Bone and Extracellular Signal-Related Kinase 5 (ERK5)
Abstract
:1. Introduction
2. Overview of Bone Development and Bone Homeostasis
2.1. Bone Formation
2.2. Bone Resorption
3. ERK5 Overview
3.1. Discovery and Structure of ERK5
3.2. The Role of ERK5 in Different Tissues or Diseases
3.2.1. Heart and Vascular Endothelium
3.2.2. Nervous System
3.2.3. Lung
3.2.4. Breast
3.2.5. Kidney and Liver
3.2.6. Skin
3.2.7. Hematopoietic System
3.2.8. Prostate
3.2.9. Other Tissues
4. The Role of ERK5 in Bones
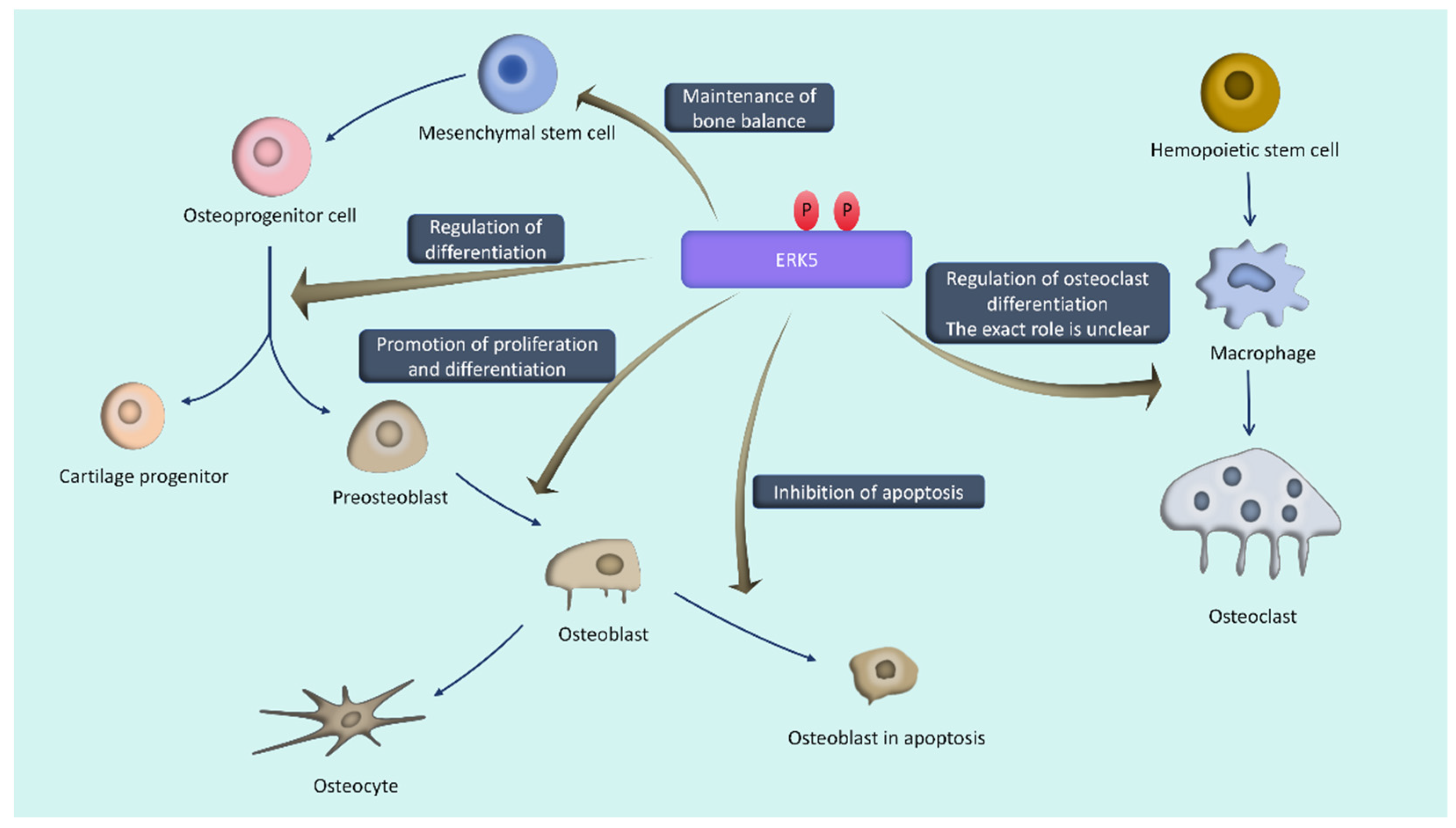
4.1. ERK5 and Bone Metabolism (Figure 2)
4.1.1. Bone Formation
4.1.2. Bone Resorption
4.1.3. Osteoporosis
4.2. ERK5 and Bone Neoplasms
4.3. Drugs
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011, 13, 27–38. [Google Scholar] [CrossRef]
- Soltanoff, C.S.; Yang, S.; Chen, W.; Li, Y.P. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 1–46. [Google Scholar] [CrossRef]
- Nithianandarajah-Jones, G.N.; Wilm, B.; Goldring, C.E.; Müller, J.; Cross, M.J. ERK5: Structure, regulation and function. Cell. Signal. 2012, 24, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Malemud, C.J. Extracellular Signal-Regulated Kinase: A Regulator of Cell Growth, Inflammation, Chondrocyte and Bone Cell Receptor-Mediated Gene Expression. Int. J. Mol. Sci. 2019, 20, 3792. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Kim, P.; Park, J.; Lee, D.J.; Mizuno, S.; Shinohara, M.; Hong, C.P.; Jeong, Y.; Yun, R.; Park, H.; Park, S.; et al. Mast4 determines the cell fate of MSCs for bone and cartilage development. Nat. Commun. 2022, 13, 3960. [Google Scholar] [CrossRef]
- Ko, F.C.; Sumner, D.R. How faithfully does intramembranous bone regeneration recapitulate embryonic skeletal development? Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2021, 250, 377–392. [Google Scholar] [CrossRef]
- Compton, J.T.; Lee, F.Y. A review of osteocyte function and the emerging importance of sclerostin. J. Bone Jt. Surg. Am. Vol. 2014, 96, 1659–1668. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Martin, T.J. Modulation of osteoclast differentiation. Endocr. Rev. 1992, 13, 66–80. [Google Scholar] [CrossRef]
- Jacome-Galarza, C.E.; Lee, S.K.; Lorenzo, J.A.; Aguila, H.L. Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 1203–1213. [Google Scholar] [CrossRef]
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Zhou, G.; Bao, Z.Q.; Dixon, J.E. Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 1995, 270, 12665–12669. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Ulevitch, R.J.; Han, J. Primary structure of BMK1: A new mammalian map kinase. Biochem. Biophys. Res. Commun. 1995, 213, 715–724. [Google Scholar] [CrossRef]
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Erazo, T.; Moreno, A.; Ruiz-Babot, G.; Rodríguez-Asiain, A.; Morrice, N.A.; Espadamala, J.; Bayascas, J.R.; Gómez, N.; Lizcano, J.M. Canonical and kinase activity-independent mechanisms for extracellular signal-regulated kinase 5 (ERK5) nuclear translocation require dissociation of Hsp90 from the ERK5-Cdc37 complex. Mol. Cell. Biol. 2013, 33, 1671–1686. [Google Scholar] [CrossRef] [PubMed]
- Paudel, R.; Fusi, L.; Schmidt, M. The MEK5/ERK5 Pathway in Health and Disease. Int. J. Mol. Sci. 2021, 22, 7594. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.L.; Sidaway, J.E.; Cross, M.J. Statin regulated ERK5 stimulates tight junction formation and reduces permeability in human cardiac endothelial cells. J. Cell. Physiol. 2018, 233, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Itoh, S.; Baines, C.P.; Zhang, C.; Ohta, S.; Che, W.; Glassman, M.; Lee, J.D.; Yan, C.; Yang, J.; et al. Activation of big MAP kinase 1 (BMK1/ERK5) inhibits cardiac injury after myocardial ischemia and reperfusion. FEBS Lett. 2004, 566, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Velasco, A.; Zi, M.; Hille, S.S.; Azam, T.; Kaur, N.; Jiang, J.; Nguyen, B.; Sekeres, K.; Binder, P.; Collins, L.; et al. Targeting mir128-3p alleviates myocardial insulin resistance and prevents ischemia-induced heart failure. eLife 2020, 9, e54298. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Ture, S.K.; Mickelsen, D.; Chakrabarti, E.; Modjeski, K.L.; McNitt, S.; Seaberry, M.; Field, D.J.; Le, N.T.; Abe, J.; et al. Platelet Extracellular Regulated Protein Kinase 5 Is a Redox Switch and Triggers Maladaptive Platelet Responses and Myocardial Infarct Expansion. Circulation 2015, 132, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Shishido, T.; Woo, C.H.; Ding, B.; McClain, C.; Molina, C.A.; Yan, C.; Yang, J.; Abe, J. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: Implications for diabetic ventricular dysfunction after myocardial infarction. Circ. Res. 2008, 102, 1416–1425. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhou, J.; Liang, C.; Hong, K.; Cheng, X.S.; Wu, Z.G. ERK5 knock down aggravates detrimental effects of hypothermal stimulation on cardiomyocytes via Bim upregulation. Environ. Toxicol. Pharmacol. 2013, 36, 724–731. [Google Scholar] [CrossRef]
- Liu, W.; Ruiz-Velasco, A.; Wang, S.; Khan, S.; Zi, M.; Jungmann, A.; Dolores Camacho-Muñoz, M.; Guo, J.; Du, G.; Xie, L.; et al. Metabolic stress-induced cardiomyopathy is caused by mitochondrial dysfunction due to attenuated Erk5 signaling. Nat. Commun. 2017, 8, 494. [Google Scholar] [CrossRef]
- Le, N.T.; Takei, Y.; Shishido, T.; Woo, C.H.; Chang, E.; Heo, K.S.; Lee, H.; Lu, Y.; Morrell, C.; Oikawa, M.; et al. p90RSK targets the ERK5-CHIP ubiquitin E3 ligase activity in diabetic hearts and promotes cardiac apoptosis and dysfunction. Circ. Res. 2012, 110, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Regan, C.P.; Li, W.; Boucher, D.M.; Spatz, S.; Su, M.S.; Kuida, K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc. Natl. Acad. Sci. USA 2002, 99, 9248–9253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Merritt, A.J.; Seyfried, J.; Guo, C.; Papadakis, E.S.; Finegan, K.G.; Kayahara, M.; Dixon, J.; Boot-Handford, R.P.; Cartwright, E.J.; et al. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol. Cell. Biol. 2005, 25, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kim, S.W.; Imanaka-Yoshida, K.; Yoshida, T.; Abel, E.D.; Eliceiri, B.; Yang, Y.; Ulevitch, R.J.; Lee, J.D. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J. Clin. Investig. 2004, 113, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Roberts, O.L.; Holmes, K.; Müller, J.; Cross, D.A.; Cross, M.J. ERK5 is required for VEGF-mediated survival and tubular morphogenesis of primary human microvascular endothelial cells. J. Cell Sci. 2010, 123, 3189–3200. [Google Scholar] [CrossRef] [PubMed]
- Ohnesorge, N.; Viemann, D.; Schmidt, N.; Czymai, T.; Spiering, D.; Schmolke, M.; Ludwig, S.; Roth, J.; Goebeler, M.; Schmidt, M. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4). J. Biol. Chem. 2010, 285, 26199–26210. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.; Lim, J.H.; Lee, C.; Choi, H.C.; Woo, C.H. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J. Biol. Chem. 2012, 287, 40722–40731. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Yan, C.; Berk, B.C. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ. Res. 2004, 94, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Vanchin, B.; Offringa, E.; Friedrich, J.; Brinker, M.G.; Kiers, B.; Pereira, A.C.; Harmsen, M.C.; Moonen, J.A.; Krenning, G. MicroRNA-374b induces endothelial-to-mesenchymal transition and early lesion formation through the inhibition of MAPK7 signaling. J. Pathol. 2019, 247, 456–470. [Google Scholar] [CrossRef]
