The Influence of Lysosomal Stress on Dental Pulp Stem Cell-Derived Schwann Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results
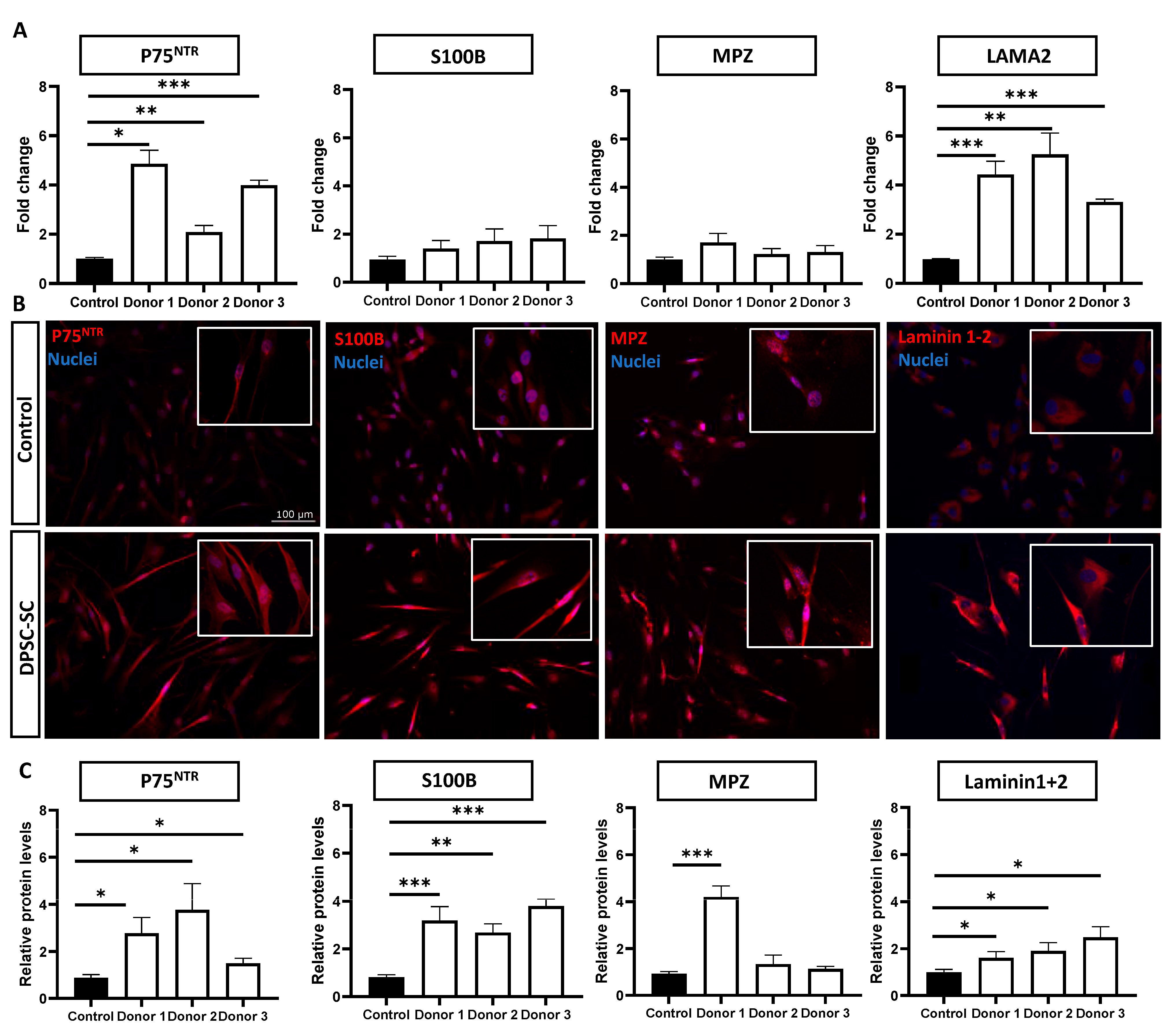
3.1. DPSCs Successfully Differentiate towards Schwann Cells
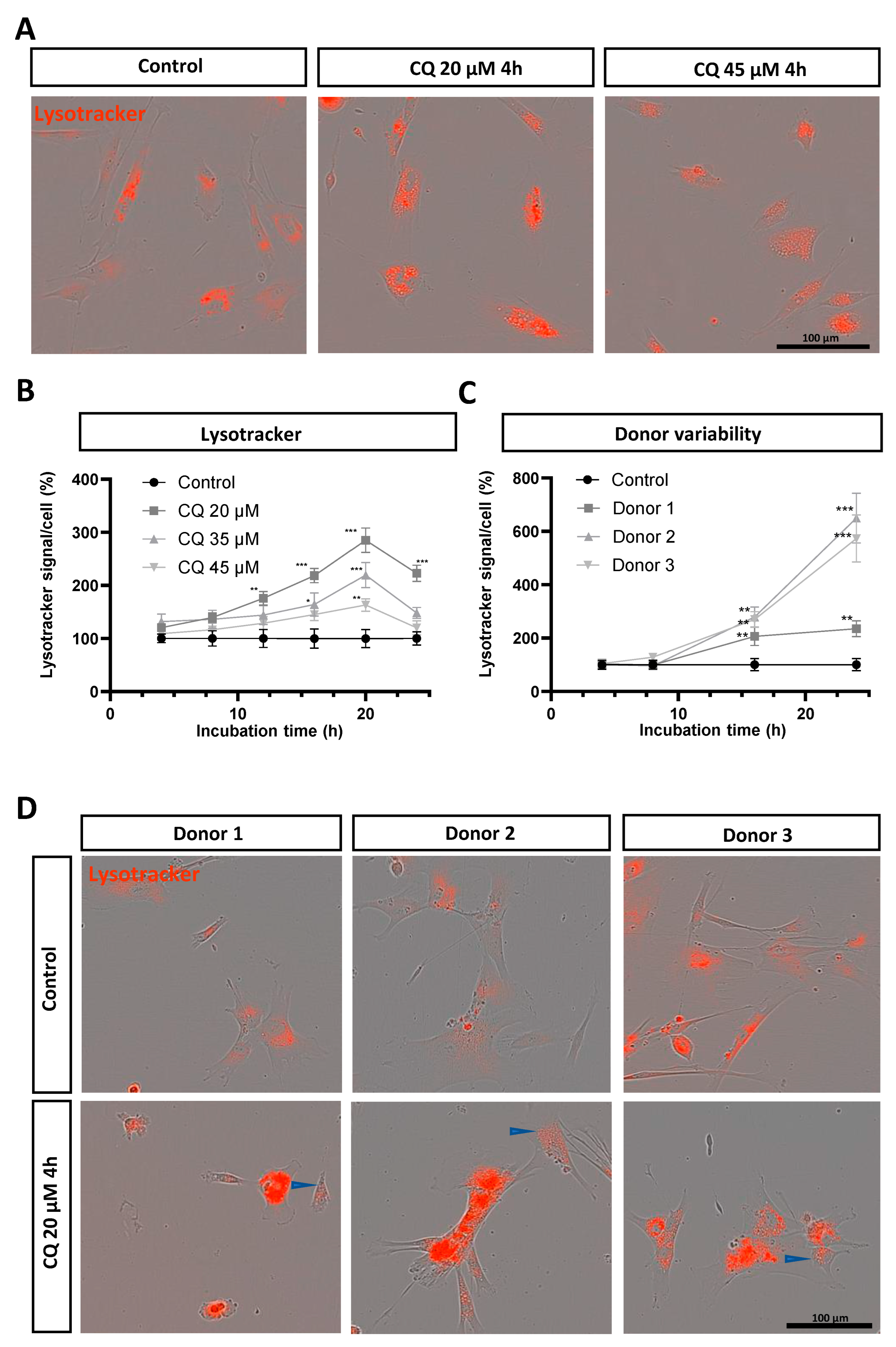
3.2. Chloroquine Induces Lysosomal Upregulation in DPSC-Derived Schwann Cells
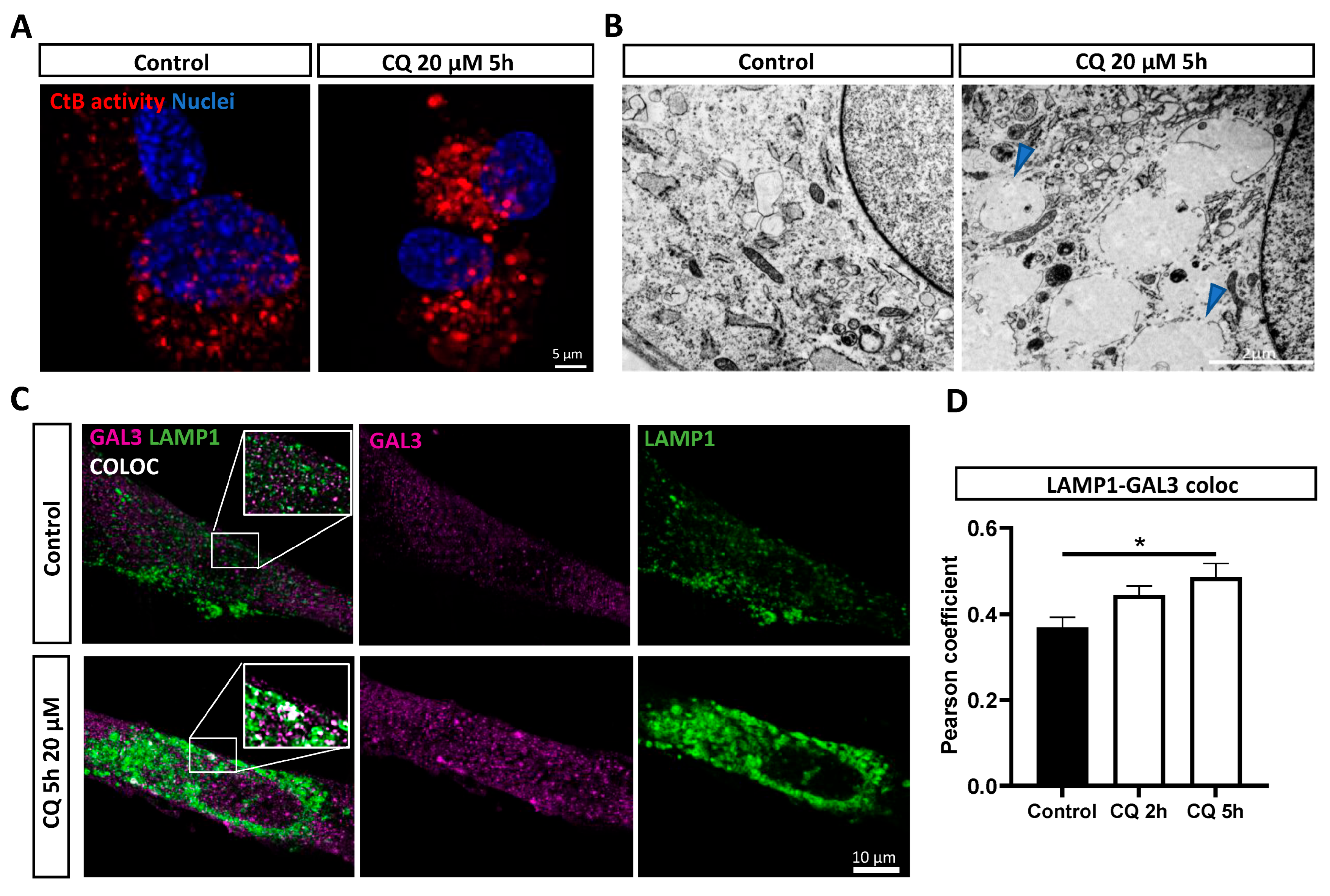
3.3. Chloroquine Induces Lysosomal Permeability in DPSC-Derived Schwann Cells
3.4. Reduced Viability and Apoptosis in Chloroquine-Exposed DPSC-Derived Schwann Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coffey, J.W.; de Duve, C. Digestive Activity of Lysosomes. J. Biol. Chem. 1968, 243, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Udayar, V.; Chen, Y.; Sidransky, E.; Jagasia, R. Lysosomal dysfunction in neurodegeneration: Emerging concepts and methods. Trends Neurosci. 2022, 45, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Appelqvist, H.; Waster, P.; Kagedal, K.; Ollinger, K. The lysosome: From waste bag to potential therapeutic target. J. Mol. Cell Biol. 2013, 5, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Popko, B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat. Neurosci. 2009, 12, 379–385. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, M.; Feltri, M.L.; Wrabetz, L. Myelin under stress. J. Neurosci. Res. 2009, 87, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Libberecht, K.; Vangansewinkel, T.; Van Den Bosch, L.; Lambrichts, I.; Wolfs, E. Proteostasis plays an important role in demyelinating Charcot Marie Tooth disease. Biochem. Pharmacol. 2023, 216, 115760. [Google Scholar] [CrossRef] [PubMed]
- Fortun, J.; Verrier, J.D.; Go, J.C.; Madorsky, I.; Dunn, W.A.; Notterpek, L. The formation of peripheral myelin protein 22 aggregates is hindered by the enhancement of autophagy and expression of cytoplasmic chaperone. Neurobiol. Dis. 2007, 25, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Usenovic, M.; Krainc, D. Lysosomal dysfunction in neurodegeneration: The role of ATP13A2/PARK9. Autophagy 2012, 8, 987–988. [Google Scholar] [CrossRef] [PubMed]
