Reelin Regulates Developmental Desynchronization Transition of Neocortical Network Activity
Abstract
:1. Introduction
2. Material and Methods
2.1. Reelin Conditional Knockout Mice (RelncKO)
2.2. PCR and Genotyping
2.3. Organotypic Cultures and Pharmacological Treatment
2.4. Ca2+ Imaging Using Spinning Disc Laser Microscopy
2.5. Analysis of Imaging Data
2.6. Statistical Analysis
3. Results
3.1. Developmental Desynchronization of Cortical Network Activity in the Somatosensory Cortex
3.2. Developmental Network Desynchronization Is Disrupted in Reelin-Deficient Mice
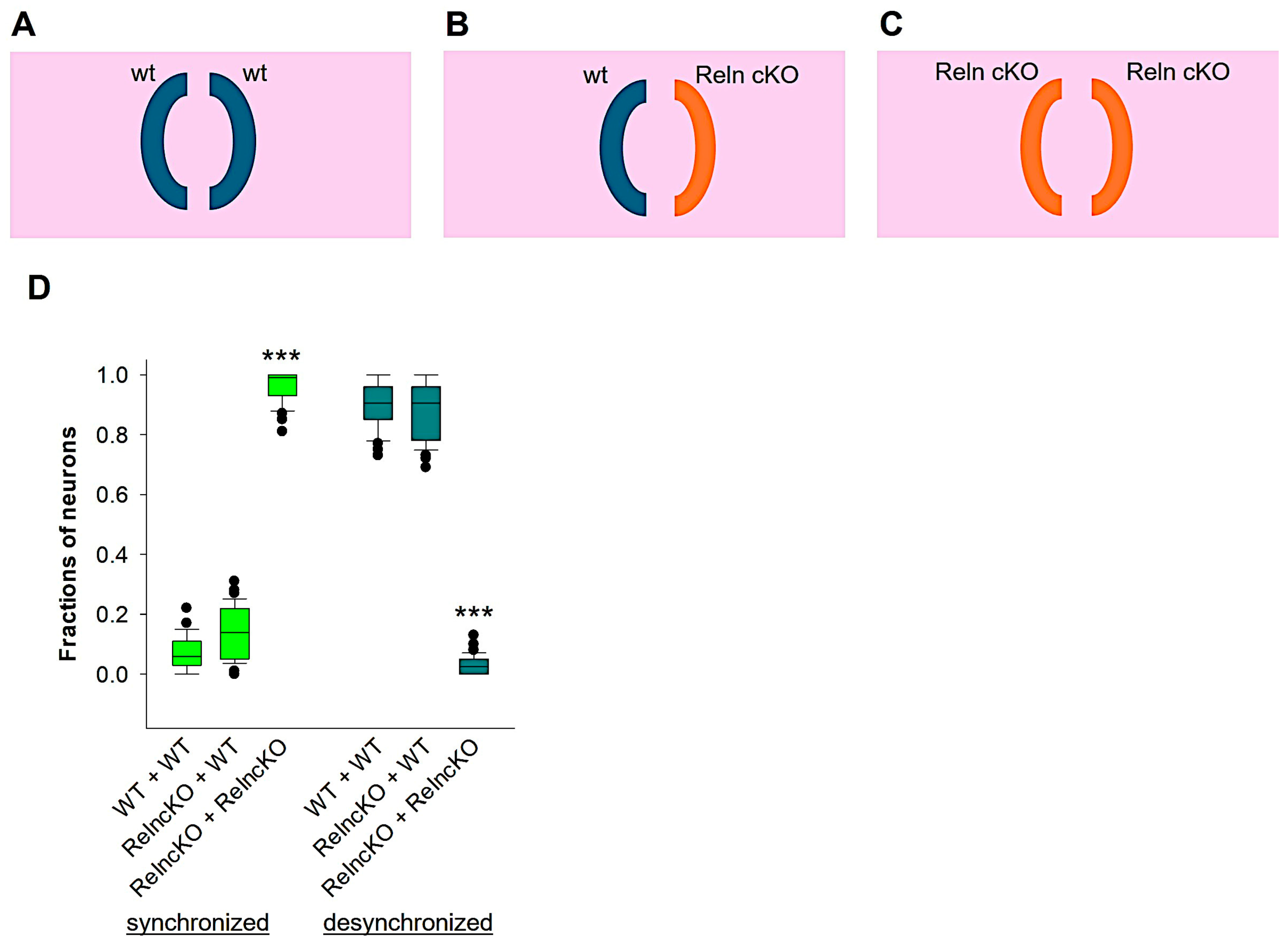
3.3. Wildtype Reelin Rescues Developmental Desynchronization in RelncKO Neurons
3.4. Developmental Network Desynchronization Is Controlled by Reelin Signaling
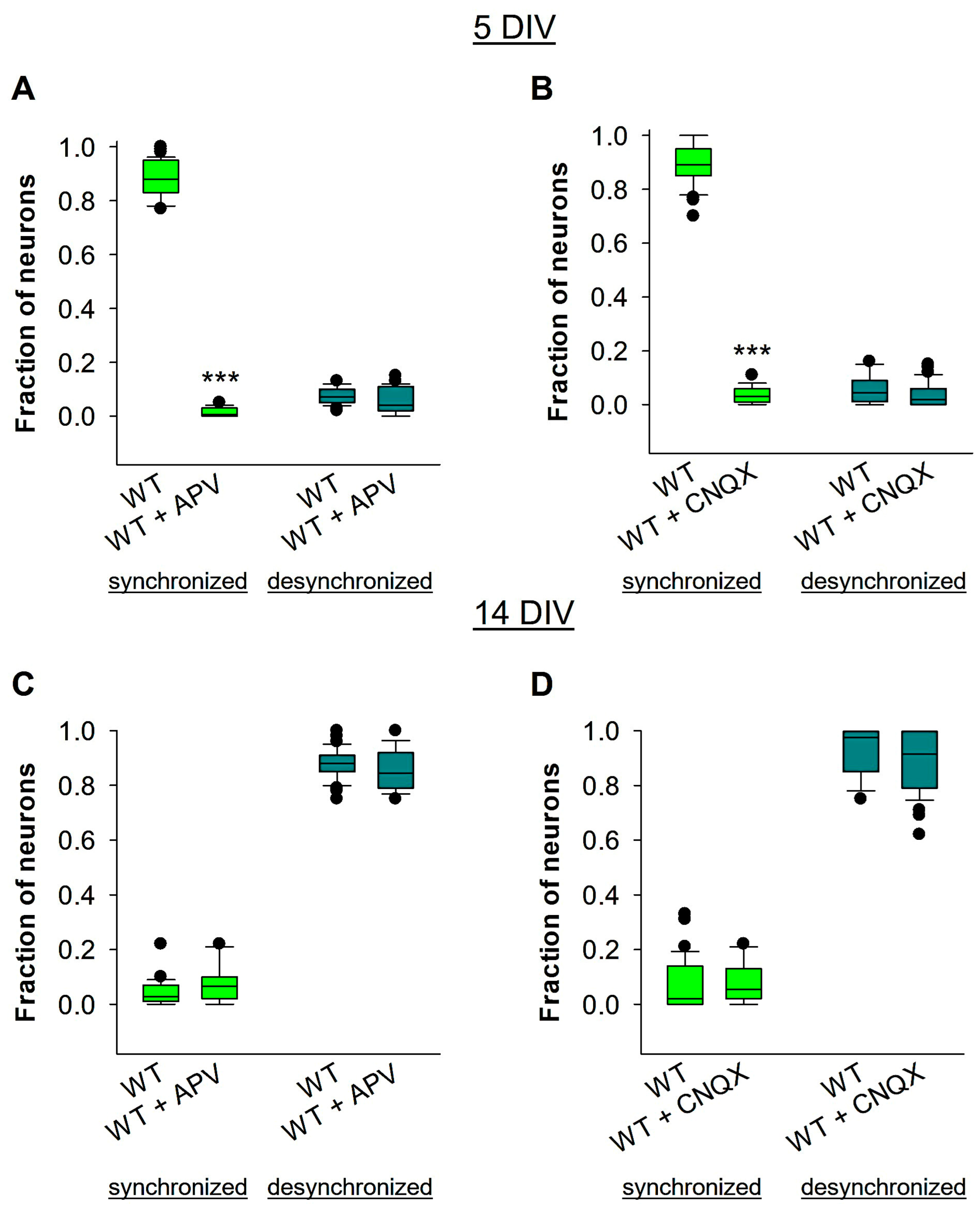
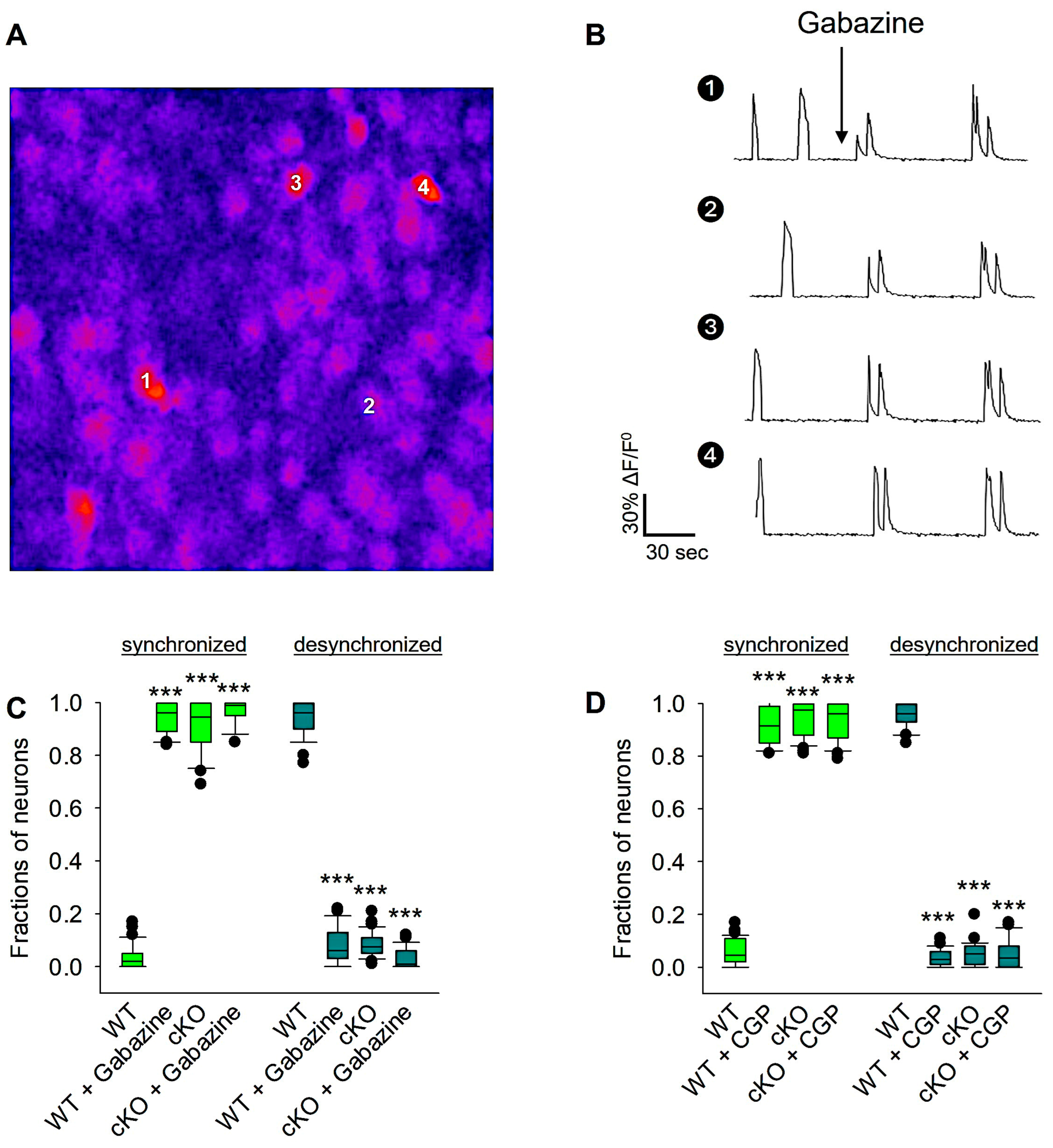
3.5. GABAergic, but Not Glutamatergic, Signaling Is Involved in Reelin-Mediated Developmental Desynchronization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khazipov, R.; Luhmann, H.J. Early Patterns of Electrical Activity in the Developing Cerebral Cortex of Humans and Rodents. Trends Neurosci. 2006, 29, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Garaschuk, O.; Linn, J.; Eilers, J.; Konnerth, A. Large-Scale Oscillatory Calcium Waves in the Immature Cortex. Nat. Neurosci. 2000, 3, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Moody, W.J.; Bosma, M.M. Ion Channel Development, Spontaneous Activity, and Activity-Dependent Development in Nerve and Muscle Cells. Physiol. Rev. 2005, 85, 883–941. [Google Scholar] [CrossRef] [PubMed]
- Allene, C.; Cossart, R. Early NMDA Receptor-Driven Waves of Activity in the Developing Neocortex: Physiological or Pathological Network Oscillations? J. Physiol. 2010, 588, 83–91. [Google Scholar] [CrossRef] [PubMed]
- McCabe, A.K.; Chisholm, S.L.; Picken-Bahrey, H.L.; Moody, W.J. The Self-Regulating Nature of Spontaneous Synchronized Activity in Developing Mouse Cortical Neurones. J. Physiol. 2006, 577, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Reyes-Puerta, V.; Kilb, W.; Luhmann, H.J. Spindle Bursts in Neonatal Rat Cerebral Cortex. Neural Plast. 2016, 2016, 3467832. [Google Scholar] [CrossRef] [PubMed]
- Golshani, P.; Gonçalves, J.T.; Khoshkhoo, S.; Mostany, R.; Smirnakis, S.; Portera-Cailliau, C. Internally Mediated Developmental Desynchronization of Neocortical Network Activity. J. Neurosci. 2009, 29, 10890–10899. [Google Scholar] [CrossRef]
