Feature Matching of Microsecond-Pulsed Magnetic Fields Combined with Fe3O4 Particles for Killing A375 Melanoma Cells
Abstract
:1. Introduction
- MNP volume and the critical volume of Brownian relaxation;
- MNP relaxation time and pulse width;
- MNP shape and the intermittence of PMF.
2. Materials and Methods
2.1. Cell Culture
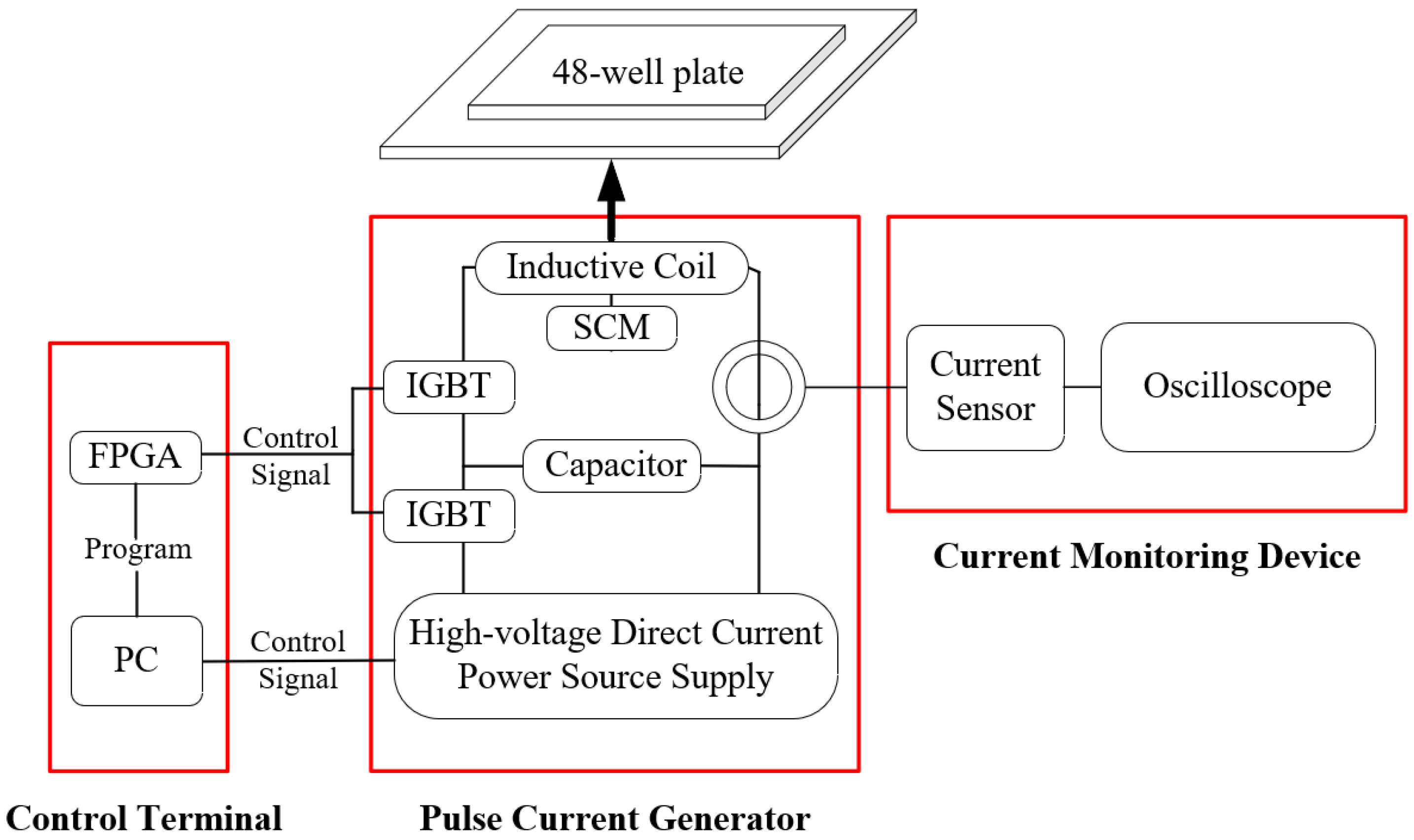
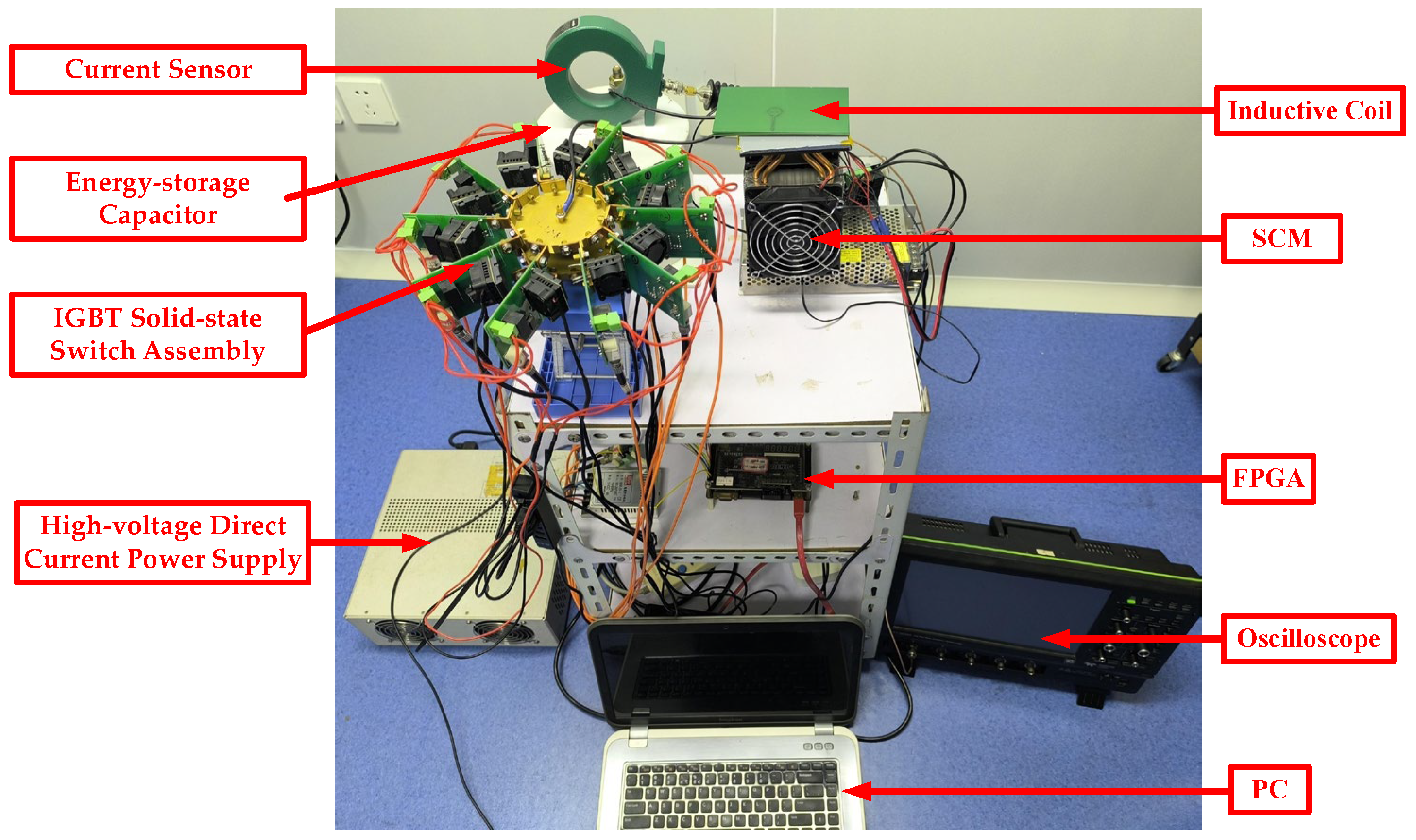
2.2. μs-PMF Generator
2.3. MNPs
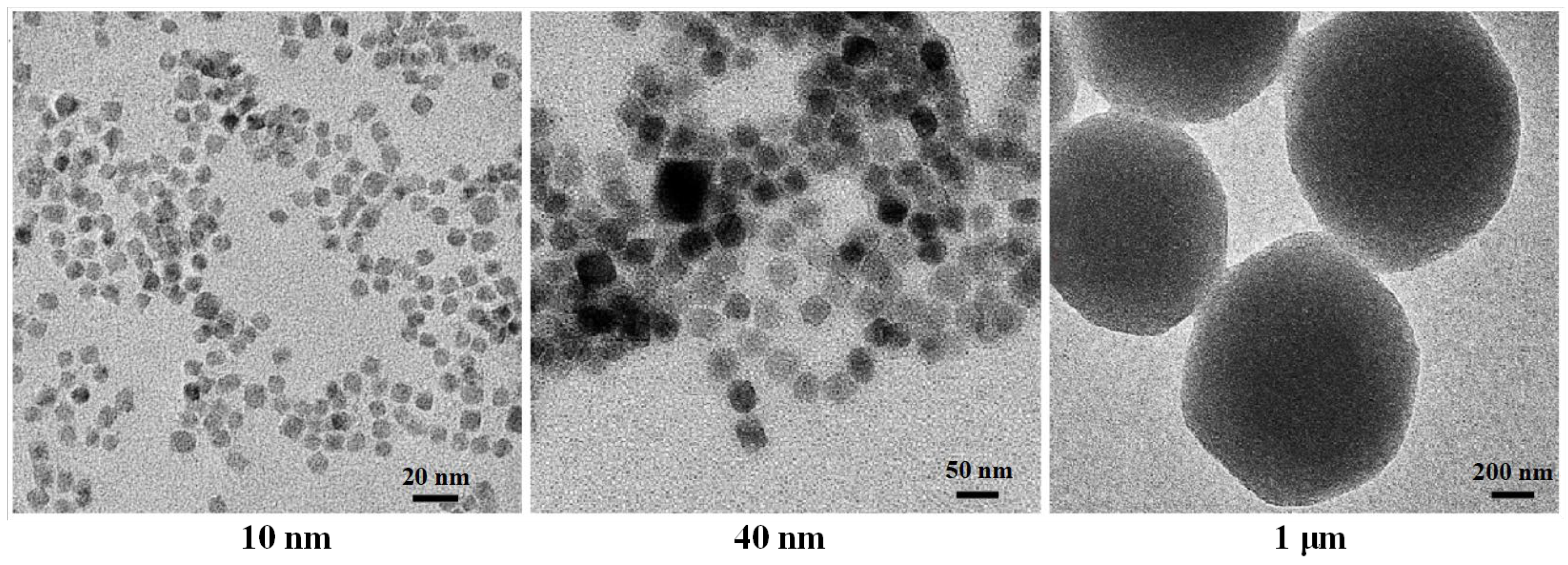
2.3.1. Type of MNPs
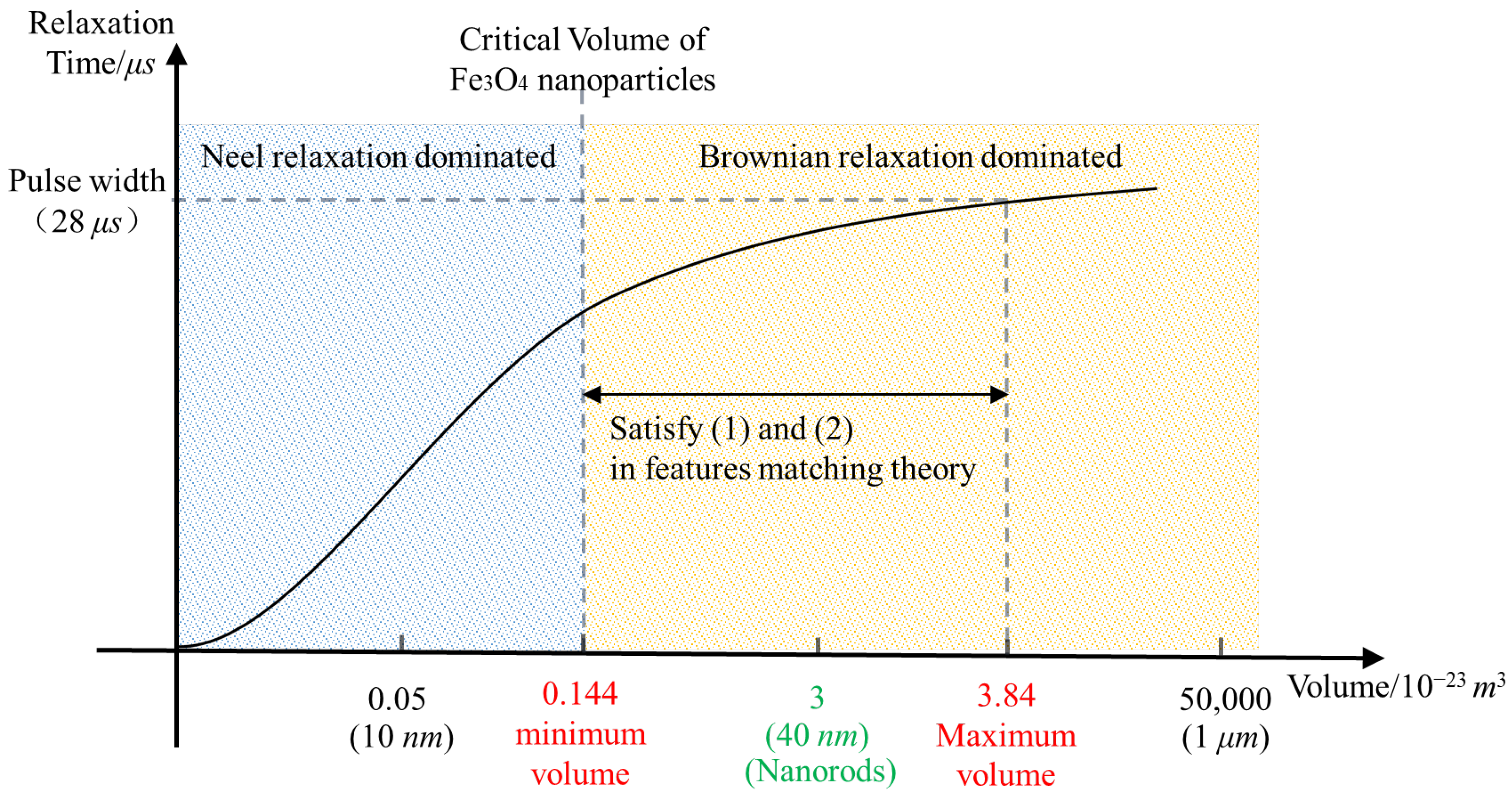
2.3.2. Volume of MNPs
- Matching between MNP volume and the critical volume of Brownian relaxation;
- Matching between MNP relaxation time and the pulse width;
- The influence of MNP volume on MMF.
- Matching between MNP volume and the critical volume of Brownian relaxation
- 2.
- Matching between MNP relaxation time and the pulse width
- 3.
- The influence of MNP volume on MMF
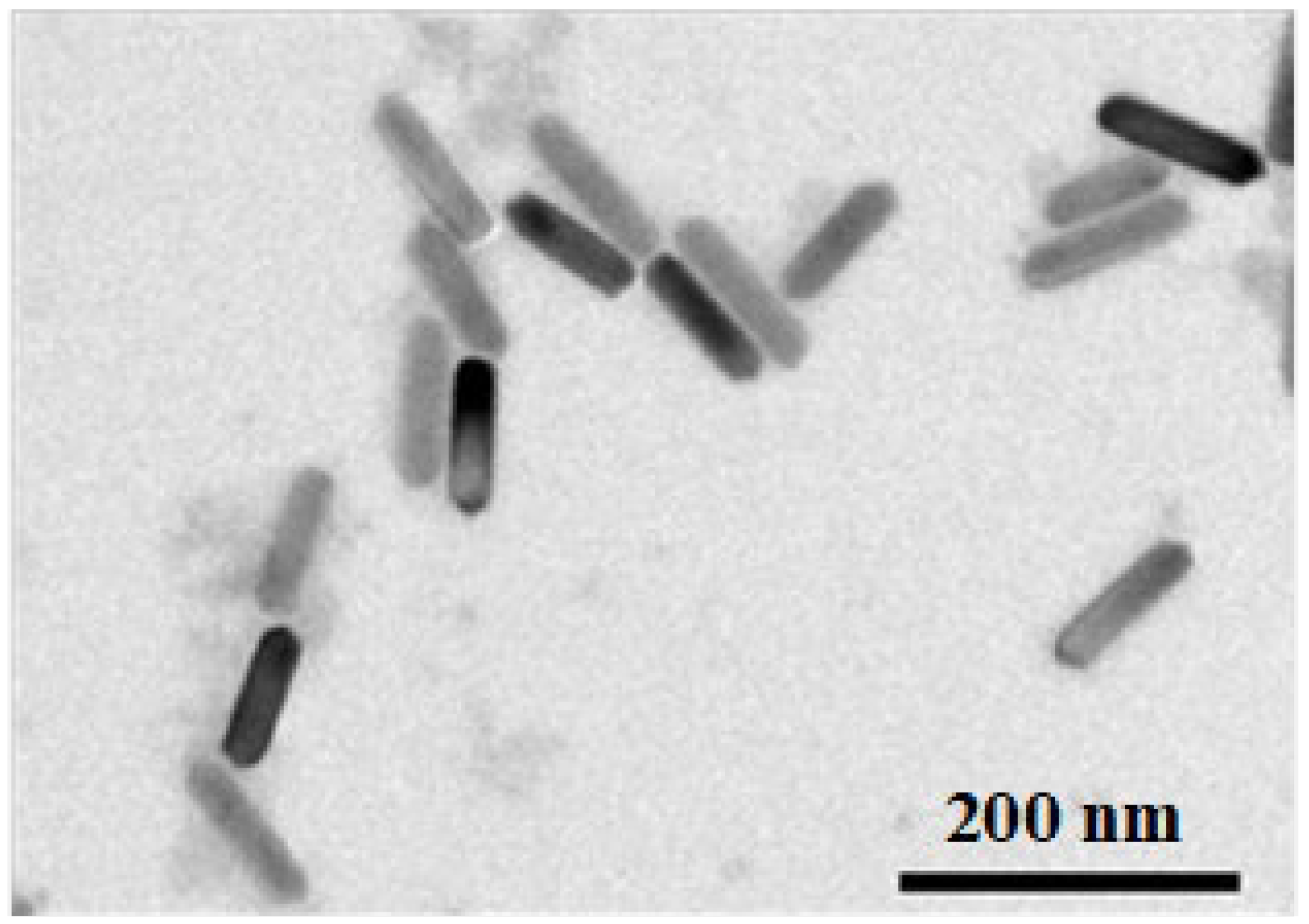
2.3.3. Shape of MNPs
2.4. Simulation Method for Magnetic Field Distribution
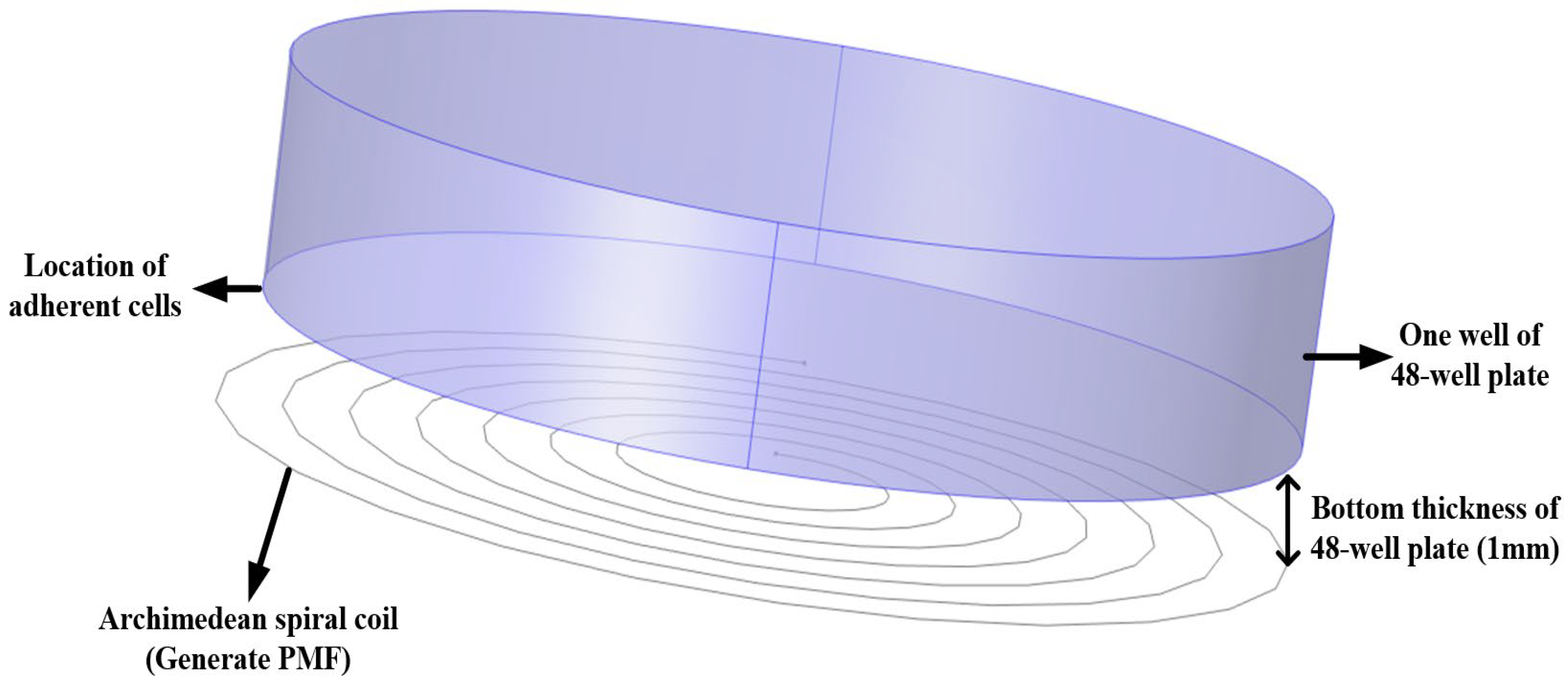
2.5. In Vitro Cell Experiment
2.5.1. Toxicity Experiment
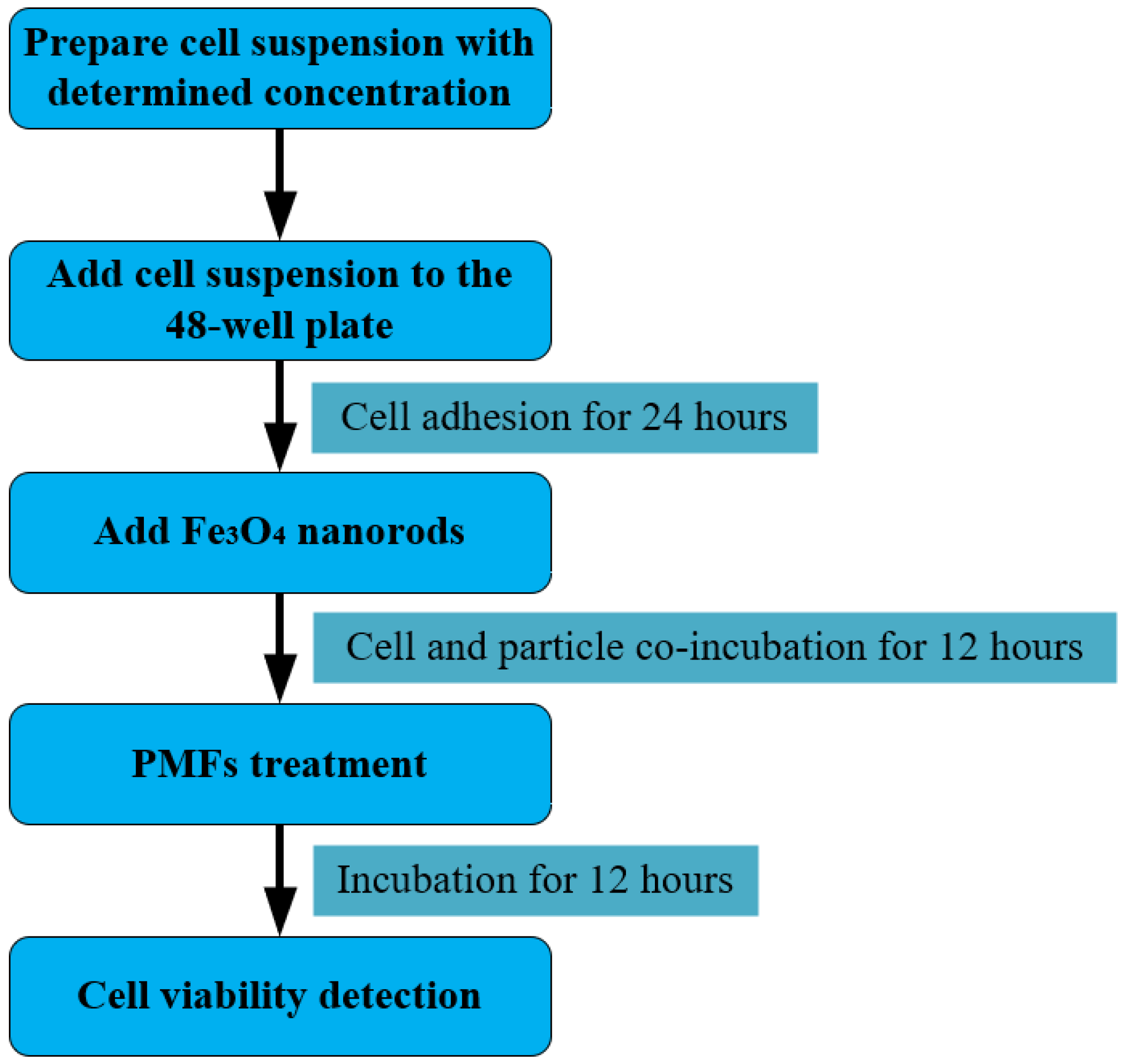
2.5.2. μs-PMF Treatment Experiment
2.5.3. MMT Experiment
2.6. Cell Viability Detection
2.7. Data Processing and Statistical Analysis
3. Results
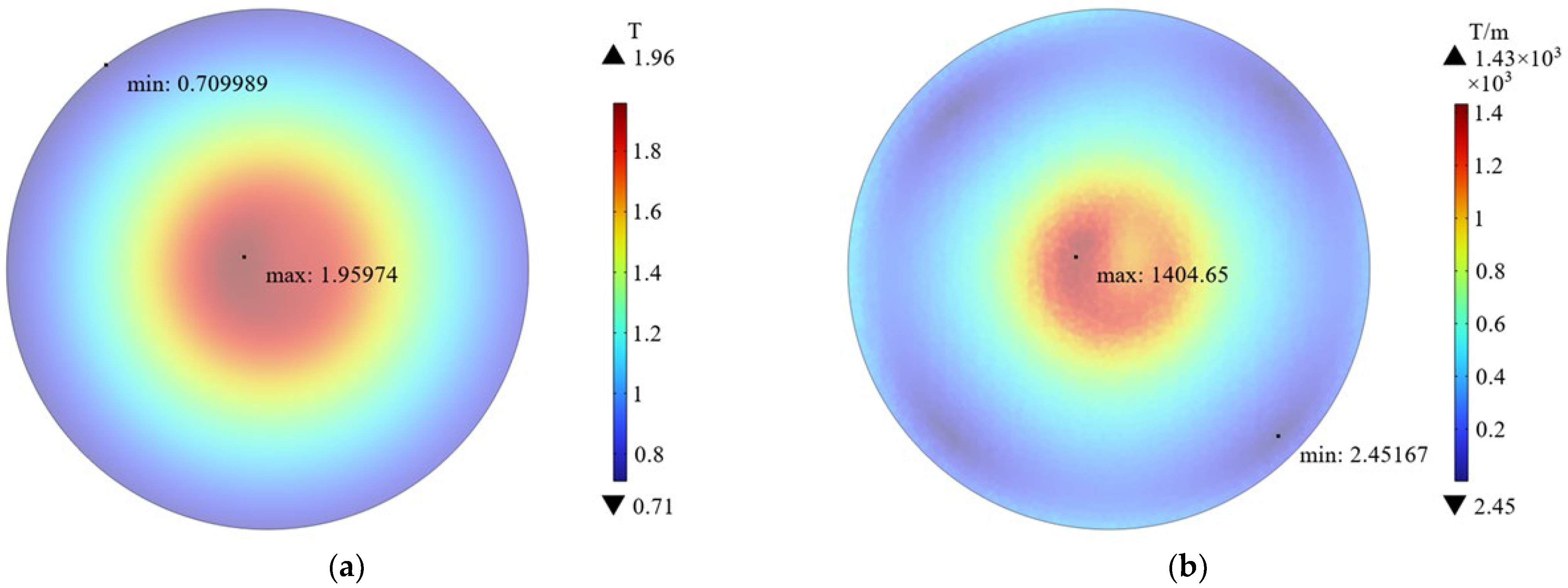
3.1. Magnetic Field Simulation
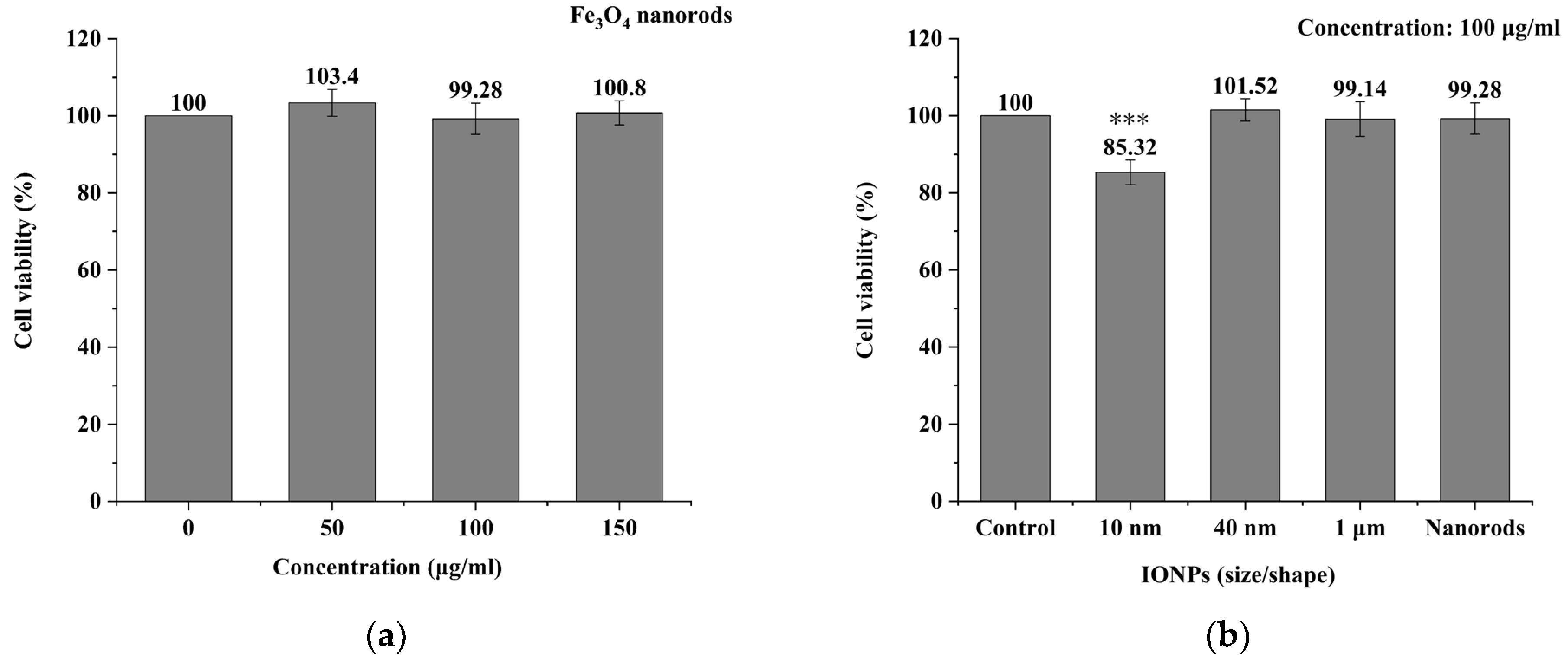
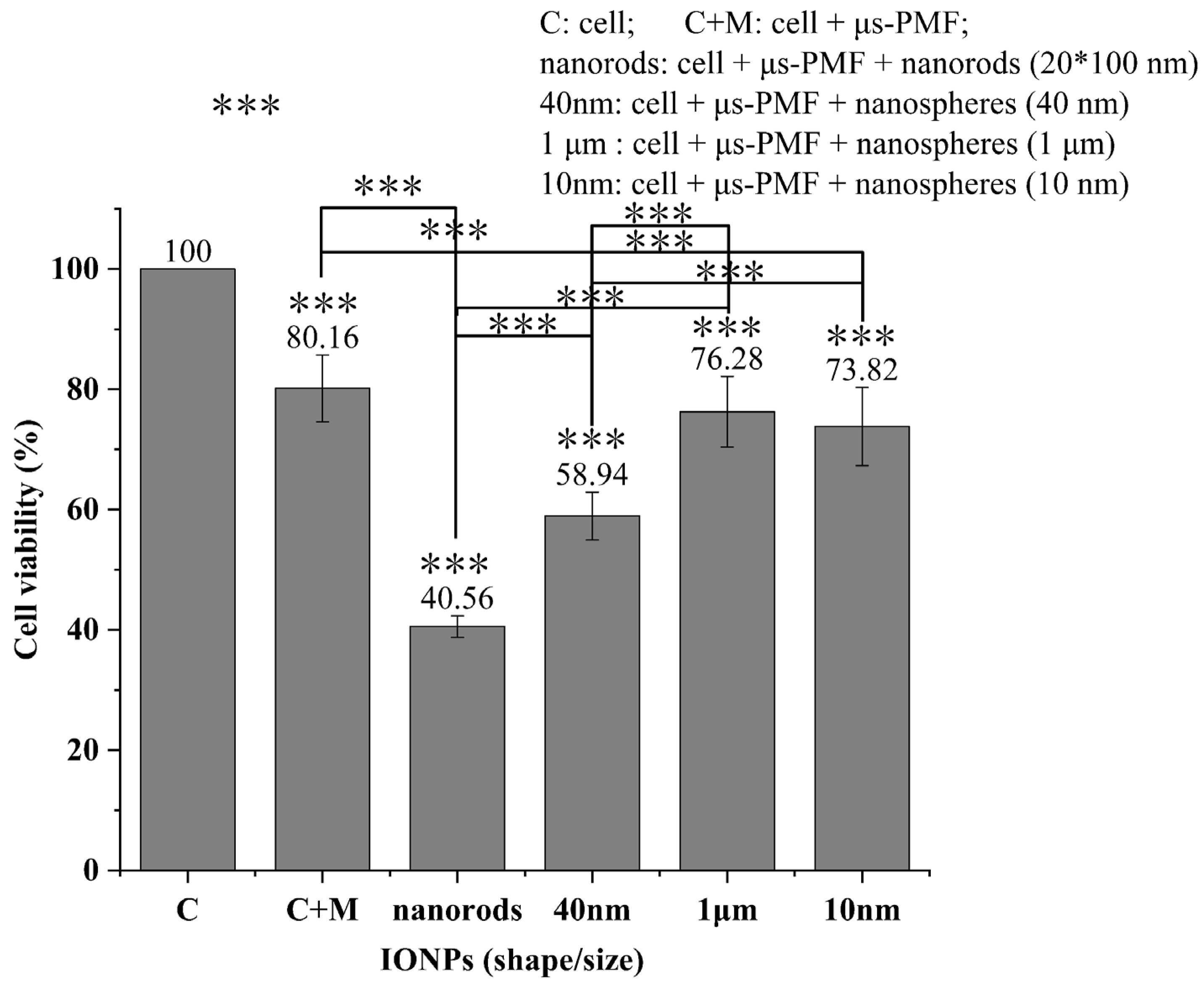
3.2. MNPs Toxicity Detection
3.3. μs-PMF Treatment and MMT
4. Discussion
4.1. μs-PMF Treatment
4.2. Feature-Matching Theory of MMT
4.2.1. MNP Volume and Brownian Relaxation Critical Volume
4.2.2. MNP Relaxation Time and the Pulse Width
4.2.3. MNPs Shape and the Intermittence of PMF
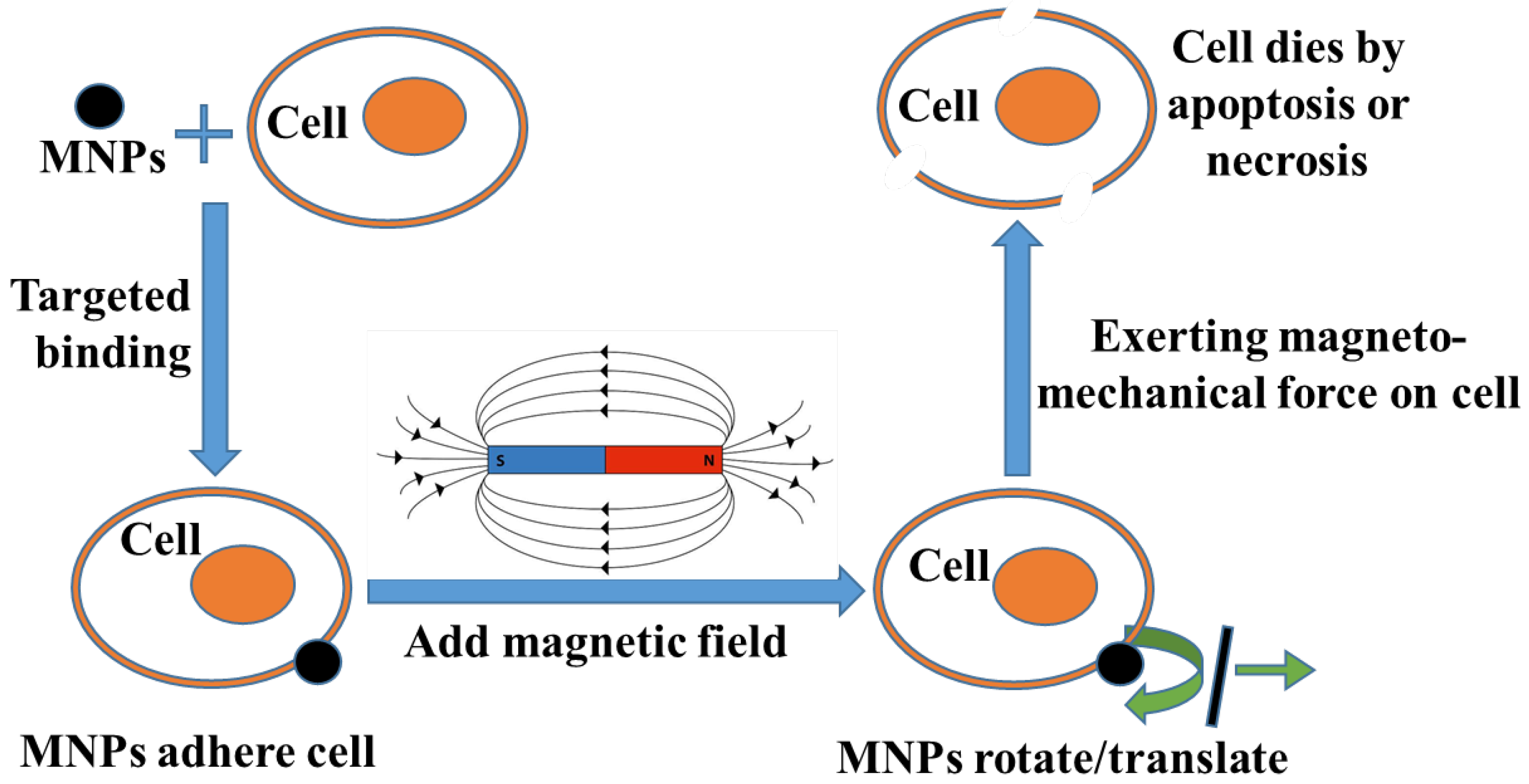
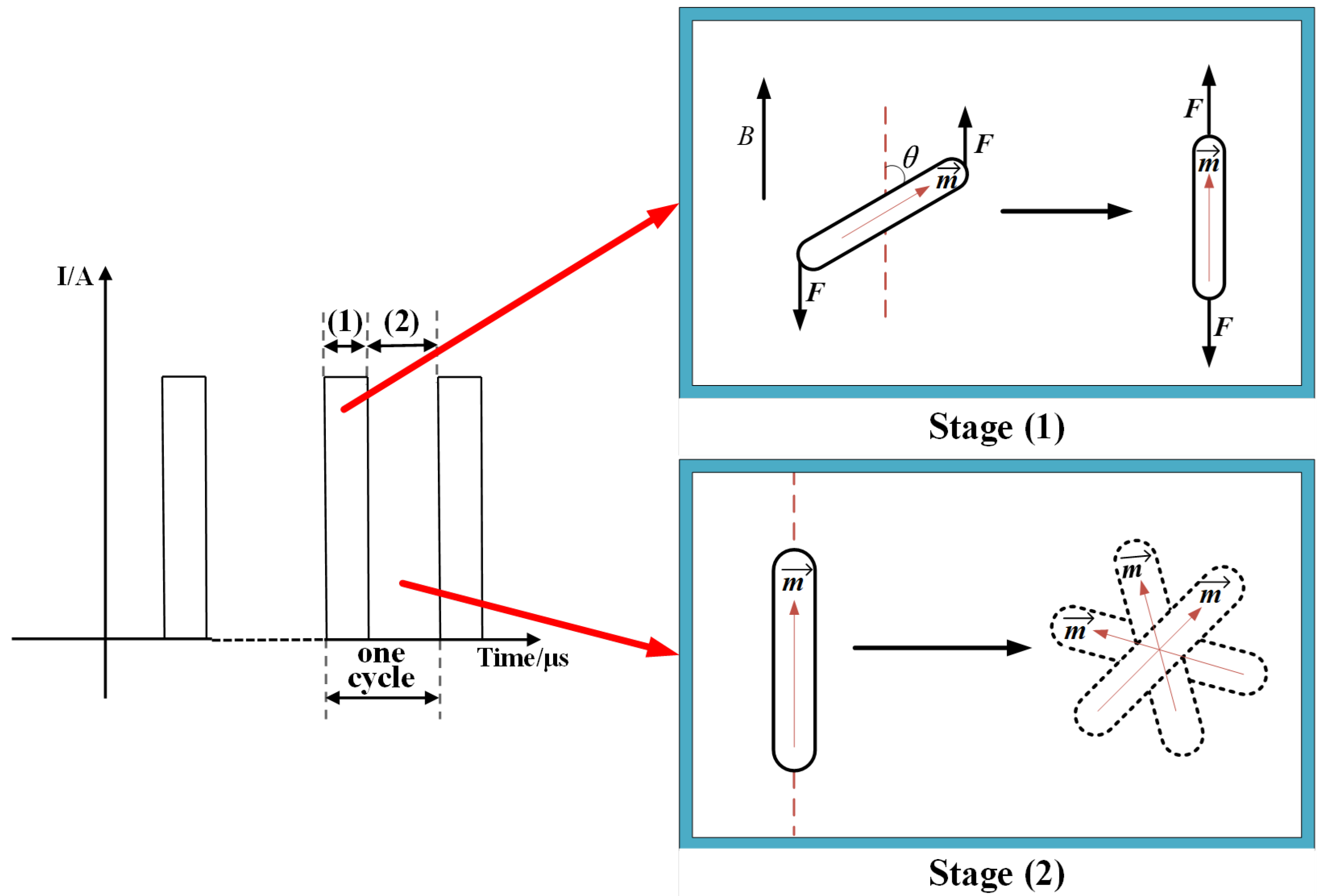
4.3. Mechanism of MMT
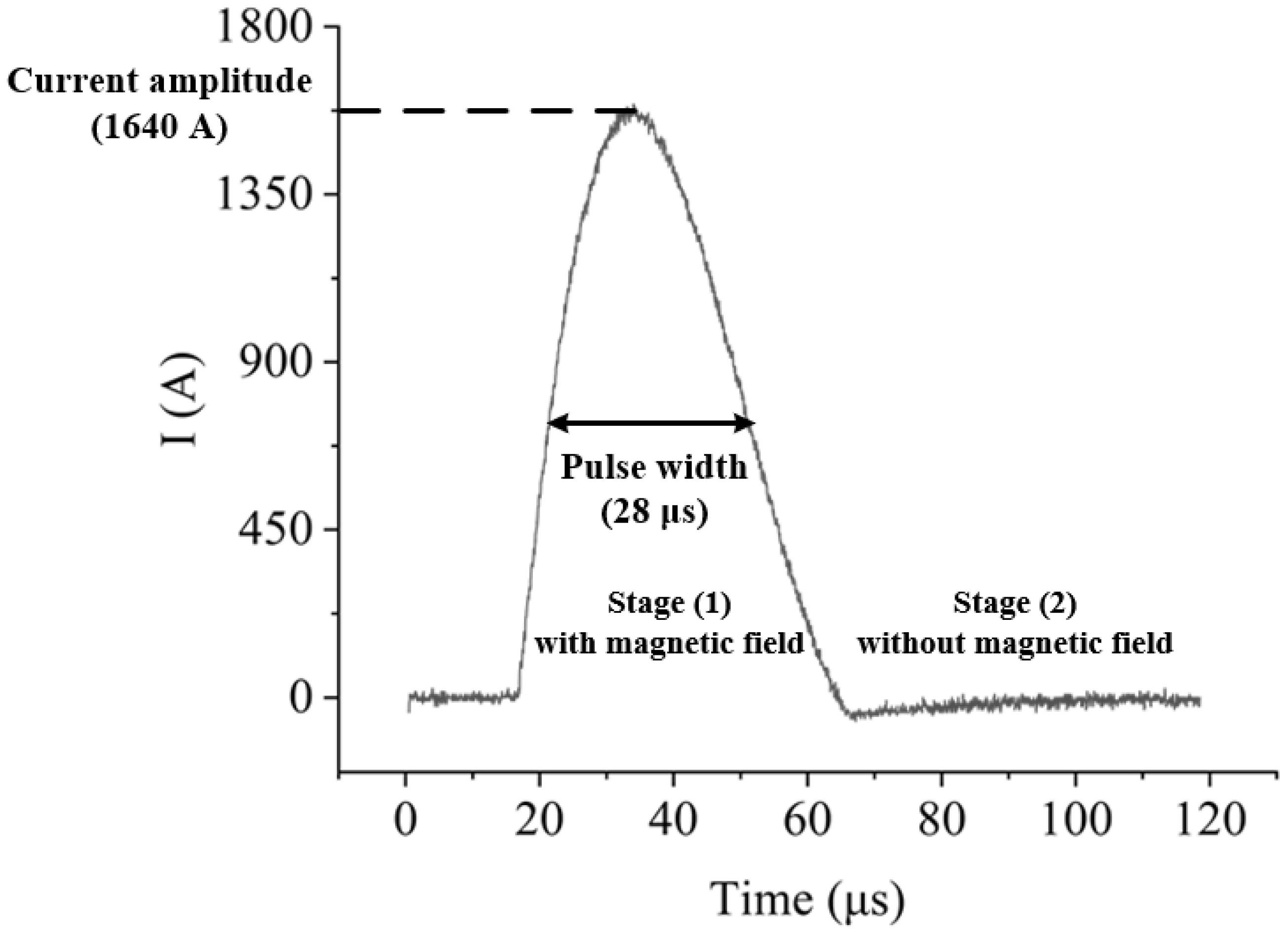
4.3.1. Stage (1) with Magnetic Field
4.3.2. Stage (2) without Magnetic Field
4.4. Limitations of Feature-Matching Theory
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vegerhof, A.; Barnoy, E.A.; Motiei, M.; Malka, D.; Danan, Y.; Zalevsky, Z.; Popovtzer, R. Targeted Magnetic Nanoparticles for Mechanical Lysis of Tumor Cells by Low-Amplitude Alternating Magnetic Field. Materials 2016, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Ogiue-Ikeda, M.; Sato, Y.; Ueno, S. Destruction of targeted cancer cells using magnetizable beads and pulsed magnetic forces. IEEE Trans. Magn. 2004, 40, 3018–3020. [Google Scholar] [CrossRef]