- Lakpa, K.L.; Khan, N.; Afghah, Z.; Chen, X.; Geiger, J.D. Lysosomal Stress Response (LSR): Physiological Importance and Pathological Relevance. J. Neuroimmune Pharmacol. 2021, 16, 219–237. [Google Scholar] [CrossRef]
- Lin, J.; Shi, S.S.; Zhang, J.Q.; Zhang, Y.J.; Zhang, L.; Liu, Y.; Jin, P.P.; Wei, P.F.; Shi, R.H.; Zhou, W.; et al. Giant Cellular Vacuoles Induced by Rare Earth Oxide Nanoparticles are Abnormally Enlarged Endo/Lysosomes and Promote mTOR-Dependent TFEB Nucleus Translocation. Small 2016, 12, 5759–5768. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.; Tan, L.; Wang, Y.; Wang, L.; Zhang, Z.; Zhang, L. Polystyrene Nanoparticles Reduced ROS and Inhibited Ferroptosis by Triggering Lysosome Stress and TFEB Nucleus Translocation in a Size-Dependent Manner. Nano Lett. 2019, 19, 7781–7792. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Y.; Alamri, A.H.; Valapala, M. Nutrient deprivation and lysosomal stress induce activation of TFEB in retinal pigment epithelial cells. Cell. Mol. Biol. Lett. 2019, 24, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-E.; Lin, J.-F.; Lin, Y.-C.; Wen, S.-I.; Yang, S.-C.; Tsai, T.-F.; Chou, K.-Y.; Hwang, I.S.T. Chloroquine induces lysosomal membrane permeability-mediated cell death in bladder cancer cells. Formos. J. Surg. 2018, 51, 133–141. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Cho, K.S.; Hwang, J.J.; Lee, S.J.; Choi, J.A.; Koh, J.Y. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Funakawa, K.; Komori, T.; Sakurai, M.; Yoshii, G.; Eguchi, T.; Fukuda, M.; Iwatsubo, T. Roles of lysosomotropic agents on LRRK2 activation and Rab10 phosphorylation. Neurobiol. Dis. 2020, 145, 105081. [Google Scholar] [CrossRef]
