Unlocking Genetic Mysteries during the Epic Sperm Journey toward Fertilization: Further Expanding Cre Mouse Lines
Abstract
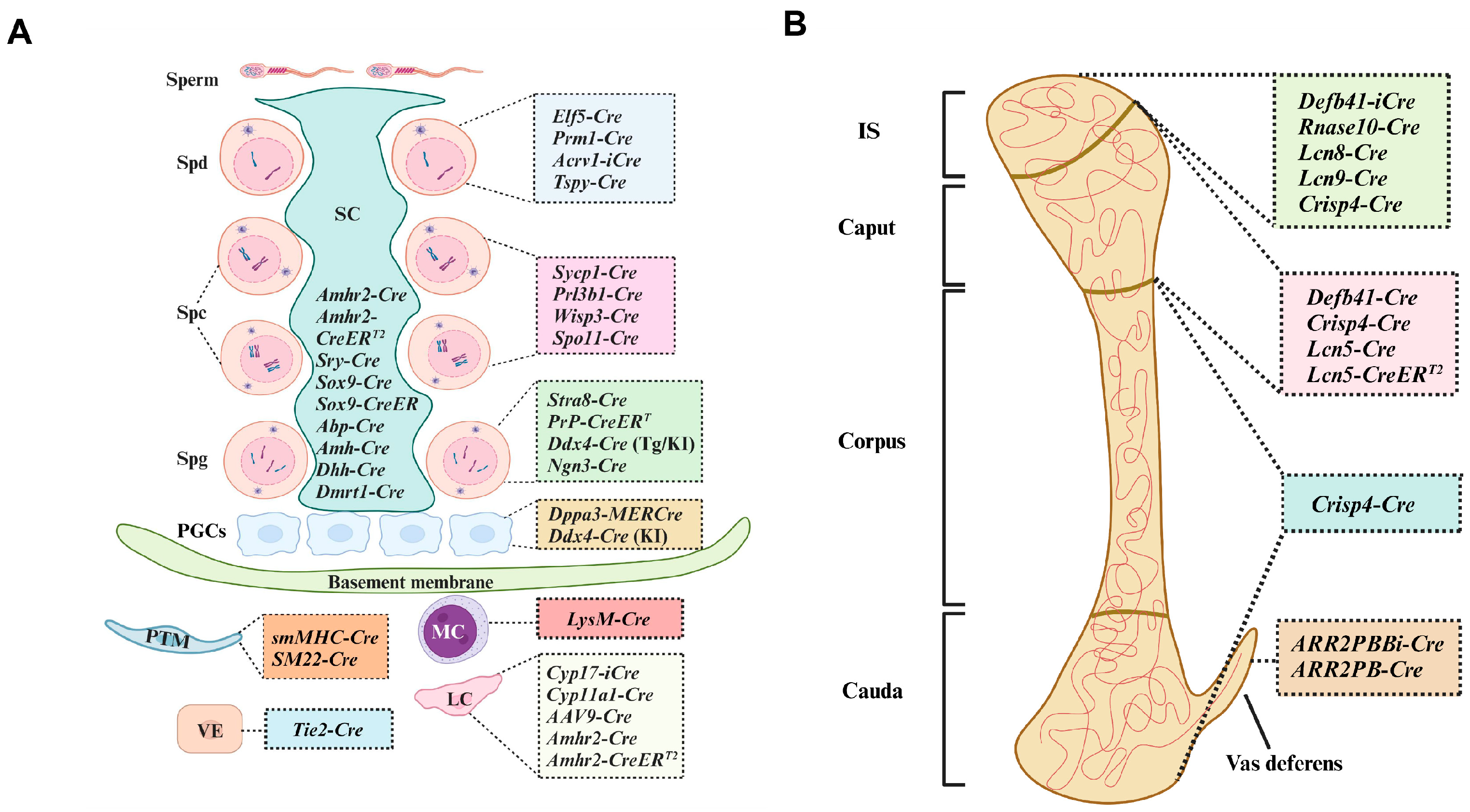
:1. Introduction
2. Cre Lines for PGCs and Spermatogonia
2.1. Ddx4-Cre
2.2. Stra8-Cre
2.3. PrP-CreERT
2.4. Dppa3-MERCre
2.5. Eomes-CreERT
2.6. Ngn3-Cre
2.7. Other Cre Lines for PGCs and Spermatogonia
3. Cre Recombination Lines for Spermatocytes
3.1. Sycp1-Cre
3.2. Prl3b1-Cre
3.3. Wisp3-Cre
3.4. Spo11-Cre
4. Cre Recombination Strains for Spermatids
4.1. Tspy-Cre
4.2. Prm1-Cre
4.3. Acrv1-iCre
4.4. Elf5-Cre
| Marker Strains | Germline Specific | Expression Outside of the Reproductive System | Initial Expression Phase | Transgenic (Tg)/Knock-In (KI) |
|---|---|---|---|---|
| Oct4-MerCreMer [78,79] (Strain #016829) | PGCs and undifferentiated spermatogonia | Pancreas, skin, intestine, kidney, etc. [80] | E7.5–8 [77] | KI |
| Tnap-Cre [66] | PGCs (around 50%) | Placenta, intestine and neural tube, labyrinthine region | E9.5–10.5 | KI |
| Nanos3-Cre [67] | PGCs (11–25%) | NR | E7.75 | KI |
| Nanos2-MerCreMer [111,112] | undifferentiated spermatogonia | NR | E13.5 [113] | Tg |
| Blimp1-Cre [68,114] Strain #008827 | PGCs (55–78%) | B and T lymphocytes, retina, limbs, pharynx, and heart [70] | E6.25 | Tg |
| Tex101-iCre [115,116] Strain #019893 | Pro-spermatogonia and subsequent germ cells | NR | 1 DPP | Tg |
| Gfra1-CreERT2 [117] | Undifferentiated spermatogonia | Kidney [118] | E9.5 | KI |
| UBC-CreERT2 [71,72,74,75,119] Strain #:007001 | Spermatogonia, testis, and somatic cells | Thymus, spleen, heart, muscle, brain, kidney, bone marrow [119] | NR | Tg |
| Rosa26-CreERT2 [73] | Spermatogonia | Other tissues in embryo and adult [120] | NR | KI |
| Aqp2-Cre [121] Strain #:006881 | Spermatids | Kidney | NR | Tg |
| Hspa2-Cre [122,123] Strain #:008870 | Spermatocyte and spermatids | Brain and embryo | Leptotene | Tg |
| Pgk2-Cre [124,125] | Spermatocyte and spermatids | Tissues in embryo [125] | NR | Tg |
| Wnt7a-Cre [126,127] Strain #036637-JAX | Spermatocyte | Uterine epithelium [127] | Mid-pachynema (12 DPP) | Tg |
| cKit-Cre [128] | Spermatocytes and spermatids | Mosaicism (20–100%) | NR | Tg |
| CaMKIIα-Cre [129,130] Strain #:005359 | Testis germ cells | Brain | NR | Tg |
| Syn1-Cre [131,132] Strain #:003966 | Spermatocytes | Neurons [132] | E12.5 | Tg |
5. Cre Transgenic Mice for Sertoli and Leydig Cells
6. Cre Transgenic Models for Other Cells in the Testes
7. Cre Models for Epididymis
7.1. Defb41-iCre
7.2. Rnase10-Cre
7.3. Crisp4-Cre
7.4. Lipocalin-Cre
8. The Cre Models Generated for the Prostate, Seminal Vesicle, and Seminiferous Duct
9. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Karimova, M.; Abi-Ghanem, J.; Berger, N.; Surendranath, V.; Pisabarro, M.T.; Buchholz, F. Vika/vox, a novel efficient and specific Cre/loxP-like site-specific recombination system. Nucleic Acids Res. 2013, 41, e37. [Google Scholar] [CrossRef] [PubMed]
- Karimova, M.; Baker, O.; Camgoz, A.; Naumann, R.; Buchholz, F.; Anastassiadis, K. A single reporter mouse line for Vika, Flp, Dre, and Cre-recombination. Mater. Sci. Eng. A 2018, 8, 14453. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M. VCre/VloxP and SCre/SloxP as Reliable Site-Specific Recombination Systems for Genome Engineering. Methods Mol. Biol. 2023, 2637, 161–180. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nomura, T.; Tsujita, M.; Suzuki, M.; Fuse, T.; Mori, H.; Mishina, M. Flp recombinase transgenic mice of C57BL/6 strain for conditional gene targeting. Biochem. Biophys. Res. Commun. 2002, 293, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Nakayama, M. VCre/VloxP and SCre/SloxP: New site-specific recombination systems for genome engineering. Nucleic Acids Res. 2011, 39, e49. [Google Scholar] [CrossRef] [PubMed]
- Sauer, B.; Henderson, N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA 1988, 85, 5166–5170. [Google Scholar] [CrossRef] [PubMed]
- Sauer, B.; Henderson, N. Cre-stimulated recombination at loxP-containing DNA sequences placed into the mammalian genome. Nucleic Acids Res. 1989, 17, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Cre transgenic mouse lines. Transgenesis Tech. Princ. Protoc. 2009, 561, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rashbrook, V.S.; Brash, J.T.; Ruhrberg, C. Cre toxicity in mouse models of cardiovascular physiology and disease. Nat. Cardiovasc. Res. 2022, 1, 806–816. [Google Scholar] [CrossRef]
- Garrick, D.; Fiering, S.; Martin, D.I.; Whitelaw, E. Repeat-induced gene silencing in mammals. Nat. Genet. 1998, 18, 56–59. [Google Scholar] [CrossRef]
- Gurumurthy, C.B.; Saunders, T.L.; Ohtsuka, M. Designing and generating a mouse model: Frequently asked questions. J. Biomed. Res. 2021, 35, 76. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef] [PubMed]
- Young, S.A.; Aitken, R.J.; Ikawa, M. Advantages of using the CRISPR/Cas9 system of genome editing to investigate male reproductive mechanisms using mouse models. Asian J. Androl. 2015, 17, 623. [Google Scholar] [PubMed]
- Neto, F.T.L.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Burnet, G.; Feng, C.A. Generation and characterization of a Ddx4-iCre transgenic line for deletion in the germline beginning at genital ridge colonization. Genesis 2023, 61, e23511. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Sage, J.; Cuzin, F.; Rassoulzadegan, M. Cre expression in primary spermatocytes: A tool for genetic engineering of the germ line. Mol. Reprod. Dev. 1998, 51, 274–280. [Google Scholar] [CrossRef]
- O’Gorman, S.; Dagenais, N.A.; Qian, M.; Marchuk, Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA 1997, 94, 14602–14607. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.S.; Matin, A. Tools for the genetic analysis of germ cells. Genesis 2009, 47, 617–627. [Google Scholar] [CrossRef]
- Smith, L. Good planning and serendipity: Exploiting the Cre/Lox system in the testis. Reproduction 2011, 141, 151–161. [Google Scholar] [CrossRef]
