Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Nippostrongylus Brasiliensis and Excretory–Secretory Products (ES)
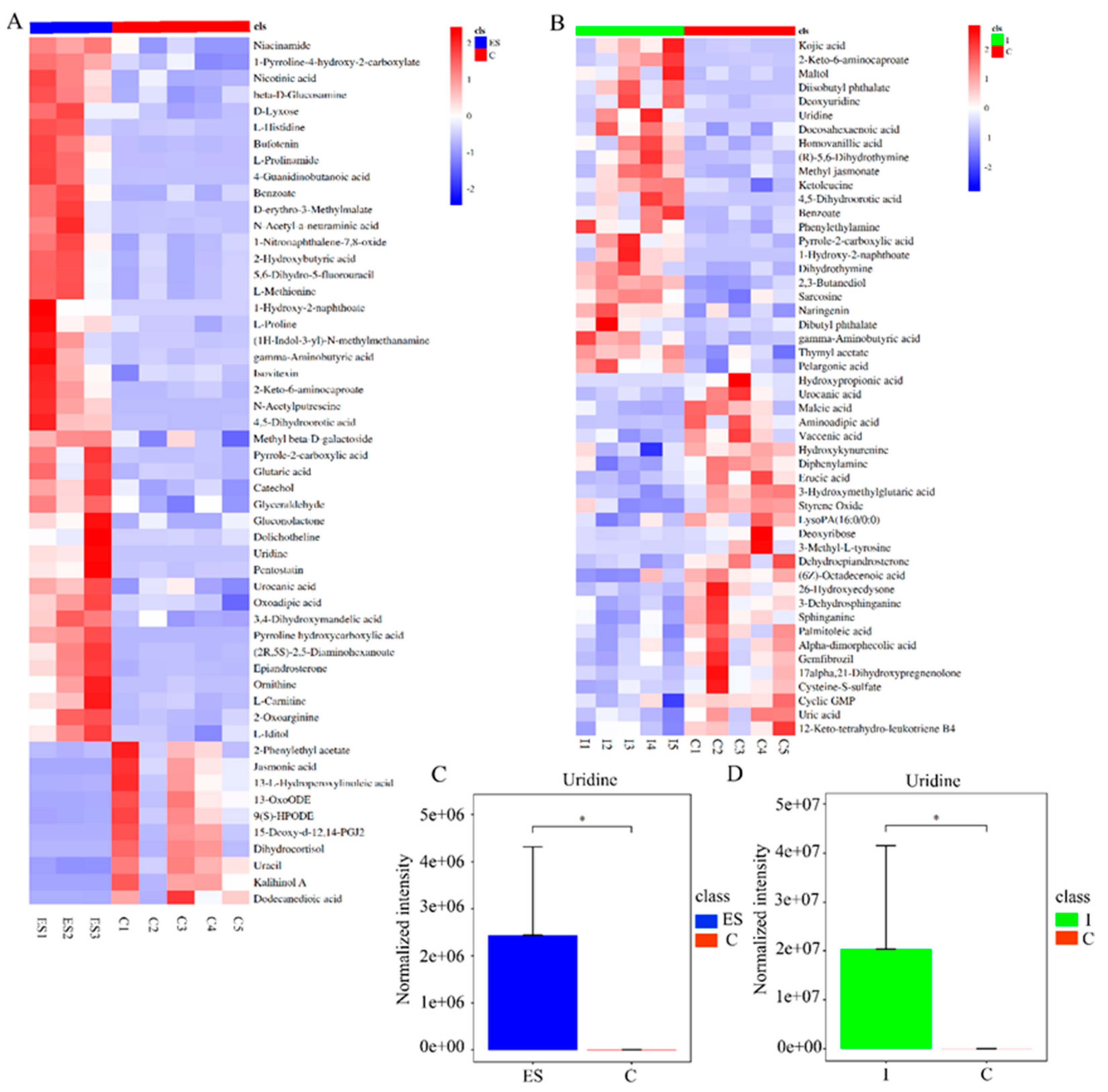
2.3. Metabolomics Analysis
2.4. Anti-Inflammatory Responses Detection In Vitro and In Vivo
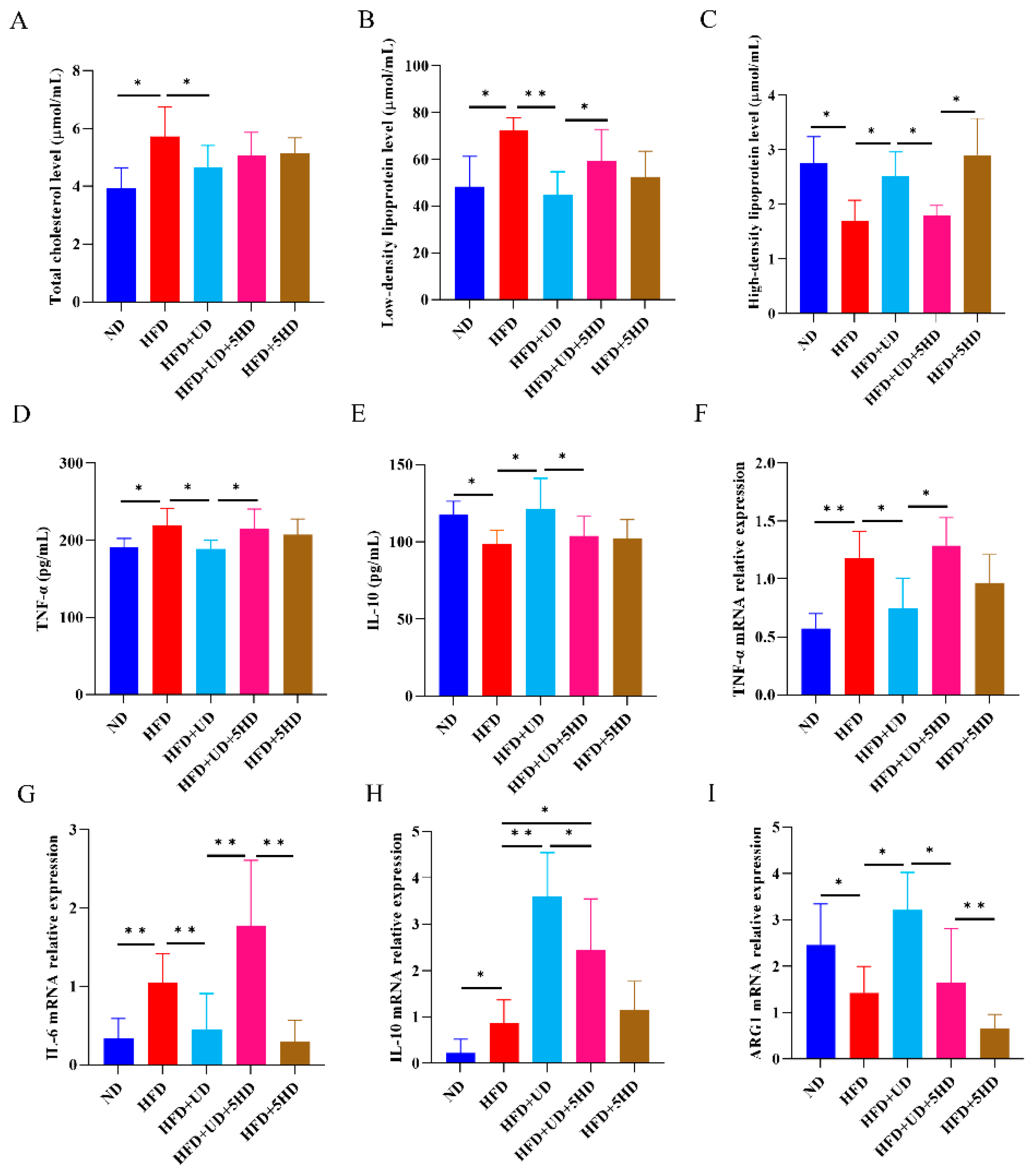
2.5. Anti-Atherosclerosis Effects Evaluation in ApoE−/− Mouse Model
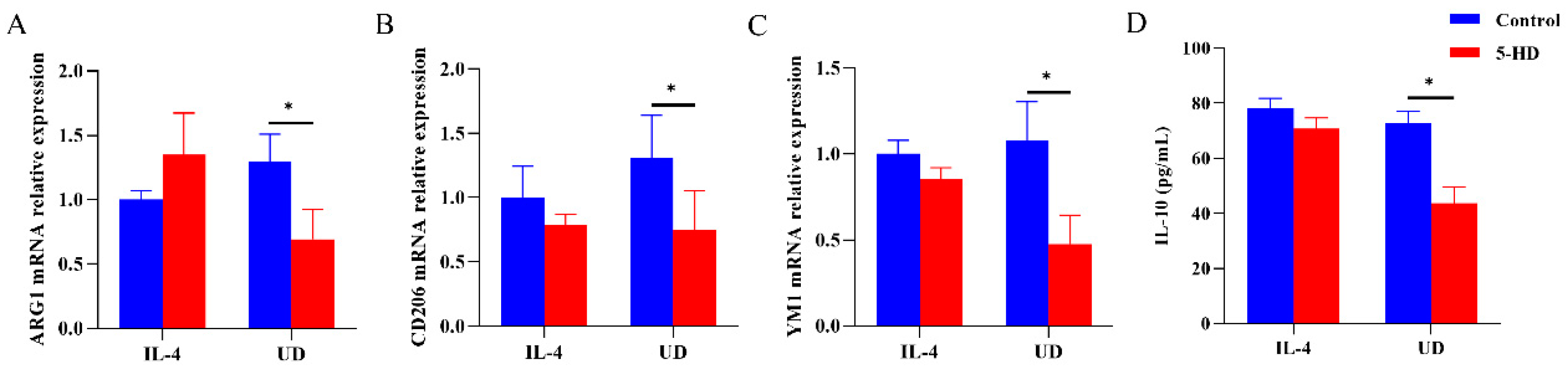
2.6. Mitochondrial ATP-Sensitive Potassium Channel Blockage In Vitro and In Vivo
2.7. Statistically Analysis
3. Results
3.1. Uridine, a Key Metabolite Derived from Nippostrongylus brasiliensis through Metabolomics Analysis
3.2. Uridine Showed Anti-Inflammatory Effects In Vivo and Could Induce an M2 Macrophage Polarization Phenotype In Vitro
3.3. Uridine Showed Anti-Atherosclerosis Effects in ApoE−/− Mouse Model through Inducing Anti-Inflammatory Responses
3.4. Anti-Inflammatory Responses and Anti-Atherosclerosis Effects Generated by Uridine Were Partially Dependent on Mitochondrial ATP-Sensitive Potassium Channel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, M.I.; Cheong, C.; Robbins, C.S. Macrophages and Dendritic Cells: Partners in Atherogenesis. Circ. Res. 2016, 118, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Sorci-Thomas, M.G.; Thomas, M.J. Microdomains, Inflammation, and Atherosclerosis. Circ. Res. 2016, 118, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Velasco, M.; González-Ramos, S.; Boscá, L. Involvement of monocytes/macrophages as key factors in the development and progression of cardiovascular diseases. Biochem. J. 2014, 458, 187–193. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, F.; Staels, B.; Chinetti-Gbaguidi, G. Macrophage phenotypes and their modulation in atherosclerosis. Circ. J. 2014, 78, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, M.; Matricardi, P.M. Parasites and the hygiene hypothesis: Regulating the immune system? Clin. Rev. Allergy Immunol. 2004, 26, 15–24. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Bach, J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Stanley, R.G.; Jackson, C.L.; Griffiths, K.; Doenhoff, M.J. Effects of Schistosoma mansoni worms and eggs on circulating cholesterol and liver lipids in mice. Atherosclerosis 2009, 207, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Magen, E.; Bychkov, V.; Ginovker, A.; Kashuba, E. Chronic Opisthorchis felineus infection attenuates atherosclerosis--an autopsy study. Int. J. Parasitol. 