- Naud, C.; Thebault, C.; Carrière, M.; Hou, Y.X.; Morel, R.; Berger, F.; Diény, B.; Joisten, H. Cancer treatment by magneto-mechanical effect of particles, a review. Nanoscale Adv. 2020, 2, 3632–3655. [Google Scholar] [CrossRef]
- Gorobets, O.; Gorobets, S.; Sharai, I.; Polyakova, T.; Zablotskii, V. Interaction of magnetic fields with biogenic magnetic nanoparticles on cell membranes: Physiological consequences for organisms in health and disease. Bioelectrochemistry 2023, 151, 108390. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, Q.; Huang, S.; Fang, Y.; Kong, X.; Kaur, P.; Zhang, J.; Wang, Y.; Liu, D.; Wu, H.; et al. Zwitterionic Injectable Hydrogel-Combined Chemo- and Immunotherapy Medicated by Monomolecular Micelles to Effectively Prevent the Recurrence of Tumor Post Operation. ACS Appl. Mater. Interfaces 2024, 16, 4071–4088. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Bakr, M.A.; Rasmy, A.H. The efficacy of using metformin and/or quercetin for amelioration of gamma-irradiation induced tongue toxicity in diabetic rats. BMC Oral Health 2024, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Manuweera, T.; Wagenknecht, A.; Kleckner, A.S.; Dorsey, S.G.; Zhu, S.; Tivarus, M.E.; Kesler, S.R.; Ciner, A.; Kleckner, I.R. Preliminary evaluation of novel Bodily Attention Task to assess the role of the brain in chemotherapy-induced peripheral neurotoxicity (CIPN). Behav. Brain Res. 2024, 460, 114803. [Google Scholar] [CrossRef] [PubMed]
- Leulmi, S.; Chauchet, X.; Morcrette, M.; Ortiz, G.; Joisten, H.; Sabon, P.; Livache, T.; Hou, Y.X.; Carrière, M.; Lequien, S.; et al. Triggering the apoptosis of targeted human renal cancer cells by the vibration of anisotropic magnetic particles attached to the cell membrane. Nanoscale 2015, 7, 15904–15914. [Google Scholar] [CrossRef]
- Ju, H.X.; Cui, Y.B.; Chen, Z.Q.; Fu, Q.P.; Sun, M.Z.; Zhou, Y. Effects of combined delivery of extremely low frequency electromagnetic field and magnetic Fe3O4 nanoparticles on hepatic cell lines. Am. J. Transl. Res. 2016, 8, 1838–1847. [Google Scholar]
- Li, W.T.; Liu, Y.Y.; Qian, Z.Y.; Yang, Y.M. Evaluation of Tumor Treatment of Magnetic Nanoparticles Driven by Extremely Low Frequency Magnetic Field. Sci. Rep. 2017, 7, 9. [Google Scholar] [CrossRef]
- Wang, B.R.; Bienvenu, C.; Mendez-Garza, J.; Lançon, P.; Madeira, A.; Vierling, P.; Di Giorgio, C.; Bossis, G. Necrosis of HepG2 cancer cells induced by the vibration of magnetic particles. J. Magn. Magn. Mater. 2013, 344, 193–201. [Google Scholar] [CrossRef]
