IFN-γ-Preconditioned Human Gingival-Derived Mesenchymal Stromal Cells Inhibit Plasmacytoid Dendritic Cells via Adenosine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Isolation of hGMSCs
2.3. In Vitro Differentiation of hGMSCs
2.4. Peripheral Blood Mononuclear Cells (PBMCs) and pDCs Isolation
2.5. Flow Cytometry
2.6. IFN-γ Preconditioning of hGMSCs (hGMSC-γ)
2.7. Validation of hGMSCs’ Regulatory Effect on PBMCs Activation
2.8. Evaluation of hGMSCs’ Regulatory Effect on pDC Activation and Functionality
2.9. Statistical Analysis
3. Results
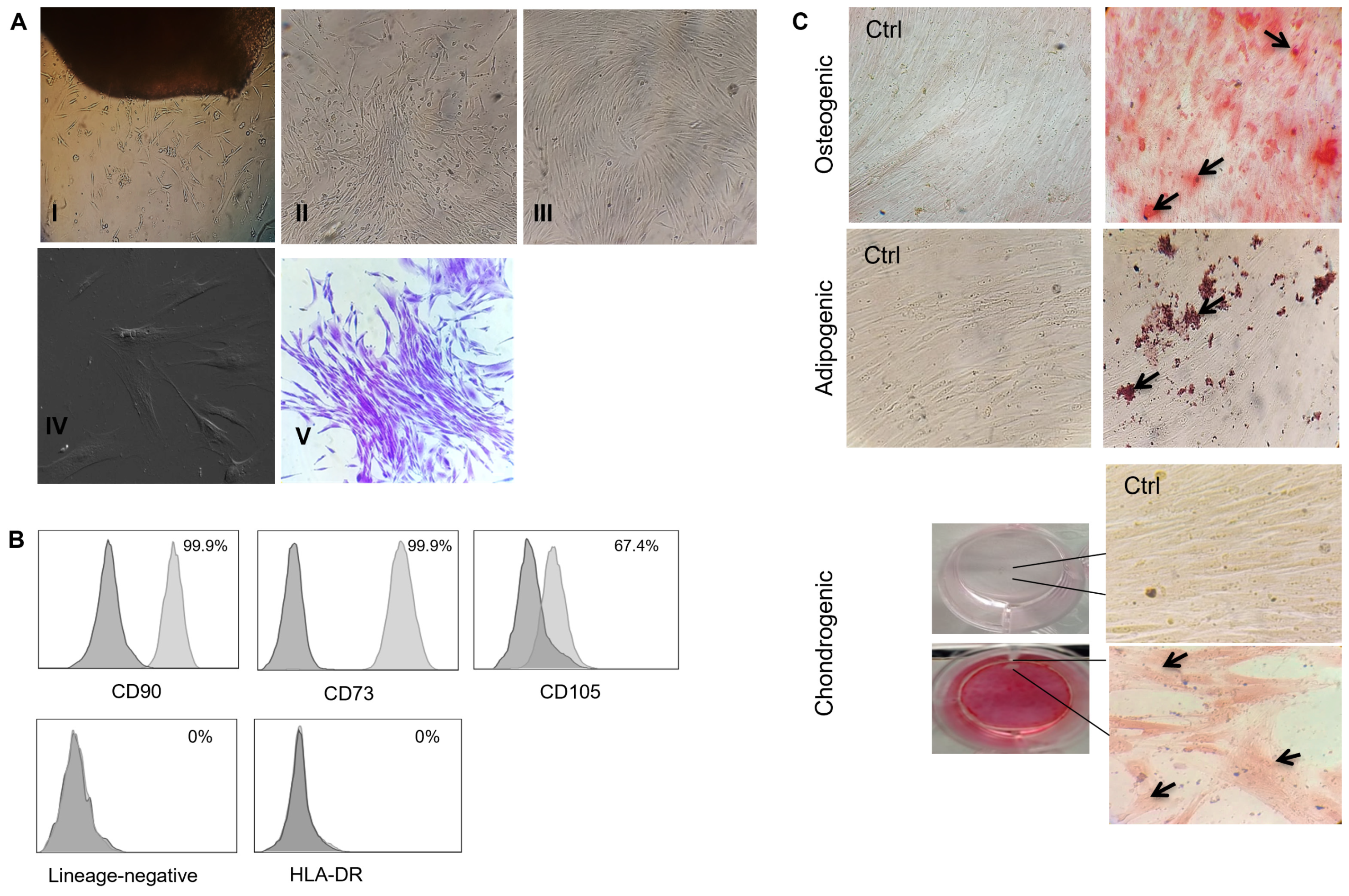
3.1. The Human Gingival Tissue-Isolated MSCs Fulfilled the International Society for Cell and Gene Therapy (ISCT) Criteria for MSC Characterization
3.2. hGMSCs Constitutively Co-Express CD73 and CD39
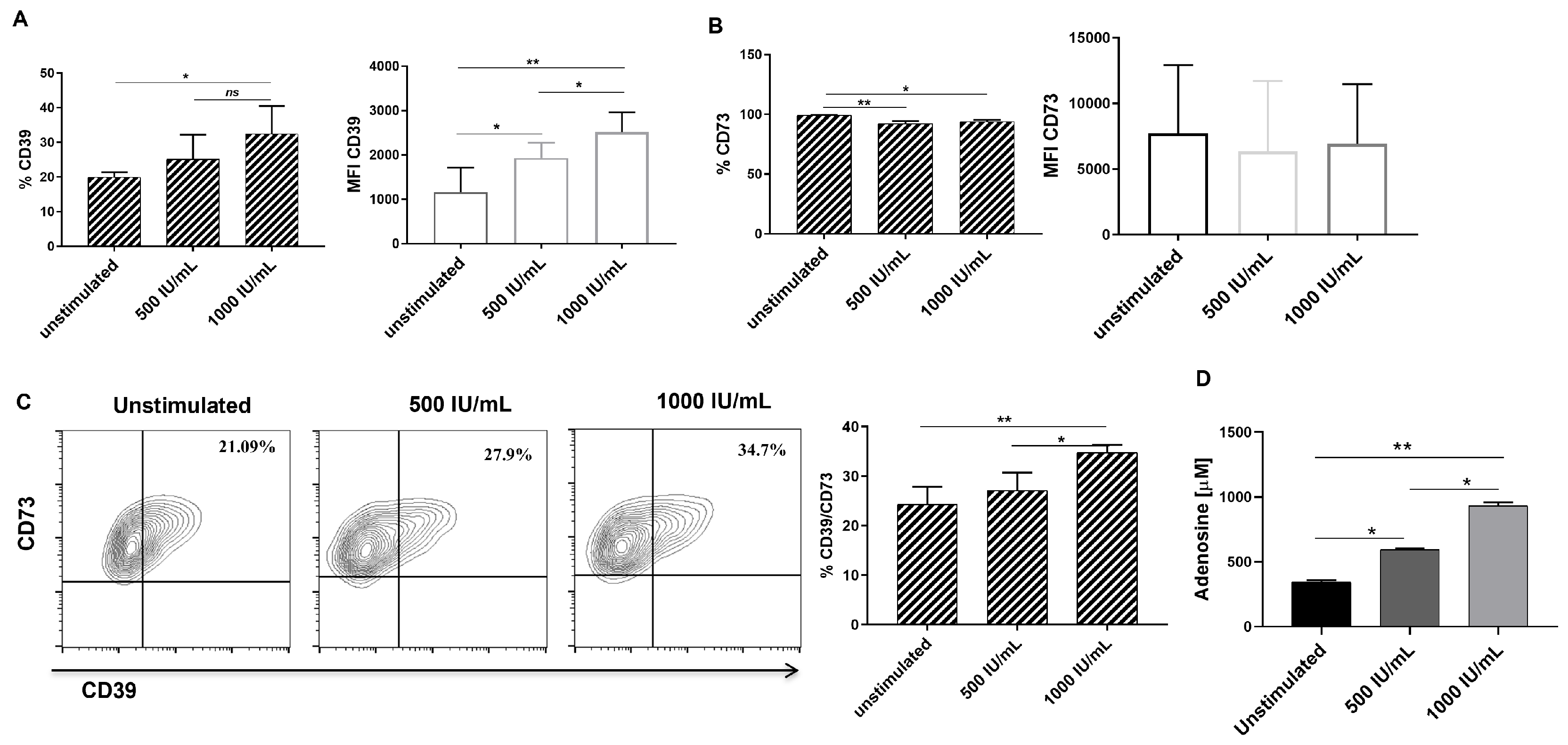
3.3. IFN-γ Stimulation Increases CD39 Expression, CD39/CD73 Double-Positive Cell Frequency and Adenosine Production in hGSMCs
3.4. Primary Cultured hGMSCs Possess Modulatory Effects on PBMC Activation, and IFN-γ-Preconditioning Increases Their Regulatory Properties
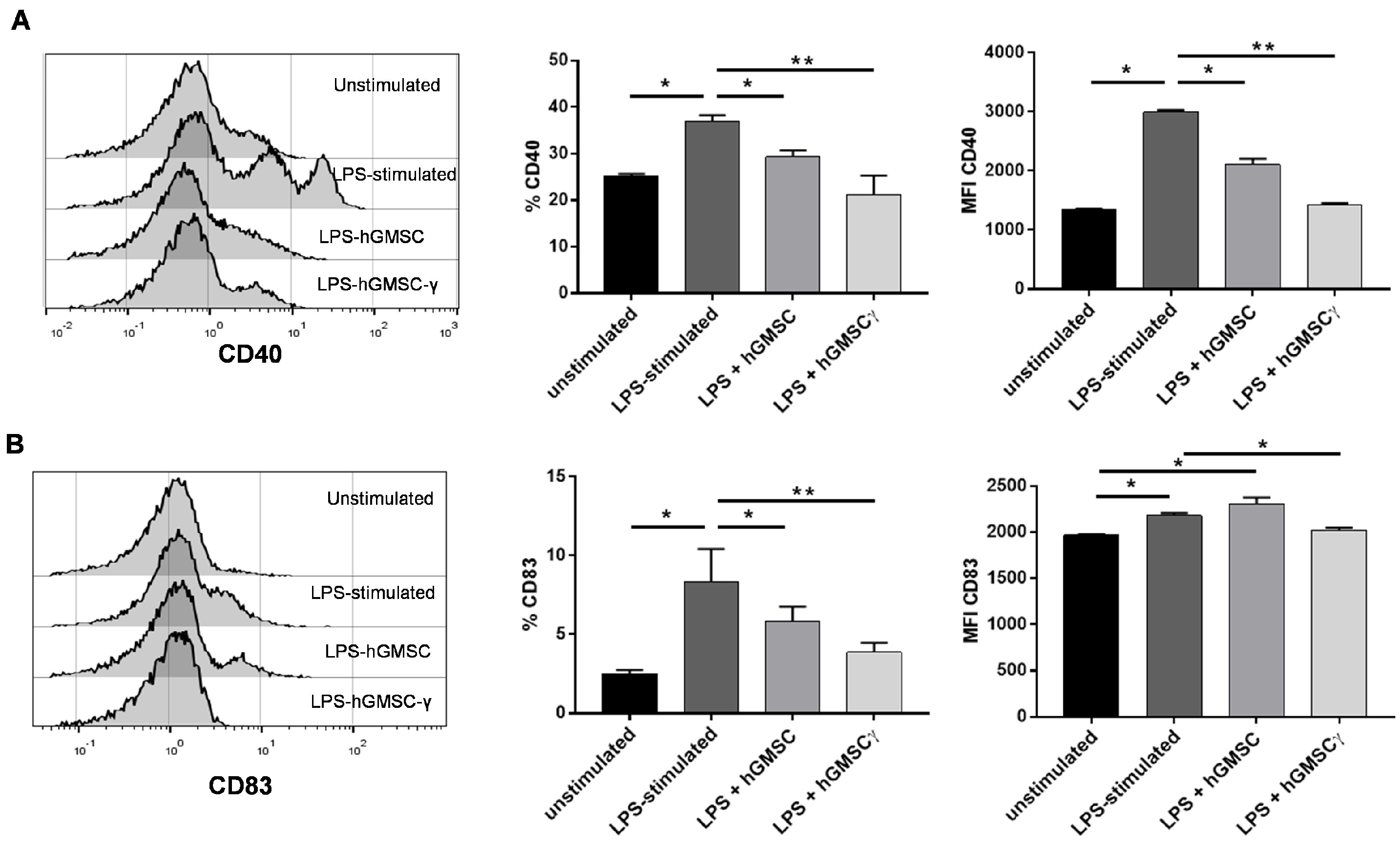
3.5. hGMSC-γ Inhibit pDC Activation and IFN-α Secretion via Adenosine
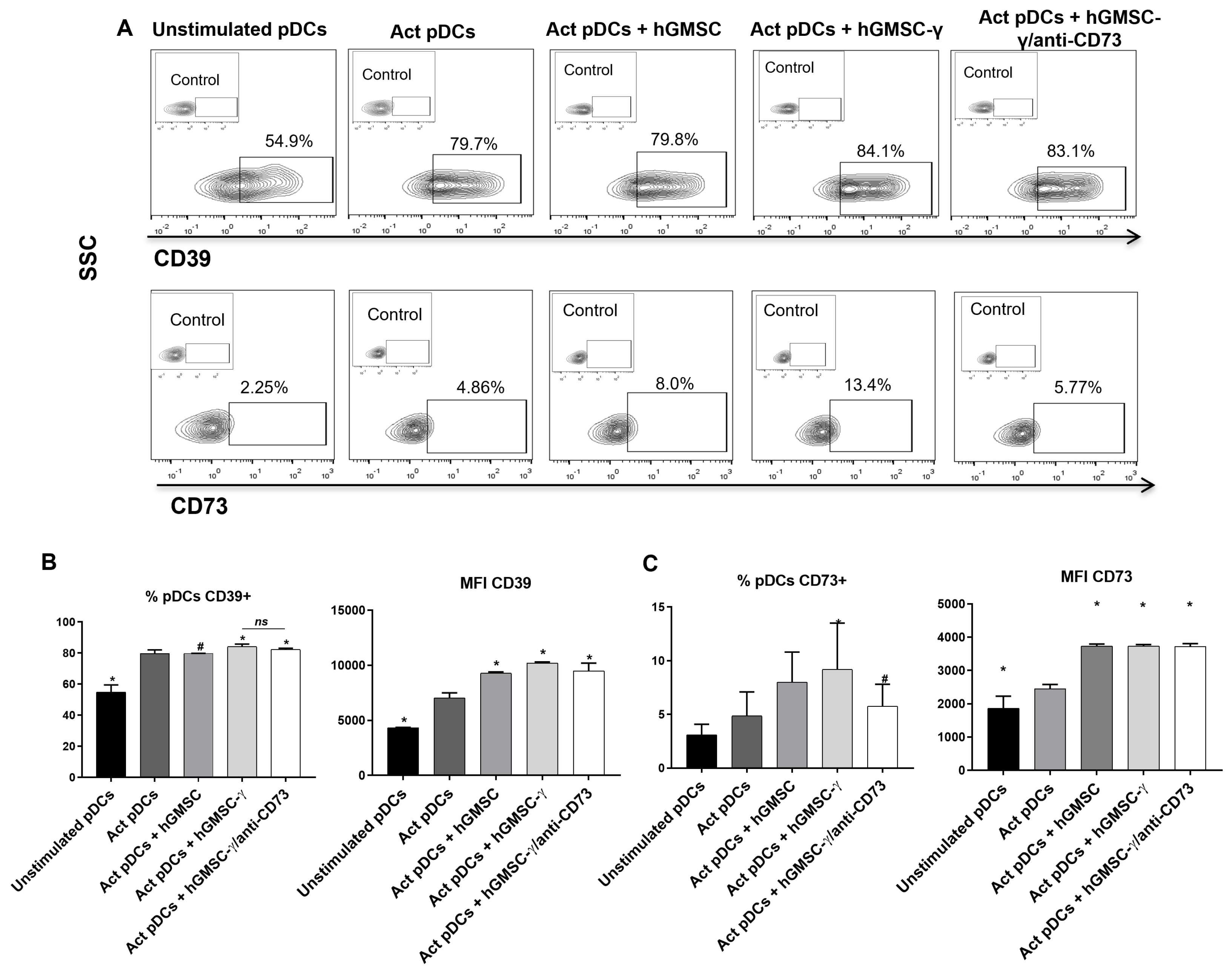
3.6. hGMSC-γ Increase the Frequency of CD73+ pDC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Melki, R.; Khalife, F.; Lagneaux, L.; Bouhtit, F.; Agha, D.M.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Merimi, M. Therapeutic Mesenchymal Stem/Stromal Cells: Value, Challenges and Optimization. Front. Cell Dev. Biol. 2022, 9, 716853. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2021, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.; Schönbeck, K.; Hoff, H.; Tran, C.L.; Strehl, C.; Lang, A.; Ohrndorf, S.; Pfeiffenberger, M.; Röhner, E.; Matziolis, G.; et al. CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2018, 19, 2312. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Galgaro, B.C.; Beckenkamp, L.R.; van den MNunnenkamp, M.; Korb, V.G.; Naasani, L.I.S.; Roszek, K.; Wink, M.R. The adenosinergic pathway in mesenchymal stem cell fate and functions. Med. Res. Rev. 2021, 41, 2316–2349. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Pacher, P.; Haskó, G. Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett. 2019, 205, 9–15. [Google Scholar] [CrossRef]
- Challier, J.; Bruniquel, D.; Sewell, A.K.; Laugel, B. Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8+ T-cell priming capacity. Immunology 2013, 138, 402–410. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Extracellular Adenosine-Mediated Modulation of Regulatory T Cells. Front. Immunol. 2014, 5, 304. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa-Ruiz, M.d.P.; Álvarez-Pérez, M.A.; Cortés-Morales, V.A.; Monroy-García, A.; Mayani, H.; Fragoso-González, G.; Caballero-Chacón, S.; Diaz, D.; Candanedo-González, F.; Montesinos, J.J. Mesenchymal Stem/Stromal Cells Derived from Dental Tissues: A Comparative In Vitro Evaluation of Their Immunoregulatory Properties Against T cells. Cells 2019, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Xia, Y.; Zhou, S.; Peng, H.; Wu, X.; Lu, H.; Wang, H.; Liu, R.; Blazar, B.R.; Gu, J.; et al. Reduction in murine acute GVHD severity by human gingival tissue-derived mesenchymal stem cells via the CD39 pathways. Cell Death Dis. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Noronha, N.