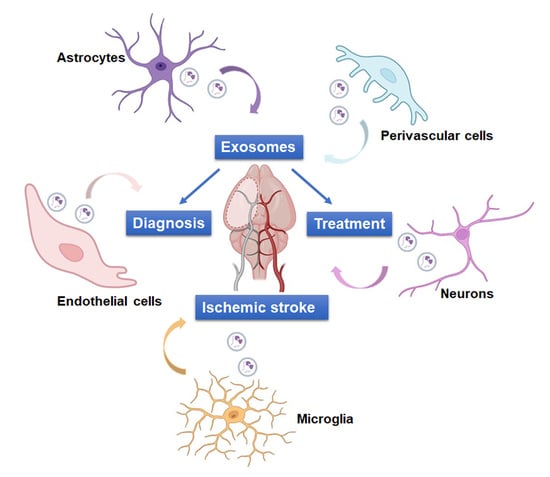
Revolutionizing Ischemic Stroke Diagnosis and Treatment: The Promising Role of Neurovascular Unit-Derived Extracellular Vesicles
Abstract
:1. Introduction
2. Neurovascular Unit
2.1. Function of the Neurovascular Unit in Central Nervous System Physiology
2.2. Function of the Neurovascular Unit in Central Nervous System Diseases
2.3. Role of the Neurovascular Unit in IS
3. Extracellular Vesicles
3.1. Extracellular Vesicles
3.2. Extracellular Vesicles and IS
3.3. Extracellular Vesicles Derived from the NVU
4. Role of NVU-EVs in IS
4.1. NVU-EVs and IS Diagnosis
4.2. NVU-EVs and IS Treatment
4.2.1. Neuron-Derived EVs
4.2.2. Astrocyte-Derived EVs
4.2.3. Microglia-Derived EVs
4.2.4. Endothelial Cell-Derived EVs
4.2.5. Perivascular Cell-Derived EVs
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, X.; Yu, X.; Liu, Z.; Jiang, Y.; Fang, Y.; Zong, M.; Suo, C.; Man, Q.; Xiong, L. Global Burden, Risk Factor Analysis, and Prediction Study of Ischemic Stroke, 1990–2030. Neurology 2023, 101, e137–e150. [Google Scholar] [CrossRef] [PubMed]
- Timpone, V.M.; Jensen, A.; Poisson, S.N.; Trivedi, P.S. Compliance with Imaging Guidelines for Workup of Transient Ischemic Attack: Evidence from the Nationwide Emergency Department Sample. Stroke 2020, 51, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. Jama 2021, 325, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Donnan, G.A.; Lees, K.R.; Hacke, W.; Khatri, P.; Hill, M.D.; Goyal, M.; Mitchell, P.J.; Saver, J.L.; Diener, H.C.; et al. Endovascular stent thrombectomy: The new standard of care for large vessel ischaemic stroke. Lancet. Neurol. 2015, 14, 846–854. [Google Scholar] [CrossRef]
- Patil, S.; Rossi, R.; Jabrah, D.; Doyle, K. Detection, Diagnosis and Treatment of Acute Ischemic Stroke: Current and Future Perspectives. Front. Med. Technol. 2022, 4, 748949. [Google Scholar] [CrossRef]
- Luo, G.; Mo, D.; Tong, X.; Liebeskind, D.S.; Song, L.; Ma, N.; Gao, F.; Sun, X.; Zhang, X.; Wang, B.; et al. Factors Associated with 90-Day Outcomes of Patients with Acute Posterior Circulation Stroke Treated By Mechanical Thrombectomy. World Neurosurg. 2018, 109, e318–e328. [Google Scholar] [CrossRef]
- Bivard, A.; Krishnamurthy, V.; Stanwell, P.; Levi, C.; Spratt, N.J.; Davis, S.; Parsons, M. Arterial spin labeling versus bolus-tracking perfusion in hyperacute stroke. Stroke 2014, 45, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.A.; Sanaie, S.; Kuchaki Rafsanjani, M.; Hosseini, M.-S. Role of imaging in early diagnosis of acute ischemic stroke: A literature review. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 175. [Google Scholar] [CrossRef]
- Tao, J.; Xie, X.; Luo, M.; Sun, Q. Identification of key biomarkers in ischemic stroke: Single-cell sequencing and weighted co-expression network analysis. Aging 2023, 15, 6346–6360. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Rossi, D.; Ricciardi, A.; Morasso, C.; Brambilla, L.; Albasini, S.; Vanna, R.; Fassio, C.; Begenisic, T.; Loi, M.; et al. Quantification and prospective evaluation of serum NfL and GFAP as blood-derived biomarkers of outcome in acute ischemic stroke patients. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2023, 43, 1601–1611. [Google Scholar] [CrossRef]
- Block, H.S.; Biller, J. Commonly asked questions: Thrombolytic therapy in the management of acute stroke. Expert Rev. Neurother. 2013, 13, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Murray, V.; Norrving, B.; Sandercock, P.A.; Terént, A.; Wardlaw, J.M.; Wester, P. The molecular basis of thrombolysis and its clinical application in stroke. J. Intern. Med. 2010, 267, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Röther, J.; Ford, G.A.; Thijs, V.N. Thrombolytics in acute ischaemic stroke: Historical perspective and future opportunities. Cerebrovasc. Dis. 2013, 35, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Golovoy, D.; Nimjee, S.; Ferrell, A.; Smith, T.; Britz, G. Mechanical thrombectomy devices for endovascular management of acute ischemic stroke: Duke stroke center experience. Asian J. Neurosurg. 2012, 7, 166–170. [Google Scholar] [CrossRef]
- Deng, L.; Qiu, S.; Wang, L.; Li, Y.; Wang, D.; Liu, M. Comparison of Four Food and Drug Administration-Approved Mechanical Thrombectomy Devices for Acute Ischemic Stroke: A Network Meta-Analysis. World Neurosurg. 2019, 127, e49–e57. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Lutsep, H.L.; Gupta, R.; Jovin, T.G.; Albers, G.W.; Walker, G.A.; Liebeskind, D.S.; Smith, W.S. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. Lancet 2012, 380, 1231–1240. [Google Scholar] [CrossRef]
- Saver, J.L.; Jahan, R.; Levy, E.I.; Jovin, T.G.; Baxter, B.; Nogueira, R.G.; Clark, W.; Budzik, R.; Zaidat, O.O. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet 2012, 380, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Occhipinti, G.; Giacoppo, D.; Agnello, F.; Laudani, C.; Spagnolo, M.; Mauro, M.S.; Rochira, C.; Finocchiaro, S.; Mazzone, P.M.; et al. Antithrombotic Therapy for Primary and Secondary Prevention of Ischemic Stroke: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 1538–1557. [Google Scholar] [CrossRef]
