Irisin Ameliorate Acute Pancreatitis and Acinar Cell Viability through Modulation of the Unfolded Protein Response (UPR) and PPARγ-PGC1α-FNDC5 Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Primary Pancreatic Acinar Cells
2.3. Experimental Protocol
2.4. Protein Extraction
2.5. Western Blot Analysis
2.6. RNA Isolation and cDNA Synthesis
2.7. Quantitative RT-PCR (qPCR)
2.8. Immunocytochemistry (ICC) Staining
2.9. Statistical Analysis
3. Results
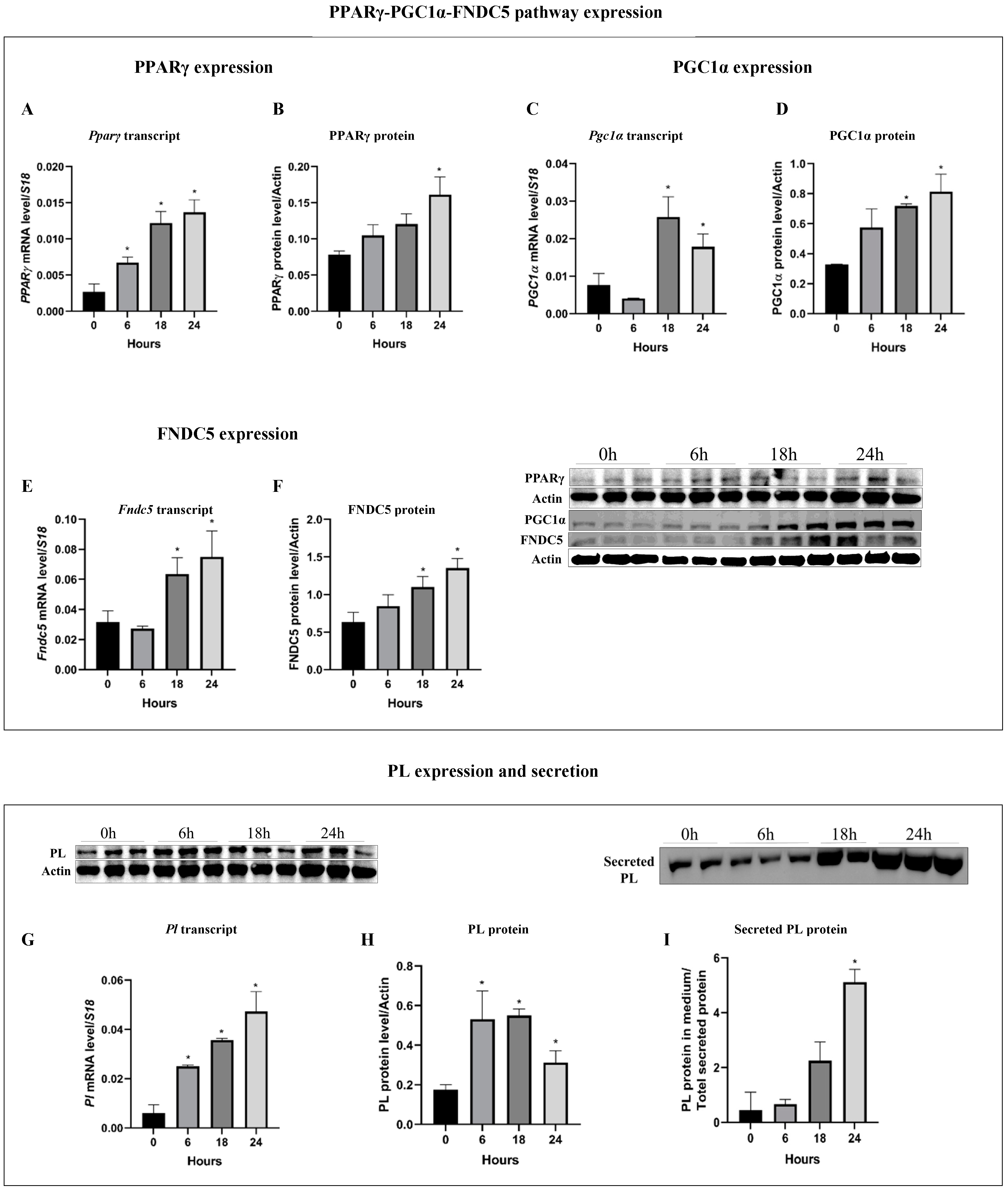
3.1. Pancreatitis Induction (Cer-Treatment) Activate the PPARγ-PGC1α-FNDC5 Pathway and Induce PL Expression and Secretion
3.2. Irisin Dulls the Increase in PPARγ-PGC1α-FNDC5 Axis and PL Production under Cer-Pancreatitis Conditions
3.3. Irisin Treatment Suppresses Inflammation Markers following Pancreatitis
3.4. Irisin Treatment Suppresses Anti-Survival URP Agents Parallel to Activation of Pro-Survival URP Agents under Cer-Pancreatitis Treatment
3.5. Irisin Tilts the Apoptosis Status towards Survival and Improved Cells Viability under Cer-Pancreatitis Condition
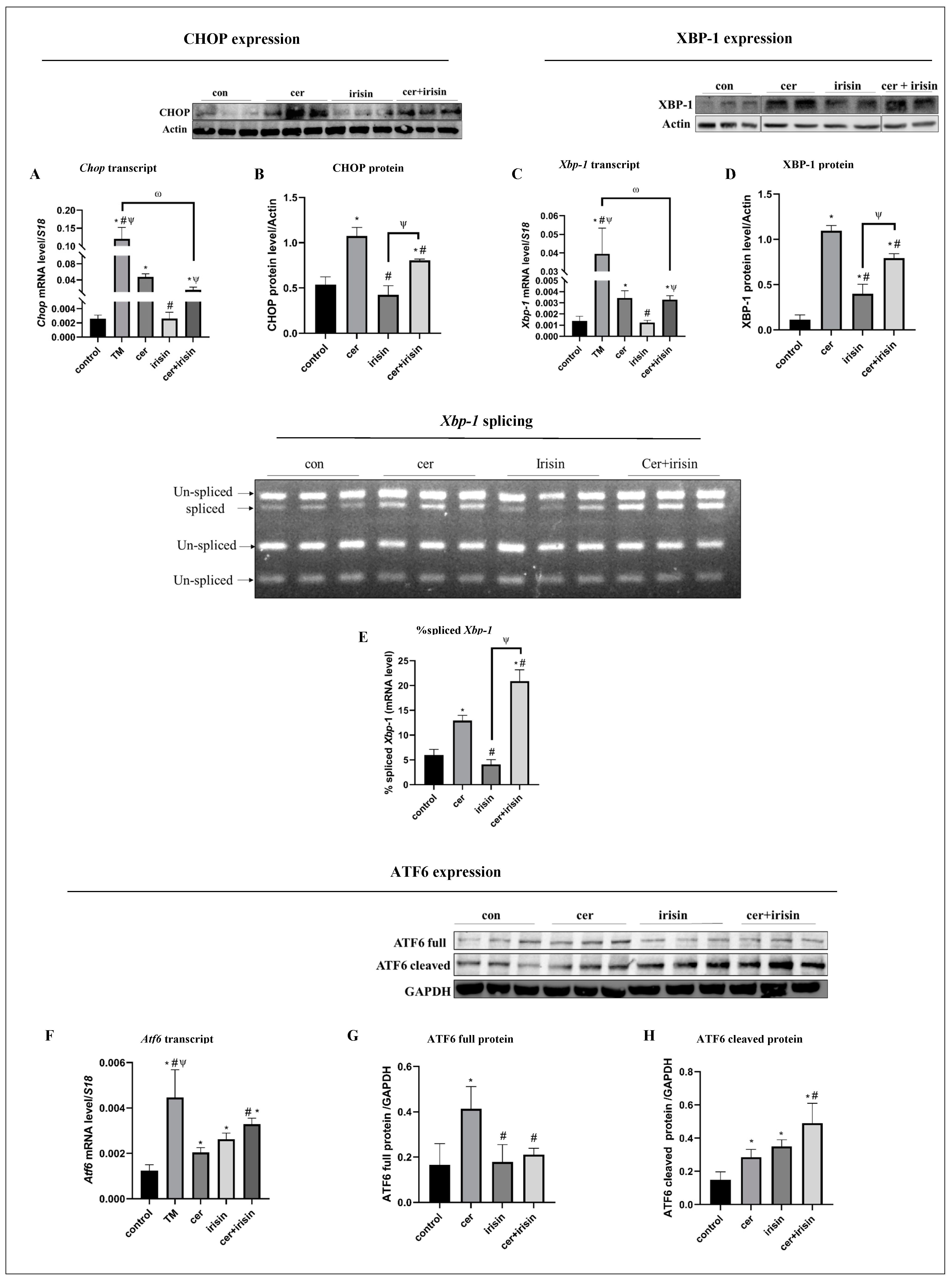
3.6. The PPARγ-PGC1α-FNDC5 Pathway Is Activated in ER Stress Condtions and Inhibited in Response to Exogenous Irisin
3.7. Irisin Spacificaly Activae Pro-Survival UPR Agents under ER Stress Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Sarvepalli, S.; Campbell, J.P.; Obaitan, I.; Singh, D.; Bazerbachi, F.; Singh, R.; Sanaka, M.R. Incidence, Admission Rates, and Predictors, and Economic Burden of Adult Emergency Visits for Acute Pancreatitis: Data from the National Emergency Department Sample, 2006 to 2012. J. Clin. Gastroenterol. 2019, 53, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of Acute Pancreatitis—2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cai, B.; Zheng, S.; Liu, X.; Cai, H.; Li, H. Effect of Emodin on Endoplasmic Reticulum Stress in Rats with Severe Acute Pancreatitis. Inflammation 2013, 36, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tang, X.; Huang, J.; Li, J.; Chen, Q.; Sun, Y.; Wu, J. Melatonin Attenuates Endoplasmic Reticulum Stress in Acute Pancreatitis. Pancreas 2018, 47, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Zhao, Q.; Chen, Q.; Sun, Y.; Jin, Y.; Wu, J. Melatonin Induces Anti-Inflammatory Effects to Play a Protective Role via Endoplasmic Reticulum Stress in Acute Pancreatitis. Cell Physiol. Biochem. 2016, 40, 1094–1104. [Google Scholar] [CrossRef]
- Antonucci, L.; Fagman, J.B.; Kim, J.Y.; Todoric, J.; Gukovsky, I.; Mackey, M.; Ellisman, M.H.; Karin, M. Basal Autophagy Maintains Pancreatic Acinar Cell Homeostasis and Protein Synthesis and Prevents ER Stress. Proc. Natl. Acad. Sci. USA 2015, 112, E6166–E6174. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Gukovskaya, A.S.; Pandol, S.J. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology 2019, 156, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Ronai, Z.A. UPR, Autophagy, and Mitochondria Crosstalk Underlies the ER Stress Response. Trends Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef]
