Scorpion Venom Antimicrobial Peptide Derivative BmKn2-T5 Inhibits Enterovirus 71 in the Early Stages of the Viral Life Cycle In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Reagents and Antibodies
2.3. MTS Assay
2.4. Circular Dichroism (CD) Analysis
2.5. qRT-PCR
2.6. Western Blotting
2.7. Time of Addition Assay
2.8. Plaque Assay
2.9. Statistical Analysis
3. Results
3.1. BmKn2 and Its Derivative BmKn2-T5 Show Significant Inhibitory Activities against EV71 Infection
3.2. BmKn2 and BmKn2-T5 Share a Typical Amphiphilic α-Helical Structure
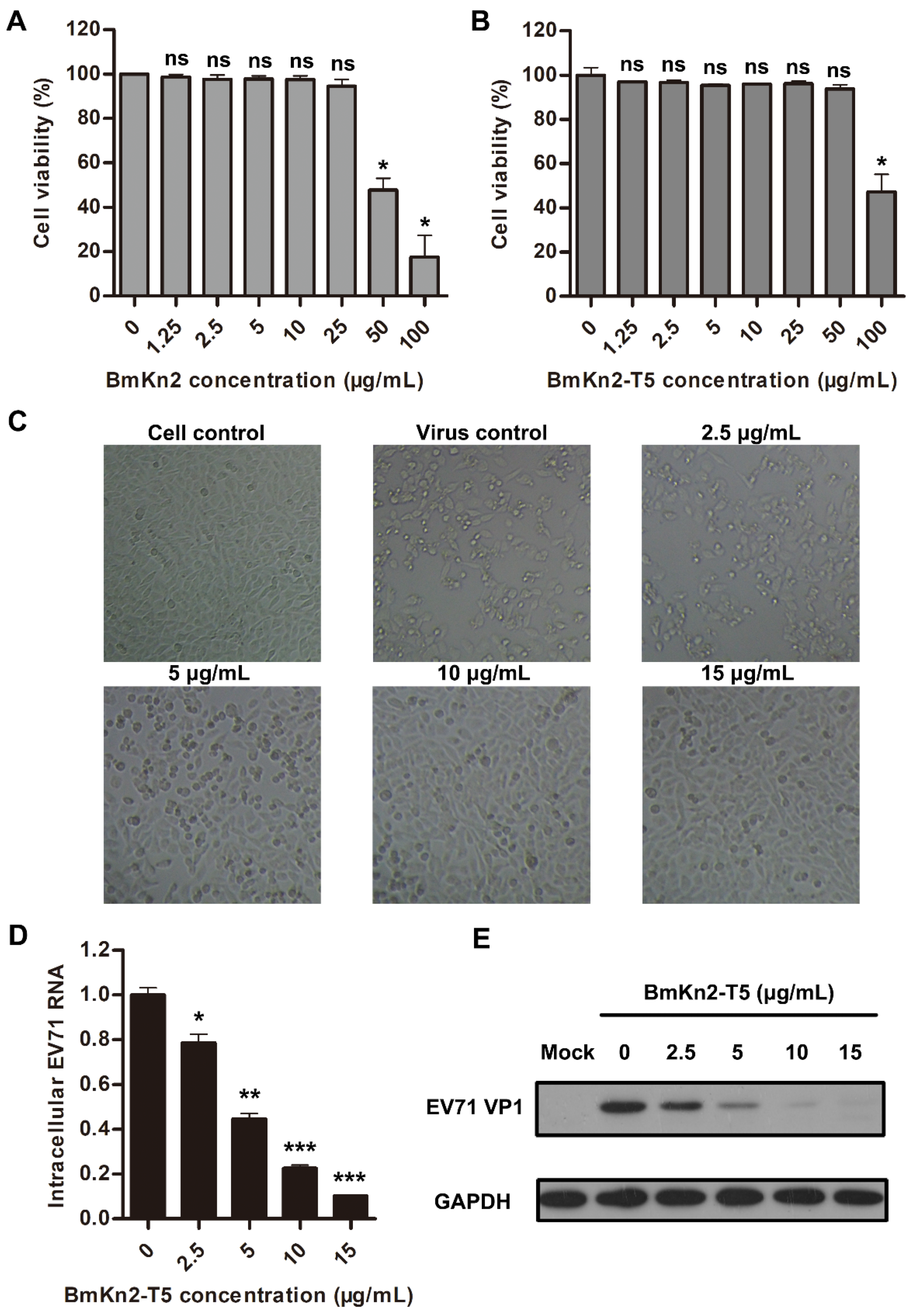
3.3. BmKn2-T5 Dose-Dependently Inhibits EV71 Infection at Noncytotoxic Concentrations
3.4. BmKn2-T5 Affects EV71 at the Early Stages of Its Life Cycle
3.5. BmKn2-T5 Shows Broad-Spectrum Antiviral Activity against DENV, ZIKV, and HSV-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial Peptides |
| CC50 | 50% Cytotoxicity Concentrations |
| CD | Circular Dichroism |
| CPE | Cytopathic Effect |
| DENV | Dengue Virus |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EV71 | Enterovirus 71 |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| HFMD | Hand, Foot, and Mouth Disease |
| HIV | Human Immunodeficiency Virus |
| HSV | Herpes Simplex Virus |
| IC50 | 50% Inhibitory Concentration |
| MEM | Minimum Essential Medium |
| MOI | Multiplicity of Infection |
| PBS | Phosphate-Buffered Saline |
| PSGL-1 | P-Selectin Glycoprotein Ligand-1 |
| qRT-PCR | Quantitative Real Time PCR |
| RD | Rhabdomyosarcoma |
| SCARB2 | Scavenger Receptor B2 |
| TFE | Trifluoroethanol |
| WB | Western Blotting |
| ZIKV | Zika Virus |
References
- Nayak, G.; Bhuyan, S.K.; Bhuyan, R.; Sahu, A.; Kar, D.; Kuanar, A. Global emergence of Enterovirus 71: A systematic review. Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Perera, R.; Cardosa, J.; Kuhn, R.J.; Rossmann, M.G. Crystal structure of human enterovirus 71. Science 2012, 336, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Onintsoa Diarimalala, R.; Yao, C.; Li, H.; Wei, Y. EV-A71 mechanism of entry: Receptors/co-receptors, related pathways and inhibitors. Viruses 2023, 15, 785–808. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.K.; Gadnayak, A.; Mohanty, J.N.; Sarangi, R.; Das, J. Does enterovirus 71 urge for effective vaccine control strategies? Challenges and current opinion. Rev. Med. Virol. 2022, 32, e2322. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Tsao, K.C.; Hsia, S.H.; Shih, S.R.; Huang, C.G.; Chan, W.K.; Hsu, K.H.; Fang, T.Y.; Huang, Y.C.; Lin, T.Y. Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA 2004, 291, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.K.; Zainol, M.I.; Sam, I.C.; Chan, Y.F. Recent advances in the understanding of enterovirus A71 infection: A focus on neuropathogenesis. Expert Rev. Anti Infect. Ther. 2021, 19, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.T.; Chang, Z.; Liu, F.; Fang, V.J.; Zheng, Y.; et al. Hand, foot, and mouth disease in China, 2008-12: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, I.; Takayama, R.; Hagiwara, A. A large-scale epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Japan in 1978. Jpn. J. Med. Sci. Biol. 1981, 34, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Jiang, L.; Ma, S.; James, L.; Ang, L.W. Basic reproduction number of coxsackievirus type A6 and A16 and enterovirus 71: Estimates from outbreaks of hand, foot and mouth disease in Singapore, a tropical city-state. Epidemiol. Infect. 2016, 144, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Puenpa, J.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y. The history of enterovirus A71 outbreaks and molecular epidemiology in the Asia-Pacific region. J. Biomed. Sci. 2019, 26, 75–86. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zeng, S.; Meng, X.; Lei, N.; Yang, H.; Li, R.; Mu, X.; Guo, X. Inhibition of EV71 replication by an interferon-stimulated gene product L3HYPDH. Virus Res. 2024, 342, 199336. [Google Scholar] [CrossRef]
- Liu, H.; Xue, Q.; Yang, F.; Cao, W.; Liu, P.; Liu, X.; Zhu, Z.; Zheng, H. Foot-and-mouth disease virus VP1 degrades YTHDF2 through autophagy to regulate IRF3 activity for viral replication. Autophagy 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Liu, B.; Yao, H.; Chen, X.; Yang, H.; Guo, S.; Wu, B.; Li, X.; Li, X.; Xun, M.; et al. Cathelicidin peptide analogues inhibit EV71 infection through blocking viral entry and uncoating. PLoS Pathog. 2024, 20, e1011967. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wang, Y.; Li, Y.; Chen, Y.; Wong, Y.T.; He, J.; He, M.L. Secreted LRPAP1 binds and triggers IFNAR1 degradation to facilitate virus evasion from cellular innate immunity. Signal Transduct. Target. Ther. 2023, 8, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, H.; Hu, D.; He, Q.; Yao, C.; Li, H.; Hu, K.; Wang, J. Recent advances in enterovirus A71 infection and antiviral agents. Lab. Investig. 2024, 104, 100298. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675–2704. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Ling, C.; Zhang, Y.; Li, J.; Chen, W.; Ling, C. Clinical use of toxic proteins and peptides from Tian Hua Fen and scorpion venom. Curr. Protein Pept. Sci. 2019, 20, 285–295. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Chen, G.; Wu, C.; Wang, X.; Zhang, Y. Bioactive constituents of animal-derived traditional Chinese medicinal materials for breast cancer: Opportunities and challenges. J. Zhejiang Univ. Sci. B 2022, 23, 547–563. [Google Scholar] [CrossRef]
- Cain, S.; Loria, S.F.; Ben-Shlomo, R.; Prendini, L.; Gefen, E. Dated phylogeny and ancestral range estimation of sand scorpions (Buthidae: Buthacus) reveal Early Miocene divergence across land bridges connecting Africa and Asia. Mol. Phylogenet. Evol. 2021, 164, 107212. [Google Scholar] [CrossRef]