- Le, H.T.; Hasegawa, Y.; Daitoku, Y.; Kato, K.; Miznuo-Iijima, S.; Dinh, T.T.H.; Kuba, Y.; Osawa, Y.; Mikami, N.; Morimoto, K.; et al. Generation of B6-Ddx4(em1(CreERT2)Utr), a novel CreERT2 knock-in line, for germ cell lineage by CRISPR/Cas9. Genesis 2020, 58, e23367. [Google Scholar] [CrossRef]
- Sheldon, C.; Kessinger, C.W.; Sun, Y.; Kontaridis, M.I.; Ma, Q.Y.; Hammoud, S.S.; Gao, Z.B.; Zhang, H.; Lin, Z.Q. Myh6 promoter-driven Cre recombinase excises floxed DNA fragments in a subset of male germline cells. J. Mol. Cell Cardiol. 2023, 175, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Rassoulzadegan, M.; Magliano, M.; Cuzin, F. Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J. 2002, 21, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.; Lu, L.; Williams, S.A.; Stanley, P. Complex N-glycans are essential, but core 1 and 2 mucin O-glycans, O-fucose glycans, and NOTCH1 are dispensable, for mammalian spermatogenesis. Biol. Reprod. 2012, 86, 179. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Campioli, E.; Sottas, C.; Zirkin, B.; Papadopoulos, V. Amhr2-Cre-Mediated Global Tspo Knockout. J. Endocr. Soc. 2020, 4, bvaa001. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, Y.; Bao, J. Building RNA-protein germ granules: Insights from the multifaceted functions of DEAD-box helicase Vasa/Ddx4 in germline development. Cell Mol. Life Sci. 2022, 79, 4. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascenção, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene expression across mammalian organ development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.S.; Toyooka, Y.; Akasu, R.; Katoh-Fukui, Y.; Nakahara, Y.; Suzuki, R.; Yokoyama, M.; Noce, T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000, 14, 841–853. [Google Scholar] [CrossRef]
- Toyooka, Y.; Tsunekawa, N.; Takahashi, Y.; Matsui, Y.; Satoh, M.; Noce, T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 2000, 93, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Ma, W.; Huang, C.; Ding, J.; Cui, D.; Zhang, M. Expression Pattern of Mouse Vasa Homologue (MVH) in the Ovaries of C57BL/6 Female Mice. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2656–2663. [Google Scholar] [CrossRef]
- Gallardo, T.; Shirley, L.; John, G.B.; Castrillon, D.H. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 2007, 45, 413–417. [Google Scholar] [CrossRef]
- Hayashi, S.; McMahon, A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002, 244, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Brocard, J.; Mascrez, B.; LeMeur, M.; Metzger, D.; Chambon, P. Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 10887–10890. [Google Scholar] [CrossRef] [PubMed]
- Metzger, D.; Clifford, J.; Chiba, H.; Chambon, P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc. Natl. Acad. Sci. USA 1995, 92, 6991–6995. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Wagner, J.; Metzger, D.; Chambon, P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 1997, 237, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.Q.; Junker, J.P.; Gill, M.E.; Mueller, J.L.; van Oudenaarden, A.; Page, D.C. A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary. PLoS Genet. 2015, 11, e1005531. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Niu, C.M.; Xia, J.; Shen, X.Y.; Xia, M.M.; Hu, Y.Q.; Zheng, Y. Stimulated by retinoic acid gene 8 (Stra8) plays important roles in many stages of spermatogenesis. Asian J. Androl. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Sadate-Ngatchou, P.I.; Payne, C.J.; Dearth, A.T.; Braun, R.E. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 2008, 46, 738–742. [Google Scholar] [CrossRef]
- Bao, J.; Ma, H.Y.; Schuster, A.; Lin, Y.M.; Yan, W. Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1(lox/lox) and Stra8-iCre; Mov10l1(lox/Δ) mice. Genesis 2013, 51, 481–490. [Google Scholar] [CrossRef]
- Blanco, M.; El Khattabi, L. DOT1L regulates chromatin reorganization and gene expression during sperm differentiation. EMBO Rep. 2023, 24, e56316. [Google Scholar] [CrossRef]
- Feng, C.W.; Burnet, G. Identification of regulatory elements required for Stra8 expression in fetal ovarian germ cells of the mouse. Development 2021, 148, 194977. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hsu, P.J.; Xing, X.; Fang, J.; Lu, Z.; Zou, Q.; Zhang, K.J.; Zhang, X.; Zhou, Y.; Zhang, T.; et al. Mettl3-/Mettl14-mediated mRNA N(6)-methyladenosine modulates murine spermatogenesis. Cell Res. 2017, 27, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Salas, E.; Lanza, D.G.; Heaney, J.D.; Pangas, S.A. Generation of a novel Stra8-driven Cre recombinase strain for use in pre-meiotic germ cells in mice. Biol. Reprod. 2023, 109, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cheng, Y.; Zhu, Y.; Xu, C.; Li, Q.; Xing, X.; Li, W.; Zou, J.; Meng, L.; Azhar, M.; et al. Maternal NAT10 orchestrates oocyte meiotic cell-cycle progression and maturation in mice. Nat. Commun. 2023, 14, 3729. [Google Scholar] [CrossRef] [PubMed]
- Lemaire-Vieille, C.; Schulze, T.; Podevin-Dimster, V.; Follet, J.; Bailly, Y.; Blanquet-Grossard, F.; Decavel, J.-P.; Heinen, E.; Cesbron, J.-Y. Epithelial and endothelial expression of the green fluorescent protein reporter gene under the control of bovine prion protein (PrP) gene regulatory sequences in transgenic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 5422–5427. [Google Scholar] [CrossRef]
- Weber, P.; Schuler, M.; Gérard, C.; Mark, M.; Metzger, D.; Chambon, P. Temporally controlled site-specific mutagenesis in the germ cell lineage of the mouse testis. Biol. Reprod. 2003, 68, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Barton, S.C.; Surani, M.A. A molecular programme for the specification of germ cell fate in mice. Nature 2002, 418, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kimura, T.; Kurokawa, K.; Fujita, Y.; Abe, K.; Masuhara, M.; Yasunaga, T.; Ryo, A.; Yamamoto, M.; Nakano, T. Identification of PGC7, a new gene expressed specifically in preimplantation embryos and germ cells. Mech. Dev. 2002, 113, 91–94. [Google Scholar] [CrossRef]