- Suzaki, Y.; Yoshizumi, M.; Kagami, S.; Koyama, A.H.; Taketani, Y.; Houchi, H.; Tsuchiya, K.; Takeda, E.; Tamaki, T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: Potential role in cell survival following oxidative insults. J. Biol. Chem. 2002, 277, 9614–9621. [Google Scholar] [CrossRef]
- Wang, S.; Liu, A.; Xu, C.; Hou, J.; Hong, J. GLP-1(7-36) protected against oxidative damage and neuronal apoptosis in the hippocampal CA region after traumatic brain injury by regulating ERK5/CREB. Mol. Biol. Rep. 2024, 51, 313. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Y.W.; Zou, J.; Li, T.; Abel, G.M.; Palmiter, R.D.; Storm, D.R.; Xia, Z. Genetic activation of ERK5 MAP kinase enhances adult neurogenesis and extends hippocampus-dependent long-term memory. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 2130–2147. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Xiao, C.; Lu, B.; Zhang, J.; Yuan, X.Z.; Chen, W.; Yu, L.N.; Zhang, F.J.; Chen, G.; Yan, M. CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J. Neurosci. Res. 2013, 91, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Sun, F.; Cunningham, R.L.; Rybalchenko, N.; Singh, M. ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. Age 2014, 36, 9685. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.L.; Heerssen, H.M.; Bhattacharyya, A.; Klesse, L.; Lin, M.Z.; Segal, R.A. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 2001, 4, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, B.; Guo, W.; Gao, L.; Shi, L.; Li, H.; Lu, S.; Liu, Y.; Li, X. miR-429 inhibits glioma invasion through BMK1 suppression. J. Neuro-Oncol. 2015, 125, 43–54. [Google Scholar] [CrossRef]
- Li, J.; Shao, R.; Xie, Q.; Qin, K.; Ming, S.; Xie, Y.; Du, X. Ulinastatin promotes macrophage efferocytosis and ameliorates lung inflammation via the ERK5/Mer signaling pathway. FEBS Open Bio 2022, 12, 1498–1508. [Google Scholar] [CrossRef]
- Kim, S.; Lim, J.H.; Woo, C.H. ERK5 inhibition ameliorates pulmonary fibrosis via regulating Smad3 acetylation. Am. J. Pathol. 2013, 183, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Gavine, P.R.; Wang, M.; Yu, D.; Hu, E.; Huang, C.; Xia, J.; Su, X.; Fan, J.; Zhang, T.; Ye, Q.; et al. Identification and validation of dysregulated MAPK7 (ERK5) as a novel oncogenic target in squamous cell lung and esophageal carcinoma. BMC Cancer 2015, 15, 454. [Google Scholar] [CrossRef]
- He, S.; Dong, D.; Lin, J.; Wu, B.; Nie, X.; Cai, G. Overexpression of TRAF4 promotes lung cancer growth and EGFR-dependent phosphorylation of ERK5. FEBS Open Bio 2022, 12, 1747–1760. [Google Scholar] [CrossRef]
- Cristea, S.; Coles, G.L.; Hornburg, D.; Gershkovitz, M.; Arand, J.; Cao, S.; Sen, T.; Williamson, S.C.; Kim, J.W.; Drainas, A.P.; et al. The MEK5-ERK5 Kinase Axis Controls Lipid Metabolism in Small-Cell Lung Cancer. Cancer Res. 2020, 80, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xie, W.; Wu, R.; Geng, H.; Zhao, L.; Xie, C.; Li, X.; Huang, C.; Zhu, J.; Zhu, M.; et al. ERK5 negatively regulates tobacco smoke-induced pulmonary epithelial–mesenchymal transition. Oncotarget 2015, 6, 19605–19618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.Q.; Ge, M.M.; Zhang, Q.; Wang, X.Q.; Zhu, J.Y.; Xie, C.F.; Li, X.T.; Zhong, C.Y.; Han, H.Y. Sulforaphane inhibits epithelial–mesenchymal transition by activating extracellular signal-regulated kinase 5 in lung cancer cells. J. Nutr. Biochem. 2019, 72, 108219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cai, F.; Xu, H.; Lu, Y.; Chen, J.; Liu, J.; Cao, N.; Zhang, X.; Chen, X.; Huang, Q.; et al. Extracellular signal regulated kinase 5 promotes cell migration, invasion and lung metastasis in a FAK-dependent manner. Protein Cell 2020, 11, 825–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, D.; Chen, Z.; Yao, W.; Yang, J.; Ramalingam, S.S.; Sun, S.Y. Inhibition of MEK5/ERK5 signaling overcomes acquired resistance to the third generation EGFR inhibitor, osimertinib, via enhancing Bim-dependent apoptosis. Cancer Lett. 2021, 519, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, G.; Cai, F.; Chen, X.; Cao, N.; Zhang, X.; Liu, J.; Chen, F.; Wang, F.; Dong, W.; et al. Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response. Exp. Mol. Med. 2019, 51, 1–20. [Google Scholar] [CrossRef]
- Riegel, K.; Yurugi, H.; Schlöder, J.; Jonuleit, H.; Kaulich, M.; Kirschner, F.; Arnold-Schild, D.; Tenzer, S.; Schild, H.; Rajalingam, K. ERK5 modulates IL-6 secretion and contributes to tumor-induced immune suppression. Cell Death Dis. 2021, 12, 969. [Google Scholar] [CrossRef]
- Xia, C.; Yang, Y.; Kong, F.; Kong, Q.; Shan, C. MiR-143-3p inhibits the proliferation, cell migration and invasion of human breast cancer cells by modulating the expression of MAPK7. Biochimie 2018, 147, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.C.; Ocaña, A.; Abad, M.; Ortiz-Ruiz, M.J.; Pandiella, A.; Esparís-Ogando, A. Expression of Erk5 in early stage breast cancer and association with disease free survival identifies this kinase as a potential therapeutic target. PLoS ONE 2009, 4, e5565. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, M.J.; Álvarez-Fernández, S.; Parrott, T.; Zaknoen, S.; Burrows, F.J.; Ocaña, A.; Pandiella, A.; Esparís-Ogando, A. Therapeutic potential of ERK5 targeting in triple negative breast cancer. Oncotarget 2014, 5, 11308–11318. [Google Scholar] [CrossRef]
- Monlish, D.A.; Cavanaugh, J.E. Age-Related Changes in ERK5 Signaling and Crosstalk with ERK1/2 and PI3K in Breast Cancer. Cancer Res. 2010, 70, P2-07-05. [Google Scholar] [CrossRef]
- Mulloy, R.; Salinas, S.; Philips, A.; Hipskind, R.A. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene 2003, 22, 5387–5398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pearson, A.J.; Sabherwal, N.; Telfer, B.A.; Ali, N.; Kan, K.; Xu, Q.; Zhang, W.; Chen, F.; Li, S.; et al. Inhibiting ERK5 overcomes breast cancer resistance to anti-HER2 therapy by targeting the G1/S cell cycle transition. Cancer Res. Commun. 2022, 2, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Javaid, S.; Zhang, J.; Smolen, G.A.; Yu, M.; Wittner, B.S.; Singh, A.; Arora, K.S.; Madden, M.W.; Desai, R.; Zubrowski, M.J.; et al. MAPK7 Regulates EMT Features and Modulates the Generation of CTCs. Mol. Cancer Res. 2015, 13, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Ma, C.; Li, W.; Yang, S.; Liu, Z. miR-143 suppresses epithelial–mesenchymal transition and inhibits tumor growth of breast cancer through down-regulation of ERK5. Mol. Carcinog. 2016, 55, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Madak-Erdogan, Z.; Ventrella, R.; Petry, L.; Katzenellenbogen, B.S. Novel roles for ERK5 and cofilin as critical mediators linking ERα-driven transcription, actin reorganization, and invasiveness in breast cancer. Mol. Cancer Res. 2014, 12, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, J.; Telfer, B.A.; Zhang, H.; Ali, N.; Chen, F.; Risa, B.; Pearson, A.J.; Zhang, W.; Finegan, K.G.; et al. The extracellular-regulated protein kinase 5 (ERK5) enhances metastatic burden in triple-negative breast cancer through focal adhesion protein kinase (FAK)-mediated regulation of cell adhesion. Oncogene 2021, 40, 3929–3941. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, H.; Song, H. Upregulation of MEK5 by Stat3 promotes breast cancer cell invasion and metastasis. Oncol. Rep. 2017, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.T.; Matossian, M.D.; Ucar, D.A.; Elliott, S.; La, J.; Wright, M.K.; Burks, H.E.; Perles, A.; Hossain, F.; King, C.T.; et al. ERK5 Is Required for Tumor Growth and Maintenance Through Regulation of the Extracellular Matrix in Triple Negative Breast Cancer. Front. Oncol. 2020, 10, 1164. [Google Scholar] [CrossRef]