- Diener, H.C.; Hankey, G.J. Primary and Secondary Prevention of Ischemic Stroke and Cerebral Hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1804–1818. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Amarenco, P.; Denison, H.; Evans, S.R.; Himmelmann, A.; James, S.; Knutsson, M.; Ladenvall, P.; Molina, C.A.; Wang, Y. Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA. N. Engl. J. Med. 2020, 383, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Shehjar, F.; Maktabi, B.; Rahman, Z.A.; Bahader, G.A.; James, A.W.; Naqvi, A.; Mahajan, R.; Shah, Z.A. Stroke: Molecular mechanisms and therapies: Update on recent developments. Neurochem. Int. 2023, 162, 105458. [Google Scholar] [CrossRef]
- Andjus, P.; Kosanović, M.; Milićević, K.; Gautam, M.; Vainio, S.J.; Jagečić, D.; Kozlova, E.N.; Pivoriūnas, A.; Chachques, J.C.; Sakaj, M.; et al. Extracellular Vesicles as Innovative Tool for Diagnosis, Regeneration and Protection against Neurological Damage. Int. J. Mol. Sci. 2020, 21, 6859. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Dar, G.H.; Mendes, C.C.; Kuan, W.L.; Speciale, A.A.; Conceição, M.; Görgens, A.; Uliyakina, I.; Lobo, M.J.; Lim, W.F.; El Andaloussi, S.; et al. GAPDH controls extracellular vesicle biogenesis and enhances the therapeutic potential of EV mediated siRNA delivery to the brain. Nat. Commun. 2021, 12, 6666. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro-oncology 2022, 24, 1871–1883. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Bang, O.Y.; Kim, J.E. Stem cell-derived extracellular vesicle therapy for acute brain insults and neurodegenerative diseases. BMB Rep. 2022, 55, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Yousif, G.; Qadri, S.; Haik, M.; Haik, Y.; Parray, A.S.; Shuaib, A. Circulating Exosomes of Neuronal Origin as Potential Early Biomarkers for Development of Stroke. Mol. Diagn. Ther. 2021, 25, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Cheng, T.; Lai, X. Mechanism of ischemic brain injury repair by endothelial progenitor cell-derived exosomes. Mol. Med. Rep. 2022, 26, 269. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, H.; Yue, K.; Cao, X.; Yang, E.; Zhang, Z.; Huang, Y.; Li, X.; Ding, D.; Luo, P.; et al. Observing Extracellular Vesicles Originating from Endothelial Cells in Vivo Demonstrates Improved Astrocyte Function Following Ischemic Stroke via Aggregation-Induced Emission Luminogens. ACS Nano 2023, 17, 16174–16191. [Google Scholar] [CrossRef]
- Tiedt, S.; Buchan, A.M.; Dichgans, M.; Lizasoain, I.; Moro, M.A.; Lo, E.H. The neurovascular unit and systemic biology in stroke-implications for translation and treatment. Nat. Rev. Neurol. 2022, 18, 597–612. [Google Scholar] [CrossRef]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Nehme, Z.; Roehlen, N.; Dhawan, P.; Baumert, T.F. Tight Junction Protein Signaling and Cancer Biology. Cells 2023, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Hadjisavva, R.; Anastasiou, O.; Ioannou, P.S.; Zheltkova, M.; Skourides, P.A. Adherens junctions stimulate and spatially guide integrin activation and extracellular matrix deposition. Cell Rep. 2022, 40, 111091. [Google Scholar] [CrossRef]
- Li, P.; Fan, H. Pericyte Loss in Diseases. Cells 2023, 12, 1931. [Google Scholar] [CrossRef]
- Mehrabadi, A.R.; Korolainen, M.A.; Odero, G.; Miller, D.W.; Kauppinen, T.M. Poly(ADP-ribose) polymerase-1 regulates microglia mediated decrease of endothelial tight junction integrity. Neurochem. Int. 2017, 108, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, G.; Ohtomo, R.; Takase, H.; Lok, J.; Arai, K. White-matter repair: Interaction between oligodendrocytes and the neurovascular unit. Brain Circ. 2018, 4, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef]
- Bogale, T.A.; Faustini, G.; Longhena, F.; Mitola, S.; Pizzi, M.; Bellucci, A. Alpha-Synuclein in the Regulation of Brain Endothelial and Perivascular Cells: Gaps and Future Perspectives. Front. Immunol. 2021, 12, 611761. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Haller, E.; Navarro, S.; Besong, T.E.; Boccio, K.J.; Hailu, S.; Khatib, M.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Transplantation of human bone marrow stem cells into symptomatic ALS mice enhances structural and functional blood-spinal cord barrier repair. Exp. Neurol. 2018, 310, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.J.; Lim, R.G.; Onur, T.; Dane, M.A.; Smith, R.; Wang, K.; Jean, G.E.; Reyes-Ortiz, A.; Devlin, K.; Miramontes, R.; et al. An altered extracellular matrix-integrin interface contributes to Huntington’s disease-associated CNS dysfunction in glial and vascular cells. Hum. Mol. Genet. 2023, 32, 1483–1496. [Google Scholar] [CrossRef]
- Garcia, V.J.; Rushton, D.J.; Tom, C.M.; Allen, N.D.; Kemp, P.J.; Svendsen, C.N.; Mattis, V.B. Huntington’s Disease Patient-Derived Astrocytes Display Electrophysiological Impairments and Reduced Neuronal Support. Front. Neurosci. 2019, 13, 669. [Google Scholar] [CrossRef]