- Payer, B.; Saitou, M.; Barton, S.C.; Thresher, R.; Dixon, J.P.C.; Zahn, D.; Colledge, W.H.; Carlton, M.B.L.; Nakano, T.; Surani, M.A. is a maternal effect gene required for normal early development in mice. Curr. Biol. 2003, 13, 2110–2117. [Google Scholar] [CrossRef]
- Hirota, T.; Ohta, H.; Shigeta, M.; Niwa, H.; Saitou, M. Drug-inducible gene recombination by the Dppa3-MER Cre MER transgene in the developmental cycle of the germ cell lineage in mice. Biol. Reprod. 2011, 85, 367–377. [Google Scholar] [CrossRef]
- Thelen, B.; Schipperges, V.; Knörlein, P.; Hummel, J.F.; Arnold, F.; Kupferschmid, L.; Klose, C.S.N.; Arnold, S.J.; Boerries, M.; Tanriver, Y. Eomes is sufficient to regulate IL-10 expression and cytotoxic effector molecules in murine CD4+ T cells. Front. Immunol. 2023, 14, 1058267. [Google Scholar] [CrossRef] [PubMed]
- Russ, A.P.; Wattler, S.; Colledge, W.H.; Aparicio, S.A.; Carlton, M.B.; Pearce, J.J.; Barton, S.C.; Surani, M.A.; Ryan, K.; Nehls, M.C.; et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature 2000, 404, 95–99. [Google Scholar] [CrossRef]
- Raab, S.; Klingenstein, M.; Möller, A.; Illing, A.; Tosic, J.; Breunig, M.; Kuales, G.; Linta, L.; Seufferlein, T.; Arnold, S.J. Reprogramming to pluripotency does not require transition through a primitive streak-like state. Sci. Rep. 2017, 7, 16543. [Google Scholar] [CrossRef]
- Sharma, M.; Srivastava, A.; Fairfield, H.E.; Bergstrom, D.; Flynn, W.F.; Braun, R.E. Identification of EOMES-expressing spermatogonial stem cells and their regulation by PLZF. Elife 2019, 8, e43352. [Google Scholar] [CrossRef]
- Legrand, J.M.; Hobbs, R.M. Defining Gene Function in Spermatogonial Stem Cells Through Conditional Knockout Approaches. In Spermatogonial Stem Cells: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2023; pp. 261–307. [Google Scholar]
- Sommer, L.; Ma, Q.; Anderson, D.J.J.M. Neuroscience, C. neurogenins, a novel family ofatonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol. Cell. Neurosci. 1996, 8, 221–241. [Google Scholar] [CrossRef]
- Yoshida, S.; Takakura, A.; Ohbo, K.; Abe, K.; Wakabayashi, J.; Yamamoto, M.; Suda, T.; Nabeshima, Y.I. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev. Biol. 2004, 269, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Sada, A.; Hasegawa, K.; Pin, P.H.; Saga, Y. NANOS2 acts downstream of glial cell line-derived neurotrophic factor signaling to suppress differentiation of spermatogonial stem cells. J. Stem Cells 2012, 30, 280–291. [Google Scholar] [CrossRef]
- Yoshida, S.; Sukeno, M.; Nakagawa, T.; Ohbo, K.; Nagamatsu, G.; Suda, T.; Nabeshima, Y.-i. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006, 133, 1495–1505. [Google Scholar] [CrossRef]
- Schonhoff, S.E.; Giel-Moloney, M.; Leiter, A.B. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 2004, 270, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.M.; Meikar, O.; Yadav, R.P.; Papaioannou, M.D.; Romero, Y.; Da Ros, M.; Herrera, P.L.; Toppari, J.; Nef, S.; Kotaja, N. Dicer is required for haploid male germ cell differentiation in mice. PLoS ONE 2011, 6, e24821. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, P.J. Blockade of pachytene piRNA biogenesis reveals a novel requirement for maintaining post-meiotic germline genome integrity. PLoS Genet. 2012, 8, e1003038. [Google Scholar] [CrossRef]
- Bai, S.; Cheng, L.; Zhang, Y.; Zhu, C.; Zhu, Z.; Zhu, R.; Cheng, C.Y.; Ye, L.; Zheng, K. A germline-specific role for the mTORC2 component Rictor in maintaining spermatogonial differentiation and intercellular adhesion in mouse testis. Mol. Hum. Reprod. 2018, 24, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhang, Y.; Wang, Z.-P.; Wang, X.-X.; Sun, T.-C.; Li, X.-Y.; Tang, J.-X.; Cheng, J.-M.; Li, J.; Chen, S.-R. EZH2 deletion promotes spermatogonial differentiation and apoptosis. Reproduction 2017, 154, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Hogarth, C.; Mitchell, D.; Griswold, M. Riding the spermatogenic wave: Profiling gene expression within neonatal germ and sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol. Reprod. 2014, 90, 108. [Google Scholar] [CrossRef] [PubMed]
- Lomelí, H.; Ramos-Mejía, V.; Gertsenstein, M.; Lobe, C.G.; Nagy, A. Targeted insertion of Cre recombinase into the TNAP gene: Excision in primordial germ cells. Genesis 2000, 26, 116–117. [Google Scholar] [CrossRef]
- Suzuki, H.; Tsuda, M.; Kiso, M.; Saga, Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Dev. Biol. 2008, 318, 133–142. [Google Scholar] [CrossRef]
- Ohinata, Y.; Payer, B.; O’Carroll, D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A.; et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005, 436, 207–213. [Google Scholar] [CrossRef]
- Chang, D.H.; Calame, K.L. The dynamic expression pattern of B lymphocyte induced maturation protein-1 (Blimp-1) during mouse embryonic development. Mech. Dev. 2002, 117, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.J.; Charatsi, I.; Joyner, C.J.; Koonce, C.H.; Morgan, M.; Islam, A.; Paterson, C.; Lejsek, E.; Arnold, S.J.; Kallies, A.; et al. Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development 2007, 134, 4335–4345. [Google Scholar] [CrossRef]
- Legrand, J.M.; Chan, A.-L.; La, H.M.; Rossello, F.J.; Änkö, M.-L.; Fuller-Pace, F.V.; Hobbs, R.M. DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia. Nat. Commun. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, S.-i.; Kobayashi, Y.; Shirakawa, T.; Watanabe, K.; Mizoguchi, K.; Hoshi, I.; Nakajima, K.; Nakabayashi, J.; Singh, S.; Dahl, A. Kmt2b conveys monovalent and bivalent H3K4me3 in mouse spermatogonial stem cells at germline and embryonic promoters. Development 2018, 145, dev169102. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Boens, S.; Winkler, C.; Szekér, K.; Verbinnen, I.; Van Eynde, A.; Fardilha, M.; Bollen, M. The protein phosphatase 1 regulator NIPP1 is essential for mammalian spermatogenesis. Sci. Rep. 2017, 7, 13364. [Google Scholar] [CrossRef] [PubMed]