- Chen, P.M.; Gombart, Z.J.; Chen, J.W. Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: Possible implications of lysosomal dysfunction in macular degeneration. Cell Biosci. 2011, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Harhaji-Trajkovic, L.; Arsikin, K.; Kravic-Stevovic, T.; Petricevic, S.; Tovilovic, G.; Pantovic, A.; Zogovic, N.; Ristic, B.; Janjetovic, K.; Bumbasirevic, V.; et al. Chloroquine-mediated lysosomal dysfunction enhances the anticancer effect of nutrient deprivation. Pharm. Res. 2012, 29, 2249–2263. [Google Scholar] [CrossRef]
- Gregorio, P.; da Cunha, R.S.; Biagini, G.; Bosquetti, B.; Budag, J.; Ortiz, A.; Sanchez-Nino, M.D.; Barreto, F.C.; Stinghen, A.E.M. Chloroquine may induce endothelial injury through lysosomal dysfunction and oxidative stress. Toxicol. Appl. Pharmacol. 2021, 414, 115412. [Google Scholar] [CrossRef]
- Stratton, J.A.; Kumar, R.; Sinha, S.; Shah, P.; Stykel, M.; Shapira, Y.; Midha, R.; Biernaskie, J. Purification and Characterization of Schwann Cells from Adult Human Skin and Nerve. eNeuro 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Andersen, N.D.; Monje, P.V. Isolation, Culture, and Cryopreservation of Adult Rodent Schwann Cells Derived from Immediately Dissociated Teased Fibers. Methods Mol. Biol. 2018, 1739, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; Shi, S.; Fan, M.; Jansen, J.A. Multilineage Differentiation Potential of Stem Cells Derived from Human Dental Pulp after Cryopreservation. Tissue Eng. 2006, 12, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Woods, E.J.; Perry, B.C.; Hockema, J.J.; Larson, L.; Zhou, D.; Goebel, W.S. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 2009, 59, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J.; Meinhardt, A.; Katona, I.; Senderek, J.; Kammerer-Gassler, E.K.; Roos, A.; Ferbert, A.; Schroder, J.M.; Nikolin, S.; Nolte, K.; et al. Myelin protein zero mutation-related hereditary neuropathies: Neuropathological insight from a new nerve biopsy cohort. Brain Pathol. 2024, 34, e13200. [Google Scholar] [CrossRef] [PubMed]
- Stefano, C.P.; Lawrence, W.; Alessandro, N.; Angelo, Q.; Carla, T.; Pardini, C.; M Laura, F. Expression of Laminin Receptors in Schwann Cell Differentiation: Evidence for Distinct Roles. J. Neurosci. 2003, 23, 5520–5530. [Google Scholar]
