Over 30 Years of DiI Use for Human Neuroanatomical Tract Tracing: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
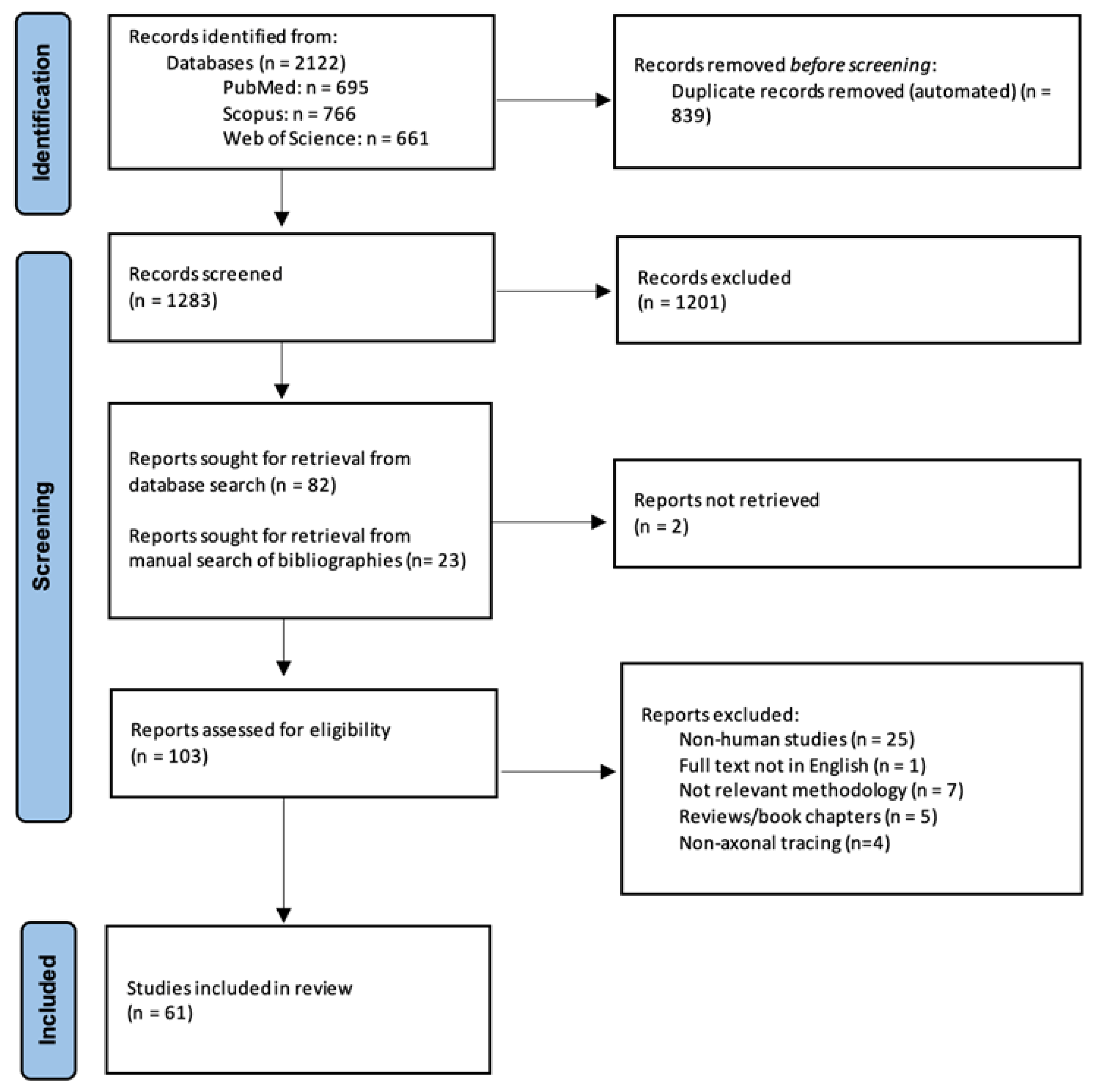
3. Results
3.1. Study Selection
3.2. Classification of Included Studies
3.3. Unique Applications
4. Discussion
4.1. Overview
4.2. Initial Application
4.3. Protocols for Carbocyanine Tract Tracing
4.3.1. Basic Methodological Steps
- Step 1: Tissue preparation for dye application
- Step 2: Dye application
- Step 3: Dye incubation
- Step 4: Tissue preparation/sectioning, counterstaining, and microscopy
4.3.2. Gastrointestinal Tract Plexuses: Methodological Considerations
4.4. Investigations Aimed at Optimizing or Broadening the Scope of Carbocyanine Tract Tracing
4.4.1. Use of Archival Specimens
4.4.2. Tracer Re-application
4.4.3. Delayed-Fixation Method
4.4.4. Application of Electrical Fields
4.4.5. Photoconversion of Labeled Tissues
4.4.6. Optical Clearing in DiI Tracing Applications
4.4.7. Double-Labeling Methods: Combination of DiI with Immunohistochemistry
4.4.8. Particle-Mediated Labeling
4.5. Limitations of the Present Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Database: PubMed Date of last search: 18 March 2024 Number of results: 695 Algorithm used: ((“Di-I”[All Fields] OR “DiI”[All Fields] OR “1 1 dioctadecyl 3 3 3 3 tetramethylindocarbocyanine perchlorate”[All Fields] OR “diic18 3”[All Fields] OR “D 282”[All Fields] OR “FAST DiI”[All Fields]) AND ((“neuron*”[All Fields] OR “axon*”[All Fields] OR “nerv*”[All Fields] OR (“brain”[MeSH Terms] OR “brain”[All Fields] OR “brains”[All Fields]) OR (“spine”[MeSH Terms] OR “spine”[All Fields] OR “spines”[All Fields]) OR “plexus”[All Fields]) AND “trac*”[All Fields])) Filter: English language |
| Database: Scopus Date of last search: 18 March 2024 Number of results: 766 Algorithm used: ((TITLE-ABS-KEY (“Di-I” OR “DiI” OR “1 1 dioctadecyl 3 3 3 3 tetramethylindocarbocyanine perchlorate” OR “diic18 3” OR “D 282” OR “FAST DiI”)) AND (TITLE-ABS-KEY (“neuron*” OR “axon*” OR “nerv*” OR “brain*” OR “spine*” OR “plexus”) AND TITLE-ABS-KEY (“trac*”))) AND (LIMIT-TO (LANGUAGE, “English”)) |
| Database: Web of Science Date of last search: 18 March 2024 Number of results: 661 Algorithm used: ((ALL = (“Di-I” OR “DiI” OR “1 1 dioctadecyl 3 3 3 3 tetramethylindocarbocyanine perchlorate” OR “diic18 3” OR “D 282” OR “FAST DiI”)) AND ALL = (((“neuron*” OR “axon*” OR “nerv*” OR “brain*” OR “spine*” OR “plexus”) AND “trac*”))) Filter: English language |
References
- Lanciego, J.L.; Wouterlood, F.G. Neuroanatomical Tract-Tracing Techniques That Did Go Viral. Brain Struct. Funct. 2020, 225, 1193–1224. [Google Scholar] [CrossRef] [PubMed]
- Rushmore, R.J.; Bouix, S.; Kubicki, M.; Rathi, Y.; Yeterian, E.H.; Makris, N. How Human Is Human Connectional Neuroanatomy? Front. Neuroanat. 2020, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Axer, M.; Amunts, K. Scale Matters: The Nested Human Connectome. Science 2022, 378, 500–504. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.J.; Daducci, A.; Wassermann, D.; Lenglet, C. Advances in Computational and Statistical Diffusion MRI. NMR Biomed. 2019, 32, e3805. [Google Scholar] [CrossRef]
- Charvet, C.J. Closing the Gap from Transcription to the Structural Connectome Enhances the Study of Connections in the Human Brain. Dev. Dyn. 2020, 249, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J. Erleichterung der makroskopischen Praeparation des Gehirns durch den Gefrierprozess. Schweiz. Arch. Neurol. Psychiatr. 1935, 36, 247–256. [Google Scholar]
- Dziedzic, T.A.; Balasa, A.; Jeżewski, M.P.; Michałowski, Ł.; Marchel, A. White Matter Dissection with the Klingler Technique: A Literature Review. Brain Struct. Funct. 2021, 226, 13–47. [Google Scholar] [CrossRef] [PubMed]
- Komaitis, S.; Skandalakis, G.P.; Kalyvas, A.V.; Drosos, E.; Lani, E.; Emelifeonwu, J.; Liakos, F.; Piagkou, M.; Kalamatianos, T.; Stranjalis, G.; et al. Dorsal Component of the Superior Longitudinal Fasciculus Revisited: Novel Insights from a Focused Fiber Dissection Study. J. Neurosurg. 2019, 132, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Koutsarnakis, C.; Kalyvas, A.V.; Skandalakis, G.P.; Karavasilis, E.; Christidi, F.; Komaitis, S.; Velonakis, G.; Liakos, F.; Emelifeonwu, J.; Giavri, Z.; et al. Sledge Runner Fasciculus: Anatomic Architecture and Tractographic Morphology. Brain Struct. Funct. 2019, 224, 1051–1066. [Google Scholar] [CrossRef]
- Komaitis, S.; Kalyvas, A.V.; Skandalakis, G.P.; Drosos, E.; Lani, E.; Liouta, E.; Liakos, F.; Kalamatianos, T.; Piagkou, M.; Emelifeonwu, J.A.; et al. The Frontal Longitudinal System as Revealed through the Fiber Microdissection Technique: Structural Evidence Underpinning the Direct Connectivity of the Prefrontal-Premotor Circuitry. J. Neurosurg. 2019, 133, 1503–1515. [Google Scholar] [CrossRef]
- Yendiki, A.; Aggarwal, M.; Axer, M.; Howard, A.F.D.; van Walsum, A.-M.v.C.; Haber, S.N. Post Mortem Mapping of Connectional Anatomy for the Validation of Diffusion MRI. NeuroImage 2022, 256, 119146. [Google Scholar] [CrossRef]
- Mollink, J.; Kleinnijenhuis, M.; Cappellen van Walsum, A.-M.v.; Sotiropoulos, S.N.; Cottaar, M.; Mirfin, C.; Heinrich, M.P.; Jenkinson, M.; Pallebage-Gamarallage, M.; Ansorge, O.; et al. Evaluating Fibre Orientation Dispersion in White Matter: Comparison of Diffusion MRI, Histology and Polarized Light Imaging. Neuroimage 2017, 157, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Zeineh, M.M.; Palomero-Gallagher, N.; Axer, M.; Gräßel, D.; Goubran, M.; Wree, A.; Woods, R.; Amunts, K.; Zilles, K. Direct Visualization and Mapping of the Spatial Course of Fiber Tracts at Microscopic Resolution in the Human Hippocampus. Cereb. Cortex 2017, 27, 1779–1794. [Google Scholar] [CrossRef]