- López, I.P.; Rodriguez-de la Rosa, L.; Pais, R.S.; Pineiro-Hermida, S.; Torrens, R.; Contreras, J.; Varela-Nieto, I.; Pichel, J.G. Differential organ phenotypes after postnatal Igf1r gene conditional deletion induced by tamoxifen in UBC-CreERT2; Igf1r fl/fl double transgenic mice. Transgenic Res. 2015, 24, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Riesterer, C.; Ayrall, A.-M.; Sablitzky, F.; Littlewood, T.D.; Reth, M. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 1996, 24, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Schöler, H.; Dressler, G.R.; Balling, R.; Rohdewohld, H.; Gruss, P. Oct-4: A germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990, 9, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Oatley, J.; Bardwell, V.J.; Zarkower, D. DMRT1 is required for mouse spermatogonial stem cell maintenance and replenishment. PLoS Genet. 2016, 12, e1006293. [Google Scholar] [CrossRef] [PubMed]
- Greder, L.V.; Gupta, S.; Li, S.N.; Abedin, M.J.; Sajini, A.; Segal, Y.; Slack, J.M.W.; Dutton, J.R. Brief Report: Analysis of Endogenous Oct4 Activation during Induced Pluripotent Stem Cell Reprogramming Using an Inducible Oct4 Lineage Label. Stem Cells 2012, 30, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Lengner, C.J.; Camargo, F.D.; Hochedlinger, K.; Welstead, G.G.; Zaidi, S.; Gokhale, S.; Scholer, H.R.; Tomilin, A.; Jaenisch, R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 2007, 1, 403–415. [Google Scholar] [CrossRef]
- de Vries, F.A.; de Boer, E.; van den Bosch, M.; Baarends, W.M.; Ooms, M.; Yuan, L.; Liu, J.-G.; van Zeeland, A.A.; Heyting, C.; Pastink, A. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 2005, 19, 1376–1389. [Google Scholar] [CrossRef]
- Chung, S.S.; Cuzin, F.; Rassoulzadegan, M.; Wolgemuth, D.J. Primary spermatocyte-specific Cre recombinase activity in transgenic mice. Transgenic Res. 2004, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Rasoulpour, R.J.; Boekelheide, K. The Sycp1-Cre Transgenic Mouse and Male Germ Cell Inhibition of NF-κB. J. Androl. 2006, 27, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Kraus, P.; Xing, X.; Lim, S.L.; Fun, M.E.; Sivakamasundari, V.; Yap, S.P.; Lee, H.; Karuturi, R.K.M.; Lufkin, T. Mouse strain specific gene expression differences for illumina microarray expression profiling in embryos. BMC Res. Notes 2012, 5, 232. [Google Scholar] [CrossRef]
- Al-Soudy, A.S.; Nakanishi, T.; Mizuno, S.; Hasegawa, Y.; Shawki, H.H.; Katoh, M.C.; Basha, W.A.; Ibrahim, A.E.; El-Shemy, H.A.; Iseki, H.; et al. Germline recombination in a novel Cre transgenic line, Prl3b1-Cre mouse. Genesis 2016, 54, 389–397. [Google Scholar] [CrossRef]
- Zou, Y.D.; Yao, H.H.; Li, J.C.; Zhang, K.; Li, Z.G. A novel WISP3 mutation in a Chinese patient with progressive pseudorheumatoid dysplasia. QJM Mon. J. Assoc. Physicians 2023, 116, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Cui, Y.; Fernando, C.; Kutz, W.E.; Warman, M.L. Normal growth and development in mice over-expressing the CCN family member WISP3. J. Cell Commun. Signal. 2009, 3, 105–113. [Google Scholar] [CrossRef]
- Hann, S.; Kvenvold, L.; Newby, B.N.; Hong, M.; Warman, M.L. A Wisp3 Cre-knockin allele produces efficient recombination in spermatocytes during early prophase of meiosis I. PLoS ONE 2013, 8, e75116. [Google Scholar] [CrossRef] [PubMed]
- Lester, W.C.; Johnson, T.; Hale, B.; Serra, N.; Elgart, B.; Wang, R.; Geyer, C.B.; Sperry, A.O. Aurora A Kinase (AURKA) is required for male germline maintenance and regulates sperm motility in the mouse. Biol. Reprod. 2021, 105, 1603–1616. [Google Scholar] [CrossRef]
- Romanienko, P.J.; Camerini-Otero, R.D. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 2000, 6, 975–987. [Google Scholar] [CrossRef]
- Pellegrini, M.; Claps, G.; Orlova, V.V.; Barrios, F.; Dolci, S.; Geremia, R.; Rossi, P.; Rossi, G.; Arnold, B.; Chavakis, T.; et al. Targeted JAM-C deletion in germ cells by Spo11-controlled Cre recombinase. J. Cell Sci. 2011, 124, 91–99. [Google Scholar] [CrossRef]
- Lyndaker, A.M.; Lim, P.X.; Mleczko, J.M.; Diggins, C.E.; Holloway, J.K.; Holmes, R.J.; Kan, R.; Schlafer, D.H.; Freire, R.; Cohen, P.E. Conditional inactivation of the DNA damage response gene Hus1 in mouse testis reveals separable roles for components of the RAD9-RAD1-HUS1 complex in meiotic chromosome maintenance. PLoS Genet. 2013, 9, e1003320. [Google Scholar] [CrossRef] [PubMed]
- Faieta, M.; Di Cecca, S.; de Rooij, D.G.; Luchetti, A.; Murdocca, M.; Di Giacomo, M.; Di Siena, S.; Pellegrini, M.; Rossi, P.; Barchi, M. A surge of late-occurring meiotic double-strand breaks rescues synapsis abnormalities in spermatocytes of mice with hypomorphic expression of SPO11. Chromosoma 2016, 125, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Wellard, S.R.; Zhang, Y.; Shults, C.; Zhao, X.; McKay, M.; Murray, S.A.; Jordan, P.W. Overlapping roles for PLK1 and Aurora A during meiotic centrosome biogenesis in mouse spermatocytes. EMBO Rep. 2021, 22, e51023. [Google Scholar] [CrossRef] [PubMed]
- Jakubiczka, S.; Schnieders, F.; Schmidtke, J. A bovine homologue of the human TSPY gene. Genomics 1993, 17, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Dechend, F.; Skawran, B.; Kunze, B.; Winking, H.; Weile, C.; Römer, I.; Hemberger, M.; Fundele, R.; Sharma, T.; et al. Silencing of the Y-chromosomal gene tspy during murine evolution. Mamm. Genome 2000, 11, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Manz, E.; Vogel, T.; Glatzel, P.; Schmidtke, J. Identification of an equine Y chromosome specific gene locus (eTSPY) with potential in preimplantation sex diagnosis. Theriogenology 1998, 1, 364. [Google Scholar] [CrossRef]
- Frank, S.; Thilo, D.; Joachim, A.; Tanja, V.; Martin, W.; Jörg, S. Testis-Specific Protein, Y-Encoded (TSPY) Expression in Testicular Tissues. Hum. Mol. Genet. 1996, 5, 1801–1807. [Google Scholar]