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Saldanha-Araujo, F.; Ferreira, F.I.S.; Palma, P.V.; Araujo, A.G.; Queiroz, R.H.; Covas, D.T.; Zago, M.A.; Panepucci, R.A. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011, 7, 66–74. [Google Scholar] [CrossRef]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, W.C.W. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016, 2016, 3924858. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Gaugler, B.; Mohty, M.; Malard, F. Plasmacytoid dendritic cell biology and its role in immune-mediated diseases. Clin. Transl. Immunol. 2020, 9, e1139. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Nakayamada, S.; Miyazaki, Y.; Kubo, S.; Ishii, A.; Nakano, K.; Tanaka, Y. Up-Regulation of TLR7-Mediated IFN-α Production by Plasmacytoid Dendritic Cells in Patients With Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 1957. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Yu, Q. Plasmacytoid dendritic cells promote the pathogenesis of Sjögren’s syndrome. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166302. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, J.H.; Ludwig, N.; Braganhol, E.; Whiteside, T.L. Inhibition of the Adenosinergic Pathway in Cancer Rejuvenates Innate and Adaptive Immunity. Int. J. Mol. Sci. 2019, 20, 5698. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Winzer, R.; Rissiek, A.; Ricklefs, I.; Meyer-Schwesinger, C.; Ricklefs, F.L.; Bauche, A.; Behrends, J.; Reimer, R.; Brenna, S.; et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat. Commun. 2021, 12, 5911. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, E.; Laitinen, A.; Räbinä, J.; Valkonen, S.; Takatalo, M.; Larjo, A.; Veijola, J.; Lampinen, M.; Siljander, P.; Lehenkari, P.; et al. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation with T cells. Stem Cells 2016, 34, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Gerner, M.C.; Ziegler, L.S.; Schmidt, R.L.J.; Krenn, M.; Zimprich, F.; Uyanik-Ünal, K.; Konstantopoulou, V.; Derdak, S.; Del Favero, G.; Schwarzinger, I.; et al. The TGF-b/SOX4 axis and ROS-driven autophagy co-mediate CD39 expression in regulatory T-cells. FASEB J. 2020, 34, 8367–8384. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Bopp, T. Cyclic AMP Represents a Crucial Component of Treg Cell-Mediated Immune Regulation. Front. Immunol. 2016, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Gomez, N.; Medina-Ruiz, L.; Graham, G.J.; Campbell, J.D.M. IL-6 and TGF-β-Secreting Adoptively-Transferred Murine Mesenchymal Stromal Cells Accelerate Healing of Psoriasis-like Skin Inflammation and Upregulate IL-17A and TGF-β. Int. J. Mol. Sci. 2023, 24, 10132. [Google Scholar] [CrossRef] [PubMed]
- Mandapathil, M.; Lang, S.; Gorelik, E.; Whiteside, T.L. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J. Immunol. Methods 2009, 346, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Sánchez, N.; Cruz-Chamorro, I.; Díaz-Sánchez, M.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Peripheral CD39-expressing T regulatory cells are increased and associated with relapsing-remitting multiple sclerosis in relapsing patients. Sci. Rep. 2019, 9, 2302. [Google Scholar] [CrossRef]
- Kurte, M.; Vega-Letter, A.M.; Luz-Crawford, P.; Djouad, F.; Noël, D.; Khoury, M.; Carrión, F. Time-dependent LPS exposure commands MSC immunoplasticity through TLR4 activation leading to opposite therapeutic outcome in EAE. Stem Cell Res. Ther. 2020, 11, 416. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; François, M.; Boivin, M.