- Schmitz, D.; Muenzing, S.E.A.; Schober, M.; Schubert, N.; Minnerop, M.; Lippert, T.; Amunts, K.; Axer, M. Derivation of Fiber Orientations from Oblique Views through Human Brain Sections in 3D-Polarized Light Imaging. Front. Neuroanat. 2018, 12, 75. [Google Scholar] [CrossRef]
- Axer, M.; Amunts, K.; Grässel, D.; Palm, C.; Dammers, J.; Axer, H.; Pietrzyk, U.; Zilles, K. A Novel Approach to the Human Connectome: Ultra-High Resolution Mapping of Fiber Tracts in the Brain. Neuroimage 2011, 54, 1091–1101. [Google Scholar] [CrossRef]
- Heilingoetter, C.L.; Jensen, M.B. Histological Methods for Ex Vivo Axon Tracing: A Systematic Review. Neurol. Res. 2016, 38, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.R.; Aigner, M.; Denk, M.; Heinzl, H.; Burian, M.; Mayr, R. Carbocyanine Postmortem Neuronal Tracing: Influence of Different Parameters on Tracing Distance and Combination with Immunocytochemistry. J. Histochem. Cytochem. 1998, 46, 901–910. [Google Scholar] [CrossRef]
- Molnár, Z.; Blakey, D.; Bystron, I.; Carney, R.S.E. Tract-Tracing in Developing Systems and in Postmortem Human Material Using Carbocyanine Dyes. In Neuroanatomical Tract-Tracing 3: Molecules, Neurons, and Systems; Zaborszky, L., Wouterlood, F.G., Lanciego, J.L., Eds.; Springer: Boston, MA, USA, 2006; pp. 366–393. ISBN 978-0-387-28942-7. [Google Scholar]
- Mohtasebi, M.S.; Nasri, F.; Kamali Sarvestani, E. Effect of DiD Carbocyanine Dye Labeling on Immunoregulatory Function and Differentiation of Mice Mesenchymal Stem Cells. Stem Cells Int. 2014, 2014, 457614. [Google Scholar] [CrossRef]
- Godement, P.; Vanselow, J.; Thanos, S.; Bonhoeffer, F. A Study in Developing Visual Systems with a New Method of Staining Neurones and Their Processes in Fixed Tissue. Development 1987, 101, 697–713. [Google Scholar] [CrossRef]
- Molecular Probes; Invitrogen. Lipophilic Tracers—Dil, DiO, DiD, DiA, and DiR; Molecular Probes, Inc.: Eugene, OR, USA, 2008. [Google Scholar]
- Sparks, D.L.; Lue, L.-F.; Martin, T.A.; Rogers, J. Neural Tract Tracing Using Di-I: A Review and a New Method to Make Fast Di-I Faster in Human Brain. J. Neurosci. Methods 2000, 103, 3–10. [Google Scholar] [CrossRef]
- Hofmann, M.H.; Bleckmann, H. Effect of Temperature and Calcium on Transneuronal Diffusion of DiI in Fixed Brain Preparations. J. Neurosci. Methods 1999, 88, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Abel, R.M.; Bishop, A.E.; Moscoso, G.; Spitz, L.; Polak, J.M. The Ontogeny of Innervation of the Human Pylorus. J. Pediatr. Surg. 1998, 33, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Axer, H.; Keyserlingk, D.G. Mapping of Fiber Orientation in Human Internal Capsule by Means of Polarized Light and Confocal Scanning Laser Microscopy. J. Neurosci. Methods 2000, 94, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Axer, H.; Lippitz, B.E.; Keyserlingk, D.G.V. Morphological Asymmetry in Anterior Limb of Human Internal Capsule Revealed by Confocal Laser and Polarized Light Microscopy. Psychiatry Res. Neuroimaging 1999, 91, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Belichenko, P.V.; Oldfors, A.; Hagberg, B.; Dahlström, A. Rett Syndrome: 3-D Confocal Microscopy of Cortical Pyramidal Dendrites and Afferents. Neuroreport 1994, 5, 1509–1513. [Google Scholar] [CrossRef]
- Bose, S.; Dhillon, N.; Ross-Cisneros, F.N.; Carelli, V. Relative Post-Mortem Sparing of Afferent Pupil Fibers in a Patient with 3460 Leber’s Hereditary Optic Neuropathy. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 1175–1179. [Google Scholar] [CrossRef]
- Burkhalter, A. Development of Forward and Feedback Connections between Areas V1 and V2 of Human Visual Cortex. Cereb. Cortex 1993, 3, 476–487. [Google Scholar] [CrossRef]
- Burkhalter, A.; Bernardo, K.L. Organization of Corticocortical Connections in Human Visual Cortex. Proc. Natl. Acad. Sci. USA 1989, 86, 1071–1075. [Google Scholar] [CrossRef]
- Burkhalter, A.; Bernardo, K.L.; Charles, V. Development of Local Circuits in Human Visual Cortex. J. Neurosci. 1993, 13, 1916–1931. [Google Scholar] [CrossRef] [PubMed]
- Bystron, I.; Molnár, Z.; Otellin, V.; Blakemore, C. Tangential Networks of Precocious Neurons and Early Axonal Outgrowth in the Embryonic Human Forebrain. J. Neurosci. 2005, 25, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhou, X.; Qu, J.; Ashwell, K.W.S.; Paxinos, G. Central Vagal Sensory and Motor Connections: Human Embryonic and Fetal Development. Auton. Neurosci. 2004, 114, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Chen, D.; Callor, W.B.; Christensen, E.; Coon, H.; Williams, M.E. DiI-Mediated Analysis of Presynaptic and Postsynaptic Structures in Human Postmortem Brain Tissue. J. Comp. Neurol. 2019, 527, 3087–3098. [Google Scholar] [CrossRef]
- Deazevedo, L.C.; Hedin-Pereira, C.; Lent, R. Callosal Neurons in the Cingulate Cortical Plate and Subplate of Human Fetuses. J. Comp. Neurol. 1997, 386, 60–70. [Google Scholar] [CrossRef]
- FitzGibbon, T. The Human Fetal Retinal Nerve Fiber Layer and Optic Nerve Head: A DiI and DiA Tracing Study. Vis. Neurosci. 1997, 14, 433–447. [Google Scholar] [CrossRef] [PubMed]
- FitzGibbon, T.; Taylor, S.F. Retinotopy of the Human Retinal Nerve Fibre Layer and Optic Nerve Head. J. Comp. Neurol. 1996, 375, 238–251. [Google Scholar] [CrossRef]