- Ariyasu, D.; Yoshida, H.; Hasegawa, Y. Endoplasmic Reticulum (ER) Stress and Endocrine Disorders. Int. J. Mol. Sci. 2017, 18, 382. [Google Scholar] [CrossRef]
- Di Conza, G.; Ho, P.-C. ER Stress Responses: An Emerging Modulator for Innate Immunity. Cells 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ryoo, H.D.; Qi, Y.; Jasper, H. PERK Limits Drosophila Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress. PLoS Genet. 2015, 11, e1005220. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; LaVail, M.M.; Walter, P. IRE1 Signaling Affects Cell Fate During the Unfolded Protein Response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Dadey, D.Y.A.; Kapoor, V.; Khudanyan, A.; Urano, F.; Kim, A.H.; Thotala, D.; Hallahan, D.E. The ATF6 Pathway of the ER Stress Response Contributes to Enhanced Viability in Glioblastoma. Oncotarget 2016, 7, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA Is Induced by ATF6 and Spliced by IRE1 in Response to ER Stress to Produce a Highly Active Transcription Factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Rutkowski, D.T.; Dubois, M.; Swathirajan, J.; Saunders, T.; Wang, J.; Song, B.; Yau, G.D.-Y.; Kaufman, R.J. ATF6α Optimizes Long-Term Endoplasmic Reticulum Function to Protect Cells from Chronic Stress. Dev. Cell 2007, 13, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.P.; Bhopale, K.K.; Caracheo, A.A.; Kaphalia, L.; Loganathan, G.; Balamurugan, A.N.; Rastellini, C.; Kaphalia, B.S. Differential Cytotoxicity, ER/Oxidative Stress, Dysregulated AMPKα Signaling, and Mitochondrial Stress by Ethanol and Its Metabolites in Human Pancreatic Acinar Cells. Alcohol. Clin. Amp; Exp. Res. 2021, 45, 961–978. [Google Scholar] [CrossRef] [PubMed]
- Tumor-Secreted Lipids Induce ER Stress Response to Polarize Macrophages. Cancer Discov. 2022, 12, OF5. [CrossRef]
- Logsdon, C.D.; Ji, B. The Role of Protein Synthesis and Digestive Enzymes in Acinar Cell Injury. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 362–370. [Google Scholar] [CrossRef]
- Wong, H.; Schotz, M.C. The Lipase Gene Family. J. Lipid Res. 2002, 43, 993–999. [Google Scholar] [CrossRef]
- Ricketts, J.; Brannon, P.M. Amount and Type of Dietary Fat Regulate Pancreatic Lipase Gene Expression in Rats. J. Nutr. 1994, 124, 1166–1171. [Google Scholar] [CrossRef]
- Birk, R.Z.; Brannon, P.M. Regulation of Pancreatic Lipase by Dietary Medium Chain Triglycerides in the Weanling Rat. Pediatr. Res. 2004, 55, 921–926. [Google Scholar] [CrossRef]