-N.; Bouchentouf, M.; Spaner, D.E.; Galipeau, J. Cytokine Modulation of TLR Expression and Activation in Mesenchymal Stromal Cells Leads to a Proinflammatory Phenotype. J. Immunol. 2009, 182, 7963–7973. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, Z.; Zhang, R.; Yan, K.; Chen, L.; Chen, F.; Huang, W.; Lv, B.; Sun, C.; Jiang, X. MSCs inhibit bone marrow-derived DC maturation and function through the release of TSG-6. Biochem. Biophys. Res. Commun. 2014, 450, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ge, W.; Li, C.; You, S.; Liao, L.; Han, Q.; Deng, W.; Zhao, R.C. Effects of Mesenchymal Stem Cells on Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells. Stem Cells Dev. 2004, 13, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liu, Y.-J. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell 2013, 4, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Peng, J.; Xie, Q.; Xiao, N.; Su, X.; Mei, H.; Lu, Y.; Zhou, J.; Dai, Y.; Wang, S.; et al. Mesenchymal Stem Cells Alleviate Moderate-to-Severe Psoriasis by Reducing the Production of Type I Interferon (IFN-I) by Plasmacytoid Dendritic Cells (pDCs). Stem Cells Int. 2019, 2019, 6961052. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Luis, S.A.; Ríos-Ríos, W.J.; Almaraz-Arreortua, A.; Romero-Tlalolini, M.A.; Aguilar-Ruiz, S.R.; Valle-Ríos, R.; Sánchez-Torres, C.; Torres-Aguilar, H. Human plasmacytoid dendritic cells express the functional purinergic halo (CD39/CD73). Purinergic Signal 2024, 20, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shao, H.; Zhi, Y.; Xiao, Q.; Su, C.; Dong, L.-J.; Liu, X.; Li, X.; Zhang, X. CD73 Pathway Contributes to the Immunosuppressive Ability of Mesenchymal Stem Cells in Intraocular Autoimmune Responses. Stem Cells Dev. 2016, 25, 337–346. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Dang, J.; Zhang, X.; Wang, J.; Chen, Y.; Liang, J.; Li, D.; Ma, J.; Yuan, J.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Ameliorate Streptozoticin-induced T1DM in mice via Suppression of T effector cells and Up-regulating Treg Subsets. Sci. Rep. 2017, 7, 15249. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Ríos, W.d.J.; Sosa-Luis, S.A.; Almaraz-Arreortua, A.; Vargas-Benitez, P.; Bernardino-Hernández, H.U.; Vargas-Arzola, J.; Hernández-Osorio, L.A.; Romero-Tlalolini, M.d.l.Á.; Aguilar-Ruiz, S.R.; Torres-Aguilar, H. IFN-γ-Preconditioned Human Gingival-Derived Mesenchymal Stromal Cells Inhibit Plasmacytoid Dendritic Cells via Adenosine. Biomolecules 2024, 14, 658. https://doi.org/10.3390/biom14060658
Ríos-Ríos WdJ, Sosa-Luis SA, Almaraz-Arreortua A, Vargas-Benitez P, Bernardino-Hernández HU, Vargas-Arzola J, Hernández-Osorio LA, Romero-Tlalolini MdlÁ, Aguilar-Ruiz SR, Torres-Aguilar H. IFN-γ-Preconditioned Human Gingival-Derived Mesenchymal Stromal Cells Inhibit Plasmacytoid Dendritic Cells via Adenosine. Biomolecules. 2024; 14(6):658. https://doi.org/10.3390/biom14060658
Chicago/Turabian StyleRíos-Ríos, William de Jesús, Sorely Adelina Sosa-Luis, Alexia Almaraz-Arreortua, Patricia Vargas-Benitez, Héctor Ulises Bernardino-Hernández, Jaime Vargas-Arzola, Luis Alberto Hernández-Osorio, María de los Ángeles Romero-Tlalolini, Sergio Roberto Aguilar-Ruiz, and Honorio Torres-Aguilar. 2024. "IFN-γ-Preconditioned Human Gingival-Derived Mesenchymal Stromal Cells Inhibit Plasmacytoid Dendritic Cells via Adenosine" Biomolecules 14, no. 6: 658. https://doi.org/10.3390/biom14060658