- Friedman, D.I.; Kelly Johnson, J.; Chorsky, R.L.; Stopa, E.G. Labeling of Human Retinohypothalamic Tract with the Carbocyanine Dye, DiI. Brain Res. 1991, 560, 297–302. [Google Scholar] [CrossRef]
- Galuske, R.A.; Schlote, W.; Bratzke, H.; Singer, W. Interhemispheric Asymmetries of the Modular Structure in Human Temporal Cortex. Science 2000, 289, 1946–1949. [Google Scholar] [CrossRef]
- Hannan, A.J.; Servotte, S.; Katsnelson, A.; Sisodiya, S.; Blakemore, C.; Squier, M.; Molnar, Z. Characterization of Nodular Neuronal Heterotopia in Children. Brain 1999, 122, 219–238. [Google Scholar] [CrossRef]
- Hayaran, A.; Bijlani, V. Polyacrylamide as an Infiltrating and Embedding Medium for Vibratome Sectioning of Human Fetal Cerebellum Containing DiI-Filled Axons. J. Neurosci. Methods 1992, 42, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Hens, J.; Vanderwinden, J.M.; De Laet, M.H.; Scheuermann, D.W.; Timmermans, J.P. Morphological and Neurochemical Identification of Enteric Neurones with Mucosal Projections in the Human Small Intestine. J. Neurochem. 2001, 76, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Hevner, R.F. Development of Connections in the Human Visual System during Fetal Mid-Gestation: A DiI-Tracing Study. J. Neuropathol. Exp. Neurol. 2000, 59, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hevner, R.F.; Kinney, H.C. Reciprocal Entorhinal-Hippocampal Connections Established by Human Fetal Midgestation. J. Comp. Neurol. 1996, 372, 384–394. [Google Scholar] [CrossRef]
- Hildebrand, S.; Schueth, A.; Wangenheim, K.V.; Mattheyer, C.; Pampaloni, F.; Bratzke, H.; Roebroeck, A.F.; Galuske, R.A.W. hFRUIT: An Optimized Agent for Optical Clearing of DiI-Stained Adult Human Brain Tissue. Sci. Rep. 2020, 10, 9950. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, K.; Horn, A.; Derfuss, T.; Glon, C.; Sinicina, I.; Arbusow, V.; Strupp, M.; Brandt, T.; Theil, D. Fewer Latent Herpes Simplex Virus Type 1 and Cytotoxic T Cells Occur in the Ophthalmic Division than in the Maxillary and Mandibular Divisions of the Human Trigeminal Ganglion and Nerve. J. Virol. 2009, 83, 3696–3703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Humenick, A.; Chen, B.N.; Lauder, C.I.W.; Wattchow, D.A.; Zagorodnyuk, V.P.; Dinning, P.G.; Spencer, N.J.; Costa, M.; Brookes, S.J.H. Characterization of Projections of Longitudinal Muscle Motor Neurons in Human Colon. Neurogastroenterol. Motil. 2019, 31, e13685. [Google Scholar] [CrossRef] [PubMed]
- Humenick, A.; Chen, B.N.; Wattchow, D.A.; Zagorodnyuk, V.P.; Dinning, P.G.; Spencer, N.J.; Costa, M.; Brookes, S.J.H. Characterization of Putative Interneurons in the Myenteric Plexus of Human Colon. Neurogastroenterol. Motil. 2021, 33, e13964. [Google Scholar] [CrossRef]
- Kakita, A.; Hayashi, S.; Moro, F.; Guerrini, R.; Ozawa, T.; Ono, K.; Kameyama, S.; Walsh, C.A.; Takahashi, H. Bilateral Periventricular Nodular Heterotopia Due to Filamin 1 Gene Mutation: Widespread Glomeruloid Microvascular Anomaly and Dysplastic Cytoarchitecture in the Cerebral Cortex. Acta Neuropathol. 2002, 104, 649–657. [Google Scholar] [CrossRef]
- Konstantinidou, A.D.; Silos-Santiago, I.; Flaris, N.; Snider, W.D. Development of the Primary Afferent Projection in Human Spinal Cord. J. Comp. Neurol. 1995, 354, 1–12. [Google Scholar] [CrossRef]
- Krassioukov, A.V.; Bygrave, M.A.; Puckett, W.R.; Bunge, R.P.; Rogers, K.A. Human Sympathetic Preganglionic Neurons and Motoneurons Retrogradely Labelled with DiI. J. Auton. Nerv. Syst. 1998, 70, 123–128. [Google Scholar] [CrossRef]
- Lai, H.M.; Liu, A.K.L.; Ng, H.H.M.; Goldfinger, M.H.; Chau, T.W.; DeFelice, J.; Tilley, B.S.; Wong, W.M.; Wu, W.; Gentleman, S.M. Next Generation Histology Methods for Three-Dimensional Imaging of Fresh and Archival Human Brain Tissues. Nat. Commun. 2018, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Mufson, E.J.; Kordower, J.H.; Blume, H.W.; Madsen, J.R.; Saper, C.B. Connections of the Hippocampal Formation in Humans: II. The Endfolial Fiber Pathway. J. Comp. Neurol. 1997, 385, 352–371. [Google Scholar] [CrossRef]
- Loeliger, M.; Tolcos, M.; Leditschke, J.; Campbell, P.; Rees, S. Tracing Cranial Nerve Pathways (Glossopharyngeal, Vagus, and Hypoglossal) in SIDS and Control Infants: A Dil Study. J. Neuropathol. Exp. Neurol. 2000, 59, 822–829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, G.; González-Hernández, T. Developmental Changes in Layer I of the Human Neocortex during Prenatal Life: A DiI-Tracing and AChE and NADPH-d Histochemistry Study. J. Comp. Neurol. 1993, 338, 317–336. [Google Scholar] [CrossRef]
- Meyer-Rüsenberg, B.; Pavlidis, M.; Stupp, T.; Thanos, S. Pathological Changes in Human Retinal Ganglion Cells Associated with Diabetic and Hypertensive Retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1009–1018. [Google Scholar] [CrossRef]
- Mufson, E.J.; Brady, D.R.; Kordower, J.H. Tracing Neuronal Connections in Postmortem Human Hippocampal Complex with the Carbocyanine Dye DiI. Neurobiol. Aging 1990, 11, 649–653. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Vogt, B.A.; Morrison, J.H.; Hof, P.R. Spindle Neurons of the Human Anterior Cingulate Cortex. J. Comp. Neurol. 1995, 355, 27–37. [Google Scholar] [CrossRef]
- Onodera, S.; Hicks, T.P. Carbocyanine Dye Usage in Demarcating Boundaries of the Aged Human Red Nucleus. PLoS ONE 2010, 5, e14430. [Google Scholar] [CrossRef]