- Contreras, M.F.; Sougrat, R.; Zaher, A.; Ravasi, T.; Kosel, J. Non-chemotoxic induction of cancer cell death using magnetic nanowires. Int. J. Nanomed. 2015, 10, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.; Gan, W.L.; Teo, Y.K.; Lew, W.S. Interplay of cell death signaling pathways mediated by alternating magnetic field gradient. Cell Death Discov. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Uzhytchak, M.; Lynnyk, A.; Zablotskii, V.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Lunova, M.; Jirsa, M.; Kubinova, S.; Lunov, O.; et al. The use of pulsed magnetic fields to increase the uptake of iron oxide nanoparticles by living cells. Appl. Phys. Lett. 2017, 111, 5. [Google Scholar] [CrossRef]
- Lunov, O.; Uzhytchak, M.; Smolkova, B.; Lunova, M.; Jirsa, M.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Hof, M.; Jurkiewicz, P.; et al. Remote Actuation of Apoptosis in Liver Cancer Cells via Magneto-Mechanical Modulation of Iron Oxide Nanoparticles. Cancers 2019, 11, 1873. [Google Scholar] [CrossRef]
- Shen, Y.J.; Zhang, W.; Li, G.; Ning, P.; Li, Z.G.; Chen, H.T.; Wei, X.Y.; Pan, X.; Qin, Y.; He, B.; et al. Adaptive Control of Nanomotor Swarms for Magnetic-Field-Programmed Cancer Cell Destruction. ACS Nano 2021, 15, 20020–20031. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Ning, P.; Gao, R.; Feng, Q.S.; Shen, Y.J.; Zhang, Y.F.; Li, Y.Z.; Xu, C.; Qin, Y.; Plaza, G.R.; et al. Programmable ROS-Mediated Cancer Therapy via Magneto-Inductions. Adv. Sci. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.A.; Prishchepa, A.V.; Rytov, R.A.; Chekhonin, V.P.; Abakumov, M.A. Unveiling the Role of the Properties of Magnetic Nanoparticles for Highly Efficient Low-Frequency Magneto-Mechanical Actuation of Biomolecules. J. Phys. Chem. Lett. 2023, 14, 9112–9117. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.; Gan, W.L.; Liu, N.; Lew, W.S. Magneto-actuated cell apoptosis by biaxial pulsed magnetic field. Sci. Rep. 2017, 7, 8. [Google Scholar] [CrossRef]
- Mi, Y.; Tang, X.F.; Rui, S.Q.; Chu, Y.D.; Bian, C.H.; Yao, C.G.; Li, C.X. Development of High dB/dt Pulsed Magnetic Field Generator Based on Printed Circuit Board Archimedes Spiral Coil for Biomedical Applications. IEEE Trans. Plasma Sci. 2016, 44, 1879–1887. [Google Scholar] [CrossRef]
- Ma, C.; Li, Z.M.; Zhu, Y.K.; Zhang, M.N.; Wang, J.L.; Mi, Y. Pulsed Magnetic Field Generator for Enhancing Cell Membrane Permeability In Vitro. IEEE Trans. Plasma Sci. 2023, 8, 3647–3654. [Google Scholar] [CrossRef]