- Harrison, P.L.; Abdel-Rahman, M.A.; Miller, K.; Strong, P.N. Antimicrobial peptides from scorpion venoms. Toxicon 2014, 88, 115–137. [Google Scholar] [CrossRef]
- Rincon-Cortes, C.A.; Bayona-Rojas, M.A.; Reyes-Montano, E.A.; Vega-Castro, N.A. Antimicrobial activity developed by scorpion venoms and its peptide component. Toxins 2022, 14, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; He, D.; Wu, Y.; Kwok, H.F.; Cao, Z. Scorpion venom peptides: Molecular diversity, structural characteristics, and therapeutic use from channelopathies to viral infections and cancers. Pharmacol. Res. 2023, 197, 106978. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, C.; Du, Q.; Wei, R.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: Evaluation of their antimicrobial and anticancer activities. Biochimie 2013, 95, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel antimicrobial peptides from the venom of the scorpion, Androctonus aeneas: Structural characterisation, molecular cloning of biosynthetic precursor-encoding cDNAs and engineering of analogues with enhanced antimicrobial and anticancer activities. Toxins 2015, 7, 219–237. [Google Scholar] [CrossRef]
- Rawson, K.M.; Lacey, M.M.; Strong, P.N.; Miller, K. Improving the therapeutic index of Smp24, a venom-derived antimicrobial peptide: Increased activity against gram-negative bacteria. Int. J. Mol. Sci. 2022, 23, 7979–7993. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Smidt, K.S.; de Oliveira, M.A.; da Cunha Morales Alvares, A.; Rigonatto, M.C.; da Silva Costa, P.H.; Tavares, A.H.; de Freitas, S.M.; Nicola, A.M.; et al. Activity of scorpion venom-derived antifungal peptides against planktonic cells of Candida spp. and Cryptococcus neoformans and Candida albicans biofilms. Front. Microbiol. 2016, 7, 1844–1858. [Google Scholar] [CrossRef]
- Parente, A.M.S.; Daniele-Silva, A.; Furtado, A.A.; Melo, M.A.; Lacerda, A.F.; Queiroz, M.; Moreno, C.; Santos, E.; Rocha, H.A.O.; Barbosa, E.G.; et al. Analogs of the scorpion venom peptide stigmurin: Structural assessment, toxicity, and increased antimicrobial activity. Toxins 2018, 10, 161–177. [Google Scholar] [CrossRef]
- Amorim-Carmo, B.; Daniele-Silva, A.; Parente, A.M.S.; Furtado, A.A.; Carvalho, E.; Oliveira, J.W.F.; Santos, E.C.G.; Silva, M.S.; Silva, S.R.B.; Silva-Junior, A.A.; et al. Potent and broad-spectrum antimicrobial activity of analogs from the scorpion peptide stigmurin. Int. J. Mol. Sci. 2019, 20, 623–644. [Google Scholar] [CrossRef]
- El-Bitar, A.M.H.; Sarhan, M.; Abdel-Rahman, M.A.; Quintero-Hernandez, V.; Aoki-Utsubo, C.; Moustafa, M.A.; Possani, L.D.; Hotta, H. Smp76, a scorpine-like peptide isolated from the venom of the scorpion Scorpio maurus palmatus, with a potent antiviral activity against hepatitis C virus and dengue virus. Int. J. Pept. Res. Ther. 2020, 26, 811–821. [Google Scholar] [CrossRef]