- Chernousov, M.A.; Yu, W.M.; Chen, Z.L.; Carey, D.J.; Strickland, S. Regulation of Schwann cell function by the extracellular matrix. Glia 2008, 56, 1498–1507. [Google Scholar] [CrossRef]
- Wang, F.; Gomez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Eriksson, I.; Waster, P.; Ollinger, K. Restoration of lysosomal function after damage is accompanied by recycling of lysosomal membrane proteins. Cell Death Dis. 2020, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Claude-Taupin, A.; Gu, Y.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2020, 52, 69–87.e8. [Google Scholar] [CrossRef] [PubMed]
- Oberle, C.; Huai, J.; Reinheckel, T.; Tacke, M.; Rassner, M.; Ekert, P.G.; Buellesbach, J.; Borner, C. Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ. 2010, 17, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. Adapting the proteostasis capacity to sustain brain healthspan. Cell 2021, 184, 1545–1560. [Google Scholar] [CrossRef]
- Fortun, J.; Go, J.C.; Li, J.; Amici, S.A.; Dunn, W.A., Jr.; Notterpek, L. Alterations in degradative pathways and protein aggregation in a neuropathy model based on PMP22 overexpression. Neurobiol. Dis. 2006, 22, 153–164. [Google Scholar] [CrossRef]
- Shi, L.; Huang, L.; He, R.; Huang, W.; Wang, H.; Lai, X.; Zou, Z.; Sun, J.; Ke, Q.; Zheng, M.; et al. Modeling the Pathogenesis of Charcot-Marie-Tooth Disease Type 1A Using Patient-Specific iPSCs. Stem Cell Rep. 2018, 10, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.; Wolfs, E.; Struys, T.; Politis, C.; Bronckaers, A.; Lambrichts, I. Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs 2012, 196, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Fedele, A.O.; Proud, C.G. Chloroquine and bafilomycin A mimic lysosomal storage disorders and impair mTORC1 signalling. Biosci. Rep. 2020, 40, BSR20200905. [Google Scholar] [CrossRef]