- Schubert, S.; Skawran, B.; Dechend, F.; Nayernia, K.; Meinhardt, A.; Nanda, I.; Schmid, M.; Engel, W.; Schmidtke, J. Generation and characterization of a transgenic mouse with a functional human TSPY. Biol. Reprod. 2003, 69, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Lau, Y.F. A Cre gene directed by a human TSPY promoter is specific for germ cells and neurons. Genesis 2005, 42, 263–275. [Google Scholar] [CrossRef]
- Hecht, N.B.; Bower, P.A.; Waters, S.H.; Yelick, P.C.; Distel, R.J. Evidence for haploid expression of mouse testicular genes. Exp. Cell Res. 1986, 164, 183–190. [Google Scholar] [CrossRef]
- Peschon, J.J.; Behringer, R.R.; Palmiter, R.D.; Brinster, R.L. Expression of mouse protamine 1 genes in transgenic mice. Ann. N. Y. Acad. Sci. 1989, 564, 186–197. [Google Scholar] [CrossRef]
- Peschon, J.J.; Behringer, R.R.; Brinster, R.L.; Palmiter, R.D. Spermatid-specific expression of protamine 1 in transgenic mice. Proc. Natl. Acad. Sci. USA 1987, 84, 5316–5319. [Google Scholar] [CrossRef] [PubMed]
- Behringer, R.R.; Peschon, J.J.; Messing, A.; Gartside, C.L.; Hauschka, S.D.; Palmiter, R.D.; Brinster, R.L. Heart and bone tumors in transgenic mice. Proc. Natl. Acad. Sci. USA 1988, 85, 2648–2652. [Google Scholar] [CrossRef]
- Schmidt, E.E.; Taylor, D.S.; Prigge, J.R.; Barnett, S.; Capecchi, M.R. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc. Natl. Acad. Sci. USA 2000, 97, 13702–13707. [Google Scholar] [CrossRef] [PubMed]
- Gobé, C.; Ialy-Radio, C. Generation and Characterization of a Transgenic Mouse That Specifically Expresses the Cre Recombinase in Spermatids. Genes 2023, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Palanisamy, V. Horizontal transfer of RNAs: Exosomes as mediators of intercellular communication. Wiley Interdiscip. Rev. RNA 2012, 3, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Liang, G.; Tu, Z.; Chen, D.; Wang, H. Generation of Elf5-Cre knockin mouse strain for trophoblast-specific gene manipulation. Genesis 2018, 56, e23101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ng, A.Y.; Tymms, M.J.; Jermiin, L.S.; Seth, A.K.; Thomas, R.S.; Kola, I. A novel transcription factor, ELF5, belongs to the ELF subfamily of ETS genes and maps to human chromosome 11p13–15, a region subject to LOH and rearrangement in human carcinoma cell lines. Oncogene 1998, 17, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, P.; Kas, K.; Dube, A.; Gu, X.; Grall, F.; Thamrongsak, U.; Akbarali, Y.; Finger, E.; Boltax, J.; Endress, G. Characterization of ESE-2, a novel ESE-1-related Ets transcription factor that is restricted to glandular epithelium and differentiated keratinocytes. J. Biol. Chem. 1999, 274, 29439–29452. [Google Scholar] [CrossRef]
- Sada, A.; Suzuki, A.; Suzuki, H.; Saga, Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 2009, 325, 1394–1398. [Google Scholar] [CrossRef]
- Saga, Y. Function of Nanos2 in the male germ cell lineage in mice. Cell Mol. Life Sci. 2010, 67, 3815–3822. [Google Scholar] [CrossRef]
- Tsuda, M.; Sasaoka, Y.; Kiso, M.; Abe, K.; Haraguchi, S.; Kobayashi, S.; Saga, Y. Conserved role of nanos proteins in germ cell development. Science 2003, 301, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, J.A.t.; Uoon Park, K.; Reh, T.A. Blimp1 (Prdm1) prevents re-specification of photoreceptors into retinal bipolar cells by restricting competence. Dev. Biol. 2013, 384, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Lin, J.; Li, X.; Li, S.; Zhou, H.; Araki, Y.; Lan, Z.J. Postnatal male germ-cell expression of cre recombinase in Tex101-iCre transgenic mice. Genesis 2010, 48, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lan, Z.J.; Li, X.; Lin, J.; Lei, Z. Role of postnatal expression of fgfr1 and fgfr2 in testicular germ cells on spermatogenesis and fertility in mice. J. Reprod. Infertil. 2014, 15, 122–133. [Google Scholar] [PubMed]
- Hara, K.; Nakagawa, T.; Enomoto, H.; Suzuki, M.; Yamamoto, M.; Simons, B.D.; Yoshida, S. Mouse Spermatogenic Stem Cells Continually Interconvert between Equipotent Singly Isolated and Syncytial States. Cell Stem Cell 2014, 14, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.K.; Hoshi, M.; Jain, S. Stage specific requirement of Gfrα1 in the ureteric epithelium during kidney development. Mech. Dev. 2013, 130, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Ruzankina, Y.; Pinzon-Guzman, C.; Asare, A.; Ong, T.; Pontano, L.; Cotsarelis, G.; Zediak, V.P.; Velez, M.; Bhandoola, A.; Brown, E. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 2007, 1, 113–126. [Google Scholar] [CrossRef]
- Seibler, J.; Zevnik, B.; Küter-Luks, B.; Andreas, S.; Kern, H.; Hennek, T.; Rode, A.; Heimann, C.; Faust, N.; Kauselmann, G. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 2003, 31, e12. [Google Scholar] [CrossRef]
- Nelson, R.D.; Stricklett, P.; Gustafson, C.; Stevens, A.; Ausiello, D.; Brown, D.; Kohan, D.E. Expression of an AQP2 Cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am. J. Physiol. 1998, 275, C216–C226. [Google Scholar] [CrossRef]
- Inselman, A.L.; Nakamura, N.; Brown, P.R.; Willis, W.D.; Goulding, E.H.; Eddy, E.M. Heat shock protein 2 promoter drives Cre expression in spermatocytes of transgenic mice. Genesis 2010, 48, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Rupik, W.; Stawierej, A.; Stolarczyk, I.; Widłak, W. Promoter of the heat shock testis-specific Hsp70.2/Hst70 gene is active in nervous system during embryonic development of mice. Anat. Embryol. 2006, 211, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Haruna, Y.; Miyazaki, J.; Okabe, M.; Nakanishi, Y. Spermatocyte-specific gene excision by targeted expression of Cre recombinase. Biochem. Biophys. Res. Commun. 2000, 272, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, B.; Schmidt, J.V.; Truong, T.; Rancourt, D.; van der Hoorn, F.A. Germ cell specific promoter drives ectopic transgene expression during embryogenesis. Mol. Reprod. Dev. Inc. Gamete Res. 2001, 59, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.A.; Xu, X.; Li, J.L.; Xu, X.; Hu, G.; Brown, P.; Willson, C.; Kirsanov, O.; Geyer, C.; Huang, C.L.; et al. WNK1 is required during male pachynema to sustain fertility. iScience 2023, 26, 107616. [Google Scholar] [CrossRef] [PubMed]