- Dulinska-Litewka, J.; Lazarczyk, A.; Halubiec, P.; Szafranski, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide NanoparticlesCurrent and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M.Q. Magnetite nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.R.; Lin, B.B.; Li, D.Y.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, X.X.; Zhang, Y.J.; Wei, J.; Wang, W.; Dong, C.Z.; Xue, Y.A.; Liu, M.; Pei, R.J. Engineered Fe3O4-based nanomaterials for diagnosis and therapy of cancer. New J. Chem. 2021, 45, 7918–7941. [Google Scholar] [CrossRef]
- Dallet, L.; Stanicki, D.; Voisin, P.; Miraux, S.; Ribot, E.J. Micron-sized iron oxide particles for both MRI cell tracking and magnetic fluid hyperthermia treatment. Sci. Rep. 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, T.; Xu, R.R.; Gao, W.J.; Zhao, H.; Shapter, J.G.; Wang, K.; Shen, Y.L.; Huang, P.; Gao, G.; et al. Large-scale immuno-magnetic cell sorting of T cells based on a self-designed high-throughput system for potential clinical application. Nanoscale 2017, 9, 13592–13599. [Google Scholar] [CrossRef]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Gao, Y.; Lim, J.; Teoh, S.H.; Xu, C.J. Emerging translational research on magnetic nanoparticles for regenerative medicine. Chem. Soc. Rev. 2015, 44, 6306–6329. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, S.H.; Keramane, A.; Kirkham, G.R.; Verschueren, S.B.; Magnay, J.L.; El Haj, A.J.; Dobson, J. Use of magnetic particles to apply mechanical forces for bone tissue engineering purposes. In Proceedings of the 5th International Conference on Fine Particle Magnetism, University College London, London, UK, 20–22 September 2004; pp. 77–80. [Google Scholar]
- Silva, A.H.; Lima, E.; Mansilla, M.V.; Zysler, R.D.; Troiani, H.; Pisciotti, M.L.M.; Locatelli, C.; Benech, J.C.; Oddone, N.; Zoldan, V.C.; et al. Superparamagnetic iron-oxide nanoparticles mPEG350-and mPEG2000-coated: Cell uptake and biocompatibility evaluation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Lassenberger, A.; Scheberl, A.; Stadlbauer, A.; Stiglbauer, A.; Helbich, T.; Reimhult, E. Individually Stabilized, Superparamagnetic Nanoparticles with Controlled Shell and Size Leading to Exceptional Stealth Properties and High Relaxivities. Acs Appl. Mater. Interfaces 2017, 9, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.C.; Gazeau, F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Torres-Diaz, I.; Rinaldi, C. Recent progress in ferrofluids research: Novel applications of magnetically controllable and tunable fluids. Soft Matter 2014, 10, 8584–8602. [Google Scholar] [CrossRef] [PubMed]