- Jlassi, A.; Mekni-Toujani, M.; Ferchichi, A.; Gharsallah, C.; Malosse, C.; Chamot-Rooke, J.; ElAyeb, M.; Ghram, A.; Srairi-Abid, N.; Daoud, S. BotCl, the first chlorotoxin-like peptide inhibiting newcastle disease virus: The emergence of a new scorpion venom AMPs family. Molecules 2023, 28, 4355–4374. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, A.; Clarke, D.; Abdel-Rahman, M.A.; Ribeiro, A.; Collie-Duguid, E.; Pattinson, C.; Burgoyne, K.; Muhammad, T.; Alfadhel, S.; Heidari, Z.; et al. Venomous gland transcriptome and venom proteomic analysis of the scorpion Androctonus amoreuxi reveal new peptides with anti-SARS-CoV-2 activity. Peptides 2024, 173, 171139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, L.; Zhong, M.; Zhang, Y.; Han, C.; Li, Q.; Yang, J.; Zhou, D.; Shi, W.; He, B.; et al. Anti-HIV-1 activity of a new scorpion venom peptide derivative Kn2-7. PLoS ONE 2012, 7, e34947. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Bagheri-Ziari, S.; Shahbazzadeh, D.; Sardari, S.; Sabatier, J.M.; Pooshang Bagheri, K. Discovery of a new analgesic peptide, leptucin, from the Iranian scorpion, Hemiscorpius lepturus. Molecules 2021, 26, 2580–2597. [Google Scholar] [CrossRef] [PubMed]
- Daelemans, D.; Pauwels, R.; De Clercq, E.; Pannecouque, C. A time-of-drug addition approach to target identification of antiviral compounds. Nat. Protoc. 2011, 6, 925–933. [Google Scholar] [CrossRef]
- Li, F.; Lang, Y.; Ji, Z.; Xia, Z.; Han, Y.; Cheng, Y.; Liu, G.; Sun, F.; Zhao, Y.; Gao, M.; et al. A scorpion venom peptide Ev37 restricts viral late entry by alkalizing acidic organelles. J. Biol. Chem. 2019, 294, 182–194. [Google Scholar] [CrossRef]
- Li, M.L.; Hsu, T.A.; Chen, T.C.; Chang, S.C.; Lee, J.C.; Chen, C.C.; Stollar, V.; Shih, S.R. The 3C protease activity of enterovirus 71 induces human neural cell apoptosis. Virology 2002, 293, 386–395. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, D.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Their physicochemical properties and therapeutic application. Arch. Pharm. Res. 2012, 35, 409–413. [Google Scholar] [CrossRef]
- Mercurio, F.A.; Scaloni, A.; Caira, S.; Leone, M. The antimicrobial peptides casocidins I and II: Solution structural studies in water and different membrane-mimetic environments. Peptides 2019, 114, 50–58. [Google Scholar] [CrossRef]
- Pei, Z.; Wang, H.; Zhao, Z.; Chen, X.; Huan, C.; Zhang, W. Chemokine PF4 Inhibits EV71 and CA16 Infections at the Entry Stage. J. Virol. 2022, 96, e0043522. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Wang, X.; Wang, Q.; Wang, Y.; Lin, J.; Sun, Y.; Li, X.; Zhang, L.; Lou, Z.; Wang, J.; et al. Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71. Protein Cell 2014, 5, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Rinkenberger, N.; Schoggins, J.W. Mucolipin-2 cation channel increases trafficking efficiency of endocytosed viruses. mBio 2018, 9, e02314–e02317. [Google Scholar] [CrossRef] [PubMed]