- Winuthayanon, W.; Hewitt, S.C.; Orvis, G.D.; Behringer, R.R.; Korach, K.S. Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc. Natl. Acad. Sci. USA 2010, 107, 19272–19277. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, I.; Eriksson, B.; Eriksson, M.; Holmberg, D. Transgenic Cre recombinase expression in germ cells and early embryogenesis directs homogeneous and ubiquitous deletion of loxP-flanked gene segments. FEBS Lett. 1998, 438, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.I.; Yoon, S.P.; Choi, J.M.; Kim, S.S.; Lee, Y.D.; Birnbaumer, L.; Suh-Kim, H. Simultaneous deletion of floxed genes mediated by CaMKIIα-Cre in the brain and in male germ cells: Application to conditional and conventional disruption of Goα. Exp. Mol. Med. 2014, 46, e93. [Google Scholar] [CrossRef] [PubMed]
- Tsien, J.Z.; Chen, D.F.; Gerber, D.; Tom, C.; Mercer, E.H.; Anderson, D.J.; Mayford, M.; Kandel, E.R.; Tonegawa, S. Subregion-and cell type–restricted gene knockout in mouse brain. Cell 1996, 87, 1317–1326. [Google Scholar] [CrossRef]
- Rempe, D.; Vangeison, G.; Hamilton, J.; Li, Y.; Jepson, M.; Federoff, H. Synapsin I Cre transgene expression in male mice produces germline recombination in progeny. Genesis 2006, 44, 44–49. [Google Scholar] [CrossRef]
- Zhu, Y.; Romero, M.I.; Ghosh, P.; Ye, Z.; Charnay, P.; Rushing, E.J.; Marth, J.D.; Parada, L.F. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001, 15, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Larney, C.; Bailey, T.L.; Koopman, P. Switching on sex: Transcriptional regulation of the testis-determining gene. Development 2014, 141, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yokouchi, K.; Yoshida, K.; Kano, K.; Naito, K.; Miyazaki, J.I.; Tojo, H. Investigation of the fate of Sry-expressing cells using an in vivo Cre/loxP system. Dev. Growth Differ. 2006, 48, 41–47. [Google Scholar] [CrossRef]
- Ito, M.; Yokouchi, K.; Naito, K.; Endo, H.; Hakamata, Y.; Miyazaki, J.i.; Tojo, H. In vitro Cre/loxP system in cells from developing gonads: Investigation of the Sry promoter. Dev. Growth Differ. 2002, 44, 549–557. [Google Scholar] [CrossRef]
- Sargent, K.M.; McFee, R.M.; Spuri Gomes, R.; Cupp, A.S. Vascular endothelial growth factor A: Just one of multiple mechanisms for sex-specific vascular development within the testis? J. Endocrinol. 2015, 227, R31–R50. [Google Scholar] [CrossRef]
- Akiyama, H.; Kim, J.-E.; Nakashima, K.; Balmes, G.; Iwai, N.; Deng, J.M.; Zhang, Z.; Martin, J.F.; Behringer, R.R.; Nakamura, T. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 2005, 102, 14665–14670. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tagami, A.; Maekawa, M.; Nagai, A. The conditional deletion of steroidogenic factor 1 (Nr5a1) in Sox9-Cre mice compromises testis differentiation. Sci. Rep. 2021, 11, 4486. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, W.; Jiang, K.; Yu, Z.; Huang, H.; Wang, F.; Zhou, B.; Chen, T. Embryonic attenuated Wnt/β-catenin signaling defines niche location and long-term stem cell fate in hair follicle. eLife 2015, 4, e10567. [Google Scholar] [CrossRef]
- Buaas, F.W.; Gardiner, J.R.; Clayton, S.; Val, P.; Swain, A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development 2012, 139, 4561. [Google Scholar] [CrossRef]
- O’Hara, L.; York, J.P.; Zhang, P.; Smith, L.B. Targeting of GFP-Cre to the mouse Cyp11a1 locus both drives cre recombinase expression in steroidogenic cells and permits generation of Cyp11a1 knock out mice. PLoS ONE 2014, 9, e84541. [Google Scholar] [CrossRef]
- Darbey, A.; Rebourcet, D.; Curley, M.; Kilcoyne, K.; Jeffery, N.; Reed, N.; Milne, L.; Roesl, C.; Brown, P.; Smith, L.B. A comparison of in vivo viral targeting systems identifies adeno-associated virus serotype 9 (AAV9) as an effective vector for genetic manipulation of Leydig cells in adult mice. Andrology 2021, 9, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Gannon, A.L.; Darbey, A.L.; Chensee, G.; Lawrence, B.M. A Novel Model Using AAV9-Cre to Knockout Adult Leydig Cell Gene Expression Reveals a Physiological Role of Glucocorticoid Receptor Signalling in Leydig Cell Function. Int. J. Mol. Sci. 2022, 23, 5015. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, J.; Luo, Y.; Zou, D.; Han, R.; Zhong, S.; Zhao, Q.; Mang, X.; Li, M.; Si, Y.; et al. Panoramic transcriptome analysis and functional screening of long noncoding RNAs in mouse spermatogenesis. Genome Res. 2021, 31, 13–26. [Google Scholar] [CrossRef]
- Mullen, R.D.; Behringer, R.R. Molecular genetics of Müllerian duct formation, regression and differentiation. Sex. Dev. 2014, 8, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Jamin, S.P.; Arango, N.A.; Mishina, Y.; Hanks, M.C.; Behringer, R.R. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat. Genet. 2002, 32, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Sargent, K.M.; Lu, N.; Clopton, D.T.; Pohlmeier, W.E.; Brauer, V.M.; Ferrara, N.; Silversides, D.W.; Cupp, A.S. Loss of vascular endothelial growth factor A (VEGFA) isoforms in granulosa cells using pDmrt-1-Cre or Amhr2-Cre reduces fertility by arresting follicular development and by reducing litter size in female mice. PLoS ONE 2015, 10, e0116332. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lin, H.-Y.; Yeh, S.-D.; Yu, I.-C.; Wang, R.-S.; Chen, Y.-T.; Zhang, C.; Altuwaijri, S.; Chen, L.-M.; Chuang, K.-H. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine 2007, 32, 96–106. [Google Scholar] [CrossRef]
- Fang, X.; Ni, N.; Gao, Y.; Vincent, D.F.; Bartholin, L.; Li, Q.L. A novel mouse model of testicular granulosa cell tumors. Mol. Hum. Reprod. 2018, 24, 343–356. [Google Scholar] [CrossRef]