2013, 43, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Gurven, M.D.; Trumble, B.C.; Stieglitz, J.; Blackwell, A.D.; Michalik, D.E.; Finch, C.E.; Kaplan, H.S. Cardiovascular disease and type 2 diabetes in evolutionary perspective: A critical role for helminths? Evol. Med. Public Health 2016, 2016, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Camberis, M.; Le Gros, G.; Urban, J., Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. 2003, 55, 12–19. [Google Scholar] [CrossRef]
- McSorley, H.J.; Maizels, R.M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef]
- Scrivener, S.; Yemaneberhan, H.; Zebenigus, M.; Tilahun, D.; Girma, S.; Ali, S.; McElroy, P.; Custovic, A.; Woodcock, A.; Pritchard, D.; et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: A nested case-control study. Lancet 2001, 358, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Loukas, A. The immunology of human hookworm infections. Parasite Immunol. 2010, 32, 549–559. [Google Scholar] [CrossRef]
- Wu, X.M.; Zhang, Q.; Ding, X.; Mao, F.-Z.; Wang, X.-T.; Dai, Y.; Wang, J.-H.; Cao, J. Polarization of human acute monocytic leukemia THP-1 cells-derived macprophages induced by Nippostrongylus brasiliensis proteins in vitro. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2020, 32, 367–373. [Google Scholar]
- Yang, Y.; Ding, X.; Chen, F.; Wu, X.; Chen, Y.; Zhang, Q.; Cao, J.; Wang, J.; Dai, Y. Inhibition Effects of Nippostrongylus brasiliensis and Its Derivatives against Atherosclerosis in ApoE−/− Mice through Anti-Inflammatory Response. Pathogens 2022, 11, 1208. [Google Scholar] [CrossRef]
- Cobos, C.; Bansal, P.S.; Wilson, D.T.; Jones, L.; Zhao, G.; Field, M.A.; Eichenberger, R.M.; Pickering, D.A.; Ryan, R.Y.; Ratnatunga, C.N.; et al. Peptides derived from hookworm anti-inflammatory proteins suppress inducible colitis in mice and inflammatory cytokine production by human cells. Front. Med. 2022, 9, 934852. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Kouremenos, K.; Eichenberger, R.M.; Pearson, M.; Susianto, A.; Wishart, D.S.; McConville, M.J.; Loukas, A. Metabolomic profiling of the excretory-secretory products of hookworm and whipworm. Metabolomics 2019, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Kahl, J.; Brattig, N.; Liebau, E. The Untapped Pharmacopeic Potential of Helminths. Trends Parasitol. 2018, 34, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Ding, X.; Yang, Y.; Chen, Y.; Zhang, Q.; Fan, Y.; Dai, Y.; Wang, J. Mining Anti-Inflammation Molecules from Nippostrongylus brasiliensis-Derived Products Through the Metabolomics Approach. Front. Cell Infect. Microbiol. 2021, 11, 781132. [Google Scholar] [CrossRef] [PubMed]
- Assouvie, A.; Daley-Bauer, L.P.; Rousselet, G. Growing Murine Bone Marrow-Derived Macrophages. Methods Mol. Biol. 2018, 1784, 29–33. [Google Scholar] [PubMed]
- Mironova, G.D.; Khrenov, M.O.; Talanov, E.Y.; Glushkova, O.V.; Parfenyuk, S.B.; Novoselova, T.V.; Lunin, S.M.; Belosludtseva, N.V.; Novoselova, E.G.; Lemasters, J.J. The role of mitochondrial KATP channel in anti-inflammatory effects of uridine in endotoxemic mice. Arch. Biochem. Biophys. 2018, 654, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xuan, Y.; Cui, J.; Liu, X.; Shao, Z.; Yu, B. Nicorandil modulated macrophages activation and polarization via NF-κb signaling pathway. Mol. Immunol. 2017, 88, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cicko, S.; Grimm, M.; Ayata, K.; Beckert, J.; Meyer, A.; Hossfeld, M.; Zissel, G.; Idzko, M.; Müller, T. Uridine supplementation exerts anti-inflammatory and anti-fibrotic effects in an animal model of pulmonary fibrosis. Respir. Res. 2015, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Park, T.J.; Ikram, M.; Ahmad, S.; Ahmad, R.; Jo, M.G.; Kim, M.O. Antioxidative and Anti-inflammatory Effects of Kojic Acid in Aβ-Induced Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 5127–5140. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Kargutkar, S.; Brijesh, S. Anti-inflammatory evaluation and characterization of leaf extract of Ananas comosus. Inflammopharmacology 2018, 26, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Krylova, I.B.; Kachaeva, E.V.; Rodionova, O.M.; Negoda, A.E.; Evdokimova, N.R.; Balina, M.I.; Sapronov, N.S.; Mironova, G.D. The cardioprotective effect of uridine and uridine-5’-monophosphate: The role of the mitochondrial ATP-dependent potassium channel. Exp. Gerontol. 