- Sawhney, R.S.; Liu, W.; Brattain, M.G. A novel role of ERK5 in integrin-mediated cell adhesion and motility in cancer cells via Fak signaling. J. Cell. Physiol. 2009, 219, 152–161. [Google Scholar] [CrossRef]
- Pavan, S.; Meyer-Schaller, N.; Diepenbruck, M.; Kalathur, R.K.R.; Saxena, M.; Christofori, G. A kinome-wide high-content siRNA screen identifies MEK5-ERK5 signaling as critical for breast cancer cell EMT and metastasis. Oncogene 2018, 37, 4197–4213. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, Q.; Lee, J.D. BMK1 kinase suppresses epithelial–mesenchymal transition through the Akt/GSK3β signaling pathway. Cancer Res. 2012, 72, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Wu, Y.; Wehrli, B.; Chakrabarti, S.; Chakraborty, C. Modulation of ERK5 is a novel mechanism by which Cdc42 regulates migration of breast cancer cells. J. Cell. Biochem. 2015, 116, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.Z. Dual Inhibition of the PI3K/Akt and MEK5/ERK5 Pathways for the Treatment of Breast Cancer. Ph.D. Thesis, Duquesne Universit, Pittsburgh, PA, USA, 2019. [Google Scholar]
- Dorado, F.; Velasco, S.; Esparís-Ogando, A.; Pericacho, M.; Pandiella, A.; Silva, J.; López-Novoa, J.M.; Rodríguez-Barbero, A. The mitogen-activated protein kinase Erk5 mediates human mesangial cell activation. Nephrol. Dial. Transplant. 2008, 23, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Badshah, I.I.; Baines, D.L.; Dockrell, M.E. Erk5 is a mediator to TGFβ1-induced loss of phenotype and function in human podocytes. Front. Pharmacol. 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Park, S.W.; Kaku, R.; Yang, J. Extracellular-regulated-kinase 5-mediated renal protection against ischemia–reperfusion injury. Biochem. Biophys. Res. Commun. 2012, 418, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, K.; Dorjsuren, N.; Izawa-Ishizawa, Y.; Sugimoto, R.; Ikeda, Y.; Kihira, Y.; Kawazoe, K.; Tomita, S.; Tsuchiya, K.; Minakuchi, K.; et al. Inhibitory effects of adiponectin on platelet-derived growth factor-induced mesangial cell migration. J. Endocrinol. 2009, 202, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Naito, S.; Obara, Y.; Ito, H.; Ichiyanagi, O.; Narisawa, T.; Kato, T.; Nagaoka, A.; Tsuchiya, N. Effect of Extracellular Signal-Regulated Protein Kinase 5 Inhibition in Clear Cell Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 8448. [Google Scholar] [CrossRef] [PubMed]
- Rovida, E.; Di Maira, G.; Tusa, I.; Cannito, S.; Paternostro, C.; Navari, N.; Vivoli, E.; Deng, X.; Gray, N.S.; Esparís-Ogando, A.; et al. The mitogen-activated protein kinase ERK5 regulates the development and growth of hepatocellular carcinoma. Gut 2015, 64, 1454–1465. [Google Scholar] [CrossRef]
- Arias-González, L.; Moreno-Gimeno, I.; del Campo, A.R.; Serrano-Oviedo, L.; Valero, M.L.; Esparís-Ogando, A.; de la Cruz-Morcillo, M.; Melgar-Rojas, P.; García-Cano, J.; Cimas, F.J.; et al. ERK5/BMK1 is a novel target of the tumor suppressor VHL: Implication in clear cell renal carcinoma. Neoplasia 2013, 15, 649–659. [Google Scholar] [CrossRef]
- Nagai, T.; Urushihara, M.; Kinoshita, Y.; Jamba, A.; Kondo, S.; Kagami, S. Differential regulation of angiotensin II-induced extracellular signal regulated kinase-1/2 and -5 in progressive glomerulonephritis. Nephrology 2016, 21, 950–958. [Google Scholar] [CrossRef]
- Rovida, E.; Navari, N.; Caligiuri, A.; Dello Sbarba, P.; Marra, F. ERK5 differentially regulates PDGF-induced proliferation and migration of hepatic stellate cells. J. Hepatol. 2008, 48, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, F.; Consalvi, V.; Noce, V.; Battistelli, C.; Cicchini, C.; Tripodi, M.; Amicone, L.; Marchetti, A. Extracellular signal-Regulated Kinase 5 (ERK5) is required for the Yes-associated protein (YAP) co-transcriptional activity. Cell Death Dis. 2023, 14, 32. [Google Scholar] [CrossRef]
- Zen, K.; Yasui, K.; Nakajima, T.; Zen, Y.; Zen, K.; Gen, Y.; Mitsuyoshi, H.; Minami, M.; Mitsufuji, S.; Tanaka, S.; et al. ERK5 is a target for gene amplification at 17p11 and promotes cell growth in hepatocellular carcinoma by regulating mitotic entry. Genes Chromosomes Cancer 2009, 48, 109–120. [Google Scholar] [CrossRef]
- Savage, H.; Pareek, S.; Lee, J.; Ballarò, R.; Conterno Minussi, D.; Hayek, K.; Sadullozoda, M.; Lochmann, B.S.; McQuade, J.L.; LaVoy, E.C.; et al. Aerobic Exercise Alters the Melanoma Microenvironment and Modulates ERK5 S496 Phosphorylation. Cancer Immunol. Res. 2023, 11, 1168–1183. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Zhao, J.; Martin, B.; Zepp, J.A.; Ko, J.S.; Gu, C.; Cai, G.; Ouyang, W.; Sen, G.; et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 2015, 212, 1571–1587. [Google Scholar] [CrossRef]
- Giurisato, E.; Xu, Q.; Lonardi, S.; Telfer, B.; Russo, I.; Pearson, A.; Finegan, K.G.; Wang, W.; Wang, J.; Gray, N.S.; et al. Myeloid ERK5 deficiency suppresses tumor growth by blocking protumor macrophage polarization via STAT3 inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, E2801–E2810. [Google Scholar] [CrossRef] [PubMed]
- Tusa, I.; Gagliardi, S.; Tubita, A.; Pandolfi, S.; Menconi, A.; Lulli, M.; Dello Sbarba, P.; Stecca, B.; Rovida, E. The Hedgehog-GLI Pathway Regulates MEK5-ERK5 Expression and Activation in Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 11259. [Google Scholar] [CrossRef] [PubMed]
- Tubita, A.; Lombardi, Z.; Tusa, I.; Lazzeretti, A.; Sgrignani, G.; Papini, D.; Menconi, A.; Gagliardi, S.; Lulli, M.; Dello Sbarba, P.; et al. Inhibition of ERK5 Elicits Cellular Senescence in Melanoma via the Cyclin-Dependent Kinase Inhibitor p21. Cancer Res. 2022, 82, 447–457. [Google Scholar] [CrossRef]
- Buschbeck, M.; Hofbauer, S.; Di Croce, L.; Keri, G.; Ullrich, A. Abl-kinase-sensitive levels of ERK5 and its intrinsic basal activity contribute to leukaemia cell survival. EMBO Rep. 2005, 6, 63–69. [Google Scholar] [CrossRef]
- Wang, X.; Pesakhov, S.; Harrison, J.S.; Danilenko, M.; Studzinski, G.P. ERK5 pathway regulates transcription factors important for monocytic differentiation of human myeloid leukemia cells. J. Cell. Physiol. 2014, 229, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Tusa, I.; Cheloni, G.; Poteti, M.; Gozzini, A.; DeSouza, N.H.; Shan, Y.; Deng, X.; Gray, N.S.; Li, S.; Rovida, E.; et al. Targeting the Extracellular Signal-Regulated Kinase 5 Pathway to Suppress Human Chronic Myeloid Leukemia Stem Cells. Stem Cell Rep. 2018, 11, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pesakhov, S.; Weng, A.; Kafka, M.; Gocek, E.; Nguyen, M.; Harrison, J.S.; Danilenko, M.; Studzinski, G.P. ERK 5/MAPK pathway has a major role in 1α,25-(OH)2 vitamin D3-induced terminal differentiation of myeloid leukemia cells. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.U.; Bräuer-Hartmann, D.; Kardosova, M.; Wurm, A.A.; Wilke, F.; Schödel, C.; Gerloff, D.; Katzerke, C.; Krakowsky, R.; Namasu, C.Y.; et al. MicroRNA-143 targets ERK5 in granulopoiesis and predicts outcome of patients with acute myeloid leukemia. Cell Death Dis. 2018, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yang, J. Hydrogen peroxide activation of ERK5 confers resistance to Jurkat cells against apoptosis induced by the extrinsic pathway. Biochem. Biophys. Res. Commun. 