Silva, F.; Freitas, D.F.S.; Gutierrez-Galhardo, M.C.; Almeida-Paes, R.; Zancopé-Oliveira, R.M. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front. Cell. Infect. Microbiol. 2022, 12, 893501. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.A.; Marks, L.R.; Roche-Håkansson, H.; Håkansson, A.P. Monitoring changes in membrane polarity, membrane integrity, and intracellular ion concentrations in Streptococcus pneumoniae using fluorescent dyes. Jove-J. Vis. Exp. 2014, 84, e51008. [Google Scholar]
- Rai, G.K.; Rai, N.P.; Rathaur, S.; Kumar, S.; Singh, M. Expression of rd29A::AtDREB1A/CBF3 in tomato alleviates drought induced oxidative stress by regulating key enzymatic and non-enzymatic antioxidants. Plant Physiol. Biochem. 2013, 69, 90–100. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Kuang, J.; Ge, Q.; Zhang, Y.; Wang, Z. Overexpression of SmLEA enhances salt and drought tolerance in Escherichia coli and Salvia miltiorrhiza. Protoplasma 2014, 251, 1191–1199. [Google Scholar] [CrossRef]
- Li, L.; Song, S.; Nirasawa, S.; Hung, Y.C.; Jiang, Z.; Liu, H. Slightly acidic electrolyzed water treatment enhances the main bioactive phytochemicals content in broccoli sprouts via changing metabolism. J. Agric. Food Chem. 2019, 67, 606–614. [Google Scholar] [CrossRef]
- Coutinho, L.L.; Junior, T.C.T.; Rangel, M.C. Sulforaphane: An emergent anti-cancer stem cell agent. Front. Oncol. 2023, 13, 1089115. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Bueno-Solano, C.; Martínez-Ibarra, D.M.; Camacho-Gil, F.; Villa-Lerma, A.G.; Rodríguez-Núñez, J.R.; Lóez-Cervantes, J.; Sánchez-Machado, D.I. Sulforaphane (1-isothiocyanato-4-(methylsulfinyl)-butane) content in cruciferous vegetables. Arch. Latinoam. Nutr. 2009, 59, 95–100. [Google Scholar]
- Wu, X.; Huang, H.; Childs, H.; Wu, Y.; Yu, L.; Pehrsson, P.R. Glucosinolates in Brassica vegetables: Characterization and factors that influence distribution, content, and intake. Annu. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef]
- Wu, Y.F.; Lv, C.Z.; Zou, L.G.; Sun, J.; Song, X.J.; Zhao, Y.; Mao, J.W. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar]
- Liang, H.; Yuan, Q.P.; Dong, H.R.; Liu, Y.M. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J. Food Compos. Anal. 2006, 19, 473–476. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, Q.; Liu, M. Simultaneous determination of glucoraphanin and sulforaphane in Brassica oleracea seeds by high-performance liquid chromatography with evaporative light-scattering detector. Nat. Prod. Res. 2013, 27, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Meng, G.; Li, W.; Fan, D.; Wang, X.; Espinoza-Pinochet, C.A.; Cespedes-Acuña, C.L. Sulforaphane and its antioxidative effects in broccoli seeds and sprouts of different cultivars. Food Chem. 2020, 316, 126216. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Yang, Y.; Ávila, F.W.; Fish, T.; Yuan, H.; Hui, M.; Pan, S.; Thannhauser, T.W.; Li, L. Effects of selenium supplementation on glucosinolate biosynthesis in broccoli. J. Agric. Food Chem. 2018, 66, 8036–8044. [Google Scholar] [CrossRef]
- Mitreiter, S.; Gigolashvili, T. Regulation of glucosinolate biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef]
- Grubb, C.D.; Zipp, B.J.; Kopycki, J.; Schubert, M.; Quint, M.; Lim, E.K.; Bowles, D.J.; Pedras, M.S.; Abel, S. Comparative analysis of Arabidopsis UGT74 glucosyltransferases reveals a special role of UGT74C1 in glucosinolate biosynthesis. Plant J. 2014, 79, 92–105. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.; Xie, Z.; Liu, Y.; Wang, P.; Dai, L.; Zhang, X.; Wang, Z.; Wang, Z.; Wan, L.; et al. Natural variation and artificial selection at the BnaC2.MYB28 locus modulate Brassica napus seed glucosinolate. Plant Physiol. 2023, 191, 352–368. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from broccoli, sulforaphane, and its properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.F.; Wang, J.; Sun, G.X. Sulforaphane regulates apoptosis- and proliferation-related signaling pathways and synergizes with cisplatin to suppress human ovarian cancer. Int. J. Mol. Med. 2018, 42, 2447–2458. [Google Scholar] [CrossRef]
- Zheng, Z.; Lin, K.; Hu, Y.; Zhou, Y.; Ding, X.; Wang, Y.; Wu, W. Sulforaphane metabolites inhibit migration and invasion via microtubule-mediated Claudins dysfunction or inhibition of autolysosome formation in human non-small cell lung cancer cells. Cell Death Dis. 2019, 10, 259. [Google Scholar] [CrossRef]
- Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.K.; Blaheta, R.A. Sulforaphane reduces prostate cancer cell growth and proliferation in vitro by modulating the Cdk-Cyclin axis and expression of the CD44 variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Dong, N.; Su, X.; Duan, M.; Wei, Y.; Wei, J.; Liu, G.; Peng, Q.; Zhao, Y. Sulforaphane induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells. Sci. Rep. 2021, 11, 2504. [Google Scholar] [CrossRef]