- Juopperi, T.A.; Kim, W.R.; Chiang, C.H.; Yu, H.; Margolis, R.L.; Ross, C.A.; Ming, G.L.; Song, H. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol. Brain 2012, 5, 17. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Chen, Y.C.; Huang, C.H.; Chen, C.C.; Hsu, Y.H.; Chen, H.M.; Chiu, F.L.; Kuo, H.C.; Chang, C.; Chern, Y. Aberrant astrocytes impair vascular reactivity in Huntington disease. Ann. Neurol. 2015, 78, 178–192. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J.; Sawiak, S.J.; Cisbani, G.; Lagacé, M.; Kuan, W.L.; Saint-Pierre, M.; Dury, R.J.; Alata, W.; St-Amour, I.; Mason, S.L.; et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann. Neurol. 2015, 78, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Wang, S.J. Pathophysiology of reversible cerebral vasoconstriction syndrome. J. Biomed. Sci. 2022, 29, 72. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F.; Andjelkovic, A.V. Involvement of Epigenetic Mechanisms and Non-coding RNAs in Blood-Brain Barrier and Neurovascular Unit Injury and Recovery After Stroke. Front. Neurosci. 2019, 13, 864. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhou, J.; Liu, H.; Wu, X.; Li, F.; Zhao, J.; Zhang, Y.; Wang, L.; Chao, M.; Wang, Q.; et al. Astrocytic NDRG2-PPM1A interaction exacerbates blood-brain barrier disruption after subarachnoid hemorrhage. Sci. Adv. 2022, 8, eabq2423. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hui, Y.N.; Guo, B.; Ma, J.X. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye 2007, 21, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Sano, Y.; Saito, K.; Abe, M.A.; Maeda, T.; Haruki, H.; Kanda, T. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem. Res. 2012, 37, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, Z.; Wang, L.; Zhang, L.; Liu, Y.; Cao, J.; Fu, Q.; Liu, Y.; Li, H.; Lou, J.; et al. Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J. Neuroinflamm. 2020, 17, 270. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.X.; Shen, H.; Huang, S.X.; Wang, L.P.; Li, Z.W.; Peng, P.; Mamtilahun, M.; Tang, Y.H.; Shen, F.X.; Tian, H.L.; et al. P2Y6 receptor inhibition aggravates ischemic brain injury by reducing microglial phagocytosis. CNS Neurosci. Ther. 2020, 26, 416–429. [Google Scholar] [CrossRef]
- Liu, Z.J.; Ran, Y.Y.; Qie, S.Y.; Gong, W.J.; Gao, F.H.; Ding, Z.T.; Xi, J.N. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci. Ther. 2019, 25, 1353–1362. [Google Scholar] [CrossRef]
- Ay, H.; Koroshetz, W.J.; Vangel, M.; Benner, T.; Melinosky, C.; Zhu, M.; Menezes, N.; Lopez, C.J.; Sorensen, A.G. Conversion of ischemic brain tissue into infarction increases with age. Stroke 2005, 36, 2632–2636. [Google Scholar] [CrossRef]
- Lindner, M.D.; Gribkoff, V.K.; Donlan, N.A.; Jones, T.A. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 10913–10922. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Popa-Wagner, A.; Kleinschnitz, C.; Doeppner, T.R. Animal models of ischemic stroke and their impact on drug discovery. Expert Opin. Drug Discov. 2019, 14, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef] [PubMed]
- Pirisinu, M.; Pham, T.C.; Zhang, D.X.; Hong, T.N.; Nguyen, L.T.; Le, M.T. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: Recent advances, current obstacles, and challenges for clinical translation. Semin. Cancer Biol. 2022, 80, 340–355. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Dieudé, M.; Bell, C.; Turgeon, J.; Beillevaire, D.; Pomerleau, L.; Yang, B.; Hamelin, K.; Qi, S.; Pallet, N.; Béland, C.; et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci. Transl. Med. 2015, 7, 318ra200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Mikami, K.; Venugopal, S.; Li, Y.; Török, N.J. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J. Hepatol. 2009, 51, 139–148. [Google Scholar] [CrossRef]
- Pavlyukov, M.S.; Yu, H.; Bastola, S.; Minata, M.; Shender, V.O.; Lee, Y.; Zhang, S.; Wang, J.; Komarova, S.; Wang, J.; et al. Apoptotic Cell-Derived Extracellular Vesicles Promote Malignancy of Glioblastoma Via Intercellular Transfer of Splicing Factors. Cancer Cell 2018, 34, 119–135.e110. [Google Scholar] [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything-The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles: Toward a clinical application in urological cancer treatment. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2018, 25, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Kosaka, N.; Yoshioka, Y.; Egawa, S.; Ochiya, T. The small vesicular culprits: The investigation of extracellular vesicles as new targets for cancer treatment. Clin. Transl. Med. 2017, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Rao, D.; Xi, X.; Zhang, Z.; Zhong, T. Application of extracellular vesicles proteins in cancer diagnosis. Front. Cell Dev. Biol. 2022, 10, 1007360. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Kotani, A. Recent advances in extracellular vesicles in gastrointestinal cancer and lymphoma. Cancer Sci. 2023, 114, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Liu, Q.; Xu, Y.; Zhang, Q. Extracellular Vesicles: The Landscape in the Progression, Diagnosis, and Treatment of Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 2022, 10, 842898. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yuan, S.; Fang, C.; Hu, X.; Zhang, Y.S.; Zhang, L.L.; Zeng, X.T. Nanomaterials-Based Urinary Extracellular Vesicles Isolation and Detection for Non-invasive Auxiliary Diagnosis of Prostate Cancer. Front. Med. 2021, 8, 800889. [Google Scholar] [CrossRef] [PubMed]
- Ogata-Kawata, H.; Izumiya, M.; Kurioka, D.; Honma, Y.; Yamada, Y.; Furuta, K.; Gunji, T.; Ohta, H.; Okamoto, H.; Sonoda, H.; et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS ONE 2014, 9, e92921. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F.; et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015, 67, 33–41. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y.; et al. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): A platform for glioblastoma biomarker development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef]