- Redmann, M.; Benavides, G.A.; Berryhill, T.F.; Wani, W.Y.; Ouyang, X.; Johnson, M.S.; Ravi, S.; Barnes, S.; Darley-Usmar, V.M.; Zhang, J. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2017, 11, 73–81. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017, 5, e00293. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.; Mateuszuk, L.; Orleanska, J.; Baranska, M.; Chlopicki, S.; Majzner, K. Chloroquine-Induced Accumulation of Autophagosomes and Lipids in the Endothelium. Int. J. Mol. Sci. 2021, 22, 2401. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, B.; Farber, H.; Assaraf, Y.G. LysoTracker and MitoTracker Red are transport substrates of P-glycoprotein: Implications for anticancer drug design evading multidrug resistance. J. Cell. Mol. Med. 2018, 22, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, Y. Biphasic Activity of Chloroquine in Human Colorectal Cancer Cells. Dev. Reprod. 2014, 18, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Valadez, K.; Sauvat, A.; Fohrer-Ting, H.; Klein, C.; Kepp, O.; Kroemer, G.; Djavaheri-Mergny, M. A novel tool for detecting lysosomal membrane permeabilization by high-throughput fluorescence microscopy. Methods Cell Biol. 2021, 165, 1–12. [Google Scholar]
- Beauvarlet, J.; Nath Das, R.; Alvarez-Valadez, K.; Martins, I.; Muller, A.; Darbo, E.; Richard, E.; Soubeyran, P.; Kroemer, G.; Guillon, J.; et al. Triarylpyridine Compounds and Chloroquine Act in Concert to Trigger Lysosomal Membrane Permeabilization and Cell Death in Cancer Cells. Cancers 2020, 12, 1621. [Google Scholar] [CrossRef]
- Koyanagi, M.; Imai, S.; Matsumoto, M.; Iguma, Y.; Kawaguchi-Sakita, N.; Kotake, T.; Iwamitsu, Y.; Ntogwa, M.; Hiraiwa, R.; Nagayasu, K.; et al. Pronociceptive Roles of Schwann Cell-Derived Galectin-3 in Taxane-Induced Peripheral Neuropathy. Cancer Res. 2021, 81, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Sagulenko, V.; Vitak, N.; Vajjhala, P.R.; Vince, J.E.; Stacey, K.J. Caspase-1 Is an Apical Caspase Leading to Caspase-3 Cleavage in the AIM2 Inflammasome Response, Independent of Caspase-8. J. Mol. Biol. 2018, 430, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Xue, Y.; Yan, X.; Li, J.; Wu, Y.; Guo, R.; Zhang, J.; Zhang, L.; Li, Y.; Liu, Y.; et al. Autophagy inhibitor chloroquine induces apoptosis of cholangiocarcinoma cells via endoplasmic reticulum stress. Oncol. Lett. 2018, 16, 3509–3516. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.L.; Deng, X.; Yang, S.; Mao, Y.; Li, Z.; Jiang, P.; Zhao, X.; Wei, Y. Chloroquine inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis. Cancer Investig. 2009, 27, 286–292. [Google Scholar] [CrossRef]
- Boya, P.; Gonzalez-Polo, R.A.; Poncet, D.; Andreau, K.; Vieira, H.L.; Roumier, T.; Perfettini, J.L.; Kroemer, G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 2003, 22, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- Hsin, I.L.; Wang, S.C.; Li, J.R.; Ciou, T.C.; Wu, C.H.; Wu, H.M.; Ko, J.L. Immunomodulatory proteins FIP-gts and chloroquine induce caspase-independent cell death via autophagy for resensitizing cisplatin-resistant urothelial cancer cells. Phytomedicine 2016, 23, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Cruz, D.; Asamoah, N.; Buxbaum, A.; Sohar, I.; Lobel, P.; Maxfield, F.R. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol. Biol. Cell 2007, 18, 1490–1496. [Google Scholar] [CrossRef]
- Koh, J.Y.; Kim, H.N.; Hwang, J.J.; Kim, Y.H.; Park, S.E. Lysosomal dysfunction in proteinopathic neurodegenerative disorders: Possible therapeutic roles of cAMP and zinc. Mol. Brain 2019, 12, 18. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Reigada, D.; Nguyen, J.; Laties, A.M.; Mitchell, C.H. Restoration of lysosomal pH in RPE cells from cultured human and ABCA4−/− mice: Pharmacologic approaches and functional recovery. Investig. Ophthalmol. Vis. Sci. 2008, 49, 772–780. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, Y.H.; Kim, J.Y.; Lee, M.O. RORalpha Enhances Lysosomal Acidification and Autophagic Flux in the Hepatocytes. Hepatol. Commun. 2021, 5, 2121–2138. [Google Scholar] [CrossRef]