- Ozturk, N.C.; Koc, T. Testing the Suitability of Neuroanatomical Tracing Method in Human Fetuses with Long Years of Postmortem Delay. Surg. Radiol. Anat. 2022, 44, 769–783. [Google Scholar] [CrossRef]
- Pavlidis, M.; Stupp, T.; Naskar, R.; Cengiz, C.; Thanos, S. Retinal Ganglion Cells Resistant to Advanced Glaucoma: A Postmortem Study of Human Retinas with the Carbocyanine Dye DiI. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5196–5205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porter, A.J.; Wattchow, D.A.; Brookes, S.J.; Costa, M. The Neurochemical Coding and Projections of Circular Muscle Motor Neurons in the Human Colon. Gastroenterology 1997, 113, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.J.; Wattchow, D.A.; Brookes, S.J.; Costa, M. Projections of Nitric Oxide Synthase and Vasoactive Intestinal Polypeptide-Reactive Submucosal Neurons in the Human Colon. J. Gastroenterol. Hepatol. 1999, 14, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.J.; Wattchow, D.A.; Brookes, S.J.H.; Costa, M. Cholinergic and Nitrergic Interneurones in the Myenteric Plexus of the Human Colon. Gut 2002, 51, 70–75. [Google Scholar] [CrossRef]
- Qu, J.; Zhou, X.; Zhu, H.; Cheng, G.; Ashwell, K.W.S.; Lu, F. Development of the Human Superior Colliculus and the Retinocollicular Projection. Exp. Eye Res. 2006, 82, 300–310. [Google Scholar] [CrossRef]
- Sailaja, K.; Gopinath, G. Developing Substantia Nigra in Human: A Qualitative Study. Dev. Neurosci. 1994, 16, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Schueler, M.; Neuhuber, W.L.; De Col, R.; Messlinger, K. Innervation of Rat and Human Dura Mater and Pericranial Tissues in the Parieto-Temporal Region by Meningeal Afferents. Headache 2014, 54, 996–1009. [Google Scholar] [CrossRef]
- Seehaus, A.K.; Roebroeck, A.; Chiry, O.; Kim, D.-S.; Ronen, I.; Bratzke, H.; Goebel, R.; Galuske, R.A.W. Histological Validation of DW-MRI Tractography in Human Postmortem Tissue. Cereb. Cortex 2013, 23, 442–450. [Google Scholar] [CrossRef]
- Sivukhina, E.V.; Jirikowski, G.F. Oxytocin, but Not Arginine-Vasopressin Neurons Project from the Hypothalamus to Amygdala in Human: DiI-Based Tracing Study in Postmortem Brain. J. Chem. Neuroanat. 2021, 111, 101882. [Google Scholar] [CrossRef]
- Swift, M.J.; Crago, P.E.; Grill, W.M. Applied Electric Fields Accelerate the Diffusion Rate and Increase the Diffusion Distance of DiI in Fixed Tissue. J. Neurosci. Methods 2005, 141, 155–163. [Google Scholar] [CrossRef]
- Tardif, E.; Clarke, S. Intrinsic Connectivity of Human Auditory Areas: A Tracing Study with Dil. Eur. J. Neurosci. 2001, 13, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Tardif, E.; Clarke, S. Commissural Connections of Human Superior Colliculus. Neuroscience 2002, 111, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Tardif, E.; Delacuisine, B.; Probst, A.; Clarke, S. Intrinsic Connectivity of Human Superior Colliculus. Exp. Brain Res. 2005, 166, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Tardif, E.; Probst, A.; Clarke, S. Laminar Specificity of Intrinsic Connections in Broca’s Area. Cereb. Cortex 2007, 17, 2949–2960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thal, D.R.; Capetillo-Zarate, E.; Galuske, R.A. Tracing of Temporo-Entorhinal Connections in the Human Brain: Cognitively Impaired Argyrophilic Grain Disease Cases Show Dendritic Alterations but No Axonal Disconnection of Temporo-Entorhinal Association Neurons. Acta Neuropathol. 2008, 115, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Thanos, S.; Rohrbach, J.M.; Thiel, H.J. Postmortem Preservation of Ganglion Cells in the Human Retina. A Morphometric Investigation with the Carbocyanine Dye DiI. Retina 1991, 11, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Wattchow, D.A.; Brookes, S.J.; Costa, M. The Morphology and Projections of Retrogradely Labeled Myenteric Neurons in the Human Intestine. Gastroenterology 1995, 109, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Wattchow, D.A.; Porter, A.J.; Brookes, S.J.; Costa, M. The Polarity of Neurochemically Defined Myenteric Neurons in the Human Colon. Gastroenterology 1997, 113, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, H.; Liao, L.; Dadihan, T.; Wang, X.; Kerem, G. Trigeminal Ganglion Morphology in Human Fetus. Microsc. Res. Tech. 2013, 76, 598–605. [Google Scholar] [CrossRef]
- Zec, N.; Filiano, J.J.; Kinney, H.C. Anatomic Relationships of the Human Arcuate Nucleus of the Medulla: A DiI-Labeling Study. J. Neuropathol. Exp. Neurol. 1997, 56, 509–522. [Google Scholar] [CrossRef][Green Version]
- Zec, N.; Kinney, H.C. Anatomic Relationships of the Human Nucleus Paragigantocellularis Lateralis: A DiI Labeling Study. Auton. Neurosci. 2001, 89, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Zec, N.; Kinney, H.C. Anatomic Relationships of the Human Nucleus of the Solitary Tract in the Medulla Oblongata: A DiI Labeling Study. Auton. Neurosci.-Basic Clin. 2003, 105, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Deigendesch, N.; Wittig, H.; Scheurer, E.; Lenz, C. Tissue Sample Analysis for Post Mortem Determination of Brain Edema. Forensic Sci. Int. 2021, 323, 110808. [Google Scholar] [CrossRef]