- Luczak, A.; Maclean, J.N. Default Activity Patterns at the Neocortical Microcircuit Level. Front. Integr. Neurosci. 2012, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, F.; Heimel, J.A.; Peters, J.; Lohmann, C. Peripheral and Central Inputs Shape Network Dynamics in the Developing Visual Cortex in Vivo. Curr. Biol. 2012, 22, 253–258. [Google Scholar] [CrossRef]
- Yang, J.-W.; Hanganu-Opatz, I.L.; Sun, J.-J.; Luhmann, H.J. Three Patterns of Oscillatory Activity Differentially Synchronize Developing Neocortical Networks in Vivo. J. Neurosci. 2009, 29, 9011–9025. [Google Scholar] [CrossRef]
- Khazipov, R.; Sirota, A.; Leinekugel, X.; Holmes, G.L.; Ben-Ari, Y.; Buzsáki, G. Early Motor Activity Drives Spindle Bursts in the Developing Somatosensory Cortex. Nature 2004, 432, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E.; Hanganu, I.L.; Kilb, W.; Hirsch, S.; Luhmann, H.J. Rapid Developmental Switch in the Mechanisms Driving Early Cortical Columnar Networks. Nature 2006, 439, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J.; Khazipov, R. Neuronal Activity Patterns in the Developing Barrel Cortex. Neuroscience 2018, 368, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.J.; Guillamón-Vivancos, T.; Moreno-Juan, V.; Valdeolmillos, M.; López-Bendito, G. Spontaneous Activity in Developing Thalamic and Cortical Sensory Networks. Neuron 2021, 109, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Babola, T.A.; Li, S.; Gribizis, A.; Lee, B.J.; Issa, J.B.; Wang, H.C.; Crair, M.C.; Bergles, D.E. Homeostatic Control of Spontaneous Activity in the Developing Auditory System. Neuron 2018, 99, 511–524.e5. [Google Scholar] [CrossRef]
- Dreyfus-Brisac, C.; Larroche, J.C. Discontinuous electroencephalograms in the premature newborn and at term. Electro-anatomo-clinical correlations. Rev. Electroencephalogr. Neurophysiol. Clin. 1971, 1, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, I.L.; Ben-Ari, Y.; Khazipov, R. Retinal Waves Trigger Spindle Bursts in the Neonatal Rat Visual Cortex. J. Neurosci. 2006, 26, 6728–6736. [Google Scholar] [CrossRef] [PubMed]
- Colonnese, M.T.; Kaminska, A.; Minlebaev, M.; Milh, M.; Bloem, B.; Lescure, S.; Moriette, G.; Chiron, C.; Ben-Ari, Y.; Khazipov, R. A Conserved Switch in Sensory Processing Prepares Developing Neocortex for Vision. Neuron 2010, 67, 480–498. [Google Scholar] [CrossRef]
- Allène, C.; Cattani, A.; Ackman, J.B.; Bonifazi, P.; Aniksztejn, L.; Ben-Ari, Y.; Cossart, R. Sequential Generation of Two Distinct Synapse-Driven Network Patterns in Developing Neocortex. J. Neurosci. 2008, 28, 12851–12863. [Google Scholar] [CrossRef]