- Reva, V.V.; Lyutyy, T.V. Microwave Absorption by a Rigid Dipole in a Viscous Fluid. In Proceedings of the 2nd International Young Scientists Forum on Applied Physics and Engineering (YSF), Kharkiv, Ukraine, 10–14 October 2016; pp. 104–107. [Google Scholar]
- Mi, Y.; Ma, C.; Zheng, W.; Li, Z.M.; Zhang, M.N. Magnetic losses in single-domain magnetic particles. Eur. Phys. J. -Spec. Top. 2023, 232, 1353–1368. [Google Scholar] [CrossRef]
- Odenbach, S.; Raj, K.J.M. The Influence of Large Particles and Agglomerates on the Magnetoviscous Effect in Ferrofluids. Magnetohydrodynamics 2000, 36, 312–319. [Google Scholar] [CrossRef]
- Brown, W.F. Thermal Fluctuations of a Single-Domain Particle. Phys. Rev. 1963, 130, 1677. [Google Scholar] [CrossRef]
- Abbott, J.J.; Ergeneman, O.; Kummer, M.P.; Hirt, A.M.; Nelson, B.J. Modeling magnetic torque and force for controlled manipulation of soft-magnetic bodies. In Proceedings of the IEEE/ASME International Conference on Advanced Intelligent Mechatronics, Swiss Fed Inst Technol, Zurich, Switzerland, 4–7 September 2007; p. 841. [Google Scholar]
- Yao, C.Y.; Yang, F.; Zhang, J.J.; Yao, J.L.; Cao, Y.; Peng, H.; Stanciu, S.G.; Charitidis, C.A.; Wu, A.G. Magneto-mechanical therapeutic effects and associated cell death pathways of magnetic nanocomposites with distinct geometries. Acta Biomater. 2023, 161, 238–249. [Google Scholar] [CrossRef]
- Zablotskaya, A.; Segal, I.; Lukevics, E.; Maiorov, M.; Zablotsky, D.; Blums, E.; Shestakova, I.; Domracheva, I. Water-soluble magnetic nanoparticles with biologically active stabilizers. J. Magn. Magn. Mater. 2009, 321, 1428–1432. [Google Scholar] [CrossRef]
- Frtús, A.; Smolková, B.; Uzhytchak, M.; Lunova, M.; Jirsa, M.; Kubinová, S.; Dejneka, A.; Lunov, O. Analyzing the mechanisms of iron oxide nanoparticles interactions with cells: A road from failure to success in clinical applications. J. Control. Release 2020, 328, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Motaghi, H.; Khanahmad, H.; Mehrgardi, M.A.; Farzadniya, A.; Shokrani, P. Preparation and Characterization of SPION-CDs as a Multifunctional Fluorescence/Magnetic Resonance Nanoparticle. Acta Chem. Iasi 2019, 27, 87–98. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Liu, Y.P.; Huang, J.; Chen, K.; Huang, J.X.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhao, Y.P.; Wang, X.Q. Nanoparticle-Mediated Mechanical Destruction of Cell Membranes: A Coarse-Grained Molecular Dynamics Study. Acs Appl. Mater. Interfaces 2017, 9, 26665–26673. [Google Scholar] [CrossRef] [PubMed]