2014, 444, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Sovershaev, M.A.; Egorina, E.M.; Gruber, F.X.; Bjorkoy, G.; Johansen, T. Extracellular signal-regulated protein kinase 5 mediates resistance of human chronic mycloid leukemia K562 cells to imatinib. Blood 2006, 108, 604A. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, L.; Wang, T. Phosphorylation of BMK1 induces prostatic carcinoma cell proliferation by promoting entry into the S phase of the cell cycle. Oncol. Lett. 2016, 11, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Clapé, C.; Fritz, V.; Henriquet, C.; Apparailly, F.; Fernandez, P.L.; Iborra, F.; Avancès, C.; Villalba, M.; Culine, S.; Fajas, L. miR-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS ONE 2009, 4, e7542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, C.; Ma, X.; Wu, R.; Zhu, W.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; Geng, S.; et al. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ. Toxicol. Pharmacol. 2018, 63, 29–33. [Google Scholar] [CrossRef]
- McCracken, S.R.; Ramsay, A.; Heer, R.; Mathers, M.E.; Jenkins, B.L.; Edwards, J.; Robson, C.N.; Marquez, R.; Cohen, P.; Leung, H.Y. Aberrant expression of extracellular signal-regulated kinase 5 in human prostate cancer. Oncogene 2008, 27, 2978–2988. [Google Scholar] [CrossRef]
- Ramsay, A.K.; McCracken, S.R.; Soofi, M.; Fleming, J.; Yu, A.X.; Ahmad, I.; Morland, R.; Machesky, L.; Nixon, C.; Edwards, D.R.; et al. ERK5 signalling in prostate cancer promotes an invasive phenotype. Br. J. Cancer 2011, 104, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Cheng, J.; Luan, S.; Xiao, X.; Li, X.; Fang, P.; Gu, Y.; Shang, Q.; Zhang, H.; et al. MTHFD1L confers a poor prognosis and malignant phenotype in esophageal squamous cell carcinoma by activating the ERK5 signaling pathway. Exp. Cell Res. 2023, 427, 113584. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Fu, Y.; Zhu, N.; Zhao, M.; Ding, Z.; Zhang, X.; Song, Y.; Jing, Y.; Zhang, Q.; Chen, S.; et al. OXTR(High) stroma fibroblasts control the invasion pattern of oral squamous cell carcinoma via ERK5 signaling. Nat. Commun. 2022, 13, 5124. [Google Scholar] [CrossRef] [PubMed]
- Sticht, C.; Freier, K.; Knöpfle, K.; Flechtenmacher, C.; Pungs, S.; Hofele, C.; Hahn, M.; Joos, S.; Lichter, P. Activation of MAP kinase signaling through ERK5 but not ERK1 expression is associated with lymph node metastases in oral squamous cell carcinoma (OSCC). Neoplasia 2008, 10, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, A.; Lori, G.; Caligiuri, A.; Raggi, C.; Di Maira, G.; Pastore, M.; Piombanti, B.; Lottini, T.; Arcangeli, A.; Madiai, S.; et al. Extracellular Signal-Regulated Kinase 5 Regulates the Malignant Phenotype of Cholangiocarcinoma Cells. Hepatology 2021, 74, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, H.; Jiang, X.; Cao, L.; Wen, Z.; Yang, X.; Xue, P. Role of AP-2α and MAPK7 in the regulation of autocrine TGF-β/miR-200b signals to maintain epithelial–mesenchymal transition in cholangiocarcinoma. J. Hematol. Oncol. 2017, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.R.; Taniguchi, K.; Harris, A.R.; Bertin, S.; Takahashi, N.; Duong, J.; Campos, A.D.; Powis, G.; Corr, M.; Karin, M.; et al. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat. Commun. 2016, 7, 11551. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Jin, Z.; Wang, D.; Guo, X.; Wang, H.; Yang, S. Erk5 functions in modulation of zebrafish intestinal permeability. Cell Tissue Res. 2023, 393, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, J.; Qin, Y.; Mo, X.; Huang, M.; Ru, H.; Yang, Y.; Liu, J.; Lin, Y. SATB2 suppresses gastric cancer cell proliferation and migration. Tumour Biol. 2016, 37, 4597–4602. [Google Scholar] [CrossRef]
- Dai, J.; Wang, T.; Wang, W.; Zhang, S.; Liao, Y.; Chen, J. Role of MAPK7 in cell proliferation and metastasis in ovarian cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 10444–10451. [Google Scholar]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef]
- Hayashi, M.; Fearns, C.; Eliceiri, B.; Yang, Y.; Lee, J.D. Big mitogen-activated protein kinase 1/extracellular signal-regulated kinase 5 signaling pathway is essential for tumor-associated angiogenesis. Cancer Res. 2005, 65, 7699–7706. [Google Scholar] [CrossRef]
- Vanchin, B.; Sol, M.; Gjaltema, R.A.F.; Brinker, M.; Kiers, B.; Pereira, A.C.; Harmsen, M.C.; Moonen, J.A.J.; Krenning, G. Reciprocal regulation of endothelial–mesenchymal transition by MAPK7 and EZH2 in intimal hyperplasia and coronary artery disease. Sci. Rep. 2021, 11, 17764. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.E.; Jin, J.; Zi, M.; Prehar, S.; Liu, W.; Oceandy, D.; Abe, J.; Neyses, L.; Weston, A.H.; Cartwright, E.J.; et al. Targeted deletion of the extracellular signal-regulated protein kinase 5 attenuates hypertrophic response and promotes pressure overload-induced apoptosis in the heart. Circ. Res. 2010, 106, 961–970. [Google Scholar] [CrossRef] [PubMed]
- García-Hoz, C.; Sánchez-Fernández, G.; García-Escudero, R.; Fernández-Velasco, M.; Palacios-García, J.; Ruiz-Meana, M.; Díaz-Meco, M.T.; Leitges, M.; Moscat, J.; García-Dorado, D.; et al. Protein kinase C (PKC)ζ-mediated Gαq stimulation of ERK5 protein pathway in cardiomyocytes and cardiac fibroblasts. J. Biol. Chem. 2012, 287, 7792–7802. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, J.H.; Lim, H.J.; Park, H.Y. HB-EGF induces cardiomyocyte hypertrophy via an ERK5-MEF2A-COX2 signaling pathway. Cell. Signal. 2011, 23, 1100–1109. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.W.; Wang, W.; Abel, G.; Zou, J.; Xu, L.; Storm, D.R.; Xia, Z. Targeted deletion of the ERK5 MAP kinase impairs neuronal differentiation, migration, and survival during adult neurogenesis in the olfactory bulb. PLoS ONE 2013, 8, e61948. [Google Scholar] [CrossRef] [PubMed]
- Middleton, R.C.; Liao, K.; Liu, W.; de Couto, G.; Garcia, N.; Antes, T.; Wang, Y.; Wu, D.; Li, X.; Tourtellotte, W.G.; et al. Newt A1 cell-derived extracellular vesicles promote mammalian nerve growth. Sci. Rep. 2023, 13, 11829. [Google Scholar] [CrossRef]
- Cavanaugh, J.E. Role of extracellular signal regulated kinase 5 in neuronal survival. Eur. J. Biochem. 2004, 271, 2056–2059. [Google Scholar] [CrossRef]
- van Oterendorp, C.; Sgouris, S.; Schallner, N.; Biermann, J.; Lagrèze, W.A. Retrograde neurotrophic signaling in rat retinal ganglion cells is transmitted via the ERK5 but not the ERK1/2 pathway. Investig. Ophthalmol. Vis. Sci. 2014, 55, 658–665. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Mizushima, T.; Sakurai, J.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Fukuoka, T.; Noguchi, K. Roles of extracellular signal-regulated protein kinases 5 in spinal microglia and primary sensory neurons for neuropathic pain. J. Neurochem. 2007, 102, 1569–1584. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.N.; Sun, L.H.; Wang, M.; Yan, M. Research progress of the role and mechanism of extracellular signal-regulated protein kinase 5 (ERK5) pathway in pathological pain. J. Zhejiang Univ. Sci. B 2016, 17, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, J.; Yu, W.; Li, D.; Zhang, Y.; Wan, F.; Kou, X. Biochanin A protects against PM(2.5)-induced acute pulmonary cell injury by interacting with the target protein MEK5. Food Funct. 2019, 10, 7188–7203. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fdez, A.; Re-Louhau, M.F.; Rodríguez-Núñez, P.; Ludeña, D.; Matilla-Almazán, S.; Pandiella, A.; Esparís-Ogando, A. Clinical, genetic and pharmacological data support targeting the MEK5/ERK5 module in lung cancer. NPJ Precis. Oncol. 2021, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, Y.; Yoshizumi, M.; Kagami, S.; Nishiyama, A.; Ozawa, Y.; Kyaw, M.; Izawa, Y.; Kanematsu, Y.; Tsuchiya, K.; Tamaki, T. BMK1 is activated in glomeruli of diabetic rats and in mesangial cells by high glucose conditions. Kidney Int. 2004, 65, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Urushihara, M.; Takamatsu, M.; Shimizu, M.; Kondo, S.; Kinoshita, Y.; Suga, K.; Kitamura, A.; Matsuura, S.; Yoshizumi, M.; Tamaki, T.; et al. ERK5 activation enhances mesangial cell viability and collagen matrix accumulation in rat progressive glomerulonephritis. Am. J. Physiol. Ren. Physiol. 2010, 298, F167–F176. [Google Scholar] [CrossRef] [PubMed]