- Stosiek, C.; Garaschuk, O.; Holthoff, K.; Konnerth, A. In Vivo Two-Photon Calcium Imaging of Neuronal Networks. Proc. Natl. Acad. Sci. USA 2003, 100, 7319–7324. [Google Scholar] [CrossRef]
- Kerr, J.N.D.; Greenberg, D.; Helmchen, F. Imaging Input and Output of Neocortical Networks in Vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 14063–14068. [Google Scholar] [CrossRef]
- Hromádka, T.; Deweese, M.R.; Zador, A.M. Sparse Representation of Sounds in the Unanesthetized Auditory Cortex. PLoS Biol. 2008, 6, e16. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Nelissen, R.; Mansvelder, H.D.; Meredith, R.M. Spontaneous Synchronous Network Activity in the Neonatal Development of mPFC in Mice. Dev. Neurobiol. 2021, 81, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Olshausen, B.A.; Field, D.J. Sparse Coding of Sensory Inputs. Curr. Opin. Neurobiol. 2004, 14, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, K.; Lorincz, A.; Gleeson, P.; Farinella, M.; Nusser, Z.; Silver, R.A. Rapid Desynchronization of an Electrically Coupled Interneuron Network with Sparse Excitatory Synaptic Input. Neuron 2010, 67, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. Developing Networks Play a Similar Melody. Trends Neurosci. 2001, 24, 353–360. [Google Scholar] [CrossRef]
- Peerboom, C.; Wierenga, C.J. The Postnatal GABA Shift: A Developmental Perspective. Neurosci. Biobehav. Rev. 2021, 124, 179–192. [Google Scholar] [CrossRef]
- Leinekugel, X.; Khazipov, R.; Cannon, R.; Hirase, H.; Ben-Ari, Y.; Buzsáki, G. Correlated Bursts of Activity in the Neonatal Hippocampus in Vivo. Science 2002, 296, 2049–2052. [Google Scholar] [CrossRef]
- Sipilä, S.T.; Schuchmann, S.; Voipio, J.; Yamada, J.; Kaila, K. The Cation-Chloride Cotransporter NKCC1 Promotes Sharp Waves in the Neonatal Rat Hippocampus. J. Physiol. 2006, 573, 765–773. [Google Scholar] [CrossRef]
- Murata, Y.; Colonnese, M.T. GABAergic Interneurons Excite Neonatal Hippocampus in Vivo. Sci. Adv. 2020, 6, eaba1430. [Google Scholar] [CrossRef]
- Kirmse, K.; Kummer, M.; Kovalchuk, Y.; Witte, O.W.; Garaschuk, O.; Holthoff, K. GABA Depolarizes Immature Neurons and Inhibits Network Activity in the Neonatal Neocortex in Vivo. Nat. Commun. 2015, 6, 7750. [Google Scholar] [CrossRef] [PubMed]
- Che, A.; Babij, R.; Iannone, A.F.; Fetcho, R.N.; Ferrer, M.; Liston, C.; Fishell, G.; De Marco García, N.V. Layer I Interneurons Sharpen Sensory Maps during Neonatal Development. Neuron 2018, 99, 98–116.e7. [Google Scholar] [CrossRef] [PubMed]