2006, 41, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zuo, X.; Wang, Q.; Yu, Y.; Xie, L.; Wang, H.; Wu, H.; Xie, W. Nicorandil inhibits hypoxia-induced apoptosis in human pulmonary artery endothelial cells through activation of mitoKATP and regulation of eNOS and the NF-κB pathway. Int. J. Mol. Med. 2013, 32, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, F.; Khanizadeh, A.M.; Golab, F.; Baluchnejadmojarad, T.; Vazifehkhah, S.; Moeinsadat, A. Mitochondrial ATP-sensitive potassium channel, MitoKATP, ameliorates mitochondrial dynamic disturbance induced by temporal lobe epilepsy. J. Chem. Neuroanat. 2021, 113, 101808. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Family size, infection and atopy: The first decade of the “hygiene hypothesis”. Thorax 2000, 55 (Suppl. 1), S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Pickering, D.A.; Ferreira, I.B.; Jones, L.; Ryan, S.; Troy, S.; Leech, A.; Hotez, P.J.; Zhan, B.; Laha, T.; et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci. Transl. Med. 2016, 8, 362ra143. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Rapin, A.; Lebon, L.; Dubey, L.K.; Mosconi, I.; Sarter, K.; Piersigilli, A.; Menin, L.; Walker, A.W.; Rougemont, J.; et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity 2015, 43, 998–1010. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Xie, C.; Fang, J. Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis. Biomed. Res. Int. 2020, 2020, 7091718. [Google Scholar] [CrossRef]
- Evaldsson, C.; Rydén, I.; Uppugunduri, S. Anti-inflammatory effects of exogenous uridine in an animal model of lung inflammation. Int. Immunopharmacol. 2007, 7, 1025–1032. [Google Scholar] [CrossRef]
- Chenna Narendra, S.; Chalise, J.P.; Magnusson, M.; Uppugunduri, S. Local but Not Systemic Administration of Uridine Prevents Development of Antigen-Induced Arthritis. PLoS ONE 2015, 10, e0141863. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Cardilo-Reis, L.; Gruber, S.; Schreier, S.M.; Drechsler, M.; Papac-Milicevic, N.; Weber, C.; Wagner, O.; Stangl, H.; Soehnlein, O.; Binder, C.J. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 2012, 4, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Hanley, P.J.; Mickel, M.; Löffler, M.; Brandt, U.; Daut, J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J. Physiol. 