- Edlow, B.L.; Mareyam, A.; Horn, A.; Polimeni, J.R.; Witzel, T.; Tisdall, M.D.; Augustinack, J.C.; Stockmann, J.P.; Diamond, B.R.; Stevens, A.; et al. 7 Tesla MRI of the Ex Vivo Human Brain at 100 Micron Resolution. Sci. Data 2019, 6, 244. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Morii, E.; Arai, I.; Takada, T.; Aozasa, K. Successful Treatment of Recurrent Small Bowel Adenocarcinoma by Cytoreductive Surgery and Chemotherapy: A Case Report and Review of the Literature. J. Med. Case Rep. 2010, 4, 213. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Fox, E.A. Anterograde Tracing Method Using DiI to Label Vagal Innervation of the Embryonic and Early Postnatal Mouse Gastrointestinal Tract. J. Neurosci. Methods 2007, 163, 213–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernhardt, R. Axonal Pathfinding during the Regeneration of the Goldfish Optic Pathway. J. Comp. Neurol. 1989, 284, 119–134. [Google Scholar] [CrossRef]
- Zecevic, D.; Antic, S. Fast Optical Measurement of Membrane Potential Changes at Multiple Sites on an Individual Nerve Cell. Histochem. J. 1998, 30, 197–216. [Google Scholar] [CrossRef]
- Richardson, D.S.; Lichtman, J.W. Clarifying Tissue Clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef]
- Tian, T.; Yang, Z.; Li, X. Tissue Clearing Technique: Recent Progress and Biomedical Applications. J. Anat. 2021, 238, 489–507. [Google Scholar] [CrossRef]
- Marx, V. Optimizing Probes to Image Cleared Tissue. Nat. Methods 2016, 13, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Chapleau, C.A.; Calfa, G.D.; Lane, M.C.; Albertson, A.J.; Larimore, J.L.; Kudo, S.; Armstrong, D.L.; Percy, A.K.; Pozzo-Miller, L. Dendritic Spine Pathologies in Hippocampal Pyramidal Neurons from Rett Syndrome Brain and after Expression of Rett-Associated MECP2 Mutations. Neurobiol. Dis. 2009, 35, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.-B.; Grutzendler, J.; Wong, W.T.; Wong, R.O.L.; Lichtman, J.W. Multicolor “DiOlistic” Labeling of the Nervous System Using Lipophilic Dye Combinations. Neuron 2000, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Grutzendler, J.; Helmin, K.; Tsai, J.; Gan, W.-B. Various Dendritic Abnormalities Are Associated with Fibrillar Amyloid Deposits in Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2007, 1097, 30–39. [Google Scholar] [CrossRef]
- Pham, M.T.; Rajić, A.; Greig, J.D.; Sargeant, J.M.; Papadopoulos, A.; McEwen, S.A. A Scoping Review of Scoping Reviews: Advancing the Approach and Enhancing the Consistency. Res. Synth. Methods 2014, 5, 371–385. [Google Scholar] [CrossRef]

| First Author YOP | Specimen Type, N of Subjects (DiI Tracing) | Specimen Fixation, Processing for Tracer Application | Application Method | Application Site | Tracing Distance (Max) | Incubation Solution Time Temperature | Study Objective |
|---|---|---|---|---|---|---|---|
| Axer H. 2000 [27] | Brain, 4 | Collected brains fixed in 4% aqueous formalin solution, sucrose cryoprotected, 60 µm sections | Solution covering (3–4 drops of 1 mg DiI in 1100 µL DMSO) | Internal capsule sections | NA | DMSO 7 days 37 °C | Fiber location and orientation of the internal capsule in adults |
| Axer H. 1999 [28] | Brain, 4 | Collected brains fixed in 4% aqueous formalin solution, sucrose cryoprotected, 60 µm sections | Solution covering (3–4 drops of 1 mg DiI in 1100 µL DMSO) | Internal capsule sections | NA | DMSO 7 days 37 °C | Fiber structure in the anterior limb of internal capsule in adults |
| Belichenko P. 1994 [29] | Brain, 1 | Tissue blocks following 1 month formaldehyde fixation | Crystal placement | White matter or layers I and IV of cortex | NA | 4% PFA 2 to 6 months NA | Effects of Rett syndrome on cortical architecture and afferent axons, assessed in a 16-year-old and 2 adult patients in comparison to normal subjects and patients with epilepsy |
| Bose S. 2005 [30] | Brain and optic nerve, 2 | Tissue initially fixed in 10% formalin, followed by 2% PFA and 2% glutaraldehyde for 1–3 weeks | Crystal placement | Brachium of the superior colliculus | NA | NA 4 weeks NA | Pupillary fibers of the pretectal region of an adult with LOHN compared to a healthy control |
| Burkhalter A. 1989 [32] | Brain, 4 | Tissue blocks fixed in 3% PFA solution containing 0.1 M lysine-HCI, 0.8% NaIO4, and 0.8% iodoacetic acid for 24 h at 4 °C | Crystal placement | V1 cortex | 6 mm | Phosphate buffer 2 to 8 weeks 21 °C | Circuitry of visual cortex within the occipital lobe in adults |
| Fitzgibbon T. 1996 [39] | Eye, 32 retinae/NA * | Fixed in 2–4% PFA | Crystal placement | Nerve fiber layer of macula region or various retinal locations | 8–10 mm (estimate) | 2–4% PFA 4–24 weeks 37 °C or room temperature | Development of connections in the human visual cortex at various gestational ages |
| Friedman D. 1991 [40] | Brain and optic nerve, 6 | Tissue blocks fixed in 4% PFA | Crystal placement | Distal end of optic nerve | 10 mm | 4% PFA 3–4 months 37 °C | Labeling of the human retinohypothalamic tract in adults |
| Hannan A. 1999 [42] | Brain, 4 | Formalin-fixed tissue blocks | Crystal placement | Vicinity of heterotopic nodules | 2–3 mm | 4% PFA 3–6 months 37 °C | Connectivity of heterotopic nodules in children |
| Hufner K. 2009 [48] | Trigeminal ganglion/opthalmic nerve, 11 | Delayed fixation using 4% PFA | Solution covering (17% Fast DiI in dimethylformamide) | Nerve stump | 5 mm (estimate) | Dimethylformamide 10–14 days 4 °C | HSV-1 infiltration of the trigeminal ganglion and nerve in adults |
| Kakita A. 2002 [51] | Brain, 1 | Specimens in 20% formalin | Crystal placement | Periventricular nodule surface or adjacent white matter | 2–3 mm (estimate) | NA 5 months 37 °C | Effect of bilateral periventricular nodular heterotopia due to filamin 1 gene mutation on characteristics and connectivity of nodules in an adult patient |