- Minlebaev, M.; Ben-Ari, Y.; Khazipov, R. Network Mechanisms of Spindle-Burst Oscillations in the Neonatal Rat Barrel Cortex In Vivo. J. Neurophysiol. 2007, 97, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Minlebaev, M.; Colonnese, M.; Tsintsadze, T.; Sirota, A.; Khazipov, R. Early Gamma Oscillations Synchronize Developing Thalamus and Cortex. Science 2011, 334, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Marguet, S.L.; Le-Schulte, V.T.Q.; Merseburg, A.; Neu, A.; Eichler, R.; Jakovcevski, I.; Ivanov, A.; Hanganu-Opatz, I.L.; Bernard, C.; Morellini, F.; et al. Treatment during a Vulnerable Developmental Period Rescues a Genetic Epilepsy. Nat. Med. 2015, 21, 1436–1444. [Google Scholar] [CrossRef]
- Caviness, V.S. Patterns of Cell and Fiber Distribution in the Neocortex of the Reeler Mutant Mouse. J. Comp. Neurol. 1976, 170, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; D’Arcangelo, G. Role of Reelin in the Control of Brain Development. Brain Res. Brain Res. Rev. 1998, 26, 285–294. [Google Scholar] [CrossRef]
- Faini, G.; Del Bene, F.; Albadri, S. Reelin Functions beyond Neuronal Migration: From Synaptogenesis to Network Activity Modulation. Curr. Opin. Neurobiol. 2021, 66, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Leifeld, J.; Förster, E.; Reiss, G.; Hamad, M.I.K. Considering the Role of Extracellular Matrix Molecules, in Particular Reelin, in Granule Cell Dispersion Related to Temporal Lobe Epilepsy. Front. Cell Dev. Biol. 2022, 10, 917575. [Google Scholar] [CrossRef]
- Pielecka-Fortuna, J.; Wagener, R.J.; Martens, A.-K.; Goetze, B.; Schmidt, K.-F.; Staiger, J.F.; Löwel, S. The Disorganized Visual Cortex in Reelin-Deficient Mice Is Functional and Allows for Enhanced Plasticity. Brain Struct. Funct. 2015, 220, 3449–3467. [Google Scholar] [CrossRef]
- Rogers, J.T.; Rusiana, I.; Trotter, J.; Zhao, L.; Donaldson, E.; Pak, D.T.S.; Babus, L.W.; Peters, M.; Banko, J.L.; Chavis, P.; et al. Reelin Supplementation Enhances Cognitive Ability, Synaptic Plasticity, and Dendritic Spine Density. Learn. Mem. 2011, 18, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, J.; Mi, K.; Shen, Y.; Zhang, X. Functional Role of SIL1 in Neurodevelopment and Learning. Neural Plast. 2019, 2019, 9653024. [Google Scholar] [CrossRef] [PubMed]