- Tree-Udom, T.; Seemork, J.; Shyou, K.; Hamada, T.; Sangphech, N.; Palaga, T.; Insin, N.; Pan-In, P.; Wanichwecharungruang, S. Shape Effect on Particle-Lipid Bilayer Membrane Association, Cellular Uptake, and Cytotoxicity. Acs Appl. Mater. Interfaces 2015, 7, 23993–24000. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Liu, Y.L.; Hu, W.; Gao, J.M. Modeling Particle Shape-Dependent Dynamics in Nanomedicine. J. Nanosci. Nanotechnol. 2011, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Yang, F.C.; Zhu, L.; Qiao, R.; Zhao, Y.P. Manipulation of magnetic nanorod clusters in liquid by non-uniform alternating magnetic fields. Soft Matter 2017, 13, 3750–3759. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-del-Campo, L.; Montenegro, M.F.; Cabezas-Herrera, J.; Rodríguez-López, J.N. The critical role of alpha-folate receptor in the resistance of melanoma to methotrexate. Pigment Cell Melanoma Res. 2009, 22, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Zhang, T.P.; Wu, B.J.; Zhang, X.W. Insights into the therapeutic potential of hypoxia-inducible factor-1 α small interfering RNA in malignant melanoma delivered via folate-decorated cationic liposomes. Int. J. Nanomed. 2016, 11, 991–1002. [Google Scholar] [CrossRef]
- Majidi, F.S.; Mohammadi, E.; Mehravi, B.; Nouri, S.; Ashtari, K.; Neshasteh-riz, A. Investigating the effect of near infrared photo thermal therapy folic acid conjugated gold nano shell on melanoma cancer cell line A375. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2161–2170. [Google Scholar] [CrossRef]
- Buqué, A.; Muhialdin, J.S.; Muñoz, A.; Calvo, B.; Carrera, S.; Aresti, U.; Sancho, A.; Rubio, I.; López-Vivanco, G. Molecular mechanism implicated in Pemetrexed-induced apoptosis in human melanoma cells. Mol. Cancer 2012, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S.; Hann, B.; Shapiro, E.M. On the Use of Micron-Sized Iron Oxide Particles (MPIOS) to Label Resting Monocytes in Bone Marrow. Mol. Imaging. Biol. 2011, 13, 819–824. [Google Scholar] [CrossRef]
- Raschzok, N.; Langer, C.M.; Schmidt, C.; Lerche, K.H.; Billecke, N.; Nehls, K.; Schlüter, N.B.; Leder, A.; Rohn, S.; Mogl, M.T.; et al. Functionalizable Silica-Based Micron-Sized Iron Oxide Particles for Cellular Magnetic Resonance Imaging. Cell Transplant. 2013, 22, 1959–1970. [Google Scholar] [CrossRef]
- Rafieepour, A.; Azari, M.R.; Peirovi, H.; Khodagholi, F.; Jaktaji, J.P.; Mehrabi, Y.; Naserzadeh, P.; Mohammadian, Y. Investigation of the effect of magnetite iron oxide particles size on cytotoxicity in A(549) cell line. Toxicol. Ind. Health 2019, 35, 703–713. [Google Scholar] [CrossRef]