| Krassioukov A. 1998 [53] | Spinal cord, 1 | Spinal cord fixed in 4% formalin (2 weeks) | Solution injections (80–100 μL of 4% DiI solution in 100% ethanol) | Ventral portion of spinal segments, ventral root origin | NA | Phosphate buffer 7 months 4 °C | Method for retrograde labeling of preganglionic neurons and motor-neurons in human thoracic spinal cord in adults |
| Lim C. 1997 [55] | Brain, 6 | 10% formalin-fixed brains (>2 weeks), 1 mm thick slabs, tissue blocks | Crystal placement or Solution injections (45 nL of 1% solution DiI in 100% ethanol) | Fields and layers of hippocampal formation | 8 mm | Formalin up to 1 year 37–45 °C | Connections of pyramidal neurons of hippocampal regions in adults |
| Lukas J.R. 1998 [17] | Spinal cord, sciatic nerve, brachial plexus, 6 | 4% PFA or 4% carbol and 0.5% PFA perfusion-fixed cadavers | Crystal placement | Proximodistal direction to sciatic nerves and to brachial plexus or the lateral or posterior funiculus or dorsal columns of spinal cord | 28.5 ± 2.3 mm (12–15 weeks) | 4% PFA up to >1 year 37 °C or 40 °C | Methods for optimizing tracing in adult tissue |
| Mufson E. 1990 [59] | Brain, 1 | Coronal sections (1 cm) fixed in 4% PFA for 24 h at 4 °C | Crystal placement | Separate subregions of hippocampal complex | 8 mm | 4% PFA 6 months 20 °C | Validation of DiI as a neuronal tracer in fixed brain in adults |
| Onodera S. 2010 [61] | Brain, 7 ** | 10% formalin solution-fixed cadaver, tissue blocks | DiI powder | Dorsomedial, ventrolateral parts of the rostral red nucleus or the ventro/dorso-lateral zones of the caudal red nucleus | 10 mm (estimate) | 4% PFA 7 years room temperature | Morphology and boundaries of the red nucleus in adults |
| Schueler M. 2014 [69] | Brain with dura (to use some nerve branches), 3 | Unfixed skull, nerve stumps | Crystal placement | Distal nerve stump | NA | 4% PFA 6–7 months 37 °C | Dura mater and extracranial tissue innervation in adults |
| Sivukhina E. 2020 [71] | Brain, 6 | Tissue sections (6–10 mm), immersion-fixed in 4% phosphate buffered PFA | Solution injection (1–2 μL of 1 mg/mL DiI in 100% ethanol) | Amygdalar nuclei | 20–30 mm | 4% PFA approx. 10 days room temperature | Hypothalamic projections from oxytocin and arginine-vasopressin neurons to limbic system targets in adults |
| Sparks DL. 2000 [22] | Brain, 1 | Delayed fixation: fixation in 4% PFA delayed until 36 h following DiI application | Solution injection (5 μL of 1.7 mg/mL Fast DiI in dimethylformamide) | Lateral Cerebellum | 20–40 mm | NA 36 h NA | Method of delayed-fixation for tracer diffusion in adult brain |
| Tardif E. 2001 [73] | Brain, 4 | 4% PFA perfused brain and 12 h postfixation in same fixative | Solution injection (0.1– 0.2 μL of 10% DiI in dimethylformamide) | Parts of auditory cortex | 5 mm | 4% PFA 6–12 months NA | Intrinsic connectivity of auditory areas in adults |
| Tardif E. 2002 [74] | Brain, 5 | 4% PFA perfused brain followed by 12 h postfixation in the same fixative | Crystal placement or solution injection (0.2 μL of 10% DiI in dimethylformamide) | Superior colliculus | 7.5 mm | 4% PFA 6–12 months 4 °C or room temperature | Commissural connections of the superior colliculus in adults |
| Tardif E. 2005 [75] | Brain, 5 | Tectal plate immersion-fixed in 4% PFA for 12–24 h | Crystal placement in superficial layers | Superior colliculus | 6 mm | 4% PFA 12–32 months 37 °C | Intrinsic connectivity of the superior colliculus in adults |
| Tardif E. 2007 [76] | Brain, 7 | Tissue blocks fixed in 4% PFA (1 week) or brains perfused (internal carotid and basilar arteries) with 4% PFA followed by postfixation of 12 h by immersion in the same fixative | Crystal placement or solution injection (0.2 μL of 10% DiI in dimethylformamide) | Cortical grey matter | 8.8 mm for injected DiI solution | NA 3–54 months 37 °C or room temperature | Intrinsic connectivity of the Broca area in adults |
| Thal DR. 2008 [77] | Brain, 7 | Tissues (10 mm slabs) immersion-fixed 2.6% PFA, 0.8% iodoacetic acid, 0.8% sodium periodate, and 0.1 M d-l Lysine for 5 days | Crystal placement | Entorhinal layers | 9.5 mm | 2% PFA At least 12 months 37 °C | The temporo-entorhinal connections in adults |
| Nimchinsky E. 1995 [60] | Brain, 8/NA ** | Immersion-fixed brains in 4% PFA for up to 120 h | DiI methanol paste | Cingulum bundle | NA | Phosphate buffer 2–5 months Room temperature | Morphological and anatomical features of spindle neurons in the cingulate cortex of adults |
| Galuske R. 2000 [41] | Brain, 7 | Tissue blocks fixed in solution containing 2.6% PFA, 0.8% iodacetic acid, 0.8% sodiumperiodate, and 0.1 M D-Llysine for 48 h | Crystal placement | Cortical tissue | 7 mm | 2% PFA 4–6 months 37 °C | Intrinsic connections of primary auditory cortex in adults |
| Lai HM. 2018 [54] | Brain, 1 | Formalin-fixed brain for 5 years | Crystal placement | Deep white matter of cerebellum | 3–4 mm | Phosphate buffer 10 days 37 °C | Method for clearing fresh and archived adult brain tissues for 3D visualization |
| Hildebrand S. 2020 [47] | Brain, 1 | Periodate-lysine-paraformaldehyde fixed amygdala blocks for 2–7 days | Crystal placement | Amygdala specimens | 0.4 mm | 2% PFA 6 months–several years 37 °C | Method for clearing fresh brain tissues for 3D visualization in adults |
| Meyer B. 2006 [58] | Eye, 9 | 4% PFA overnight at 4 °C, then retinas transferred in 1% PFA | Crystal placement | Flat-mounted retinas | 5 mm | 1% PFA >2 months 20 °C | Effect of diabetes and hypertension on the morphology of retinal ganglion cells in adults |