- Wu, Y.F.; Zhao, P.; Luo, X.; Xu, J.C.; Xue, L.; Zhou, Q.; Xiong, M.; Shen, J.; Peng, Y.B.; Yu, M.F.; et al. Chloroquine inhibits Ca2+ permeable ion channels-mediated Ca2+ signaling in primary B lymphocytes. Cell Biosci. 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Peng, Y.B.; Wei, M.Y.; Wu, Y.F.; Guo, D.; Qin, G.; Ji, G.; Shen, J.; Liu, Q.H. Chloroquine Inhibits Ca2+ Signaling in Murine CD4+ Thymocytes. Cell Physiol. Biochem. 2015, 36, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Duellman, T.; Burnett, J.; Shin, A.; Yang, J. LMAN1 (ERGIC-53) is a potential carrier protein for matrix metalloproteinase-9 glycoprotein secretion. Biochem. Biophys. Res. Commun. 2015, 464, 685–691. [Google Scholar] [CrossRef]
- Zaidi, A.U.; Mcdonough, J.S.; Klocke, B.J.; Latham, C.B.; Korsmeyer, S.J.; Flavell, R.A.; Schmidt, R.E.; Roth, K.A. Chloroquine-Induced Neuronal Cell Death Is p53 and Bcl-2 Family-Dependent but Caspase-Independent Caspase-Independent. J. Neuropathol. Exp. Neurol. 2001, 60, 937–945. [Google Scholar] [CrossRef]
- Pedrioli, G.; Patani, R.; Paganetti, P. Chloroquine, the Coronavirus Crisis, and Neurodegeneration: A Perspective. Front. Neurol. 2020, 11, 596528. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.M.; Gao, J.L.; Cui, Y.; Sun, L.Q.; Wang, Y.C.; Wang, K.J.; Li, R.; Tian, Y.X.; Cui, J.Z. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol. Med. Rep. 2015, 12, 2323–2328. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libberecht, K.; Dirkx, N.; Vangansewinkel, T.; Vandendries, W.; Lambrichts, I.; Wolfs, E. The Influence of Lysosomal Stress on Dental Pulp Stem Cell-Derived Schwann Cells. Biomolecules 2024, 14, 405. https://doi.org/10.3390/biom14040405
Libberecht K, Dirkx N, Vangansewinkel T, Vandendries W, Lambrichts I, Wolfs E. The Influence of Lysosomal Stress on Dental Pulp Stem Cell-Derived Schwann Cells. Biomolecules. 2024; 14(4):405. https://doi.org/10.3390/biom14040405
Chicago/Turabian StyleLibberecht, Karen, Nathalie Dirkx, Tim Vangansewinkel, Wendy Vandendries, Ivo Lambrichts, and Esther Wolfs. 2024. "The Influence of Lysosomal Stress on Dental Pulp Stem Cell-Derived Schwann Cells" Biomolecules 14, no. 4: 405. https://doi.org/10.3390/biom14040405