- Karasneh, G.A.; Shukla, D. Herpes simplex virus infects most cell types in vitro: Clues to its success. Virol. J. 2011, 8, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Hong, Y.; Jiang, C.; Wu, J.; Wu, M.; Sheng, R.; Liu, H.; Sun, J.; Xin, Y.; et al. Antiviral effects of the petroleum ether extract of Tournefortia sibirica L. against enterovirus 71 infection in vitro and in vivo. Front. Pharmacol. 2022, 13, 999798. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.D.; Bataglioli, R.A.; Oshodi, J.; Beppu, M.M. Antimicrobial peptides and their potential application in antiviral coating agents. Colloids Surf. B Biointerfaces 2022, 217, 112693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G. Insights into antimicrobial peptides from spiders and scorpions. Protein Pept. Lett. 2016, 23, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dai, Y.; Fu, Y.; Wang, K.; Yang, Y.; Li, M.; Xu, W.; Wei, L. Cathelicidin antimicrobial peptides suppress EV71 infection via regulating antiviral response and inhibiting viral binding. Antivir. Res. 2021, 187, 105021. [Google Scholar] [CrossRef]
- El Hidan, M.A.; Laaradia, M.A.; El Hiba, O.; Draoui, A.; Aimrane, A.; Kahime, K. Scorpion-derived antiviral peptides with a special focus on medically important viruses: An update. Biomed. Res. Int. 2021, 2021, 9998420. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Fujii, K.; Koike, S. Receptors for enterovirus 71. Emerg. Microbes Infect. 2014, 3, e53. [Google Scholar] [CrossRef]




| Name | Direction | Sequence (5′-3′) |
|---|---|---|
| hGAPDH | Sense | TGATGACATCAAGAAGGTGGTGAAG |
| Antisense | TCCTTGGAGGCCATGTGGGCCAT | |
| EV71 | Sense | TGAATGCCGGCTAATCCCAACT |
| Antisense | AAGAAACACGGACACCCAAAG | |
| DENV | Sense | GGCCTCGACTTCAATGAGATGG |
| Antisense | CCTGTTTCTTTGCATGGGGAT | |
| ZIKV | Sense | TTGTGGAAGGTATGTCAGGTG |
| Antisense | ATCTTACCTCCGCCATGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Wang, H.; Chen, W.; Wang, A.; Cao, Z. Scorpion Venom Antimicrobial Peptide Derivative BmKn2-T5 Inhibits Enterovirus 71 in the Early Stages of the Viral Life Cycle In Vitro. Biomolecules 2024, 14, 545. https://doi.org/10.3390/biom14050545
Xia Z, Wang H, Chen W, Wang A, Cao Z. Scorpion Venom Antimicrobial Peptide Derivative BmKn2-T5 Inhibits Enterovirus 71 in the Early Stages of the Viral Life Cycle In Vitro. Biomolecules. 2024; 14(5):545. https://doi.org/10.3390/biom14050545
Chicago/Turabian StyleXia, Zhiqiang, Huijuan Wang, Weilie Chen, Aili Wang, and Zhijian Cao. 2024. "Scorpion Venom Antimicrobial Peptide Derivative BmKn2-T5 Inhibits Enterovirus 71 in the Early Stages of the Viral Life Cycle In Vitro" Biomolecules 14, no. 5: 545. https://doi.org/10.3390/biom14050545
APA StyleXia, Z., Wang, H., Chen, W., Wang, A., & Cao, Z. (2024). Scorpion Venom Antimicrobial Peptide Derivative BmKn2-T5 Inhibits Enterovirus 71 in the Early Stages of the Viral Life Cycle In Vitro. Biomolecules, 14(5), 545. https://doi.org/10.3390/biom14050545