- Upadhya, R.; Shetty, A.K. Extracellular Vesicles for the Diagnosis and Treatment of Parkinson’s Disease. Aging Dis. 2021, 12, 1438–1450. [Google Scholar] [CrossRef]
- Grossi, I.; Radeghieri, A.; Paolini, L.; Porrini, V.; Pilotto, A.; Padovani, A.; Marengoni, A.; Barbon, A.; Bellucci, A.; Pizzi, M.; et al. MicroRNA-34a-5p expression in the plasma and in its extracellular vesicle fractions in subjects with Parkinson’s disease: An exploratory study. Int. J. Mol. Med. 2021, 47, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Y.; Faleti, O.D.; Zhao, Q.; Liu, J.; Zhang, G.; Li, M.; Qi, S.; Feng, W.; Lyu, X. A Panel of Exosome-Derived miRNAs of Cerebrospinal Fluid for the Diagnosis of Moyamoya Disease. Front. Neurosci. 2020, 14, 548278. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, Y.; Yang, Q.; Cheng, Y.; Xu, J.; Zhang, Y.; Dai, H.; Wang, B.; Ma, Q.; Chen, Y.; et al. Engineered Extracellular Vesicles with SHP2 High Expression Promote Mitophagy for Alzheimer’s Disease Treatment. Adv. Mater. 2022, 34, e2207107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Song, Y.; Pan, J.J.; Jiang, Y.; Shi, X.; Liu, C.; Ma, Y.; Luo, L.; Mamtilahun, M.; et al. M2 microglia-derived extracellular vesicles promote white matter repair and functional recovery via miR-23a-5p after cerebral ischemia in mice. Theranostics 2022, 12, 3553–3573. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Hira, K.; Miyamoto, N.; Kijima, C.; Inaba, T.; Hattori, N. Pleiotropic Effects of Exosomes as a Therapy for Stroke Recovery. Int. J. Mol. Sci. 2020, 21, 6894. [Google Scholar] [CrossRef]
- Gualerzi, A.; Picciolini, S.; Rodà, F.; Bedoni, M. Extracellular Vesicles in Regeneration and Rehabilitation Recovery after Stroke. Biology 2021, 10, 843. [Google Scholar] [CrossRef]
- Hirsch, Y.; Geraghty, J.R.; Reiter, C.R.; Katz, E.A.; Little, C.F.; Tobin, M.K.; Testai, F.D. Unpacking the Role of Extracellular Vesicles in Ischemic and Hemorrhagic Stroke: Pathophysiology and Therapeutic Implications. Transl. Stroke Res. 2023, 14, 146–159. [Google Scholar] [CrossRef]
- Jiang, Y.; He, R.; Shi, Y.; Liang, J.; Zhao, L. Plasma exosomes protect against cerebral ischemia/reperfusion injury via exosomal HSP70 mediated suppression of ROS. Life Sci. 2020, 256, 117987. [Google Scholar] [CrossRef]
- Huang, L.; Hua, L.; Zhang, X. The Exosomal MicroRNA Profile Is Responsible for the Mesenchymal Stromal Cell Transplantation-Induced Improvement of Functional Recovery after Stroke in Rats. Neuroimmunomodulation 2022, 29, 151–160. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhao, Y.; Su, Y.; Cao, B.; Yang, J.J.; Xing, Q. Serum Extracellular Vesicle-Derived miR-124-3p as a Diagnostic and Predictive Marker for Early-Stage Acute Ischemic Stroke. Front. Mol. Biosci. 2021, 8, 685088. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Alonso-López, E.; Pérez-Mato, M.; Laso-García, F.; Gómez-de Frutos, M.C.; Diekhorst, L.; García-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; de Leciñana, M.A.; et al. Circulating Extracellular Vesicle Proteins and MicroRNA Profiles in Subcortical and Cortical-Subcortical Ischaemic Stroke. Biomedicines 2021, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.B.; Li, R.Y.; Zhou, X.; Yu, D.J.; Lan, X.Y.; Li, J.P.; Liu, J.L. Diagnosis of Hyperacute and Acute Ischaemic Stroke: The Potential Utility of Exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cerebrovasc. Dis. 2018, 45, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Leggio, L.; L’Episcopo, F.; Magrì, A.; Ulloa-Navas, M.J.; Paternò, G.; Vivarelli, S.; Bastos, C.A.P.; Tirolo, C.; Testa, N.; Caniglia, S.; et al. Small Extracellular Vesicles Secreted by Nigrostriatal Astrocytes Rescue Cell Death and Preserve Mitochondrial Function in Parkinson’s Disease. Adv. Healthc. Mater. 2022, 11, e2201203. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cho, H.J.; Park, D.; Han, S. DICAM in the Extracellular Vesicles from Astrocytes Attenuates Microglia Activation and Neuroinflammation. Cells 2022, 11, 2977. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, C.; Bao, T.; Zhao, X.; Xiong, W.; Luo, C.; Yin, G.; Fan, J. Exosome-Shuttled miR-672-5p from Anti-Inflammatory Microglia Repair Traumatic Spinal Cord Injury by Inhibiting AIM2/ASC/Caspase-1 Signaling Pathway Mediated Neuronal Pyroptosis. J. Neurotrauma 2022, 39, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Prada, I.; Joshi, P.; Falcicchia, C.; D’Arrigo, G.; Rutigliano, G.; Battocchio, E.; Zenatelli, R.; Tozzi, F.; Radeghieri, A.; et al. Microglial large extracellular vesicles propagate early synaptic dysfunction in Alzheimer’s disease. Brain J. Neurol. 2022, 145, 2849–2868. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, B.; Wang, Q.; Chen, X.; Lai, W.; Xu, Y.; Deng, S.; Yu, Z.; Xie, M.; Bu, B.; et al. Astrocyte-Derived Exosomes Contribute to Pathologies of Neuromyelitis Optica Spectrum Disorder in Rodent Model. Ann. Neurol. 2023, 94, 163–181. [Google Scholar] [CrossRef]
- Kluge, A.; Bunk, J.; Schaeffer, E.; Drobny, A.; Xiang, W.; Knacke, H.; Bub, S.; Lückstädt, W.; Arnold, P.; Lucius, R.; et al. Detection of neuron-derived pathological α-synuclein in blood. Brain J. Neurol. 2022, 145, 3058–3071. [Google Scholar] [CrossRef]