- Birk, R.Z.; Regan, K.S.; Boyle-Roden, E.; Brannon, P.M. Pancreatic Lipase and Its Related Protein 2 Are Regulated by Dietary Polyunsaturated Fat during the Postnatal Development of Rats. Pediatr. Res. 2004, 56, 256–262. [Google Scholar] [CrossRef]
- Janani, C.; Ranjitha Kumari, B.D. PPAR Gamma Gene—A Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Krey, G.; Braissant, O.; L’Horset, F.; Kalkhoven, E.; Perroud, M.; Parker, M.G.; Wahli, W. Fatty Acids, Eicosanoids, and Hypolipidemic Agents Identified as Ligands of Peroxisome Proliferator-Activated Receptors by Coactivator-Dependent Receptor Ligand Assay. Mol. Endocrinol. 1997, 11, 779–791. [Google Scholar] [CrossRef]
- Quintanilla Rodriguez, B.S.; Correa, R. Rosiglitazone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Danino, H.; Naor, R.P.-; Fogel, C.; Ben-Harosh, Y.; Kadir, R.; Salem, H.; Birk, R. PPARγ Regulates Exocrine Pancreas Lipase. Biochim. Et. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2016, 1861, 1921–1928. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes Through Mitogen-Activated Protein Kinase P38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association with Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Hee Park, K.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Koulaxis, D.; Kefala, N.; Polyzos, S.A.; Upadhyay, J.; Pagkalidou, E.; Economou, F.; Anastasilakis, C.D.; Mantzoros, C.S. Circulating Irisin Levels Are Lower in Patients with Either Stable Coronary Artery Disease (CAD) or Myocardial Infarction (MI) versus Healthy Controls, Whereas Follistatin and Activin a Levels Are Higher and Can Discriminate MI from CAD with Similar to CK-MB Accuracy. Metabolism 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Liu, J.-J.; Wong, M.D.S.; Toy, W.C.; Tan, C.S.H.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower Circulating Irisin Is Associated with Type 2 Diabetes Mellitus. J. Diabetes Its Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhang, X.-F.; Ma, Z.-M.; Pan, L.-L.; Chen, Z.; Han, H.-W.; Han, C.-K.; Zhuang, X.-J.; Lu, Y.; Li, X.-J.; et al. Irisin Is Inversely Associated with Intrahepatic Triglyceride Contents in Obese Adults. J. Hepatol. 2013, 59, 557–562. [Google Scholar] [CrossRef]
- Wen, M.-S.; Wang, C.-Y.; Lin, S.-L.; Hung, K.-C. Decrease in Irisin in Patients with Chronic Kidney Disease. PLoS ONE 2013, 8, e64025. [Google Scholar] [CrossRef]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef]
- Guo, Q.; Wei, X.; Hu, H.; Yang, D.; Zhang, B.; Fan, X.; Liu, J.; He, H.; Oh, Y.; Wu, Q.; et al. The Saturated Fatty Acid Palmitate Induces Insulin Resistance through Smad3-Mediated down-Regulation of FNDC5 in Myotubes. Biochem. Biophys. Res. Commun. 2019, 520, 619–626. [Google Scholar] [CrossRef]