| Palvidis M. 2003 [63] | Eye, 4 | Retinas in 4% PFA overnight at 4 °C | Crystal placement | Flat-mounted retinas | 2–5 mm | 0.5–1% PFA 4–6 months 4 °C | Effect of glaucoma on the morphology of retinal ganglion cells in adults |
| Seehaus A. 2012 [70] | Brain, 1 | Temporal lobe fixed in 2.6% PFA, 0.8% iodoacetic acid, 0.8% sodium periodate, and 0.1 M D-L-lysine (4 days) | Crystal placement | Surface of temporal cortex block | 13 mm | NA 48 months NA | Validate diffusion-weighted MRI using lipophilic tracing in adults |
| Thanos S. 1991 [78] | Eye, 16/NA * | Retinas in 4% PFA overnight at 4 °C | Crystal placement | Flat-mounted retina | 5 mm | 2% formalin 4 weeks NA | Morphology of retinal ganglion cells (specimen unknown age) |
| Burkhalter A. 1993 [33] | Brain, 12 | Occipital lobe pieces fixed in 3% paraformaldehyde solution containing 0.1 M lysine-HCI, 0.8% NaIO4, and 0.8% iodoacetic acid for 24 h at 4 °C | Crystal placement | V1 or V2 cortex | 3 mm (estimate) | Phosphate buffer 1–6 months 21 °C | Development of connections in the visual cortex from fetal to infant period |
| Cheng G. 2004 [35] | Brain, 8 | Tissues immersion-fixed in 4% PFA for 6 months–1 year | Crystal placement | Upper right superior mediastinum or vagal rootlets from groove along the lateral rim of olive | 3 mm (estimate) | 4% PFA 6–8 weeks 37 °C | Development of vagal nerve afferents/efferents within the tractus solitarius nuclear complex from embryonic to fetal period |
| deAzevedo L.C. 1997 [37] | Brain, 4 | Brains fixed in 4% PFA for 2–3 weeks | Crystal placement | Dorsal half of corpus callosum | NA | 4% PFA 4–6 months room temperature | Development of callosal neuron architecture during fetal period |
| Fitzgibbon T. 1997 [38] | Eye, 32 retinae/NA * | Eyes fixed in 2–4% PFA | Crystal placement | Retina nerve fiber layer | Rarely over 4 mm | 2–4% PFA 3–8 weeks 37 °C | Development optic nerve head and retinal nerve fiber layer during fetal period |
| Hevner R. 2000 [45] | Brain, 4 | Brains fixed in 4% PFA/4% Sucrose for 2–7 days, tissue blocks obtained | Crystal placement | Optic tract or optic nerve or optic radiations | NA | 4% PFA/4% sucrose 17–45 weeks room temperature | Development of connections in the visual pathway in the mid-gestation fetal brain |
| Hevner R. 1996 [46] | Brain, 9 | Brains fixed in cold 4% PFA/4% sucrose for 2–7 days or 10% formalin at RT | Crystal placement | Entorhinal cortex or dentate gyrus or CA1/Subiculum or CA3 | 3 mm (estimate) | 4% PFA 17–45 weeks Room temperature | Development of connections of hippocampal formation subdivisions by fetal midgestation |
| Konstantinidou A. 1995 [52] | Spinal cord, 13 | 4% PFA for 48–72 h | Crystal placement | Spinal cord | NA | Phosphate buffer 1 week 37 °C | Development of fetal dorsal root afferent projections |
| Loeliger M. 2000 [56] | Brain, 21 | Tissue blocks immersed in 4% PFA for at least 3 months | Solution of DiI in 100% ethanol | Cranial nerve ends with brush | 15 mm | 4% PFA At least 8 months 37 °C | Afferents and efferents of lower cranial nerves in patients with sudden infant syndrome and control infants |
| Meyer G. 1993 [57] | Brain, 12 | Initial fixation in 10% formalin (2 to 24 h) followed by 4% PFA | Crystal placement | Motor and temporal cortex | NA | 4% PFA 2–6 weeks 30 °C | Development of fetal layer I in neocortex |
| Ozturk NC. 2022 [62] | Peripheral and upper and lower cranial nerves, 4 | Archival tissue in 4% formalin at room temperature | Crystal or paste placement with micro bendable pin | Peripheral and cranial nerves following microincision | 25.11 ± 9.1 mm | 4% PFA up to 16 weeks 37 °C | Method for archival fetal tissue DiI tracing |
| Qu J. 2006 [67] | Brain, 10 | Brain fixed in 4% PFA for up to 6 weeks | Crystal placement | Brachium of the superior colliculus or groove between posterior thalamus and rostrolateral rim of crus cerebri | NA | 4% PFA Up to 15 weeks 37 °C | Development of the connections between retina and superior colliculus during embryonic and fetal period |
| Sailaja K. 1994 [68] | Brain, 6 | Dissected tissue in 4% PFA or 10% formalin for up to 4 weeks | Crystal placement | Head, body, and tail of caudate nucleus | NA | 4% PFA 6 months room temperature | Development of substantia nigra during embryonic and fetal period |
| Wu L. 2013 [81] | Trigeminal ganglion/ nerves, 5 | Intracardial perfusion 10% formalin | Crystal placement | Ophthalmic, maxillary, and mandibular nerves | 5 mm | 10% formalin 3 months 37 °C | Fetal trigeminal ganglion morphology |
| Zec N. 1997 [82] | Brain, 23/NA ** | Dissected tissues fixed in 4% PFA with 4% sucrose for 48–72 h at 4°C | Crystal placement | Arcuate nucleus, raphe obscurus nucleus, pyramid, corticospinal tract | 19–28 mm | 4% PFA with 4% sucrose 7–15.5 months room temperature | Fetal arcuate and caudal raphe nuclei connectivity |
| Zec N. 2001 [83] | Brain, 9 | Dissected tissues fixed in 4% PFA with 4% sucrose at 4 °C for 24 h, tissue blocks fixed for additional 24–48 h | Crystal placement | Nucleus Paragigantocellularis lateralis | 20–28 mm | 4% PFA with 4% sucrose 8.5–15.5 months room temperature | Fetal paragigantocellularis lateralis nucelus connectivity |
| Zec N. 2003 [84] | Brain, 10 | Dissected tissues fixed in 4% PFA with 4% sucrose at 4 °C for 24 h, tissue blocks fixed for additional 24–48 h | Crystal placement | Nucleus of the solitary tract | NA | 4% PFA with 4% sucrose 6–16 months room temperature | Fetal nucleus tractus solitarius connectivity |