- Mazzucco, M.; Mannheim, W.; Shetty, S.V.; Linden, J.R. CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis. Fluids Barriers CNS 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, D.B.; Liu, J.L.; Wang, W.; Luo, X.M.; Zhou, X.; Li, J.P.; Cao, X.L.; Long, X.H.; Chen, J.G.; Qin, C. Plasma Exosomal miRNA-122-5p and miR-300-3p as Potential Markers for Transient Ischaemic Attack in Rats. Front. Aging Neurosci. 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, W.; Li, D.; Xu, C.; Liao, B.; Li, F.; Zhou, X.; Qin, W.; Liu, J. Plasma Exosomal miR-450b-5p as a Possible Biomarker and Therapeutic Target for Transient Ischaemic Attacks in Rats. J. Mol. Neurosci. 2019, 69, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.L.; Walberer, M.; Rueger, M.A.; Backes, H.; Wiedermann, D.; Hoehn, M.; Neumaier, B.; Graf, R.; Fink, G.R.; Schroeter, M. In vivo analysis of neuroinflammation in the late chronic phase after experimental stroke. Neuroscience 2015, 292, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Godbout, J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, N.; Zhang, A.; Deziel, R.A.; Tasker, R.A.; Whitehead, S.N. Prefrontal Ischemia in the Rat Leads to Secondary Damage and Inflammation in Remote Gray and White Matter Regions. Front. Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef]
- Drago, F.; Lombardi, M.; Prada, I.; Gabrielli, M.; Joshi, P.; Cojoc, D.; Franck, J.; Fournier, I.; Vizioli, J.; Verderio, C. ATP Modifies the Proteome of Extracellular Vesicles Released by Microglia and Influences Their Action on Astrocytes. Front. Pharmacol. 2017, 8, 910. [Google Scholar] [CrossRef]
- Roseborough, A.D.; Myers, S.J.; Khazaee, R.; Zhu, Y.; Zhao, L.; Iorio, E.; Elahi, F.M.; Pasternak, S.H.; Whitehead, S.N. Plasma derived extracellular vesicle biomarkers of microglia activation in an experimental stroke model. J. Neuroinflamm. 2023, 20, 20. [Google Scholar] [CrossRef]
- Picciolini, S.; Mangolini, V.; Rodà, F.; Montesano, A.; Arnaboldi, F.; Liuzzi, P.; Mannini, A.; Bedoni, M.; Gualerzi, A. Multiplexing Biosensor for the Detection of Extracellular Vesicles as Biomarkers of Tissue Damage and Recovery after Ischemic Stroke. Int. J. Mol. Sci. 2023, 24, 7937. [Google Scholar] [CrossRef]
- Simak, J.; Gelderman, M.P.; Yu, H.; Wright, V.; Baird, A.E. Circulating endothelial microparticles in acute ischemic stroke: A link to severity, lesion volume and outcome. J. Thromb. Haemost. 2006, 4, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Peña, E.; Padró, T.; Jiménez-Xarrié, E.; Martí-Fàbregas, J.; Badimon, L. Microparticle Shedding from Neural Progenitor Cells and Vascular Compartment Cells Is Increased in Ischemic Stroke. PLoS ONE 2016, 11, e0148176. [Google Scholar] [CrossRef]
- Brenna, S.; Altmeppen, H.C.; Mohammadi, B.; Rissiek, B.; Schlink, F.; Ludewig, P.; Krisp, C.; Schlüter, H.; Failla, A.V.; Schneider, C.; et al. Characterization of brain-derived extracellular vesicles reveals changes in cellular origin after stroke and enrichment of the prion protein with a potential role in cellular uptake. J. Extracell. Vesicles 2020, 9, 1809065. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Qiu, W.; Pan, Q.; Chen, Y.; Chen, Y.; Ma, X. Plasma endothelial microvesicles and their carrying miRNA-155 serve as biomarkers for ischemic stroke. J. Neurosci. Res. 2020, 98, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Du, Y.; Esposito, E.; Liu, Y.; Guo, S.; Wang, X.; Lo, E.H.; Xing, C.; Ji, X. Effects of Focal Cerebral Ischemia on Exosomal Versus Serum miR126. Transl. Stroke Res. 2015, 6, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, L.L.; Wang, X.P.; Guo, W.; Guo, R.B.; Sun, Y.Q.; Xue, T.F.; Cai, Z.Y.; Ji, J.; Cheng, H.; et al. Neuronal extracellular vesicle derived miR-98 prevents salvageable neurons from microglial phagocytosis in acute ischemic stroke. Cell Death Dis. 2021, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, X.; Chen, R.; Miao, J.; Wang, L.; Cui, L.; Ji, H.; Liu, Y. Cortical Neuron-Derived Exosomal MicroRNA-181c-3p Inhibits Neuroinflammation by Downregulating CXCL1 in Astrocytes of a Rat Model with Ischemic Brain Injury. Neuroimmunomodulation 2019, 26, 217–233. [Google Scholar] [CrossRef]
- Tian, T.; Cao, L.; He, C.; Ye, Q.; Liang, R.; You, W.; Zhang, H.; Wu, J.; Ye, J.; Tannous, B.A.; et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics 2021, 11, 6507–6521. [Google Scholar] [CrossRef]
- Zhang, L.; Graf, I.; Kuang, Y.; Zheng, X.; Haupt, M.; Majid, A.; Kilic, E.; Hermann, D.M.; Psychogios, M.N.; Weber, M.S.; et al. Neural Progenitor Cell-Derived Extracellular Vesicles Enhance Blood-Brain Barrier Integrity by NF-κB (Nuclear Factor-κB)-Dependent Regulation of ABCB1 (ATP-Binding Cassette Transporter B1) in Stroke Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1127–1145. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Li, J.; Chen, Y.; Zhong, W.; Chen, Y.; Ma, X. Combination of EPC-EXs and NPC-EXs with miR-126 and miR-210 overexpression produces better therapeutic effects on ischemic stroke by protecting neurons through the Nox2/ROS and BDNF/TrkB pathways. Exp. Neurol. 2023, 359, 114235. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell Res. 2019, 382, 111474. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes transfer miR-190b to inhibit oxygen and glucose deprivation-induced autophagy and neuronal apoptosis. Cell Cycle 2020, 19, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Heras-Romero, Y.; Morales-Guadarrama, A.; Santana-Martínez, R.; Ponce, I.; Rincón-Heredia, R.; Poot-Hernández, A.C.; Martínez-Moreno, A.; Urrieta, E.; Bernal-Vicente, B.N.; Campero-Romero, A.N.; et al. Improved post-stroke spontaneous recovery by astrocytic extracellular vesicles. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 798–815. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.W.; Li, X.X.; Tang, Y.S.; Lim-Ho Kong, B.; Wu, H.Y.; Xiao, M.J.; Cheung, C.K.; Shaw, P.C. Novel mechanistic insight on the neuroprotective effect of berberine: The role of PPARδ for antioxidant action. Free Radic. Biol. Med. 2022, 181, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Liang, Y.; Chen, H.; Ji, X.; Huang, M. Berberine mitigates cognitive decline in an Alzheimer’s Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 121, 109670. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, H.; Lin, Y.; Liu, M.; Li, Y.; Mao, H.; Zhang, Z.; Zhang, Y.; Ye, P.; Ding, L.; et al. Berberine Protects Against NLRP3 Inflammasome via Ameliorating Autophagic Impairment in MPTP-Induced Parkinson’s Disease Model. Front. Pharmacol. 2020, 11, 618787. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Dalimi, A.; Ghaffarifar, F.; Nader-Mohammadi, M.; Molavi, P.; Dadkhah, M.; Molaei, S. Berberine improves inhibitory avoidance memory impairment of Toxoplasma gondii-infected rat model of ketamine-induced schizophrenia. BMC Complement. Med. Ther. 2023, 23, 303. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Gu, Q.; Liu, M.; Zou, J.; Sun, J.; Zhu, J. Astrocytes-derived exosomes pre-treated by berberine inhibit neuroinflammation after stroke via miR-182-5p/Rac1 pathway. Int. Immunopharmacol. 2023, 118, 110047. [Google Scholar] [CrossRef]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-Derived Exosomes Treated With a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Ai, X.; Kilic, E.; Hermann, D.M.; Venkataramani, V.; Bähr, M.; Doeppner, T.R. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway. Cell Death Dis. 2021, 12, 1068. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F.; et al. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics 2021, 11, 1232–1248. [Google Scholar] [CrossRef]
- Song, Y.; Shi, R.; Liu, Y.; Cui, F.; Han, L.; Wang, C.; Chen, T.; Li, Z.; Zhang, Z.; Tang, Y.; et al. M2 Microglia Extracellular Vesicle miR-124 Regulates Neural Stem Cell Differentiation in Ischemic Stroke via AAK1/NOTCH. Stroke 2023, 54, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cai, G.; Liu, K.; Zhuang, Z.; Jia, K.; Pei, S.; Wang, X.; Wang, H.; Xu, S.; Cui, C.; et al. Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging 2021, 13, 4079–4095. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Pan, Y.; Wei, W.; Tatenhorst, L.; Graf, I.; Popa-Wagner, A.; Gerner, S.T.; Huber, S.; Kilic, E.; Hermann, D.M.; et al. Preconditioned extracellular vesicles from hypoxic microglia reduce poststroke AQP4 depolarization, disturbed cerebrospinal fluid flow, astrogliosis, and neuroinflammation. Theranostics 2023, 13, 4197–4216. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhao, H.; Wang, Y.; Chen, Z. Exosomal shuttled miR-424-5p from ischemic preconditioned microglia mediates cerebral endothelial cell injury through negatively regulation of FGF2/STAT3 pathway. Exp. Neurol. 2020, 333, 113411. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chai, Y.; Lv, S.; Ye, M.; Wu, M.; Xie, L.; Fan, Y.; Zhu, X.; Gao, Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int. J. Mol. Med. 2017, 40, 1201–1209. [Google Scholar] [CrossRef]
- Venkat, P.; Cui, C.; Chopp, M.; Zacharek, A.; Wang, F.; Landschoot-Ward, J.; Shen, Y.; Chen, J. MiR-126 Mediates Brain Endothelial Cell Exosome Treatment-Induced Neurorestorative Effects After Stroke in Type 2 Diabetes Mellitus Mice. Stroke 2019, 50, 2865–2874. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Zhang, W.; Chen, Y.; Bihl, J.C. Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci. Ther. 2020, 26, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Chen, S.; Zhang, W.; Chen, Y.; Yang, Y. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp. Neurol. 2020, 330, 113325. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, B.; Sun, C.; Bai, Y.; Cheng, D.; Zhang, Y.; Li, X.; Zhao, J.; Xu, D. Vascular Endothelial Cell-derived Exosomes Protect Neural Stem Cells Against Ischemia/reperfusion Injury. Neuroscience 2020, 441, 184–196. [Google Scholar] [CrossRef]
- Yerrapragada, S.M.; Sawant, H.; Chen, S.; Bihl, T.; Wang, J.; Bihl, J.C. The protective effects of miR-210 modified endothelial progenitor cells released exosomes in hypoxia/reoxygenation injured neurons. Exp. Neurol. 2022, 358, 114211. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, C.; Zhang, Y.; Alsrouji, O.K.; Chebl, A.B.; Ding, G.; Jiang, Q.; Mayer, S.A.; Lu, M.; Kole, M.K.; et al. Cerebral endothelial cell-derived small extracellular vesicles enhance neurovascular function and neurological recovery in rat acute ischemic stroke models of mechanical thrombectomy and embolic stroke treatment with tPA. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2021, 41, 2090–2104. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; He, C.; Liu, H.; Liao, X.; Dai, B.; Chen, Y.; Yang, Y.; Zhao, B.; Bihl, J.; Ma, X. Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow. Mol. Brain 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Seifali, E.; Hassanzadeh, G.; Mahdavipour, M.; Mortezaee, K.; Moini, A.; Satarian, L.; Shekari, F.; Nazari, A.; Movassaghi, S.; Akbari, M. Extracellular Vesicles Derived from Human Umbilical Cord Perivascular Cells Improve Functional Recovery in Brain Ischemic Rat via the Inhibition of Apoptosis. Iran. Biomed. J. 2020, 24, 347–360. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, X.; Peng, S.; Sun, J.; Chen, X.; Deng, Y.; Yi, L. Identification of novel biomarkers in ischemic stroke: A genome-wide integrated analysis. BMC Med. Genet. 2020, 21, 66. [Google Scholar] [CrossRef]