- Telese, F.; Ma, Q.; Perez, P.M.; Notani, D.; Oh, S.; Li, W.; Comoletti, D.; Ohgi, K.A.; Taylor, H.; Rosenfeld, M.G. LRP8-Reelin-Regulated Neuronal Enhancer Signature Underlying Learning and Memory Formation. Neuron 2015, 86, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Bock, H.H.; May, P. Canonical and Non-Canonical Reelin Signaling. Front. Cell Neurosci. 2016, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; D’Arcangelo, G. New Insights into Reelin-Mediated Signaling Pathways. Front. Cell Neurosci. 2016, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Renfro, A.; Quattrocchi, C.C.; Sheldon, M.; D’Arcangelo, G. Reelin Promotes Hippocampal Dendrite Development through the VLDLR/ApoER2-Dab1 Pathway. Neuron 2004, 41, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Jossin, Y.; Goffinet, A.M. Reelin Signals through Phosphatidylinositol 3-Kinase and Akt to Control Cortical Development and through mTor to Regulate Dendritic Growth. Mol. Cell Biol. 2007, 27, 7113–7124. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Pramatarova, A.; Howell, B.W. Reduction of Crk and CrkL Expression Blocks Reelin-Induced Dendritogenesis. J. Cell Sci. 2008, 121, 1869–1875. [Google Scholar] [CrossRef]
- Hamad, M.I.K.; Emerald, B.S.; Kumar, K.K.; Ibrahim, M.F.; Ali, B.R.; Bataineh, M.F. Extracellular Molecular Signals Shaping Dendrite Architecture during Brain Development. Front. Cell Dev. Biol. 2023, 11, 1254589. [Google Scholar] [CrossRef]
- Yabut, O.; Renfro, A.; Niu, S.; Swann, J.W.; Marín, O.; D’Arcangelo, G. Abnormal Laminar Position and Dendrite Development of Interneurons in the Reeler Forebrain. Brain Res. 2007, 1140, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chameau, P.; Inta, D.; Vitalis, T.; Monyer, H.; Wadman, W.J.; van Hooft, J.A. The N-Terminal Region of Reelin Regulates Postnatal Dendritic Maturation of Cortical Pyramidal Neurons. Proc. Natl. Acad. Sci. USA 2009, 106, 7227–7232. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.I.K.; Petrova, P.; Daoud, S.; Rabaya, O.; Jbara, A.; Melliti, N.; Leifeld, J.; Jakovčevski, I.; Reiss, G.; Herz, J.; et al. Reelin Restricts Dendritic Growth of Interneurons in the Neocortex. Development 2021, 148, dev199718. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Philips, G.T.; Wasser, C.R.; Durakoglugil, M.S.; Masiulis, I.; Upadhaya, A.; Pohlkamp, T.; Coskun, C.; Kotti, T.; Steller, L.; et al. Reelin Protects against Amyloid β Toxicity in Vivo. Sci. Signal 2015, 8, ra67. [Google Scholar] [CrossRef]
- Hayashi, S.; McMahon, A.P. Efficient Recombination in Diverse Tissues by a Tamoxifen-Inducible Form of Cre: A Tool for Temporally Regulated Gene Activation/Inactivation in the Mouse. Dev. Biol. 2002, 244, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.I.K.; Krause, M.; Wahle, P. Improving AM Ester Calcium Dye Loading Efficiency. J. Neurosci. Methods 2015, 240, 48–60. [Google Scholar] [CrossRef]