- Chauvin, M.; Meinsohn, M.-C.; Dasari, S.; May, P.; Iyer, S.; Nguyen, N.; Oliva, E.; Lucchini, Z.; Nagykery, N.; Kashiwagi, A. Cancer-associated mesothelial cells are regulated by the anti-Müllerian hormone axis. Cell Rep. 2023, 42, 112730. [Google Scholar] [CrossRef]
- Shami, A.N.; Zheng, X.; Munyoki, S.K.; Ma, Q.; Manske, G.L.; Green, C.D.; Sukhwani, M.; Orwig, K.E.; Li, J.Z.; Hammoud, S.S. Single-Cell RNA Sequencing of Human, Macaque, and Mouse Testes Uncovers Conserved and Divergent Features of Mammalian Spermatogenesis. Dev. Cell 2020, 54, 529–547.e12. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Q.W.; Sun, Y. The emerging role of extracellular vesicles in the testis. Hum. Reprod. 2023, 38, 334–351. [Google Scholar] [CrossRef]
- Abe, S.I. Behavior and Functional Roles of CD34+ Mesenchymal Cells in Mammalian Testes. Int. J. Mol. Sci. 2022, 23, 9585. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Brown, P.R.; Willis, W.B.; Eddy, E.M. Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance. Endocrinology 2014, 155, 4964–4974. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.B.; Deng, K.Y.; Rishniw, M.; Ji, G.; Kotlikoff, M.I. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol. Genom. 2002, 10, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Willis, W.D.; Eddy, E.M. Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc. Natl. Acad. Sci. USA 2016, 113, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Man, Y. Inactivation of Cops5 in Smooth Muscle Cells Causes Abnormal Reproductive Hormone Homeostasis and Development in Mice. Endocrinology 2023, 164, bqad062. [Google Scholar] [CrossRef] [PubMed]
- Bulut, G.B.; Alencar, G.F.; Owsiany, K.M.; Nguyen, A.T.; Karnewar, S.; Haskins, R.M.; Waller, L.K.; Cherepanova, O.A.; Deaton, R.A.; Shankman, L.S.; et al. KLF4 (Kruppel-Like Factor 4)-Dependent Perivascular Plasticity Contributes to Adipose Tissue inflammation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 284–301. [Google Scholar] [CrossRef]
- Eddy, E.M.; Chen, L.Y. Reply to Chen and Liu: Role of GDNF from peritubular myoid cells in the testis stem cell niche. Proc. Natl. Acad. Sci. USA 2016, 113, E2353. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Aliberti, J.; Graemmel, P.; Sunshine, M.J.; Kreutzberg, G.W.; Sher, A.; Littman, D.R. Analysis of Fractalkine Receptor CX3CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Mol. Cell. Biol. 2000, 20, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Kisanuki, Y.Y.; Hammer, R.E.; Miyazaki, J.-i.; Williams, S.C.; Richardson, J.A.; Yanagisawa, M. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001, 230, 230–242. [Google Scholar] [CrossRef]
- O’Hara, L.; Smith, L.B. Androgen receptor signalling in Vascular Endothelial cells is dispensable for spermatogenesis and male fertility. BMC Res. Notes 2012, 5, 16. [Google Scholar] [CrossRef]
- Meng, R.; Cai, W.K.; Xu, W.M.; Feng, Q.; Wang, P.; Huang, Y.H.; Fan, Y.X.; Zhou, T.; Yang, Q.; Li, Z.R.; et al. Generation and identification of endothelial-specific Hrh2 knockout mice. Transgenic Res. 2021, 30, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Kilani, B.; Gourdou-Latyszenok, V.; Guy, A.; Bats, M.L.; Peghaire, C.; Parrens, M.; Renault, M.A.; Duplàa, C.; Villeval, J.L.; Rautou, P.E.; et al. Comparison of endothelial promoter efficiency and specificity in mice reveals a subset of Pdgfb-positive hematopoietic cells. J. Thromb. Haemost. JTH 2019, 17, 827–840. [Google Scholar] [CrossRef]
- Rumianek, A.N.; Davies, B.; Channon, K.M.; Greaves, D.R.; Purvis, G.S.D. A Human CD68 Promoter-Driven Inducible Cre-Recombinase Mouse Line Allows Specific Targeting of Tissue Resident Macrophages. Front. Immunol. 2022, 13, 918636. [Google Scholar] [CrossRef] [PubMed]
- Rumianek, A.N. New Methods to Study Macrophage Biology In Vitro and In Vivo; University of Oxford: Oxford, UK, 2022. [Google Scholar]
- Hashimoto, M.; Kimura, S.; Kanno, C.; Yanagawa, Y.; Watanabe, T.; Okabe, J.; Takahashi, E.; Nagano, M.; Kitamura, H. Macrophage ubiquitin-specific protease 2 contributes to motility, hyperactivation, capacitation, and in vitro fertilization activity of mouse sperm. Cell Mol. Life Sci. 2021, 78, 2929–2948. [Google Scholar] [CrossRef]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A. New insights into epididymal biology and function. Human Reprod. Update 2009, 15, 213–227. [Google Scholar] [CrossRef]
- Jalkanen, J.; Huhtaniemi, I.; Poutanen, M. Discovery and characterization of new epididymis-specific beta-defensins in mice. Biochim. Biophys. Acta BBA—Gene Struct. Expr. 2005, 1730, 22–30. [Google Scholar] [CrossRef]
- Björkgren, I.; Alvarez, L.; Blank, N.; Balbach, M.; Turunen, H.; Laajala, T.D.; Toivanen, J.; Krutskikh, A.; Wahlberg, N.; Huhtaniemi, I.; et al. Targeted inactivation of the mouse epididymal beta-defensin 41 alters sperm flagellar beat pattern and zona pellucida binding. Mol. Cell. Endocrinol. 2016, 427, 143–154. [Google Scholar] [CrossRef]
- Björkgren, I.; Saastamoinen, L.; Krutskikh, A.; Huhtaniemi, I.; Poutanen, M.; Sipilä, P. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS ONE 2012, 7, e38457. [Google Scholar] [CrossRef]
- Björkgren, I.; Gylling, H.; Turunen, H.; Huhtaniemi, I.; Strauss, L.; Poutanen, M.; Sipilä, P. Imbalanced lipid homeostasis in the conditional Dicer1 knockout mouse epididymis causes instability of the sperm membrane. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, J.; Pujianto, D.A.; Sipilä, P.; Huhtaniemi, I.; Poutanen, M. Discovery in silico and characterization in vitro of novel genes exclusively expressed in the mouse epididymis. Mol. Endocrinol. 2003, 17, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- Krutskikh, A.; Poliandri, A.; Cabrera-Sharp, V.; Dacheux, J.L.; Poutanen, M.; Huhtaniemi, I. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 4198–4209. [Google Scholar] [CrossRef] [PubMed]