- Liu, D.L.; Li, J.J.; Wang, C.B.; An, L.; Lin, J.M.; Tian, Q.W.; Yang, S.P. Ultrasmall Fe@Fe3O4 nanoparticles as T-1-T-2 dual-mode MRI contrast agents for targeted tumor imaging. Nanomed.-Nanotechnol. Biol. Med. 2021, 32, 11. [Google Scholar] [CrossRef] [PubMed]
- Guichard, Y.; Schmit, J.; Darne, C.; Gate, L.; Goutet, M.; Rousset, D.; Rastoix, O.; Wrobel, R.; Witschger, O.; Martin, A.; et al. Cytotoxicity and Genotoxicity of Nanosized and Microsized Titanium Dioxide and Iron Oxide Particles in Syrian Hamster Embryo Cells. Ann. Occup. Hyg. 2012, 56, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, Y.; Xu, Y.H.; Li, J.C.; Zhang, Z.X.; Wang, H.; Shen, M.W.; Shi, X.Y.; Zhang, G.X. Conjugation of Iron Oxide Nanoparticles with RGD-Modified Dendrimers for Targeted Tumor MR Imaging. Acs Appl. Mater. Interfaces 2015, 7, 5420–5428. [Google Scholar] [CrossRef]
- Qian, A.R.; Gao, X.; Zhang, W.; Li, J.B.; Wang, Y.; Di, S.M.; Hu, L.F.; Shang, P. Large Gradient High Magnetic Fields Affect Osteoblast Ultrastructure and Function by Disrupting Collagen I or Fibronectin/αβ1 Integrin. PLoS ONE 2013, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Syrovets, T.; Schmidt, Z.W.; Dejneka, A.; Simmet, T. Modulation of monocytic leukemia cell function and survival by high gradient magnetic fields and mathematical modeling studies. Biomaterials 2014, 35, 3164–3171. [Google Scholar] [CrossRef]
- Zablotskii, V.; Lunov, O.; Kubinova, S.; Polyakova, T.; Sykova, E.; Dejneka, A. Effects of high-gradient magnetic fields on living cell machinery. J. Phys. D-Appl. Phys. 2016, 49, 23. [Google Scholar] [CrossRef]
- Sen, S.; Subramanian, S.; Discher, D.E. Indentation and adhesive probing of a cell membrane with AFM: Theoretical model and experiments. Biophys. J. 2005, 89, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.J.; Helenius, J.; Alsteens, D.; Dufrene, Y.F. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 2009, 5, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Dai, L.; Xu, N.; Zheng, W.; Ma, C.; Chen, W.; Zhang, Q.J.N. Viability inhibition of A375 melanoma cells in vitro by a high-frequency nanosecond-pulsed magnetic field combined with targeted iron oxide nanoparticles via membrane magnetoporation. Nanotechnology 2021, 32, 385101. [Google Scholar] [CrossRef]
- Raikher, Y.L.; Shliomis, M.I. The Effective-Field Method in the Orientational Kinetics of Magnetic Fluids and Liquid-Crystals. In Relaxation Phenomena in Condensed Matter; Coffey, W., Ed.; Advances in Chemical Physics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1994; Volume 87, pp. 595–751. [Google Scholar]