| Das S. 2019 [36] | Brain, 13 | Tissue blocks fixed in 4% cold paraformaldehyde for 1 h, followed by 4% PFA/0.125% glutaraldehyde for 24 h at 4 °C, 350 µm sections with vibratome | Crystal placement | CA1 sub-region and supra-pyramidal blade of the dentate gyrus | NA | Phosphate buffer 72 h 37 °C | Method for the study of pre- and postsynaptic elements in hippocampus across the lifespan (4 months to 71 years) |
| Burkhalter A. 1993 [31] | Brain, 6 | Brains fixed 2.6% paraformaldehyde solution containing 0.1 M lysine-HCI, 0.8% NaIO4, and 0.8% iodoacetic acid for 24 h at 4 °C. Blocked into 5 mm slabs | Crystal placement | V1 or V2 cortex | 3 mm (estimate) | Phosphate buffer 1–6 months 21 °C | Development of connections between V1 and V2 visual cortical areas from fetal to infant period |
| Hayaran A. 1992 [43] | Brain, 6 | Dissected tissues fixed in 4% PFA for 4–6 weeks | Crystal placement | Olive or cerebellar dentate nucleus | NA | 4% PFA 12–16 weeks room temperature | Method for polyacrylamide infiltration and embedding of DiI-stained fetal cerebellum |
| Bystron I. 2005 [34] | Brain, 21/NA * | Whole heads in 4% PBS for 7–24 h | Crystal placement | Parts of telencephalon or diencephalon | NA | Phosphate-buffered saline 3–4 weeks NA | Embryonic human forebrain early axonal outgrowth |
| Hens J. 2001 [44] | Jejunum, 4 | Unfixed in oxygenated Krebs solution | Crystal placement | Intestinal villus | “Several mm” | Culture medium 4–5 days 37 °C | Enteric neurons innervating the mucosa of the small intestine during infant life |
| Abel R.M. 1998 [26] | Pylorus, 14 | Specimens fixed in 2% formalin | Crystal placement | Severed surface of the vagus nerve or surface of pylorus (control) | NA | Phosphate buffer 6 months Room temperature | Fetal pylorus vagal innervation |
| Humenick A. 2019 [49] | Colon, 21 | Unfixed (post-DiI fixed in Zamboni’s for 24–48 h) | Tracer-covered glass beads | Serosal surface (fills from inter-tenial muscle) or incision of tenial muscle (fills of tenia) | 12 mm | Culture medium 4–5 days 37 °C | Innervation of longitudinal muscle of colon by motor neurons in adults |
| Humenick A. 2020 [50] | Colon, 36/NA ** | Unfixed (post-DiI fixed in Zamboni’s for 24–48 h) | Tracer-covered glass beads | Surface of the circular muscle or ganglia or longitudinally running internodal strands of the myenteric plexus | 70 mm | Culture medium 4–5 days 37 °C | Characterization of interneurons in myenteric plexus of colon in adults |
| Swift M. 2005 [72] | Peripheral nerves, 6 | 10% formalin-fixed cadavers | Solution (5–10 μL of 1 mg/mL in ethanol) Or paste | Median and ulnar nerves or cutaneous nerves | Electrical fields for 48 h: 53.7 ± 1.66 mm, controls: 8.1 ± 0.52 mm | Mineral oil immersion (of silicone gel coated samples) for up to 10 days Room temperature | Method to accelerate tracer diffusion and increase tracing distance in peripheral nerve tissue in adults |
| Porter A. 1997 [64] | Colon, 13 | Unfixed (post-DiI fixed with modified Zamboni’s for 16–24 h at 4 °C) | Tracer-covered glass beads | Circular muscle | 20 mm | Culture medium 5 days 37 °C | Motor neurons of the circular muscle of the human colon in adults |
| Porter A. 1999 [65] | Colon, 11 | Unfixed (post-DiI fixed with modified Zamboni’s for 16–24 h at 4 °C | Tracer-covered glass beads | Mucosa or submucosa, or circular muscle | 9.3 mm | Culture medium 5 days 37 °C | Connections within and between the mucosa, submucosa, and muscle layer of colon in adults |
| Porter A. 2002 [66] | Colon, 8 | Unfixed (post-DiI fixed with modified Zamboni’s for 16–24 h at 4 °C | Tracer-covered glass beads | Incision of myenteric plexus | 33 mm | Culture medium 5 days 37 °C | Cholinergic and nitrergic neurons in colon (myenteric plexus) in adults |
| Wattchow D. 1995 [79] | Intestine, 28 | Unfixed (post-DiI fixed with modified Zamboni’s for 16–24 h at 4 °C) | Tracer-covered glass beads | Mucosa or submucosa, or circular or longitudinal muscle layer or myenteric plexus | 68 mm | Culture medium 3–5 days 37 °C | Projections and morphology of myenteric neurons in adults |
| Wattchow D. 1997 [80] | Colon, 13 | Unfixed (post-DiI fixed with modified Zamboni’s for 16–24 h at 4 °C) | Tracer-covered glass beads | Circular muscle or incision through the myenteric plexus | 30 mm | Culture medium 5 days 37 °C | Polarity of neurochemically defined myenteric neurons in the human colon in adults |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrovounis, G.; Skouroliakou, A.; Kalatzis, I.; Stranjalis, G.; Kalamatianos, T. Over 30 Years of DiI Use for Human Neuroanatomical Tract Tracing: A Scoping Review. Biomolecules 2024, 14, 536. https://doi.org/10.3390/biom14050536
Mavrovounis G, Skouroliakou A, Kalatzis I, Stranjalis G, Kalamatianos T. Over 30 Years of DiI Use for Human Neuroanatomical Tract Tracing: A Scoping Review. Biomolecules. 2024; 14(5):536. https://doi.org/10.3390/biom14050536
Chicago/Turabian StyleMavrovounis, Georgios, Aikaterini Skouroliakou, Ioannis Kalatzis, George Stranjalis, and Theodosis Kalamatianos. 2024. "Over 30 Years of DiI Use for Human Neuroanatomical Tract Tracing: A Scoping Review" Biomolecules 14, no. 5: 536. https://doi.org/10.3390/biom14050536
APA StyleMavrovounis, G., Skouroliakou, A., Kalatzis, I., Stranjalis, G., & Kalamatianos, T. (2024). Over 30 Years of DiI Use for Human Neuroanatomical Tract Tracing: A Scoping Review. Biomolecules, 14(5), 536. https://doi.org/10.3390/biom14050536