- Askan, G.; Sahin, I.H.; Chou, J.F.; Yavas, A.; Capanu, M.; Iacobuzio-Donahue, C.A.; Basturk, O.; O’Reilly, E.M. Pancreatic cancer stem cells may define tumor stroma characteristics and recurrence patterns in pancreatic ductal adenocarcinoma. BMC Cancer 2021, 21, 385. [Google Scholar] [CrossRef]
- Kennelley, G.E.; Amaye-Obu, T.; Foster, B.A.; Tang, L.; Paragh, G.; Huss, W.J. Mechanistic review of sulforaphane as a chemoprotective agent in bladder cancer. Am. J. Clin. Exp. Urol. 2023, 11, 103–120. [Google Scholar]
- Heckler, C.; Sant’anna, V.; Brandelli, A.; Malheiros, P.S. Combined effect of carvacrol, thymol and nisin against Staphylococcus aureus and Salmonella Enteritidis. An. Acad. Bras. Cienc. 2021, 93, e20210550. [Google Scholar] [CrossRef]
- De Souza Lopes, A.; Elisabete Costa Antunes, A.; Idelça Aires Machado, K.; Sartoratto, A.; Cristina Teixeira Duarte, M. The impact of antimicrobial food additives and sweeteners on the growth and metabolite production of gut bacteria. Folia Microbiol. 2023, 68, 813–821. [Google Scholar] [CrossRef]
- Hu, Z.; Gänzle, M.G. Challenges and opportunities related to the use of chitosan as a food preservative. J. Appl. Microbiol. 2019, 126, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.G.; Ramesh, D.; Santhosh Manikandan, K.; Theresa, M.; Sethumadhavan, A.; Priyadarisini, V.B.; Radhakrishnan, E.K.; Mani, M.; Kannan, T. Chitosan with pendant (E)-5-((4-acetylphenyl) diazenyl)-6-aminouracil groups as synergetic antimicrobial agents. J. Mater. Chem. B 2022, 10, 4048–4058. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Takada, K.; Makimura, M.; Otake, S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J. Periodontal Res. 2002, 37, 433–438. [Google Scholar] [CrossRef]
- Biagi, M.; Tan, X.; Wu, T.; Jurkovic, M.; Vialichka, A.; Meyer, K.; Mendes, R.E.; Wenzler, E. Activity of potential alternative treatment agents for Stenotrophomonas maltophilia isolates nonsusceptible to levofloxacin and/or trimethoprim-sulfamethoxazole. J. Clin. Microbiol. 2020, 58, e01603-19. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, R.; Deng, R.; Zheng, S.; Shen, Z. Antibacterial activity and antibacterial mechanism of flavaspidic acid BB against Staphylococcus haemelyticus. BMC Microbiol. 2023, 23, 276. [Google Scholar] [CrossRef] [PubMed]
- Giordani, C.; Simonetti, G.; Natsagdorj, D.; Choijamts, G.; Ghirga, F.; Calcaterra, A.; Quaglio, D.; De Angelis, G.; Toniolo, C.; Pasqua, G. Antifungal activity of Mongolian medicinal plant extracts. Nat. Prod. Res. 2020, 34, 449–455. [Google Scholar] [CrossRef]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 13677. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, W.; Su, R.; Yang, M.; Zhang, N.; Li, X.; Li, L.; Sheng, J.; Tian, Y. Multi-Target antibacterial mechanism of moringin from moringa oleifera seeds against listeria monocytogenes. Front. Microbiol. 2022, 13, 925291. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signaling in plant stress responses. Nature reviews. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar]
- Apostol, I.; Heinstein, P.F.; Low, P.S. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: Role in defense and signal transduction. Plant Physiol. 1989, 90, 109–116. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Curtis, C.; Fu, Z.Q. Go in for the kill: How plants deploy effector-triggered immunity to combat pathogens. Virulence 2014, 5, 710–721. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, P.; Prasad, A.; Dogra, V.; Kumar, A. Jasmonic acid limits Rhizoctonia solani AG1-IA infection in rice by modulating reactive oxygen species homeostasis. Plant Physiol. Biochem. 2023, 196, 520–530. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Jiang, H.; Li, Y.; Zhang, X.; Sun, W.; Liu, C.; Zhao, Z.; Yun, C.; Li, H.; Wang, C. Sulforaphane-Enriched Extracts from Broccoli Exhibit Antimicrobial Activity against Plant Pathogens, Promising a Natural Antimicrobial Agent for Crop Protection. Biomolecules 2024, 14, 352. https://doi.org/10.3390/biom14030352
He L, Jiang H, Li Y, Zhang X, Sun W, Liu C, Zhao Z, Yun C, Li H, Wang C. Sulforaphane-Enriched Extracts from Broccoli Exhibit Antimicrobial Activity against Plant Pathogens, Promising a Natural Antimicrobial Agent for Crop Protection. Biomolecules. 2024; 14(3):352. https://doi.org/10.3390/biom14030352
Chicago/Turabian StyleHe, Lixia, Hanmin Jiang, Yaotong Li, Xu Zhang, Wenting Sun, Ce Liu, Zekai Zhao, Chengrong Yun, Hui Li, and Chunguo Wang. 2024. "Sulforaphane-Enriched Extracts from Broccoli Exhibit Antimicrobial Activity against Plant Pathogens, Promising a Natural Antimicrobial Agent for Crop Protection" Biomolecules 14, no. 3: 352. https://doi.org/10.3390/biom14030352