- Hamad, M.I.K.; Daoud, S.; Petrova, P.; Rabaya, O.; Jbara, A.; Melliti, N.; Stichmann, S.; Reiss, G.; Herz, J.; Förster, E. Biolistic Transfection and Expression Analysis of Acute Cortical Slices. J. Neurosci. Methods 2020, 337, 108666. [Google Scholar] [CrossRef]
- Frotscher, M.; Chai, X.; Bock, H.H.; Haas, C.A.; Förster, E.; Zhao, S. Role of Reelin in the Development and Maintenance of Cortical Lamination. J. Neural. Transm. (Vienna) 2009, 116, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.I.K.; Jbara, A.; Rabaya, O.; Petrova, P.; Daoud, S.; Melliti, N.; Meseke, M.; Lutz, D.; Petrasch-Parwez, E.; Schwitalla, J.C.; et al. Reelin Signaling Modulates GABAB Receptor Function in the Neocortex. J. Neurochem. 2021, 156, 589–603. [Google Scholar] [CrossRef]
- Yuste, R.; MacLean, J.; Vogelstein, J.; Paninski, L. Imaging Action Potentials with Calcium Indicators. Cold Spring Harb. Protoc. 2011, 2011, 985–989. [Google Scholar] [CrossRef]
- Del Río, J.A.; Heimrich, B.; Supèr, H.; Borrell, V.; Frotscher, M.; Soriano, E. Differential Survival of Cajal-Retzius Cells in Organotypic Cultures of Hippocampus and Neocortex. J. Neurosci. 1996, 16, 6896–6907. [Google Scholar] [CrossRef] [PubMed]
- Anstötz, M.; Cosgrove, K.E.; Hack, I.; Mugnaini, E.; Maccaferri, G.; Lübke, J.H.R. Morphology, Input-Output Relations and Synaptic Connectivity of Cajal-Retzius Cells in Layer 1 of the Developing Neocortex of CXCR4-EGFP Mice. Brain Struct. Funct. 2014, 219, 2119–2139. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, S.; Ruiz, M.; D’Arcangelo, G.; Ezan, F.; de Lecea, L.; Curran, T.; Sotelo, C.; Soriano, E. Regional and Cellular Patterns of Reelin mRNA Expression in the Forebrain of the Developing and Adult Mouse. J. Neurosci. 1998, 18, 7779–7799. [Google Scholar] [CrossRef] [PubMed]
- Pesold, C.; Impagnatiello, F.; Pisu, M.G.; Uzunov, D.P.; Costa, E.; Guidotti, A.; Caruncho, H.J. Reelin Is Preferentially Expressed in Neurons Synthesizing Gamma-Aminobutyric Acid in Cortex and Hippocampus of Adult Rats. Proc. Natl. Acad. Sci. USA 1998, 95, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.; Massaro, C.M.; Palop, J.J.; Thwin, M.T.; Yu, G.-Q.; Bien-Ly, N.; Bender, A.; Mucke, L. Reelin Depletion in the Entorhinal Cortex of Human Amyloid Precursor Protein Transgenic Mice and Humans with Alzheimer’s Disease. J. Neurosci. 