2002, 542, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Paggio, A.; Checchetto, V.; Campo, A.; Menabò, R.; Di Marco, G.; Di Lisa, F.; Szabo, I.; Rizzuto, R.; De Stefani, D. Identification of an ATP-sensitive potassium channel in mitochondria. Nature 2019, 572, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Prasai, K. Regulation of mitochondrial structure and function by protein import: A current review. Pathophysiology 2017, 24, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Mah, T.; Peishi, C.; Tan, S.Y.; Chawla, R.; Arumugam, T.V.; Ramasamy, A.; Mallilankaraman, K. Oxygen Glucose Deprivation Induced Prosurvival Autophagy Is Insufficient to Rescue Endothelial Function. Front. Physiol. 2020, 11, 533683. [Google Scholar] [CrossRef]
- Suarez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines 2021, 9, 258. [Google Scholar] [CrossRef]




| Primers | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| IL-1β | TGTCTTGGCCGAGGACTAAGG | TGGGCTGGACTGTTTCTAATGC |
| IL-6 | AGGGTCTGGGCCATAGAACT | CCACCACGCTCTTCTGTCTAC |
| TNF-α | CTGAACTTCGGGGTGATCGG | GGCTTGTCACTCGAATTTTGAGA |
| iNOS | CCTTCCGAAGTTTCTGGCAGCAGC | GGCTGTCAGAGCCTCGTGGCTTTGG |
| IL-10 | AGCCTTATCGGAAATGATCCAGT | GGCCTTGTAGACACCTTGGT |
| TGF-β | ATTCCTGGCGTTACCTTGG | AGCCCTGTATTCCGTCTCCT |
| ARG1 | GTGAAGAACCCACGGTCTGT | GCCAGAGATGCTTCCAACTG |
| YM1 | CAGGTCTGGCAATTCTTCTGAA | CTCTTGCTCATGTGTGTAAGTGA |
| CD206 | TCTTTGCCTTTCCCAGTCTCC | TGACACCCAGCGGAATTTC |
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTGGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ding, X.; Yuan, C.; Yang, Y.; Zhang, Q.; Yao, J.; Zhang, Y.; Wang, J.; Dai, Y. Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model. Biomolecules 2024, 14, 672. https://doi.org/10.3390/biom14060672
Zhang Y, Ding X, Yuan C, Yang Y, Zhang Q, Yao J, Zhang Y, Wang J, Dai Y. Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model. Biomolecules. 2024; 14(6):672. https://doi.org/10.3390/biom14060672
Chicago/Turabian StyleZhang, Yingshu, Xin Ding, Caiyi Yuan, Yougui Yang, Qiang Zhang, Jiakai Yao, Ying Zhang, Junhong Wang, and Yang Dai. 2024. "Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model" Biomolecules 14, no. 6: 672. https://doi.org/10.3390/biom14060672