- Sarközi, R.; Miller, B.; Pollack, V.; Feifel, E.; Mayer, G.; Sorokin, A.; Schramek, H. ERK1/2-driven and MKP-mediated inhibition of EGF-induced ERK5 signaling in human proximal tubular cells. J. Cell. Physiol. 2007, 211, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Tapping, R.I.; Huang, S.; Watson, M.H.; Ulevitch, R.J.; Lee, J.D. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 1998, 395, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Oviedo, L.; Giménez-Bachs, J.M.; Nam-Cha, S.Y.; Cimas, F.J.; García-Cano, J.; Sánchez-Prieto, R.; Salinas-Sánchez, A.S. Implication of VHL, ERK5, and HIF-1alpha in clear cell renal cell carcinoma: Molecular basis. Urol. Oncol. 2017, 35, 114.e115–114.e122. [Google Scholar] [CrossRef]
- Salinas-Sánchez, A.S.; Serrano-Oviedo, L.; Nam-Cha, S.Y.; Roche-Losada, O.; Sánchez-Prieto, R.; Giménez-Bachs, J.M. Prognostic Value of the VHL, HIF-1α, and VEGF Signaling Pathway and Associated MAPK (ERK1/2 and ERK5) Pathways in Clear-Cell Renal Cell Carcinoma. A Long-Term Study. Clin. Genitourin. Cancer 2017, 15, e923–e933. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, Z.; Wang, G.; Hao, X.; Zhang, L.; Xu, C. 6 Paths of ERK5 signaling pathway regulate hepatocyte proliferation in rat liver regeneration. Indian J. Biochem. Biophys. 2012, 49, 165–172. [Google Scholar] [PubMed]
- Finegan, K.G.; Perez-Madrigal, D.; Hitchin, J.R.; Davies, C.C.; Jordan, A.M.; Tournier, C. ERK5 is a critical mediator of inflammation-driven cancer. Cancer Res. 2015, 75, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ryu, J.; Min, E.; Oi, N.; Bai, R.; Zykova, T.A.; Yu, D.H.; Moriyama, K.; Bode, A.M.; Dong, Z. TRAF1 Is Critical for DMBA/Solar UVR-Induced Skin Carcinogenesis. J. Investig. Dermatol. 2017, 137, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sahoo, A.; Sawada, J.; Zisoulis, D.G.; Marchica, J.; Sahoo, S.; Layng, F.I.A.D.L.; Finlay, D.; Mazar, J.; Joshi, P.; et al. microRNA-211 promotes aggressive melanoma growth in vivo by epigenetic modification, and contributes to BRAFV600E inhibitor resistance via ERK5 signaling. Cancer Res. 2019, 79, 3550. [Google Scholar] [CrossRef]
- Giurisato, E.; Lonardi, S.; Telfer, B.; Lussoso, S.; Risa-Ebrí, B.; Zhang, J.; Russo, I.; Wang, J.; Santucci, A.; Finegan, K.G.; et al. Extracellular-Regulated Protein Kinase 5-Mediated Control of p21 Expression Promotes Macrophage Proliferation Associated with Tumor Growth and Metastasis. Cancer Res. 2020, 80, 3319–3330. [Google Scholar] [CrossRef] [PubMed]
- Razumovskaya, E.; Sun, J.; Rönnstrand, L. Inhibition of MEK5 by BIX02188 induces apoptosis in cells expressing the oncogenic mutant FLT3-ITD. Biochem. Biophys. Res. Commun. 2011, 412, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Tusa, I.; Cheloni, G.; Gray, N.; Gozzini, A.; Dello Sbarba, P.; Rovida, E. Inhibition of the ERK5 pathway as a novel approach to target human chronic myeloid leukemia stem cells. Cancer Res. 2017, 77, 3904. [Google Scholar] [CrossRef]
- Mehta, P.B.; Jenkins, B.L.; McCarthy, L.; Thilak, L.; Robson, C.N.; Neal, D.E.; Leung, H.Y. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene 2003, 22, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Dudderidge, T.J.; McCracken, S.R.; Loddo, M.; Fanshawe, T.R.; Kelly, J.D.; Neal, D.E.; Leung, H.Y.; Williams, G.H.; Stoeber, K. Mitogenic growth signalling, DNA replication licensing, and survival are linked in prostate cancer. Br. J. Cancer 2007, 96, 1384–1393. [Google Scholar] [CrossRef]
- Loveridge, C.J.; Mui, E.J.; Patel, R.; Tan, E.H.; Ahmad, I.; Welsh, M.; Galbraith, J.; Hedley, A.; Nixon, C.; Blyth, K.; et al. Increased T-cell Infiltration Elicited by Erk5 Deletion in a Pten-Deficient Mouse Model of Prostate Carcinogenesis. Cancer Res. 2017, 77, 3158–3168. [Google Scholar] [CrossRef]
- Takaoka, Y.; Shimizu, Y.; Hasegawa, H.; Ouchi, Y.; Qiao, S.; Nagahara, M.; Ichihara, M.; Lee, J.D.; Adachi, K.; Hamaguchi, M.; et al. Forced expression of miR-143 represses ERK5/c-Myc and p68/p72 signaling in concert with miR-145 in gut tumors of Apc(Min) mice. PLoS ONE 2012, 7, e42137. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, J.; Tang, H.; Bai, L.; Lu, C.; Wang, K.; Li, M.; Yan, Y.; Tang, L.; Wu, R.; et al. EGCG Suppresses ERK5 Activation to Reverse Tobacco Smoke-Triggered Gastric Epithelial–mesenchymal Transition in BALB/c Mice. Nutrients 2016, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Lin, X.; Su, S.; Hou, X.; Zhang, Q.; Tian, Y. The Drug Combination of SB202190 and SP600125 Significantly Inhibit the Growth and Metastasis of Olaparib-resistant Ovarian Cancer Cell. Curr. Pharm. Biotechnol. 2018, 19, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fdez, A.; Matilla-Almazán, S.; Montero, J.C.; Del Carmen, S.; Abad, M.; García-Alonso, S.; Bhattacharya, S.; Calar, K.; de la Puente, P.; Ocaña, A.; et al. The WNK1-ERK5 route plays a pathophysiological role in ovarian cancer and limits therapeutic efficacy of trametinib. Clin. Transl. Med. 2023, 13, e1217. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Rovida, E. Impact of ERK5 on the Hallmarks of Cancer. Int. J. Mol. Sci. 2019, 20, 1426. [Google Scholar] [CrossRef] [PubMed]
- Charlson, A.T.; Zeliadt, N.A.; Wattenberg, E.V. Extracellular signal regulated kinase 5 mediates signals triggered by the novel tumor promoter palytoxin. Toxicol. Appl. Pharmacol. 2009, 241, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.B.; Patel, S.; Matossian, M.D.; Ucar, D.A.; Miele, L.; Burow, M.E.; Flaherty, P.T.; Cavanaugh, J.E. Molecular Mechanisms of Epithelial to Mesenchymal Transition Regulated by ERK5 Signaling. Biomolecules 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zhang, M.; Yang, P.; Huang, Y.; Hu, X.; Cai, J.; Yang, C.; Situ, M.; Zhang, H.; Fu, L.; et al. Identification of De Novo JAK2 and MAPK7 Mutations Related to Autism Spectrum Disorder Using Whole-Exome Sequencing in a Chinese Child and Adolescent Trio-Based Sample. J. Mol. Neurosci. 2020, 70, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, K.; Xu, F.; Farrar, K.; Tran, A.; Khakpour, S.; Sundar, S.; Prakash, A.; Wang, J.; Gray, N.S.; Hellman, J. Extracellular signal-regulated kinase 5 promotes acute cellular and systemic inflammation. Sci. Signal. 2015, 8, ra86. [Google Scholar] [CrossRef]
- Yang, M.; Cooley, B.C.; Li, W.; Chen, Y.; Vasquez-Vivar, J.; Scoggins, N.O.; Cameron, S.J.; Morrell, C.N.; Silverstein, R.L. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood 2017, 129, 2917–2927. [Google Scholar] [CrossRef]