- Krutskikh, A.; De Gendt, K.; Sharp, V.; Verhoeven, G.; Poutanen, M.; Huhtaniemi, I. Targeted Inactivation of the Androgen Receptor Gene in Murine Proximal Epididymis Causes Epithelial Hypotrophy and Obstructive Azoospermia. Endocrinology 2011, 152, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.; Gibbs, G.M.; Merriner, D.J.; Kerr, J.B.; O’Bryan, M.K. Cysteine-rich secretory proteins are not exclusively expressed in the male reproductive tract. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 3313–3323. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.A.; Wu, L.; Bang, H.J.; Jelinsky, S.A.; Roberts, K.P.; Turner, T.T.; Kopf, G.S.; Johnston, D.S. Identification of rat cysteine-rich secretory protein 4 (Crisp4) as the ortholog to human CRISP1 and mouse Crisp4. Biol. Reprod. 2006, 74, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Turunen, H.T.; Sipilä, P.; Krutskikh, A.; Toivanen, J.; Mankonen, H.; Hämäläinen, V.; Björkgren, I.; Huhtaniemi, I.; Poutanen, M. Loss of cysteine-rich secretory protein 4 (Crisp4) leads to deficiency in sperm-zona pellucida interaction in mice. Biol. Reprod. 2012, 86, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.W.; Carvajal, G.; Curci, L.; Gonzalez, S.N.; Cuasnicu, P.S. Relevance of CRISP proteins for epididymal physiology, fertilization, and fertility. Andrology 2019, 7, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, G.; Brukman, N.G.; Muñoz, M.W.; Battistone, M.A.; Guazzone, V.A.; Ikawa, M.; Haruhiko, M.; Lustig, L.; Breton, S.; Cuasnicu, P.S. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Sci. Rep. 2018, 8, 17531. [Google Scholar] [CrossRef]
- Suzuki, K.; Lareyre, J.J.; Sánchez, D.; Gutierrez, G.; Araki, Y.; Matusik, R.J.; Orgebin-Crist, M.C. Molecular evolution of epididymal lipocalin genes localized on mouse chromosome 2. Gene 2004, 339, 49–59. [Google Scholar] [CrossRef]
- Lareyre, J.J.; Reid, K.; Nelson, C.; Kasper, S.; Rennie, P.S.; Orgebin-Crist, M.C.; Matusik, R.J. Characterization of an androgen-specific response region within the 5′ flanking region of the murine epididymal retinoic acid binding protein gene. Biol. Reprod. 2000, 63, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yu, X.; Chaurand, P.; Araki, Y.; Lareyre, J.J.; Caprioli, R.M.; Orgebin-Crist, M.C.; Matusik, R.J. Epididymis-specific lipocalin promoters. Asian J. Androl. 2007, 9, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, D.; Zhu, H.; Sun, X.; Xiao, Y.; Lin, Z.; Zhang, A.; Ye, C.; Gao, J. Deficiency for Lcn8 causes epididymal sperm maturation defects in mice. Biochem. Biophys. Res. Commun. 2021, 548, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Fujihara, Y. CRISPR/Cas9-mediated disruption of lipocalins, Ly6g5b, and Ly6g5c causes male subfertility in mice. Andrology 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Xu, J.; Ma, W.; Liu, Q.; Han, J.; Yao, G.; Huang, X.; Zhang, Y. Lcn5 promoter directs the region-specific expression of cre recombinase in caput epididymidis of transgenic mice. Biol. Reprod. 2013, 88, 71. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yao, G.; Ru, Y.; Xie, S. Expression of tamoxifen-inducible CRE recombinase in Lcn5-CreER(T2) transgenic mouse caput epididymis. Mol. Reprod. Dev. 2017, 84, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.Q.; Dou, Z.L.; Wang, X.; Zhang, K.Y.; Chen, H.; Gao, J.G.; Sun, X.Y. Epididymal initial segment-specific Cre recombinase activity in Lcn8-Cre knock-in mice. Mol. Biol. Rep. 2021, 48, 6015–6023. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.Q.; Wang, X.; Dou, Z.L.; Zhang, K.Y.; Liu, X.G.; Gao, J.G.; Sun, X.Y. A novel mouse line with epididymal initial segment-specific expression of Cre recombinase driven by the endogenous promoter. PLoS ONE 2021, 16, e0254802. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Qiao, F.; Chen, Y.; Chan, D.Y.L.; Yim, H.C.H.; Fok, K.L.; Chen, H. SARS-CoV-2 and male infertility: From short- to long-term impacts. J. Endocrinol. Investig. 2023, 46, 1491–1507. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Huang, J.; Powell, W.C.; Zhang, J.; Matusik, R.J.; Sangiorgi, F.O.; Maxson, R.E.; Sucov, H.M.; Roy-Burman, P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech. Dev. 2001, 101, 61–69. [Google Scholar] [CrossRef]
- Jin, C.; McKeehan, K.; Wang, F. Transgenic mouse with high Cre recombinase activity in all prostate lobes, seminal vesicle, and ductus deferens. Prostate 2003, 57, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Archambeault, D.R.; Matzuk, M.M. Disrupting the male germ line to find infertility and contraception targets. In Annales D’endocrinologie; Elsevier Masson: Amsterdam, The Netherlands, 2014; pp. 101–108. [Google Scholar]
- Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999, 21, 70–71. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, Y.; Li, Y.; Pu, W.; Huang, X.; Tian, X.; Wang, Y.; Zhang, H.; Liu, Q.; Zhang, L. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat. Med. 2017, 23, 1488–1498. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, P.; Ma, C.; Chen, C.; Liang, M.; Dong, S.; Chen, H.; Zhang, X. Unlocking Genetic Mysteries during the Epic Sperm Journey toward Fertilization: Further Expanding Cre Mouse Lines. Biomolecules 2024, 14, 529. https://doi.org/10.3390/biom14050529
Dai P, Ma C, Chen C, Liang M, Dong S, Chen H, Zhang X. Unlocking Genetic Mysteries during the Epic Sperm Journey toward Fertilization: Further Expanding Cre Mouse Lines. Biomolecules. 2024; 14(5):529. https://doi.org/10.3390/biom14050529
Chicago/Turabian StyleDai, Pengyuan, Chaoye Ma, Chen Chen, Min Liang, Shijue Dong, Hao Chen, and Xiaoning Zhang. 2024. "Unlocking Genetic Mysteries during the Epic Sperm Journey toward Fertilization: Further Expanding Cre Mouse Lines" Biomolecules 14, no. 5: 529. https://doi.org/10.3390/biom14050529
APA StyleDai, P., Ma, C., Chen, C., Liang, M., Dong, S., Chen, H., & Zhang, X. (2024). Unlocking Genetic Mysteries during the Epic Sperm Journey toward Fertilization: Further Expanding Cre Mouse Lines. Biomolecules, 14(5), 529. https://doi.org/10.3390/biom14050529