- Charos, A.E.; Reed, B.D.; Raha, D.; Szekely, A.M.; Weissman, S.M.; Snyder, M. A Highly Integrated and Complex PPARGC1A Transcription Factor Binding Network in HepG2 Cells. Genome Res. 2012, 22, 1668–1679. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin Reverses Intestinal Epithelial Barrier Dysfunction during Intestinal Injury via Binding to the Integrin αVβ5 Receptor. J. Cell Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef]
- Ohtaki, H. Irisin. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2016; pp. 329–330, e37-1–e37-3. ISBN 978-0-12-801028-0. [Google Scholar]
- Horwitz, A.; Birk, R. Irisin Regulates Pancreatic Lipases through PPARγ-PGCα-FNDC5 Pathway. Genes. Dis. 2023, 10, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and Role of Irisin in Glucose Homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Yatchenko, Y.; Horwitz, A.; Birk, R. Endocrine and Exocrine Pancreas Pathologies Crosstalk: Insulin Regulates the Unfolded Protein Response in Pancreatic Exocrine Acinar Cells. Exp. Cell Res. 2019, 375, 28–35. [Google Scholar] [CrossRef]
- Ren, Y.; Qiu, M.; Zhang, J.; Bi, J.; Wang, M.; Hu, L.; Du, Z.; Li, T.; Zhang, L.; Wang, Y.; et al. Low Serum Irisin Concentration Is Associated with Poor Outcomes in Patients with Acute Pancreatitis, and Irisin Administration Protects Against Experimental Acute Pancreatitis. Antioxid. Redox Signal. 2019, 31, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Gout, J.; Pommier, R.M.; Vincent, D.F.; Kaniewski, B.; Martel, S.; Valcourt, U.; Bartholin, L. Isolation and Culture of Mouse Primary Pancreatic Acinar Cells. JoVE 2013, 50514. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. ISBN 978-1-60327-474-6. [Google Scholar]
- Ben-Dror, K.; Birk, R. Oleic Acid Ameliorates Palmitic Acid-Induced ER Stress and Inflammation Markers in Naive and Cerulein-Treated Exocrine Pancreas Cells. Biosci. Rep. 2019, 39, BSR20190054. [Google Scholar] [CrossRef]
- Szegezdi, E.; Fitzgerald, U.; Samali, A. Caspase-12 and ER-Stress-Mediated Apoptosis: The Story So Far. Ann. N. Y. Acad. Sci. 2003, 1010, 186–194. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, Function, and Biotechnological Aspects. Biotech. App Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A Glance at the Therapeutic Potential of Irisin against Diseases Involving Inflammation, Oxidative Stress, and Apoptosis: An Introductory Review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and Underlying Mechanisms of Irisin on the Proliferation and Apoptosis of Pancreatic β Cells. PLoS ONE 2017, 12, e0175498. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Zhang, J.; Zhang, X.; Xin, C.; Zhang, F.; Lee, Y.; Zhang, L.; Lian, K.; Yan, W.; et al. Irisin Improves Endothelial Function in Type 2 Diabetes through Reducing Oxidative/Nitrative Stresses. J. Mol. Cell. Cardiol. 2015, 87, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.J.; Lee, H.S. Experimental Models of Pancreatitis. Clin. Endosc. 2014, 47, 212. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Papachristou, G.I. New Insights into Acute Pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, G.; Bertrand, M.J.M. Death by TNF: A Road to Inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.G. Acute Pancreatitis Induces Intrapancreatic Tumor Necrosis Factor Gene Expression. Arch. Surg. 1995, 130, 966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Z.; Liu, X.; Yan, L.; Hu, W.; Tian, B.; Liu, J. Intrapulmonary expression of tumor necrosis factor alpha mRNA and its significance in rats with acute pancreatitis. Hua Xi Yi Ke Da Xue Xue Bao 1999, 30, 379–383. [Google Scholar] [PubMed]
- Van Duijneveldt, G.; Griffin, M.D.W.; Putoczki, T.L. Emerging Roles for the IL-6 Family of Cytokines in Pancreatic Cancer. Clin. Sci. 2020, 134, 2091–2115. [Google Scholar] [CrossRef] [PubMed]
- Gukovskaya, A.S.; Gukovsky, I.; Zaninovic, V.; Song, M.; Sandoval, D.; Gukovsky, S.; Pandol, S.J. Pancreatic Acinar Cells Produce, Release, and Respond to Tumor Necrosis Factor-Alpha. Role in Regulating Cell Death and Pancreatitis. J. Clin. Invest. 1997, 100, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; Vasseur, S.; Bödeker, H.; Ortiz, E.M.; Dusetti, N.J.; Verrando, P.; Dagorn, J.; Iovanna, J.L. Tumor Necrosis Factor α Triggers Antiapoptotic Mechanisms in Rat Pancreatic Cells through Pancreatitis-Associated Protein I Activation. Gastroenterology 2000, 119, 816–828. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Lin, Q.; Zhou, Y.-F. Progress of Study on the Relationship Between Mediators of Inflammation and Apoptosis in Acute Pancreatitis. Dig. Dis. Sci. 2007, 52, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Gliem, N.; Ammer-Herrmenau, C.; Ellenrieder, V.; Neesse, A. Management of Severe Acute Pancreatitis: An Update. Digestion 2021, 102, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ding, Y.-X.; Qu, Y.-X.; Cao, F.; Li, F. A Narrative Review of Acute Pancreatitis and Its Diagnosis, Pathogenetic Mechanism, and Management. Ann. Transl. Med. 2021, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xie, T.; Leung, P.S. Irisin Ameliorates Glucolipotoxicity-Associated β-Cell Dysfunction and Apoptosis via AMPK Signaling and Anti-Inflammatory Actions. Cell Physiol. Biochem. 2018, 51, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhan, W.; Li, R.; Tian, T.; Yu, L.; Yang, Q. Irisin Ameliorates Endoplasmic Reticulum Stress and Liver Fibrosis through Inhibiting PERK-Mediated Destabilization of HNRNPA1 in Hepatic Stellate Cells. Biol. Chem. 2021, 402, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Lv, M.; Lan, M.; Zheng, Z.; Tan, X.; Zhao, X.; Zhang, Y.; Pu, J.; Xu, L.; Hu, H. Irisin Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury via Attenuating AMPK Mediated Endoplasmic Reticulum Stress. Sci. Rep. 2022, 12, 7415. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, J.; Xu, Y.; Gong, S.; Zhu, Y.; Zhang, H.; Dong, Y.; Zhao, B.; Li, X. Irisin Ameliorates Oxidative Stress-Induced Injury in Pancreatic Beta-Cells by Inhibiting Txnip and Inducing Stat3-Trx2 Pathway Activation. Oxidative Med. Cell. Longev. 2022, 2022, 4674215. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, N.; Kang, X.; Hu, Y.; Shi, S. Irisin Alleviates FFA Induced β-Cell Insulin Resistance and Inflammatory Response through Activating PI3K/AKT/FOXO1 Signaling Pathway. Endocrine 2022, 75, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Li, W.M.; Chen, Y.N.; Zhao, Q.; Chen, Q.F. Endoplasmic Reticulum Stress Is Activated in Acute Pancreatitis. J. Dig. Dis. 2016, 17, 295–303. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of Endoplasmic Reticulum Stress-induced Apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the Mechanisms of Apoptosis Induced by Endoplasmic Reticulum Stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Arnold, S.M.; Miller, C.N.; Wu, J.; Li, J.; Gunnison, K.M.; Mori, K.; Sadighi Akha, A.A.; Raden, D.; Kaufman, R.J. Adaptation to ER Stress Is Mediated by Differential Stabilities of Pro-Survival and Pro-Apoptotic mRNAs and Proteins. PLoS Biol. 2006, 4, e374. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New Insights into the Roles of CHOP-Induced Apoptosis in ER Stress. ABBS 2014, 46, 629–640. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, Y.; Fang, Y.; Cao, H.; Zhang, C.; Xing, C.; Guo, X.; Li, G.; Liu, P.; Hu, G.; et al. Endoplasmic Reticulum Stress Aggravates Copper-Induced Apoptosis via the PERK/ATF4/CHOP Signaling Pathway in Duck Renal Tubular Epithelial Cells. Environ. Pollut. 2021, 272, 115981. [Google Scholar] [CrossRef]
- Puthalakath, H.; O’Reilly, L.A.; Gunn, P.; Lee, L.; Kelly, P.N.; Huntington, N.D.; Hughes, P.D.; Michalak, E.M.; McKimm-Breschkin, J.; Motoyama, N.; et al. ER Stress Triggers Apoptosis by Activating BH3-Only Protein Bim. Cell 2007, 129, 1337–1349. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell Death Induced by Endoplasmic Reticulum Stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Arenzana, N.; Tirasophon, W.; Kaufman, R.J.; Prywes, R. Activation of ATF6 and an ATF6 DNA Binding Site by the Endoplasmic Reticulum Stress Response. J. Biol. Chem. 2000, 275, 27013–27020. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Tirasophon, W.; Shen, X.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IRE1-Mediated Unconventional mRNA Splicing and S2P-Mediated ATF6 Cleavage Merge to Regulate XBP1 in Signaling the Unfolded Protein Response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef]