- Nagel, S.; Burek, C.; Venturini, L.; Scherr, M.; Quentmeier, H.; Meyer, C.; Rosenwald, A.; Drexler, H.G.; MacLeod, R.A. Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood 2007, 109, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Devost, D.; Zingg, H.H.; Hébert, T.E. The MAP kinase ERK5/MAPK7 is a downstream effector of oxytocin signaling in myometrial cells. Cell. Signal. 2022, 90, 110211. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.R.; Vasquez, Y.M.; Mo, Q.; Gibbons, W.; Kovanci, E.; DeMayo, F.J. WNK lysine deficient protein kinase 1 regulates human endometrial stromal cell decidualization, proliferation, and migration in part through mitogen-activated protein kinase 7. Biol. Reprod. 2017, 97, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Fukasawa, K.; Yamada, T.; Mizuno, S.; Iezaki, T.; Tokumura, K.; Iwahashi, S.; Sakai, S.; Suzuki, A.; Kubo, T.; et al. Erk5 in Bone Marrow Mesenchymal Stem Cells Regulates Bone Homeostasis by Preventing Osteogenesis in Adulthood. Stem Cells 2022, 40, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, Y.C.; Sheng, X.Y.; Dong, H.T.; Han, H.; Wang, J.; Xia, Y.Y. Cyclic fluid shear stress promotes osteoblastic cells proliferation through ERK5 signaling pathway. Mol. Cell. Biochem. 2012, 364, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, Y.C.; Shen, H.L.; Han, H.; Wang, J.; Cheng, H.J.; Wang, C.F.; Xia, Y.Y. Cytoskeletal reorganization mediates fluid shear stress-induced ERK5 activation in osteoblastic cells. Cell Biol. Int. 2012, 36, 229–236. [Google Scholar] [CrossRef]
- Zhao, L.G.; Chen, S.L.; Teng, Y.J.; An, L.P.; Wang, J.; Ma, J.L.; Xia, Y.Y. The MEK5/ERK5 pathway mediates fluid shear stress promoted osteoblast differentiation. Connect. Tissue Res. 2014, 55, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bin, G.; Bo, Z.; Jing, W.; Jin, J.; Xiaoyi, T.; Cong, C.; Liping, A.; Jinglin, M.; Cuifang, W.; Yonggang, C.; et al. Fluid shear stress suppresses TNF-α-induced apoptosis in MC3T3-E1 cells: Involvement of ERK5-AKT-FoxO3a-Bim/FasL signaling pathways. Exp. Cell Res. 2016, 343, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, S.; Otsuki, D.; Yoshida, K.; Yoshikawa, H.; Higuchi, C. MEK5 suppresses osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2015, 463, 241–247. [Google Scholar] [CrossRef]
- Bo, Z.; Bin, G.; Jing, W.; Cuifang, W.; Liping, A.; Jinglin, M.; Jin, J.; Xiaoyi, T.; Cong, C.; Ning, D.; et al. Fluid shear stress promotes osteoblast proliferation via the Gαq-ERK5 signaling pathway. Connect. Tissue Res. 2016, 57, 299–306. [Google Scholar] [CrossRef]
- Ding, N.; Geng, B.; Li, Z.; Yang, Q.; Yan, L.; Wan, L.; Zhang, B.; Wang, C.; Xia, Y. Fluid shear stress promotes osteoblast proliferation through the NFATc1-ERK5 pathway. Connect. Tissue Res. 2019, 60, 107–116. [Google Scholar] [CrossRef]
- Zhang, B.; An, L.; Geng, B.; Ding, N.; Coalson, E.; Wan, L.; Yan, L.; Mohammed, F.H.A.; Ma, C.; Li, R.; et al. ERK5 negatively regulates Kruppel-like factor 4 and promotes osteogenic lineage cell proliferation in response to MEK5 overexpression or fluid shear stress. Connect. Tissue Res. 2021, 62, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Geng, B.; Wang, H.; Wang, S.; Zhao, D.; He, J.; Lu, F.; An, J.; Wang, C.; Xia, Y. Fluid shear stress-induced down-regulation of microRNA-140-5p promotes osteoblast proliferation by targeting VEGFA via the ERK5 pathway. Connect. Tissue Res. 2022, 63, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Bobick, B.E.; Matsche, A.I.; Chen, F.H.; Tuan, R.S. The ERK5 and ERK1/2 signaling pathways play opposing regulatory roles during chondrogenesis of adult human bone marrow-derived multipotent progenitor cells. J. Cell. Physiol. 2010, 224, 178–186. [Google Scholar] [CrossRef]
- Chang, S.F.; Huang, K.C.; Chang, H.I.; Lee, K.C.; Su, Y.P.; Chen, C.N. 2 dyn/cm2 shear force upregulates kruppel-like factor 4 expression in human chondrocytes to inhibit the interleukin-1β-activated nuclear factor-κB. J. Cell. Physiol. 2018, 234, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Iezaki, T.; Fukasawa, K.; Horie, T.; Park, G.; Robinson, S.; Nakaya, M.; Fujita, H.; Onishi, Y.; Ozaki, K.; Kanayama, T.; et al. The MAPK Erk5 is necessary for proper skeletogenesis involving a Smurf-Smad-Sox9 molecular axis. Development 2018, 145, dev164004. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Liu, W.; Meng, H.; Song, Y.; Wang, W. Sox9-induced chondrogenesis in mesenchymal stem cells was mediated by ERK5 signal pathway. Cell. Mol. Biol. 2016, 62, 1–7. [Google Scholar] [PubMed]
- Wu, C.; Liu, H.; Zhong, D.; Yang, X.; Liao, Z.; Chen, Y.; Zhang, S.; Su, D.; Zhang, B.; Li, C.; et al. Mapk7 deletion in chondrocytes causes vertebral defects by reducing MEF2C/PTEN/AKT signaling. Genes Dis. 2024, 11, 964–977. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, D.; Gao, W.; Liao, Z.; Chen, Y.; Zhang, S.; Zhou, H.; Su, P.; Xu, C. Conditional ablation of MAPK7 expression in chondrocytes impairs endochondral bone formation in limbs and adaptation of chondrocytes to hypoxia. Cell Biosci. 2020, 10, 103. [Google Scholar] [CrossRef]
- Adam, C.; Glück, L.; Ebert, R.; Goebeler, M.; Jakob, F.; Schmidt, M. The MEK5/ERK5 mitogen-activated protein kinase cascade is an effector pathway of bone-sustaining bisphosphonates that regulates osteogenic differentiation and mineralization. Bone 2018, 111, 49–58. [Google Scholar] [CrossRef]
- Ansari, N.; Ho, P.W.; Crimeen-Irwin, B.; Poulton, I.J.; Brunt, A.R.; Forwood, M.R.; Divieti Pajevic, P.; Gooi, J.H.; Martin, T.J.; Sims, N.A. Autocrine and Paracrine Regulation of the Murine Skeleton by Osteocyte-Derived Parathyroid Hormone-Related Protein. J. Bone Miner. Res. 2018, 33, 137–153. [Google Scholar] [CrossRef]
- Park-Min, K.H.; Lorenzo, J. Osteoclasts: Other functions. Bone 2022, 165, 116576. [Google Scholar] [CrossRef]
- Amano, S.; Chang, Y.T.; Fukui, Y. ERK5 activation is essential for osteoclast differentiation. PLoS ONE 2015, 10, e0125054. [Google Scholar] [CrossRef] [PubMed]
- Loveridge, C.J.; van ’t Hof, R.J.; Charlesworth, G.; King, A.; Tan, E.H.; Rose, L.; Daroszewska, A.; Prior, A.; Ahmad, I.; Welsh, M.; et al. Analysis of Nkx3.1:Cre-driven Erk5 deletion reveals a profound spinal deformity which is linked to increased osteoclast activity. Sci. Rep. 2017, 7, 13241. [Google Scholar] [CrossRef]
- Ma, C.; Geng, B.; Zhang, X.; Li, R.; Yang, X.; Xia, Y. Fluid Shear Stress Suppresses Osteoclast Differentiation in RAW264.7 Cells through Extracellular Signal-Regulated Kinase 5 (ERK5) Signaling Pathway. Med. Sci. Monit. 2020, 26, e918370. [Google Scholar] [CrossRef]
- Ramos-Junior, E.S.; Leite, G.A.; Carmo-Silva, C.C.; Taira, T.M.; Neves, K.B.; Colón, D.F.; da Silva, L.A.; Salvador, S.L.; Tostes, R.C.; Cunha, F.Q.; et al. Adipokine Chemerin Bridges Metabolic Dyslipidemia and Alveolar Bone Loss in Mice. J. Bone Miner. Res. 2017, 32, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, C.; Zhou, T.; Yang, S.; Gao, B.; Zhou, H.; Lian, C.; Wu, Z.; Qiu, X.; Yang, X.; et al. Rare coding variants in MAPK7 predispose to adolescent idiopathic scoliosis. Hum. Mutat. 2017, 38, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Guevara, J.M.; Moncayo, M.A.; Vaca-González, J.J.; Gutiérrez, M.L.; Barrera, L.A.; Garzón-Alvarado, D.A. Growth plate stress distribution implications during bone development: A simple framework computational approach. Comput. Methods Programs Biomed. 2015, 118, 59–68. [Google Scholar] [CrossRef]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2022, 110, 592–604. [Google Scholar] [CrossRef]