2007, 27, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Pohlkamp, T.; Dávid, C.; Cauli, B.; Gallopin, T.; Bouché, E.; Karagiannis, A.; May, P.; Herz, J.; Frotscher, M.; Staiger, J.F.; et al. Characterization and Distribution of Reelin-Positive Interneuron Subtypes in the Rat Barrel Cortex. Cereb. Cortex 2014, 24, 3046–3058. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Sun, C.; Martin, J.; Kitch, L.J.; Schnitzer, M.J.; Tonegawa, S. Entorhinal Cortical Ocean Cells Encode Specific Contexts and Drive Context-Specific Fear Memory. Neuron 2015, 87, 1317–1331. [Google Scholar] [CrossRef]
- Chen, Y.; Beffert, U.; Ertunc, M.; Tang, T.-S.; Kavalali, E.T.; Bezprozvanny, I.; Herz, J. Reelin Modulates NMDA Receptor Activity in Cortical Neurons. J. Neurosci. 2005, 25, 8209–8216. [Google Scholar] [CrossRef]
- Blankenship, A.G.; Feller, M.B. Mechanisms Underlying Spontaneous Patterned Activity in Developing Neural Circuits. Nat. Rev. Neurosci. 2010, 11, 18–29. [Google Scholar] [CrossRef]
- Nishikawa, S.; Goto, S.; Hamasaki, T.; Yamada, K.; Ushio, Y. Involvement of Reelin and Cajal-Retzius Cells in the Developmental Formation of Vertical Columnar Structures in the Cerebral Cortex: Evidence from the Study of Mouse Presubicular Cortex. Cereb. Cortex 2002, 12, 1024–1030. [Google Scholar] [CrossRef]
- Zluhan, E.; Enck, J.; Matthews, R.T.; Olson, E.C. Reelin Counteracts Chondroitin Sulfate Proteoglycan-Mediated Cortical Dendrite Growth Inhibition. eNeuro 2020, 7, ENEURO.0168-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.S.; Heinemann, U. Spontaneous Activity Mediated by NMDA Receptors in Immature Rat Entorhinal Cortex in Vitro. Neurosci. Lett. 1989, 104, 93–98. [Google Scholar] [CrossRef]
- Sheroziya, M.G.; von Bohlen Und Halbach, O.; Unsicker, K.; Egorov, A.V. Spontaneous Bursting Activity in the Developing Entorhinal Cortex. J. Neurosci. 2009, 29, 12131–12144. [Google Scholar] [CrossRef]
- Namiki, S.; Norimoto, H.; Kobayashi, C.; Nakatani, K.; Matsuki, N.; Ikegaya, Y. Layer III Neurons Control Synchronized Waves in the Immature Cerebral Cortex. J. Neurosci. 2013, 33, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Dawitz, J.; Kroon, T.; Hjorth, J.J.J.; Mansvelder, H.D.; Meredith, R.M. Distinct Synchronous Network Activity During the Second Postnatal Week of Medial Entorhinal Cortex Development. Front. Cell Neurosci. 2020, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Gaiarsa, J.-L.; Tyzio, R.; Khazipov, R. GABA: A Pioneer Transmitter That Excites Immature Neurons and Generates Primitive Oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Ben-Ari, Y. The GABA Excitatory/Inhibitory Developmental Sequence: A Personal Journey. Neuroscience 2014, 279, 187–219. [Google Scholar] [CrossRef]