- Dagonnier, M.; Donnan, G.A.; Davis, S.M.; Dewey, H.M.; Howells, D.W. Acute Stroke Biomarkers: Are We There Yet? Front. Neurol. 2021, 12, 619721. [Google Scholar] [CrossRef]
- Uphaus, T.; Audebert, H.J.; Graner, M.W.; Tiedt, S.; Kowalski, R.G. Editorial: Blood-Based Biomarkers in Acute Ischemic Stroke and Hemorrhagic Stroke. Front. Neurol. 2022, 13, 866166. [Google Scholar] [CrossRef]
- Wang, M.-M.; Feng, Y.-S.; Tan, Z.-X.; Xing, Y.; Dong, F.; Zhang, F. The role of exosomes in stroke. Mol. Biol. Rep. 2020, 47, 6217–6228. [Google Scholar] [CrossRef]
- Fukuta, T.; Ishii, T.; Asai, T.; Oku, N. Applications of Liposomal Drug Delivery Systems to Develop Neuroprotective Agents for the Treatment of Ischemic Stroke. Biol. Pharm. Bull. 2019, 42, 319–326. [Google Scholar] [CrossRef]
- Girnar, G.A.; Mahajan, H.S. Cerebral ischemic stroke and different approaches for treatment of stroke. Future J. Pharm. Sci. 2021, 7, 134. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Chen, Y.; Quan, X.; Han, Y.; Zheng, Y.; Zhao, Y. Extracellular Vesicle Application as a Novel Therapeutic Strategy for Ischemic Stroke. Transl. Stroke Res. 2022, 13, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Chen, Y.; Chang, J.; Yang, L.; Liu, X.; Xu, H.; Zhang, X.; Wang, S.; Han, Q.; et al. Engineered extracellular vesicles for ischemic stroke: A systematic review and meta-analysis of preclinical studies. J. Nanobiotechnol. 2023, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Just, J.; Stenz, K.T.; Yan, Y.; Sieljacks, P.; Wang, J.; Groennebaek, T.S.; Jakobsgaard, J.E.; Rindom, E.; Herskind, J.; et al. The Role of Plasma Extracellular Vesicles in Remote Ischemic Conditioning and Exercise-Induced Ischemic Tolerance. Int. J. Mol. Sci. 2022, 23, 3334. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Gao, Y.; He, R.; Jing, L.; Li, Y.; Gong, Z.; Yao, Y.; Luan, T.; Zhang, C.; Li, L.; et al. Induced Pluripotent Stem Cells for Ischemic Stroke Treatment. Front. Neurosci. 2021, 15, 628663. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Gulvin, S.M.; Flax, J.; Gaborski, T.R. Systematic Evaluation of PKH Labelling on Extracellular Vesicle Size by Nanoparticle Tracking Analysis. Sci. Rep. 2020, 10, 9533. [Google Scholar] [CrossRef]
- Sayed, S.M.; Jia, H.R.; Jiang, Y.W.; Zhu, Y.X.; Ma, L.; Yin, F.; Hussain, I.; Khan, A.; Ma, Q.; Wu, F.G.; et al. Photostable AIE probes for wash-free, ultrafast, and high-quality plasma membrane staining. J. Mater. Chem. B 2021, 9, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Liu, J.Y.; Wu, C.Y.; Liang, Y.X.; Lu, Z.L.; Ding, A.X.; Xu, M.D. Two-Photon Near-Infrared AIE Luminogens as Multifunctional Gene Carriers for Cancer Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 23384–23395. [Google Scholar] [CrossRef]
- Xu, L.; Liang, X.; Zhong, S.; Gao, Y.; Cui, X. Seeking brightness from nature: Sustainable AIE macromolecule with clustering-triggered emission of xanthan gum and its multiple applications. Colloids Surf. B Biointerfaces 2021, 206, 111961. [Google Scholar] [CrossRef]
- Xu, R.; Chi, W.; Zhao, Y.; Tang, Y.; Jing, X.; Wang, Z.; Zhou, Y.; Shen, Q.; Zhang, J.; Yang, Z.; et al. All-in-One Theranostic Platforms: Deep-Red AIE Nanocrystals to Target Dual-Organelles for Efficient Photodynamic Therapy. ACS Nano 2022, 16, 20151–20162. [Google Scholar] [CrossRef]
- Yue, K.Y.; Zhang, P.R.; Zheng, M.H.; Cao, X.L.; Cao, Y.; Zhang, Y.Z.; Zhang, Y.F.; Wu, H.N.; Lu, Z.H.; Liang, L.; et al. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death Dis. 2019, 10, 869. [Google Scholar] [CrossRef]


| Source | Content | Models | Expression in IS | Outcome | Reference |
|---|---|---|---|---|---|
| CSF | miR-122-5p | Rats | downregulation | TIA biomarkers | [102] |
| CSF | miR-300-3p | Rats | upregulation | TIA biomarkers | [102] |
| CSF | miR-450b-5p | Rats | upregulation | a high diagnostic value and may become a therapeutic target for rat TIA | [103] |
| Serum | miR-9 | Human | upregulation | NIHSS scores, serum IL-6 concentration, infarct volume | [104] |
| Serum | miR-124 | Human | upregulation | NIHSS scores, serum IL-6 concentration, infarct volume | [104] |
| Plasma, activated microglia-EVs | NA | Rats | upregulation | worse neurological and cognitive outcomes | [109] |
| Serum, activated microglia-EVs | VEGFR2 | Human | upregulation | IS biomarkers | [110] |
| Serum, neuron/microglia-EVs | TSPO | Human | upregulation | IS biomarkers | [110] |
| Plasma, EC-EVs | phosphatidylserine | Human | upregulation | IS biomarkers | [111] |
| Plasma, EC-EVs | CD105 | Human | upregulation | IS biomarkers | [111] |
| Plasma, EC-EVs | Annexin-V | Human | upregulation | IS biomarkers | [112] |
| Plasma, EC-EVs | CD41a | Human | downregulation | IS biomarkers | [111] |
| Brain | PrP | Mice | upregulation | intercellular communication at early stages after stroke | [113] |
| Plasma, EC-EVs | miR-155 | Human | upregulation | infarct volume, NIHSS scores, large artery atherosclerosis, cardioembolism subtypes | [114] |