- Kuznetsov, A.A.; Pshenichnikov, A.F. Nonlinear response of a dilute ferrofluid to an alternating magnetic field. J. Mol. Liq. 2022, 346, 7. [Google Scholar] [CrossRef]
- Lyutyy, T.V.; Reva, V.V. Energy dissipation of rigid dipoles in a viscous fluid under the action of a time-periodic field: The influence of thermal bath and dipole interaction. Phys. Rev. E 2018, 97, 15. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.O.; Camp, P.J. Magnetization relaxation dynamics in polydisperse ferrofluids. Phys. Rev. E 2023, 107, 10. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Ikeda, A.; Berthier, L. Relaxation Dynamics of Non-Brownian Spheres Below Jamming. J. Stat. Phys. 2021, 182, 19. [Google Scholar] [CrossRef]
- Kang, M.A.; Fang, J.S.; Paragodaarachchi, A.; Kodama, K.; Yakobashvi, D.; Ichiyanagi, Y.; Matsui, H. Magnetically Induced Brownian Motion of Iron Oxide Nanocages in Alternating Magnetic Fields and Their Application for Efficient siRNA Delivery. Nano Lett. 2022, 22, 8852–8859. [Google Scholar] [CrossRef]
- Barati, M.R.; Selomulya, C.; Suzuki, K. Particle size dependence of heating power in MgFe2O4 nanoparticles for hyperthermia therapy application. J. Appl. Phys. 2014, 115, 3. [Google Scholar] [CrossRef]
- Fischer, B.; Wagner, J.; Schmitt, M.; Trieu, V.; Hempelmann, R. Dependence of Brownian relaxation on the volume fraction and an external field. Z. Phys. Chem.-Int. J. Res. Phys. Chem. Chem. Phys. 2006, 220, 69–77. [Google Scholar] [CrossRef]
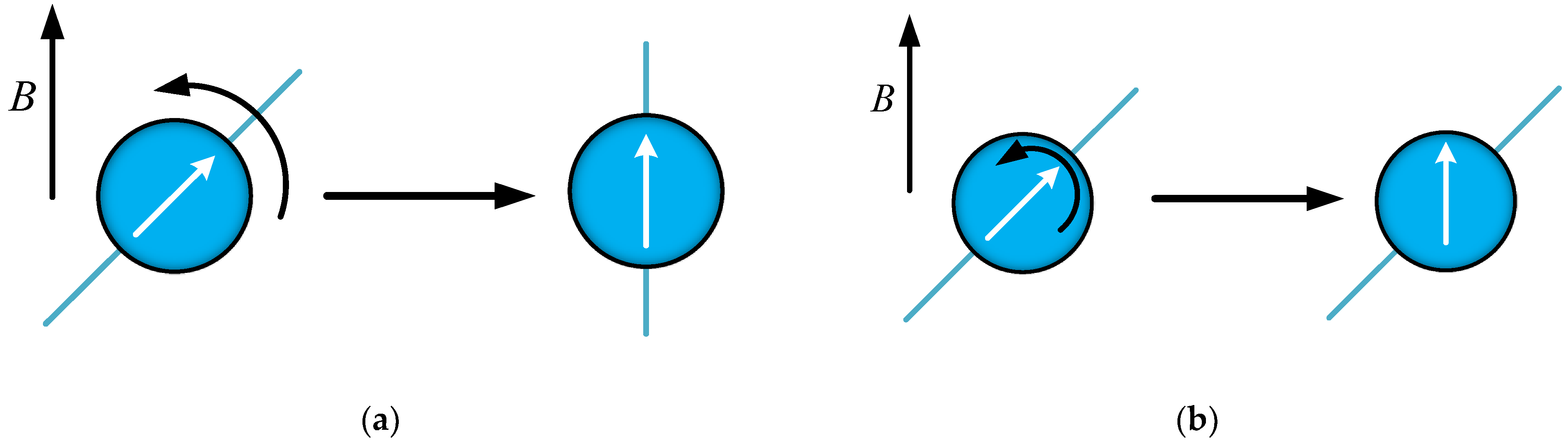
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Zhang, M.-N.; Ma, C.; Zheng, W.; Teng, F. Feature Matching of Microsecond-Pulsed Magnetic Fields Combined with Fe3O4 Particles for Killing A375 Melanoma Cells. Biomolecules 2024, 14, 521. https://doi.org/10.3390/biom14050521
Mi Y, Zhang M-N, Ma C, Zheng W, Teng F. Feature Matching of Microsecond-Pulsed Magnetic Fields Combined with Fe3O4 Particles for Killing A375 Melanoma Cells. Biomolecules. 2024; 14(5):521. https://doi.org/10.3390/biom14050521
Chicago/Turabian StyleMi, Yan, Meng-Nan Zhang, Chi Ma, Wei Zheng, and Fei Teng. 2024. "Feature Matching of Microsecond-Pulsed Magnetic Fields Combined with Fe3O4 Particles for Killing A375 Melanoma Cells" Biomolecules 14, no. 5: 521. https://doi.org/10.3390/biom14050521
APA StyleMi, Y., Zhang, M.-N., Ma, C., Zheng, W., & Teng, F. (2024). Feature Matching of Microsecond-Pulsed Magnetic Fields Combined with Fe3O4 Particles for Killing A375 Melanoma Cells. Biomolecules, 14(5), 521. https://doi.org/10.3390/biom14050521