- Stavnichuk, M.; Mikolajewicz, N.; Corlett, T.; Morris, M.; Komarova, S.V. A systematic review and meta-analysis of bone loss in space travelers. NPJ Microgravity 2020, 6, 13. [Google Scholar] [CrossRef]
- Masini, M.A.; Bonetto, V.; Manfredi, M.; Pastò, A.; Barberis, E.; Timo, S.; Vanella, V.V.; Robotti, E.; Masetto, F.; Andreoli, F.; et al. Prolonged exposure to simulated microgravity promotes stemness impairing morphological, metabolic and migratory profile of pancreatic cancer cells: A comprehensive proteomic, lipidomic and transcriptomic analysis. Cell. Mol. Life Sci. 2022, 79, 226. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Liu, Y.; Zhao, X.; Meng, C.; Zhang, Y.; Duan, Y.; Wang, G. Research progress in the mechanism and treatment of osteosarcoma. Chin. Med. J. 2023, 136, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- van Dartel, M.; Cornelissen, P.W.; Redeker, S.; Tarkkanen, M.; Knuutila, S.; Hogendoorn, P.C.; Westerveld, A.; Gomes, I.; Bras, J.; Hulsebos, T.J. Amplification of 17p11.2 approximately p12, including PMP22, TOP3A, and MAPK7, in high-grade osteosarcoma. Cancer Genet. Cytogenet. 2002, 139, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, H.; Park, Y.S.; Lee, Y.; Seo, S.W. ERK5 regulates invasiveness of osteosarcoma by inducing MMP-9. J. Orthop. Res. 2012, 30, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Ren, Q.X.; Su, T.; Wang, L.N.; Zhang, L. ERK5 silencing inhibits invasion of human osteosarcoma cell via modulating the Slug/MMP-9 pathway. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2640–2647. [Google Scholar]
- Green, D.; Eyre, H.; Singh, A.; Taylor, J.T.; Chu, J.; Jeys, L.; Sumathi, V.; Coonar, A.; Rassl, D.; Babur, M.; et al. Targeting the MAPK7/MMP9 axis for metastasis in primary bone cancer. Oncogene 2020, 39, 5553–5569. [Google Scholar] [CrossRef] [PubMed]
- Tesser-Gamba, F.; Petrilli, A.S.; Paniago, M.D.G.; Seixas Alves, M.T.; Garcia-Filho, R.J.; Toledo, S.R.C. MAPK7 gene: A target for multimodal therapies. J. Clin. Oncol. 2013, 31, 10533. [Google Scholar] [CrossRef]
- Tesser-Gamba, F.; Lopes, L.J.; Petrilli, A.S.; Toledo, S.R. MAPK7 gene controls proliferation, migration and cell invasion in osteosarcoma. Mol. Carcinog. 2016, 55, 1700–1713. [Google Scholar] [CrossRef]
- Tesser-Gamba, F.; Petrilli, A.S.; de Seixas Alves, M.T.; Filho, R.J.; Juliano, Y.; Toledo, S.R. MAPK7 and MAP2K4 as prognostic markers in osteosarcoma. Hum. Pathol. 2012, 43, 994–1002. [Google Scholar] [CrossRef]
- Silva Lopes, L.J.; Petrilli, A.S.; Tesser-Gamba, F.; Seixas Alves, M.T.; Garcia-Filho, R.J.; Toledo, S.R.C. Gene expression of the MAPK pathway in osteosarcoma. J. Clin. Oncol. 2013, 31, 10534. [Google Scholar] [CrossRef]
- Monti, M.; Celli, J.; Missale, F.; Cersosimo, F.; Russo, M.; Belloni, E.; Di Matteo, A.; Lonardi, S.; Vermi, W.; Ghigna, C.; et al. Clinical Significance and Regulation of ERK5 Expression and Function in Cancer. Cancers 2022, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.E.; Rodrigues, C.M.; Borralho, P.M. The MEK5/ERK5 signalling pathway in cancer: A promising novel therapeutic target. Drug Discov. Today 2016, 21, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lv, B.; Li, Y.; Cheng, Q.; Su, C.; Yin, G. MiR-143 regulates the proliferation and migration of osteosarcoma cells through targeting MAPK7. Arch. Biochem. Biophys. 2017, 630, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Vergara, X.; Tabera, S.; Montero, J.C.; Esparís-Ogando, A.; López-Pérez, R.; Mateo, G.; Gutiérrez, N.; Parmo-Cabañas, M.; Teixidó, J.; San Miguel, J.F.; et al. Multifunctional role of Erk5 in multiple myeloma. Blood 2005, 105, 4492–4499. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, S.; Ortiz-Ruiz, M.J.; Parrott, T.; Zaknoen, S.; Ocio, E.M.; San Miguel, J.; Burrows, F.J.; Esparís-Ogando, A.; Pandiella, A. Potent antimyeloma activity of a novel ERK5/CDK inhibitor. Clin. Cancer Res. 2013, 19, 2677–2687. [Google Scholar] [CrossRef]
- Abelson, A.; Ringe, J.D.; Gold, D.T.; Lange, J.L.; Thomas, T. Longitudinal change in clinical fracture incidence after initiation of bisphosphonates. Osteoporos. Int. 2010, 21, 1021–1029. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Synthesis and biological activities of drugs for the treatment of osteoporosis. Eur. J. Med. Chem. 2020, 197, 112313. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhong, Y.H.; Chen, L.M.; Zhuo, X.L.; Zhao, L.J.; Wang, Y.T. Recent advance of small-molecule drugs for clinical treatment of osteoporosis: A review. Eur. J. Med. Chem. 2023, 259, 115654. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Zhao, D.; Geng, B.; Xia, Y. Mangiferin promotes osteogenic differentiation and alleviates osteoporosis in the ovariectomized mouse via the AXL/ERK5 pathway. Front. Pharmacol. 2022, 13, 1028932. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhao, D.; Peng, B.; Wang, X.; Wang, S.; Zhao, X.; Xu, P.; Geng, B.; Xia, Y. A novel mechanism of Vildagliptin in regulating bone metabolism and mitigating osteoporosis. Int. Immunopharmacol. 2024, 130, 111671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Deng, X.; Lu, B.; Cameron, M.; Fearns, C.; Patricelli, M.P.; Yates, J.R., 3rd; Gray, N.S.; Lee, J.D. Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell 2010, 18, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.I.; Imanishi, M.; Li, S.; Zhang, A.; Ko, K.A.; Samanthapudi, V.S.K.; Lee, L.L.; Bojorges, A.P.; Gi, Y.J.; Hobbs, B.P.; et al. An ERK5-NRF2 Axis Mediates Senescence-Associated Stemness and Atherosclerosis. Circ. Res. 2023, 133, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Tatake, R.J.; O’Neill, M.M.; Kennedy, C.A.; Wayne, A.L.; Jakes, S.; Wu, D.; Kugler, S.Z., Jr.; Kashem, M.A.; Kaplita, P.; Snow, R.J. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem. Biophys. Res. Commun. 2008, 377, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.C.; Amantea, C.M.; Nomanbhoy, T.K.; Weissig, H.; Ishiyama, J.; Hu, Y.; Sidique, S.; Li, B.; Kozarich, J.W.; Rosenblum, J.S. ERK5 kinase activity is dispensable for cellular immune response and proliferation. Proc. Natl. Acad. Sci. USA 2016, 113, 11865–11870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Yan, H.L. MiR-24 promotes the proliferation and apoptosis of lung carcinoma via targeting MAPK7. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6845–6852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, X.; Duan, X.; Liang, H.; Gao, M.; Dong, D.; Guo, C.; Huang, L. Design, synthesis and SAR of novel 7-azaindole derivatives as potential Erk5 kinase inhibitor with anticancer activity. Bioorganic Med. Chem. 2023, 95, 117503. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.M.; Bawn, R.H.; Bisset, L.C.; Blackburn, T.J.; Cottyn, B.; Molyneux, L.; Wong, A.C.; Cano, C.; Clegg, W.; Harrington, R.W.; et al. High-Throughput Screening and Hit Validation of Extracellular-Related Kinase 5 (ERK5) Inhibitors. ACS Comb. Sci. 2016, 18, 444–455. [Google Scholar] [CrossRef]
- Nguyen, D.; Lemos, C.; Wortmann, L.; Eis, K.; Holton, S.J.; Boemer, U.; Moosmayer, D.; Eberspaecher, U.; Weiske, J.; Lechner, C.; et al. Discovery and Characterization of the Potent and Highly Selective (Piperidin-4-yl)pyrido [3,2- d]pyrimidine Based in Vitro Probe BAY-885 for the Kinase ERK5. J. Med. Chem. 2019, 62, 928–940. [Google Scholar] [CrossRef]