| Serum, EC-EVs | miR-126 | Rats | downregulation | sensitive marker for IS | [115] |
| Source | Content | Model | Effect | Mechanism | Reference |
|---|---|---|---|---|---|
| Neuron | miR-98 | tMCAO: rat/mouse OGD: neuron/microglia | Inhibit the microglial phagocytosis of neuron | PAFR | [116] |
| Neuron | miR-181c-3p | MCAO: rat OGD: neuron/astrocyte | Inhibit neuroinflammation | CXCL1 | [117] |
| NPC | let-7g-5p miR-99a-5p let-7i-5p miR-139-5p miR-98-5p miR-21-5p let-7b-5p | MCAO: mouse | Inhibit neuroinflammation | MAPK pathway | [118] |
| NPC | NA | tMCAO: mouse OGD: EC | Enhance BBB integrity Attenuate inflammatory cell recruitment | ABCB1/MMP-9 NF-κB pathway | [119] |
| NPC | miR-210 | MCAO: mouse OGD: neuron | Reduce infarct volume, NDS, neural apoptosis and ROS production Promote the spine density of dendrites | ROS/Nox2 pathways BDNF/TrkB pathways | [120] |
| Astrocyte | NA | MCAO: mouse OGD: neuron | Inhibit neurons apoptosis | autophagy | [121] |
| Astrocyte | miR-190b | OGD: neuron | Inhibit autophagy and neurons apoptosis | Atg7 | [122] |
| Astrocyte | NA | MCAO: rat | Reduce the infarct volume Protect the function of the neuronal tracts Promote axonal regeneration Enhance compound action potential recovery | NA | [123] |
| Astrocyte | miR-182-5p | MCAO: mouse OGD: neuron | Reduce neuronal injury Inhibit neuroinflammation | Rac1 pathway | [128] |
| Astrocyte | NA | MCAO: rat OGD: neuron | Promote axonal outgrowth | prostaglandin D2 synthase | [129] |
| Microglia | TGF-β1 | OGD: neuron OGD: EC | Stimulate both angiogenesis and tube formation Reduce neuronal injury | Smad2/3 pathway | [130] |
| Microglia | miR-23a-5p | tMCAO: mouse OGD: OPC | Reduce brain atrophy volume Promote functional recovery Promote oligodendrogenesis and white matter repair Increase OPC proliferation, survival, and differentiation | Olig3 | [85] |
| Microglia | miR-124 | MCAO: mouse OGD: astrocyte | Attenuate glial scar formation | STAT3 pathway glial fibrillary acidic protein Notch 1/Sox2 | [131] |
| Microglia | miR-124 | tMCAO: mouse | Promote proliferation and differentiation of NSCs | AAK1/Notch | [132] |
| Microglia | miR-137 | MCAO: mouse OGD: neuron | Inhibit neuronal apoptosis | Notch 1 | [133] |
| Microglia | NA | MCAO: mouse OGD: microglia/astrocyte | Reduce poststroke inflammation, astrogliosis, AQP4 depolarization Promete CSF flow | NA | [134] |
| EC | NA | MCAO: rat OGD: neuron/EC | Suppress neuronal apoptosis Promote migration and invasion of neuron | NA | [136] |
| EC | miR-126 | photothrombotic stroke model: mouse | Promote neurological functional recovery Improve myelin density, axon density, arterial diameter, and vascular density Induce M2 macrophage polarization in the infarct boundary zone | NA | [137] |
| EC | NA | MCAO: rat Cell scratch wound: NPC | Promote NPC proliferation and migration Reduce apoptosis of NPCs | NA | [140] |
| EC | miR-155-5p | MCAO: mouse OGD: astrocyte | Inhibit the inflammatory response of astrocytes | c-Fos/AP-1 pathway | [35] |
| EC | NA | MCAO: mouse | Promote proliferation of astrocytes Increase the expression of GFAP Inhibit apoptosis of astrocytes Reduce infarct size and BBB disruption Increase CBF and neurological functional recovery | PI3K/Akt pathway | [143] |
| EC | miR-19a, miR-21, miR-146a | tMCAO: rat | Reduce prothrombotic and BBB leakage proteins | TLR4 ICAM-1, PAI-1 TF ZO1 NF-κB | [142] |
| EPC | NA | MCAO: rat | Inhibit cell apoptosis Promote angiogenesis | Wnt3a p-GSK-3β CD31 VEGF | [34] |
| EPC | miR-126 | MCAO: mouse with diabetes | Reduce infarct size Increase CBF and MVD Promote angiogenesis, neurogenesis, and neurological functional recovery | NA | [138] |
| EPC | miR-126 | MCAO: mouse OGD: neuron | Reduce NDS, infarct size, and cell apoptosis rate Increase MVD | BDNF/TrkB/Akt signaling pathway | [139] |
| EPC | miR-126 | MCAO: mouse OGD: neuron | Reduce infarct volume, NDS, neural apoptosis, and ROS production Promote the spine density of dendrites | ROS/Nox2 pathways BDNF/TrkB pathways | [120] |
| EPC | miR-210 | OGD: neuron | Reduce neuronal apoptosis and ROS production Promote neuronal viability | BDNF/TrkB pathways Nox2/Nox4 pathways | [141] |
| PC | NA | MCAO: rat | Reduce neuronal apoptosis Promote the sensorimotor function | NA | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Liu, D.; Yue, K.; Zhang, Z.; Jiang, X.; Luo, P. Revolutionizing Ischemic Stroke Diagnosis and Treatment: The Promising Role of Neurovascular Unit-Derived Extracellular Vesicles. Biomolecules 2024, 14, 378. https://doi.org/10.3390/biom14030378
Gao X, Liu D, Yue K, Zhang Z, Jiang X, Luo P. Revolutionizing Ischemic Stroke Diagnosis and Treatment: The Promising Role of Neurovascular Unit-Derived Extracellular Vesicles. Biomolecules. 2024; 14(3):378. https://doi.org/10.3390/biom14030378
Chicago/Turabian StyleGao, Xiangyu, Dan Liu, Kangyi Yue, Zhuoyuan Zhang, Xiaofan Jiang, and Peng Luo. 2024. "Revolutionizing Ischemic Stroke Diagnosis and Treatment: The Promising Role of Neurovascular Unit-Derived Extracellular Vesicles" Biomolecules 14, no. 3: 378. https://doi.org/10.3390/biom14030378
APA StyleGao, X., Liu, D., Yue, K., Zhang, Z., Jiang, X., & Luo, P. (2024). Revolutionizing Ischemic Stroke Diagnosis and Treatment: The Promising Role of Neurovascular Unit-Derived Extracellular Vesicles. Biomolecules, 14(3), 378. https://doi.org/10.3390/biom14030378