- Iwakoshi, N.N.; Pypaert, M.; Glimcher, L.H. The Transcription Factor XBP-1 Is Essential for the Development and Survival of Dendritic Cells. J. Exp. Med. 2007, 204, 2267–2275. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, T.; Matsui, T.; Sato, M.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. Transcriptional Induction of Mammalian ER Quality Control Proteins Is Mediated by Single or Combined Action of ATF6α and XBP1. Dev. Cell 2007, 13, 365–376. [Google Scholar] [CrossRef]
- Sharma, R.B.; Darko, C.; Alonso, L.C. Intersection of the ATF6 and XBP1 ER Stress Pathways in Mouse Islet Cells. J. Biol. Chem. 2020, 295, 14164–14177. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, I.; Cebro-Márquez, M.; Rodríguez-Mañero, M.; González-Juanatey, J.R.; Lage, R. FNDC5/Irisin Counteracts Lipotoxic-Induced Apoptosis in Hypoxic H9c2 Cells. J. Mol. Endocrinol. 2019, 63, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, L.; Zhao, N.; Chang, S.; Yang, L. FNDC5/Irisin Protects Neurons through Caspase3 and Bax Pathways. Cell Biochem. Funct. 2024, 42, e3912. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wu, F.; Zhang, Y.; Zhang, Y.; Wang, F.; Jiang, M.; Wang, Z.; Zhang, M.; Li, S.; Yang, L.; et al. Irisin Promotes Human Umbilical Vein Endothelial Cell Proliferation through the ERK Signaling Pathway and Partly Suppresses High Glucose-Induced Apoptosis. PLoS ONE 2014, 9, e110273. [Google Scholar] [CrossRef] [PubMed]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, Z.; Cai, M.; Xi, Y.; Xu, Z.; Zhang, Z.; Li, H.; Zhu, W.; Tian, Z. Aerobic Exercise Alleviates Oxidative Stress-Induced Apoptosis in Kidneys of Myocardial Infarction Mice by Inhibiting ALCAT1 and Activating FNDC5/Irisin Signaling Pathway. Free Radic. Biol. Med. 2020, 158, 171–180. [Google Scholar] [CrossRef]
- Jung, T.; Choi, K. Pharmacological Modulators of Endoplasmic Reticulum Stress in Metabolic Diseases. Int. J. Mol. Sci. 2016, 17, 192. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horwitz, A.; Birk, R. Irisin Ameliorate Acute Pancreatitis and Acinar Cell Viability through Modulation of the Unfolded Protein Response (UPR) and PPARγ-PGC1α-FNDC5 Pathways. Biomolecules 2024, 14, 643. https://doi.org/10.3390/biom14060643
Horwitz A, Birk R. Irisin Ameliorate Acute Pancreatitis and Acinar Cell Viability through Modulation of the Unfolded Protein Response (UPR) and PPARγ-PGC1α-FNDC5 Pathways. Biomolecules. 2024; 14(6):643. https://doi.org/10.3390/biom14060643
Chicago/Turabian StyleHorwitz, Avital, and Ruth Birk. 2024. "Irisin Ameliorate Acute Pancreatitis and Acinar Cell Viability through Modulation of the Unfolded Protein Response (UPR) and PPARγ-PGC1α-FNDC5 Pathways" Biomolecules 14, no. 6: 643. https://doi.org/10.3390/biom14060643
APA StyleHorwitz, A., & Birk, R. (2024). Irisin Ameliorate Acute Pancreatitis and Acinar Cell Viability through Modulation of the Unfolded Protein Response (UPR) and PPARγ-PGC1α-FNDC5 Pathways. Biomolecules, 14(6), 643. https://doi.org/10.3390/biom14060643