- Khan, A.U.H.; Allende-Vega, N.; Gitenay, D.; Gerbal-Chaloin, S.; Gondeau, C.; Vo, D.N.; Belkahla, S.; Orecchioni, S.; Talarico, G.; Bertolini, F.; et al. The PDK1 Inhibitor Dichloroacetate Controls Cholesterol Homeostasis Through the ERK5/MEF2 Pathway. Sci. Rep. 2017, 7, 10654. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.J.; Lee, C.A.; Batoki, J.C.; Zapadka, T.E.; Lindstrom, S.I.; Taylor, B.E.; Taylor, P.R. Retinal Inflammation, Oxidative Stress, and Vascular Impairment Is Ablated in Diabetic Mice Receiving XMD8-92 Treatment. Front. Pharmacol. 2021, 12, 732630. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Samsonraj, R.M.; Munmun, F.; Glas, J.; Silvestros, M.; Kotlarczyk, M.P.; Rylands, R.; Dudakovic, A.; van Wijnen, A.J.; Enderby, L.T.; et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 2018, 64, e12465. [Google Scholar] [CrossRef] [PubMed]
- Kedika, S.R.; Shukla, S.P.; Udugamasooriya, D.G. Design of a dual ERK5 kinase activation and autophosphorylation inhibitor to block cancer stem cell activity. Bioorganic Med. Chem. Lett. 2020, 30, 127552. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Xie, W.; De Leon, J.P.; Burrows, F.; Feldman, E.J.; Roboz, G.J. Leukemia Stem/Progenitor Cells from AML Patients Treated with the Multi-Kinase Inhibitor TG02 Demonstrate Increased Proliferation and Are Sensitized to Chemotherapeutic Agents. Blood 2013, 122, 3892. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Liu, J.; Shang, G. Targeted therapy for osteosarcoma: A review. J. Cancer Res. Clin. Oncol. 2023, 149, 6785–6797. [Google Scholar] [CrossRef]

| Distribution | Function | Disease | References |
|---|---|---|---|
| Heart | Maintain endothelial integrity Decrease cardiac endothelial cell permeability | - | [24] |
| Protect myocardium Improve heart function | Myocardial infarction | [25,26,27] | |
| Myocardial infarction combined with diabetes | [28] | ||
| Myocardial damage caused by hypothermia | [29] | ||
| Metabolic stress-induced cardiomyopathy | [30] | ||
| Inhibit cardiomyocyte apoptosis | Myocardial infarction | [31] | |
| Vascular endothelium | Developmentally necessary | - | [32,33,34,35] |
| Protect endothelial cells | - | [36] | |
| Anti-inflammation, inhibition of apoptosis | Atherosclerosis | [36,37,38] | |
| Inhibit endothelial-to-mesenchymal transition | Atherosclerosis | [37,39] | |
| Nervous system | Protect brain cells | Ischemia–reperfusion injury | [40] |
| Inhibit oxidative damage Inhibiting neuronal apoptosis | Traumatic brain injury | [41] | |
| Promote learning ability and memory | - | [42] | |
| Mediate damage signaling | Neuropathic pain | [43] | |
| Protect neurons from cell death | Oxidative stress | [44,45] | |
| Promote tumor cell migration | Invasion glioma | [46] | |
| Lung | Promote resolution of inflammation | Acute lung injury | [47] |
| promote pulmonary fibrosis | Pulmonary fibrosis | [48] | |
| Promote proliferation | Squamous cell lung cancer | [49] | |
| Promote cell proliferation | Non-small cell lung cancer | [50] | |
| Increase cell viability | Lung cancer | [51] | |
| Inhibit epithelial–mesenchymal transition | Lung cancer | [52,53] | |
| Promote invasion and migration | Lung cancer | [54] | |
| Promote drug resistance | Lung cancer | [55] | |
| Increase radiotherapy resistance | Lung cancer | [56] | |
| Promote immune evasion | Lung cancer | [57] | |
| Breast | Promote proliferation | Breast cancer | [58,59,60,61,62,63] |
| Promote invasion and migration | Breast cancer | [63,64,65,66,67,68,69,70,71] | |
| Inhibit epithelial–mesenchymal transition | Breast cancer | [72] | |
| Inhibits migration and invasion | Breast cancer | [73] | |
| Promote drug resistance | Breast cancer | [74] | |
| Kidney | Necessary for renal tubule development | - | [35] |
| Promote proliferation and contraction of renal mesangial cells | Kidney disease | [75] | |
| Promote podocyte proliferation | Diabetic nephropathy | [76] | |
| Protect the kidneys | Renal ischemia–reperfusion injury | [77] | |
| Promote mesangial cell migration | Chronic kidney disease | [78] | |
| Promote cancer cell proliferation | Kidney cancer | [79,80,81] | |
| Promote glomerular fibrosis | Glomerulonephritis | [82] | |
| Promote invasion and migration | Kidney cancer | [80] | |
| Clear cell renal cell carcinoma | [81] | ||
| Inhibit cancer cell apoptosis | Kidney cancer | [79] | |
| Liver | Promote hepatic stellate cell proliferation and inhibit migration. | Liver damage | [83] |
| Promote cancer cell migration | Liver cancer | [84] | |
| Promote cancer cell proliferation Promote cell cycle progression | Hepatocellular carcinoma | [80,85] | |
| Skin | Promotes an inflammatory tumor microenvironment | Melanoma | [86,87,88] |
| Promote proliferation of melanoma | Melanoma | [87,89] | |
| Cutaneous squamous cell carcinoma | [87] | ||
| Promote migration, invasion and lung metastasis | Melanoma | [54] | |
| Anti-cell aging | Melanoma | [90] | |
| Hematopoietic system | Helps leukemic cells survive | Leukemia | [91] |
| Regulates monocyte differentiation of human myeloid leukemia cells | Myeloid leukemia | [92] | |
| Promote proliferation | CML | [93,94] | |
| AML | [95] | ||
| Inhibit apoptosis | AML | [95,96] | |
| Promote drug resistance | CML | [97] | |
| Prostate | promote proliferation | Prostate cancer | [98,99,100,101] |
| Promote migration and invasion | Prostate cancer | [101,102] | |
| Esophagus | Promote proliferation | Esophageal cancer | [49] |
| Prognosis related | Esophageal cancer | [103] | |
| Oral cavity | Promote cancer invasion | Oral squamous cell carcinoma | [104] |
| Associated with advanced cancer stage and metastasis | Oral squamous cell carcinoma | [105] | |
| Bile duct | Promote proliferation, migration and invasion | Cholangiocarcinoma | [106,107] |
| Intestine | Promote proliferation | Rectal cancer | [108] |
| Promote intestinal development and maintain intestinal integrity | Intestinal agenesis (zebrafish) | [109] | |
| Stomach | Promote proliferation and migration | Gastric cancer | [110] |
| Ovary | Promote cancer proliferation, invasion and migration | Ovarian cancer | [111] |
| Drug | Mechanism | Disease | Reference |
|---|---|---|---|
| Nitrogen-containing bisphosphonates | Induce ERK5 phosphorylation and ERK5-dependent gene expression | Osteoporosis | [172] |
| mangiferin | Upregulated AXL | Osteoporosis | [202] |
| Vildagliptin | Upregulated AXL | Osteoporosis | [203] |
| TG02 | block CDKs 1, 2, and 9 as well as ERK5 activity | Multiple myeloma | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Liu, Z.; Zhou, L.; Liu, Z.; Li, Q.; Geng, B.; Xia, Y. Bone and Extracellular Signal-Related Kinase 5 (ERK5). Biomolecules 2024, 14, 556. https://doi.org/10.3390/biom14050556
Wen L, Liu Z, Zhou L, Liu Z, Li Q, Geng B, Xia Y. Bone and Extracellular Signal-Related Kinase 5 (ERK5). Biomolecules. 2024; 14(5):556. https://doi.org/10.3390/biom14050556
Chicago/Turabian StyleWen, Lei, Zirui Liu, Libo Zhou, Zhongcheng Liu, Qingda Li, Bin Geng, and Yayi Xia. 2024. "Bone and Extracellular Signal-Related Kinase 5 (ERK5)" Biomolecules 14, no. 5: 556. https://doi.org/10.3390/biom14050556
APA StyleWen, L., Liu, Z., Zhou, L., Liu, Z., Li, Q., Geng, B., & Xia, Y. (2024). Bone and Extracellular Signal-Related Kinase 5 (ERK5). Biomolecules, 14(5), 556. https://doi.org/10.3390/biom14050556