- Berggaard, N.; Seifi, M.; van der Want, J.J.L.; Swinny, J.D. Spatiotemporal Distribution of GABAA Receptor Subunits Within Layer II of Mouse Medial Entorhinal Cortex: Implications for Grid Cell Excitability. Front. Neuroanat. 2018, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. GABAB Receptors: Structure, Functions, and Clinical Implications. Neurology 2012, 78, 578–584. [Google Scholar] [CrossRef]
- Pinard, A.; Seddik, R.; Bettler, B. GABAB Receptors: Physiological Functions and Mechanisms of Diversity. Adv. Pharmacol. 2010, 58, 231–255. [Google Scholar] [CrossRef]
- López-Bendito, G.; Luján, R.; Shigemoto, R.; Ganter, P.; Paulsen, O.; Molnár, Z. Blockade of GABA(B) Receptors Alters the Tangential Migration of Cortical Neurons. Cereb. Cortex 2003, 13, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Mody, I.; Prince, D.A. Differential Ontogenesis of Presynaptic and Postsynaptic GABAB Inhibition in Rat Somatosensory Cortex. J. Neurophysiol. 1993, 70, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Bony, G.; Szczurkowska, J.; Tamagno, I.; Shelly, M.; Contestabile, A.; Cancedda, L. Non-Hyperpolarizing GABAB Receptor Activation Regulates Neuronal Migration and Neurite Growth and Specification by cAMP/LKB1. Nat. Commun. 2013, 4, 1800. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, M.I.K.; Rabaya, O.; Jbara, A.; Daoud, S.; Petrova, P.; Ali, B.R.; Allouh, M.Z.; Herz, J.; Förster, E. Reelin Regulates Developmental Desynchronization Transition of Neocortical Network Activity. Biomolecules 2024, 14, 593. https://doi.org/10.3390/biom14050593
Hamad MIK, Rabaya O, Jbara A, Daoud S, Petrova P, Ali BR, Allouh MZ, Herz J, Förster E. Reelin Regulates Developmental Desynchronization Transition of Neocortical Network Activity. Biomolecules. 2024; 14(5):593. https://doi.org/10.3390/biom14050593
Chicago/Turabian StyleHamad, Mohammad I. K., Obada Rabaya, Abdalrahim Jbara, Solieman Daoud, Petya Petrova, Bassam R. Ali, Mohammed Z. Allouh, Joachim Herz, and Eckart Förster. 2024. "Reelin Regulates Developmental Desynchronization Transition of Neocortical Network Activity" Biomolecules 14, no. 5: 593. https://doi.org/10.3390/biom14050593
APA StyleHamad, M. I. K., Rabaya, O., Jbara, A., Daoud, S., Petrova, P., Ali, B. R., Allouh, M. Z., Herz, J., & Förster, E. (2024). Reelin Regulates Developmental Desynchronization Transition of Neocortical Network Activity. Biomolecules, 14(5), 593. https://doi.org/10.3390/biom14050593





